Over the last decade the field of immunotherapy has grown to be one of the most promising fields in oncology. Targeted therapies using monoclonal antibodies, kinase inhibitors or T cell therapies emerged to be an important part of cancer treatment regimens and their impact is growing (1). However, all successful clinical therapies and clinical trials focused on the treatment of pre-existing symptomatic cancers while strategies preventing cancer development or symptomatic outgrowth have remained the holy grail of cancer care. Ridker et al. recently reported that using interleukin-1β (IL-1β) blocking antibody for prevention of reoccurrence of cardiovascular events reduced lung cancer incidence and mortality (2). This was an additional finding of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) (3). The primary end point was the (re-)occurrence of defined cardiovascular events after non-fatal myocardial infarction. The study was powered and designed accordingly. By no means was cancer defined nor predicted as primary endpoint of the study.
CANTOS was a randomized, double-blinded, multi-centered trial, involving 10,061 patients from 39 countries. Patients were randomized into four study arms. This was the largest study ever conducted with an anti-inflammatory agent. There was a significant benefit on the primary endpoint, but the overall benefit for the patient was modest and came at the cost of substantial side effects. Surprisingly, a major finding of this trial was a reduction in cancer-associated mortality across the canakinumab-treated groups (50, 150 or 300 mg canakinumab, subcutaneous injection every 3 months vs. placebo), especially in lung cancer-associated mortality, and an overall decreased incidence of lung cancer.
Not surprisingly, lung cancer patients were significantly older, more likely to be current smokers and had elevated levels of high sensitivity CRP (hsCRP; 6.0 vs. 4.2 mg/L) and interleukin-6 (IL-6; 3.2 vs. 2.6 ng/L). These characteristics confirm the general notion that smokers have a higher risk of developing lung cancer based on chronic lung inflammation and might indicate that this inflammation can be suppressed or reduced by IL-1β blockade.
The importance of inflammation on the progression and development of cancer has been shown in a number of different settings, in both murine and human cancer (4,5). Chronic inflammatory diseases or states of smoldering inflammation often predispose for subsequent development of malignancies. Well-characterized examples for this phenomenon are inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis (6), or the development of NSCLC in smokers (5). A delicate and complex interplay between pro-inflammatory cytokines, chemokines and adhesion molecules seems to regulate angiogenesis, metastasis formation and distinct immune cell infiltration. This leads to a pro-neoplastic, pro-metastatic and immunosuppressive microenvironment (4). More specifically, interleukin-1 is involved in the development, progression and invasiveness of chemically induced murine fibrosarcomas (7) and the development of asbestosis-induced mesothelioma (8). Blockade of IL-1 has been shown to be able to slow tumor progression in mice and men (9,10).
Based on the short median observation and intervention time of 3.7 years compared to the estimated time required for lung cancer development, which happens across two to three decades (11), it is unlikely that IL-1β blockade prevented de novo cancer development in most cases. Canakinumab likely (I) slowed tumor progression, as suggested by the more beneficial outcome of lung cancer patients after canakinumab treatment; and (II) induced cancer clearance, as pointed by the reduced cancer incidence. However, it is uncertain as to how these effects would have been mediated.
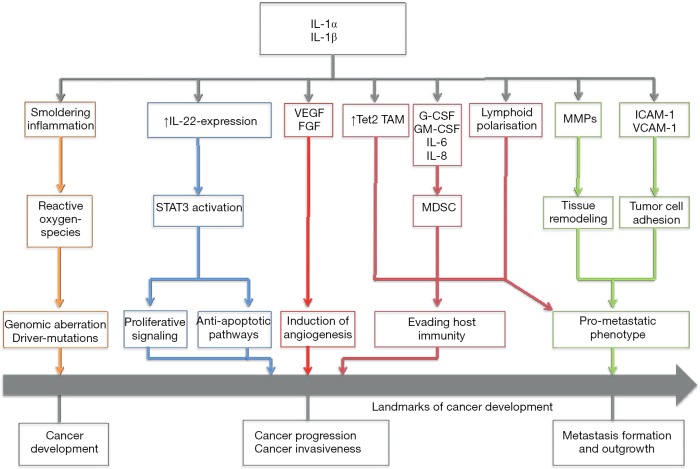
Figure 1 gives a short overview of different possible mechanisms that could mediate the anti-neoplastic effects of IL-1 blockade.
Figure 1.
Schematic overview of pro-neoplastic effects of IL-1. Tet2, Ten-Eleven-Translocation-2; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte macrophage stimulating factor; MMP, matrix metalloproteinases; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Apte et al. (12) hypothesized that IL-1-mediated induction of mutagenic reactive-oxygen-species (ROS) promotes the formation of pre-malignant lesions. Through autocrine or paracrine stimulation of tumor cells, but also stromal and immune cells, a cascade of mechanisms is set into action: IL-1 induces the expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). These pro-angiogenic molecules then lead to neo-formation of capillaries and provide a more sustainable microenvironment for the growing tumor. IL-1-deficient mice injected with a chemical carcinogen showed a remarkable change of immune cell infiltration (7): While wild-type mice exhibited dense immature leukocyte infiltration, IL-1 -/- mice showed mainly fibrotic tissue without these infiltrates. Immature myeloid cell types, such as tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC), have been shown to play a crucial role in tumor specific immune response. They have also been attributed with the capability to directly promote tumor growth and the formation of distant metastasis (13). IL-1 directly—but also IL-1 dependent secretion of pro-inflammatory cytokines and chemokines, such as interleukin-8—has been closely linked to the recruitment of MDSC. Moreover, recent studies have demonstrated the importance of IL-1 signaling for sustaining the immunosuppressive capacities of TAM (14). In the CANTOS trial, the blockade of IL-1 may have impaired the recruitment of these immature cells, thus dampening the tumor’s immune escape mechanisms and enabling the immune system to clear pre-malignant cells or small tumors more efficiently.
Pointing to another mechanism, we could recently show the involvement of IL-1-dependent up-regulation of interleukin-22 expression (IL-22) in murine and human breast and lung cancer models (9). IL-22 is, among other important functions, involved in tissue repair mechanisms and induces signal transducer and activator of transcription-3-dependent (STAT3) proliferative signaling cascades (15). Its influence on colon cancer development and outgrowth has been demonstrated (15). We were able to validate the presence of IL-22 in lavages of lung cancer patients and other pulmonary diseases (16). Along these lines, murine breast cancer cells showed a reduction in tumor growth accompanied by a decreased IL-22 expression after administration of IL-1 blocking agents (9), illustrating the close connection between these two cytokines, each representing a different cytokine family. Nevertheless, the role of IL-22 on the progression of lung cancer remains elusive and will need to be further investigated.
An important finding of the CANTOS trial is, that patients with a high serum hsCRP at 3 months, did not show a significant reduction in lung cancer incidence (2). Similar findings were reported for IL-6 concentrations. This stresses the need for effective inflammation reduction for canakinumab’s effect on cancer incidence. It also raises the important matter as to why canakinumab was not effective in some patients at reducing inflammation. Such “resistant” inflammation might be due to the pharmacological properties of canakinumab: there may be limited tissue penetration for effective decrease. On the other hand, inflammation might be dependent on other factors stepping in place of IL-1 to promote cancer progression. One possible explanation could be a more tumor necrosis factor alpha (TNF-α) driven inflammation. In a model of chemically induced carcinogenesis (17) increased levels of IL-1 in TNF-α -/- knockout mice, after administration of chemical carcinogens were demonstrated. The authors attributed the observed residual tumor promoting activity in TNF-α -/- knockout mice to these increased levels of IL-1α and IL-1β, demonstrating the possibility of different pro-inflammatory cytokines taking the lead after blockade of another. Next generation sequencing and protein expression analysis of the tumor tissue might help to unravel the underlying mechanisms involved. These methods could answer the question whether or not other pro-inflammatory cytokines were up-regulated or if specific driver mutations, possibly uncoupling inflammatory pathways, were present in these unresponsive patients.
Along these lines, the dose-dependency of canakinumab usage also suggests that an even stronger impact on tumor incidence and mortality might be possible with higher doses. However one needs to bear in mind the safety profile of canakinumab with side effects including severe infections.
Another therapeutic tool to inhibit IL-1 activity, the IL-1-receptor antagonist (IL-1Ra) anakinra, is approved for the treatment of rheumatoid arthritis. In oncologic indications it has been used to slow the progression of the precursor types monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM) and indolent multiple myeloma (IMM) into active multiple myeloma. (18). The combination therapy of dexamethasone plus anakinra was able to inhibit further disease progression more potently by addressing two different pathways. Anakinra was a stronger inhibitor of IL-6 production, while dexamethasone was able to induce apoptosis in tumor cells (19). In an end-stage disease setting, blocking the IL-1 axis in metastasized colorectal cancer, benefited patients as assessed by an increased lean body mass or improvements in the European Organization for Research and Treatment of Cancer Quality-of Life-Questionnaire Core 30 (EORTC QLQ-C30). However, blocking IL-1α only modestly ameliorated the primary end point [stable or increased lean body mass or improvement of two of three symptom measures of the EORTC-QLQ-C30 (20)].
To our knowledge, no trials, assessing the potential of IL-1 blockade in adjuvant or neoadjuvant treatment regimens in lung cancer, have been conducted or initiated. The difficulty will lay in identifying the correct setting and patient population for maximal benefit of IL-1-blockade. Should all patients with R0 resected lung cancer be treated with IL-1 blockade or only patients that are smokers or only patients with an inflammatory phenotype? Should driver mutations be selection criteria? These questions will need to be answered prospectively and, in parts, retrospectively to narrow the study population to a reasonable degree.
In symptomatic cancer patients combinatorial approaches of conventional chemotherapy plus IL-1 blockade have yet to be tested in randomized, double-blinded clinical trials. Preclinical data suggest that IL-1β, rather than IL-1α, or a full receptor blockade using IL-1Ra or IL-1-receptor antibodies might be promising targets for these approaches (7).
A particular opportunity might lay in the peculiar mechanisms of IL-1β production and secretion. The inflammasome-dependent, caspase-mediated cleavage of pro-IL-1β broadens the spectrum of possible pharmacological targets (21). It also enables the use of small molecule inhibitors of IL-1β synthesis. However these approaches come at the cost of a loss in specificity, as also the functional processing of IL-18 (and of other cytokines) depends on inflammasome activation. Shao et al. (21) give a good overview of possible new compounds and alternative options of IL-1 synthesis inhibition and IL-1 blockade. The coming years will have to show, whether the involvement of inflammasomes in cytokine maturation is a curse or a blessing, and whether these mechanisms can be exploited as means to prevent primary occurrence of malignancies or as adjuvant treatment options for clinical cancer treatment.
Future studies will also tell us, if the incidental findings of the CANTOS trial with respect to lung cancer will translate into a therapeutic strategy and possibly into a lung cancer prevention regimen for high-risk groups.
Acknowledgements
Funding: This study was supported by grants from the Wilhelm Sander Stiftung (grant number 2014.018.1 to SE and SK), the international doctoral program “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria (to SK and SE), the Melanoma Research Alliance (grant number N269626 to SE and 409510 to SK), the Marie-Sklodowska-Curie “Training Network for the Immunotherapy of Cancer (IMMUTRAIN)” funded by the H2020 program of the European Union (to SE, SK, CK and CS), the Else Kröner-Fresenius-Stiftung (to SK), the German Cancer Aid (to SK), the Ernst-Jung-Stiftung (to SK), the LMU Munich‘s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (to SE and SK), the Bundesministerium für Bildung und Forschung VIP+ grant ONKATTRACT (to SE and SK) and the European Research Council Starting Grant (grant number 756017 to SK).
Provenance: This is an invited Editorial commissioned by Section Editor Hengrui Liang (Nanshan Clinical Medicine School, Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Marin-Acevedo JA, Soyano AE, Dholaria B, et al. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol 2018;11:8. 10.1186/s13045-017-0552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833-42. 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119-31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211-7. 10.1016/j.ccr.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 5.O’Callaghan DS, O’Donnell D, O’Connell F, et al. The Role of Inflammation in the Pathogenesis of Non-small Cell Lung Cancer. J Thorac Oncol 2010;5:2024-36. 10.1097/JTO.0b013e3181f387e4 [DOI] [PubMed] [Google Scholar]
- 6.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990;323:1228-33. 10.1056/NEJM199011013231802 [DOI] [PubMed] [Google Scholar]
- 7.Krelin Y, Voronov E, Dotan S, et al. Interleukin-1β–Driven Inflammation Promotes the Development and Invasiveness of Chemical Carcinogen–Induced Tumors. Cancer Research 2007;67:1062. 10.1158/0008-5472.CAN-06-2956 [DOI] [PubMed] [Google Scholar]
- 8.Kadariya Y, Menges CW, Talarchek J, et al. Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev Res (Phila) 2016;9:406-14. 10.1158/1940-6207.CAPR-15-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voigt C, May P, Gottschlich A, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U S A 2017;114:12994-9. 10.1073/pnas.1705165114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holen I, Lefley DV, Francis SE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016;7:75571-84. 10.18632/oncotarget.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo E, Mao JT, Lam S, et al. Chemoprevention of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e40S-60S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 2006;25:387-408. 10.1007/s10555-006-9004-4 [DOI] [PubMed] [Google Scholar]
- 13.Ugel S, De Sanctis F, Mandruzzato S, et al. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest 2015;125:3365-76. 10.1172/JCI80006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev 2018;281:57-61. 10.1111/imr.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22–IL-22R1 system. Nat Rev Drug Discov 2014;13:21-38. 10.1038/nrd4176 [DOI] [PubMed] [Google Scholar]
- 16.Tufman A, Huber RM, Völk S, et al. Interleukin-22 is elevated in lavage from patients with lung cancer and other pulmonary diseases. BMC Cancer 2016;16:409. 10.1186/s12885-016-2471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suganuma M, Okabe S, Marino MW, et al. Essential Role of Tumor Necrosis Factor α (TNF-α) in Tumor Promotion as Revealed by TNF-α-deficient Mice. Cancer Res 1999;59:4516-8. [PubMed] [Google Scholar]
- 18.Lust JA, Lacy MQ, Zeldenrust SR, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol 2016;91:571-4. 10.1002/ajh.24352 [DOI] [PubMed] [Google Scholar]
- 19.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc 2009;84:114-22. 10.4065/84.2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2017;18:192-201. 10.1016/S1470-2045(17)30006-2 [DOI] [PubMed] [Google Scholar]
- 21.Shao BZ, Xu ZQ, Han BZ, et al. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 2015;6:262. 10.3389/fphar.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]