Abstract
Prophylactic drug treatment with aspirin, statins and anti-hypertensive agents has had a major impact on the incidence of cardiovascular disease and is now well established. Progress in therapeutic cancer prevention has been much slower; only recently have effective agents been clearly established. Breast cancer has led the way and endocrine agents used to treat it—notably tamoxifen and the aromatase inhibitors—have now been shown to have a substantial preventive effect as well. However, these agents carry some toxicity and thus identifying high-risk women who are likely to benefit most is a key priority. In contrast, the ability of low-dose aspirin to prevent about one-third of colorectal, gastric, and oesophageal cancers, combined with its much lower toxicity profile, make it attractive for a much larger proportion of the general population. Vaccination against the human papilloma virus is also a preventive intervention with large benefits for the whole population. Here I recall my involvement in these initiatives and offer a personal viewpoint on what has been achieved and what remains to be done.
Breast Cancer Prevention
Breast cancer has led the way in terms of developing preventive therapy for cancer. My interest began in 1979 when I first joined the Imperial Cancer Research Fund. I came to London from Oxford, to work on the Guernsey study of hormonal risk factors for breast cancer with Mick Bulbrook and John Hayward. Oestrogen had been linked, in some not fully understood way, as a major risk factor and the earliest observations go back over 100 years to Beatson,1 who reported that oophorectomy reduced recurrence rates in women with breast cancer. Increased oestrogen levels such as those associated with oral contraceptives (IARC, 1979),2 postmenopausal hormone therapy3,4 and postmenopausal obesity5 had been shown to increase breast cancer risk. However, little real progress was made in showing that reducing the oestrogen stimulus could reduce risk until Jordan6 reported that tamoxifen treatment led to lower breast cancer rates in 7,12-Dimethylbenz(a)anthracene (DMBA)-induced tumours in rats.
The first human evidence came in trials using tamoxifen for the treatment of breast cancer, where reductions in new contralateral tumours were first reported in 19857 (Fig. 1). This was followed by a range of confirmatory reports in other adjuvant trials, summarised by the Early Breast Cancer Trials Coordinating Group overview.8 A comprehensive justification for the use of tamoxifen in cancer prevention was provided in 19869 and, following a lively meeting of UK breast cancer specialists, Trevor Powles was the first to take up this proposal and he conducted a pilot study of tamoxifen in high-risk women at the Royal Marsden Hospital. Initially this was a small study of 200 women, to investigate toxicity and acceptability; however, concern about liver cancer in rats given high doses of tamoxifen and a general conservativeness about the concept of cancer prevention delayed major trials for another 6 years.
Fig. 1.
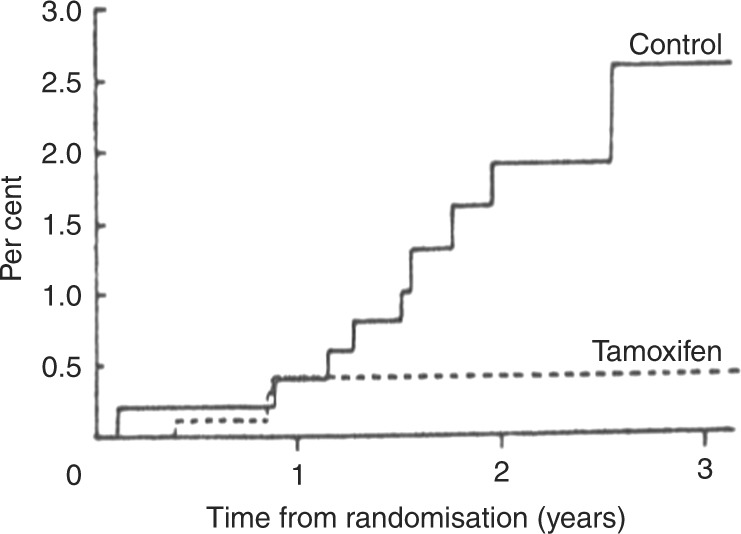
Initial data showing tamoxifen prevented contralateral tumours in an adjuvant trial of tamoxifen vs placebo (Reprinted with permission from ref.6)
Meanwhile, Powles’ study successively obtained permission to increase sample size and eventually it became a study of 2500 women—the largest ‘pilot study’ in history to my knowledge. Finally, in 1992, three large national and international studies began recruitment—our International Breast Cancer Intervention Study-1 (IBIS-I) study in the UK, Australia and New Zealand; a North American study—NSABP-P1, coordinated by the National Surgical Adjuvant Breast and Bowel Project; and a study in hysterectomised women in Italy (Table 1). The IBIS-I study was truly a trial that not even Kafka could have imagined, with scrutiny from dozens of review committees questioning the value of preventive therapy for breast cancer. Their concerns ranged from worry about liver cancer due to findings in rats (despite the fact that tamoxifen had been given to several million women for adjuvant treatment with no evidence of an increase), disbelief that a non-medically trained person could take a major role in developing such a trial, and a general reluctance to embrace the prospects of giving preventive medicine to apparently healthy women. Fortunately, I had a lot of support from a wide range of colleagues, which has been essential for this highly collaborative work. John Forbes from Australia and Tony Howell from Manchester have been with me from the beginning, and without them the work would not have been possible.
Table 1.
Breast cancer prevention trials using tamoxifen
| Trial (entry dates) | Population | Number randomised | Agents (vs placebo) and daily dose | Intended duration of treatment |
|---|---|---|---|---|
| Royal Marsden (1986–1996) | High-risk family history | 2471 | Tamoxifen 20 mg | 5–8 Years |
| NSABP-P1 (1992–1997) | High-risk women > 1.6% 5 year risk | 13,388 | Tamoxifen 20 mg | 5 Years |
| Italian (1992–1997) | Normal risk hysterectomy | 5408 | Tamoxifen 20 mg | 5 Years |
| IBIS-I (1992–2001) | > 2-fold relative risk | 7139 | Tamoxifen 20 mg | 5 Years |
| Adjuvant overview (1976–1995) | Women with ER + operable breast cancer in 11 trials | ~ 15,000 | Tamoxifen 20–40 mg with or without chemotherapy in both arms. | 3 Years or more (average ~ 5 years) |
IBIS-I, International Breast Cancer Intervention Study-1.
Adapted with permission from ref.51
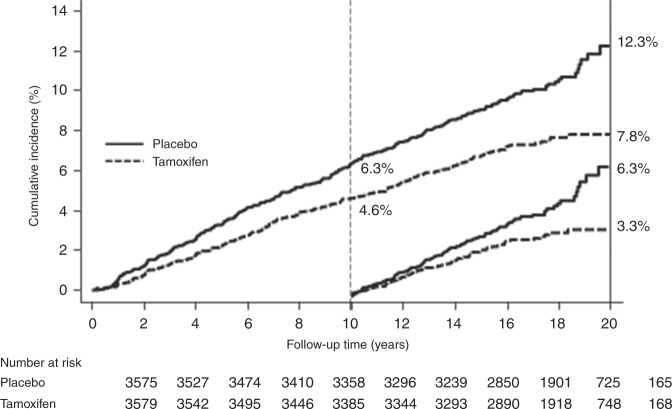
Although the IBIS-I study was the first to begin recruitment, the NSABP-P1 trial was better funded and recruited more rapidly. As a consequence, they were able to report an initial positive result first,9 but in their desire to declare success at an early stage, long-term follow-up was not carried out in this study. By 2003, all four trials had shown a clear reduction in breast cancer risk,10–14 but the most exciting results were to come from the long-term follow-up of two of these studies. Both the Marsden study15 and IBIS-I16 continued long-term follow-up and the most recent report of IBIS-I, with a 16 year median follow-up, demonstrated that the 30% reduction in all breast cancer obtained from tamoxifen continued unabated till the end of follow-up, i.e., after treatment cessation at year five, the 30% reduction in new breast cancers continued for at least another 10–15 years (Fig. 2). The reasons for this have not been established at a mechanistic level, but a likely explanation is that tamoxifen is reversing early changes in the carcinogenic process, which are known to take at least 20 years, so that—at least in some cases—the process must start again for new tumours to develop. Breast cancer mortality reductions have yet to be seen, but this reflects the fact that even longer follow-up is required to see this,17 because at that time there were 503 breast cancers but only 57 deaths from breast cancer in the IBIS-I trial. Thus, another 10 years of follow-up will probably be needed to demonstrate a reduction in breast cancer mortality.
Fig. 2.
Long-term effect of tamoxifen on breast cancer prevention in the IBIS-I trial (modified from Cuzick et al.15)
Subsequent to the tamoxifen prevention trials, clinical trials of three other selective oestrogen receptor modulators were conducted, primarily to look at the role of these agents in preventing fractures in women with osteoporosis. They all showed clear reductions in breast cancer and this subsequently became a second primary endpoint for the Continuing Outcomes Relevant to Evista (CORE) extension of the Multiple Outcomes of Raloxifene Evaluation (MORE) trial of raloxifene, in a subsequent follow-up period (Table 2). Only one trial has directly compared raloxifene with tamoxifen for cancer prevention.18,19 This was the Study of Tamoxifen and Raloxifene (STAR) trial, or NSABP-P2, in which almost 20,000 women were randomised between these two agents. Despite the indications that it might be more effective than tamoxifen, based on an indirect comparison from the MORE trial of raloxifene vs placebo with other trials of tamoxifen vs placebo, this direct comparison indicated that it was about 25% less effective, although the side-effect profile was more favourable. Both tamoxifen and raloxifene have now been approved for prevention by the Food and Drug Administration in the United States and recommended for prevention by National Institute for Health and Care Excellence (NICE) in the United Kingdom.
Table 2.
Other SERMs that have been evaluated for effect on reducing breast cancer incidence in randomised trials
| Trial (entry dates) | Population | Number randomised | Agents (vs placebo) and daily dose | Intended duration of treatment |
|---|---|---|---|---|
| MORE (1994–1999) | Normal risk, post-menopausal women with osteoporosis | 7705 | Raloxifene 60 or 120 mg (3 arm trial) | 4 Years |
| CORE (2000–2004) | Normal risk, post-menopausal women with osteoporosis | 4011 | Raloxifene 60 mg | Additional 4 years after 4 years in MORE |
| RUTH (1998–2000) | Post menopausal women ≥ 55 years with CHD or risk factors | 10,101 | Raloxifene 60 mg | 5 Years |
| STAR (2001 –2005) | High-risk post-menopausal women > 1.6% 5-year risk | 19,747 | Raloxifene 60 mg vs Tamoxifen (20 mg) | 5 Years |
| PEARL (2001–2007) | Normal risk, post-menopausal women with osteoporosis | 8556 | Lasofoxifene 0.25 mg or 0.5 mg (3 arm) | 5 Years |
| GENERATIONS (2005 – 2009) | Normal risk, post-menopausal women with osteoporosis | 9354 | Arzoxifene 20 mg | 5 Years |
CORE Continuing Outcomes Relevant to Evista, MORE Multiple Outcomes of Raloxifene Evaluation, SERM selective oestrogen receptor modulator, STAR Study of Tamoxifen and Raloxifene.
In the STAR trial, the comparator was tamoxifen and breast cancer incidence was the primary endpoint. In other listed trials, fracture prevention was the primary endpoint and the comparator was placebo. Adapted with permission from ref.51
During this period, the Anastrozole Tamoxifen Alone or in Combination (ATAC) trial and other subsequent trials showed that aromatase inhibitors were more effective at reducing recurrence from breast cancer in the adjuvant setting than tamoxifen. I had the good fortune to be the statistician for the ATAC trial and I was able to monitor the data on contralateral tumours as they developed. It was with great excitement that I was able to show this data to the steering committee at time of the first planned efficacy analysis of the trial, which indicated that anastrozole was not only more effective than tamoxifen in preventing recurrence of breast cancer but was also more effective at preventing new disease in the contralateral breast. Other trials comparing tamoxifen with aromatase inhibitors subsequently also demonstrated this superiority20 (Fig. 3).
Fig. 3.
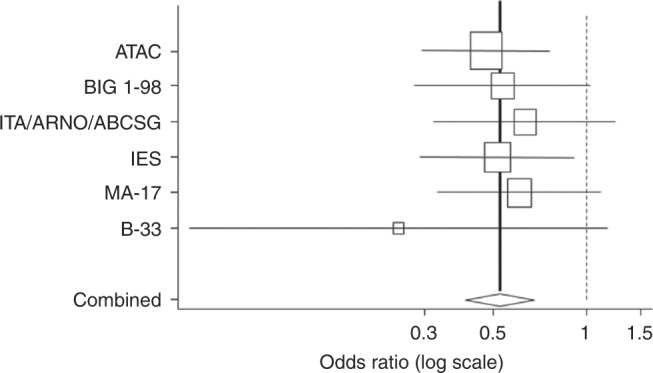
Reduced risk of contralateral breast cancer in trials comparing an aromatase inhibitor to tamoxifen. (Reprinted with permission from ref.20)
Thus, in 2003 we began the IBIS-II trial of anastrozole versus placebo in high-risk women. A major concern when planning this trial was whether the comparator should be placebo or tamoxifen and we had several long arguments about this. In the end, it was accepted that although tamoxifen clearly showed a reduction in cancer incidence, it was not widely used because of a lack of evidence for an overall long-term clinical benefit, and a full evaluation of side effects such as endometrial cancer, increased venous thromboses and a range of menopausal symptoms associated with oestrogen suppression was needed before this could be considered a “standard of care”. It was ultimately decided that the best design was to compare anastrozole to placebo. After a 5-year median follow-up, this trial showed a 53% reduction in all breast cancer,21 which was larger than the ~ 30% seen with tamoxifen in the four tamoxifen prevention trials. Another trial using the aromatase inhibitor exemestane also showed a strong reduction of 53% for all cancers and a 65% reduction for invasive cancers compared with placebo.22 Overall, the aromatase inhibitors are more active than tamoxifen, both for preventing disease recurrence and reducing the development of new cancers. An ongoing challenge, however, is to determine which individuals are more likely to respond to an aromatase inhibitor or tamoxifen, and today we still have few markers to guide that decision. The only current possibility is reduction in breast density at 6–12 months, which has been clearly established to be a predictor of response to tamoxifen24–26 and more recently appears to also be useful for aromatase inhibitors.27,28
One of the spin-offs from the prevention trials was the need to develop a model, which would predict the risk of breast cancer with sufficient accuracy to identify women at sufficient risk to be offered preventive tamoxifen or anastrozole. The model developed is known either as the IBIS model or the Tyrer–Cuzick model,29 reflecting my work with Jonathan Tyrer. Mathematically, it has several interesting features: in particular, it uses a segregation model to deal with family history of breast cancer, combined with a proportional hazards model to deal with the other known risk factors such as age, weight, reproductive history, hormone replacement therapy and prior benign breast disease. Subsequent versions of this model have been released and we are now using version 8, which was developed with Adam Brentnall. This includes a measurement of mammographic breast density and a single nucleotide polymorphism (SNP) score to look for low penetrance but common germline genetic differences.30
The model has now been widely validated and used to determine the need for preventive therapy or magnetic resonance imaging screening. More recently, it has emerged as a tool to guide the individual choice of screening intervals in so-called “risk adapted” screening algorithms. This may prove an important concept and would effectively expand screening programs to include a prevention component, with the idea being to conduct a risk assessment in all consenting women at their first screen. This would include a questionnaire to record “classical” factors as listed above, an assessment of density on their initial mammogram and a genetic SNP score based on a saliva sample. Several studies have now shown that these three factors are largely independent and contribute almost equally to the assessment of breast cancer risk31–33 (and several papers in preparation).
The biggest challenge now in therapeutic breast cancer prevention is not so much to identify high-risk women or specific effective agents, but to widely communicate this information in a way that is understandable to general practitioners and the general public.34 Uptake of preventive therapy has been very low35 and if it is to be an effective component of a comprehensive breast cancer prevention programme, e.g., as statins have been for the prevention of cardiovascular disease, we need to convince the medical profession and the public of the value of this approach.
Use of low-dose aspirin for cancer prevention
Another area in therapeutic cancer prevention which has excited me greatly in recent years is the use of low-dose aspirin. A study more than 30 years ago by Waddell and Loughry36 showed that sulindac had a preventive effect on polyps in individuals with polyposis coli. Over the years, additional studies have shown the same is true for aspirin (Table 3). A review of the available studies in 200937 concluded that more evidence was needed to make a recommendation for widespread use, and further follow-up of ongoing trials was the most useful activity. Shortly after that study was published, further follow-up, led largely by Rothwell and colleagues38 in Oxford, began to emerge and provided strong evidence for a preventive effect on a range of cancers.
Table 3.
Aspirin trials with colorectal adenoma as the primary endpoint
| Study | Arm | Treatment duration (years) | Follow-up (years) | N (participants) | N(cases) | Relative risk (95% CI) |
|---|---|---|---|---|---|---|
| Baron et al.52 | 85 mg | 3 | 3 | 377 | 140 | 0.81 (0.69–0.96) |
| 325 mg | 366 | 160 | 0.96 (0.81–1.19) | |||
| Placebo | 372 | |||||
| Sandler et al.53 | 325 mg | 1 | 2.5 | 259 | 43 | 0.65 (0.46–0.91) |
| Placebo | 258 | 60 | ||||
| APACC trial54 | 160/300 mg | 4 | 5 | 126 | 38 | 0.73 (0.52–1.04) |
| Placebo | 112 | 46 |
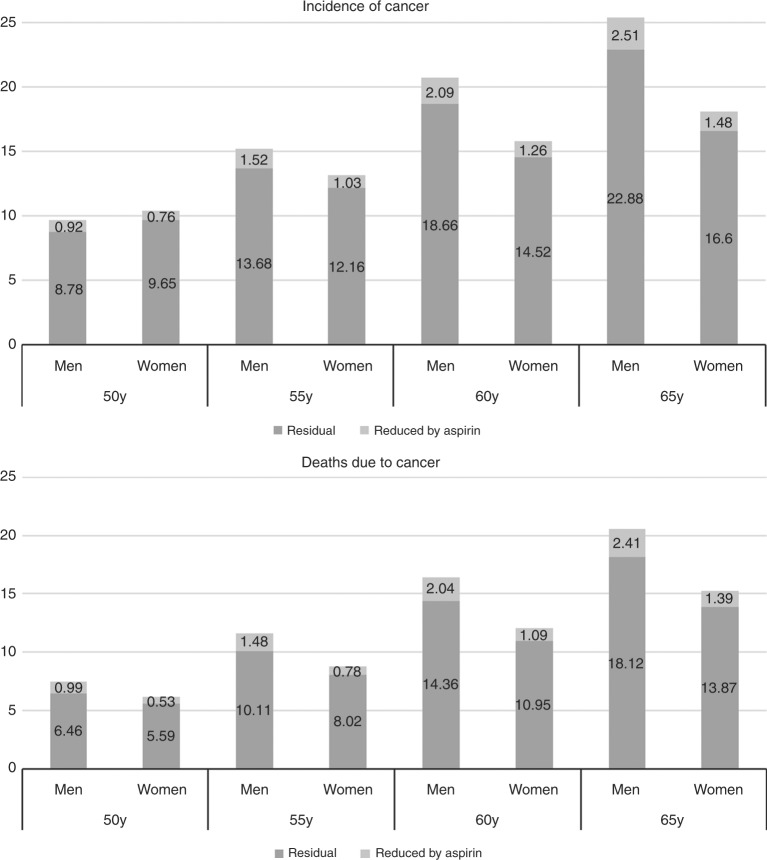
It is now apparent that aspirin has a large preventive effect on both the incidence and mortality from colorectal cancer, as well as gastric and oesophageal cancers.40–42 Small benefits are seen for lung, breast and prostate cancer in the order of 10%, but no other cancers appear to have been prevented by the use of aspirin (Table 4). Overall, a reduction of around 10% is seen for all cancers combined if aspirin is taken for 10 years between ages 50 and 60 years, with a slightly larger impact on mortality (Fig. 4). These figures put aspirin second on a population basis, only below avoidance of tobacco as a cancer prevention approach, although this should not override the need for maintaining a good level of physical activity and avoiding obesity, as they are complementary and all have other major health benefits. Recommendations are now being made to offer aspirin for individuals at high risk of colorectal cancer,43 but a risk-benefit analysis from a group of experts strongly suggests that aspirin is suitable for a large proportion of the population,39 and that routine aspirin prophylaxis for at least 5 years should be offered to those between the ages of 50 and 70 years, if they have no contraindications based on risks of gastrointestinal (GI) bleeding or haemorrhagic stroke.39
Table 4.
Benefits and harms of aspirin use that have been synthesised from more than 50 randomised trials and 100 epidemiologic cohort and case control studies
| Event | Incidence | Mortality |
|---|---|---|
| Colorectal cancer | 0.65 | 0.60 |
| Oesophageal cancer | 0.70 | 0.50 |
| Gastric cancer | 0.70 | 0.65 |
| Lung cancer | 0.95 | 0.85 |
| Prostate cancer | 0.90 | 0.85 |
| Breast cancer | 0.90 | 0.95 |
| Myocardial infarction | 0.82 | 0.95 |
| Stroke | 0.95 | 1.21 |
| Major bleeding | 1.54 | – |
| GI bleeding | – | 1.60 |
| Peptic ulcer | – | 1.60 |
Fig. 4.
Estimated impact of use of aspirin for 10 years on the incidence and mortality of all causes of cancer (% of total population) by sex and age at starting. Estimates for incidence are for 15 years after starting aspirin and are 20 years after starting for mortality. Effect size is 7–10% reduction for incidence and 9–13% for mortality, depending on sex and age at starting. (Modified with permission from39)
Aspirin also shows promise for the adjuvant treatment of these cancers and a large trial in colorectal cancer is almost fully recruited (ASCOLT).44 A multisite trial (Add-Aspirin)45 in colorectal, gastric, oesophageal, breast, lung and prostate cancer is also now underway, as well as a smaller trial in early prostate cancer in combination with vitamin D (PROVENT).
The results on cancer prevention were a serendipitous finding in trials looking at low-dose aspirin to prevent cardiovascular disease, and as yet we have no clear understanding about the mechanism by which this takes place.46 Inflammation is likely to be involved in some way but the doses mostly used are too low for the standard Cox2 anti-inflammatory processes to be responsible; a major challenge is thus to understand more clearly the mechanisms by which aspirin prevents these cancers. We also need to more clearly understand who is at increased risk of GI bleeding, which is the major side-effect of aspirin even when used at low doses.
It is surprising that it took so long for the anti-cancer effects of aspirin to be discovered; a main part of the reason for this is that very little effect occurs in the first 5 years of follow-up after starting aspirin, and only long-term use for more than 5 years shows a beneficial effect on cancer, whereas the bleeding effects occur almost immediately. Thus, long-term follow-up is essential to determine the overall risk-benefit ratio of aspirin’s prophylactic use.
I believe that the evidence for an overall beneficial effect of aspirin is already strong enough to begin to make recommendations for the general population, after excluding those at high risk of bleeding. Nevertheless, several issues remain to help refine this indication. In particular the duration of use is not clearly established, although use for at least 5 years and probably 10 years is the minimum that should be offered. Continued use depends on the ratio of continued benefits versus increasing GI bleeding side-effects as an individual ages. Some evidence from the cardiovascular trials suggests a long-term benefit of aspirin after completion of the prescribed duration within the trial. However, it is not known how many of the participants continued to take aspirin after the trial was over and direct evidence on optimal duration of use is needed. Evidence on the risks of GI bleeding associated with aspirin in the elderly also needs refinement. A recent study indicated a clear increase in serious and fatal bleeds with aspirin in individuals aged more than 75 years, but it was shown that this could be avoided by the concomitant use of a proton pump inhibitor.38 It is not clear at which age a protein pump inhibitor should be offered, but its use does offer prospects for continuing prophylactic aspirin in older ages, which is certainly likely to be beneficial to cardiovascular disease prevention as well.
HPV vaccination and treatment of precursor lesions
Another major advance in the area of ‘preventive therapy’ is the development of HPV vaccines. The newest nine-valent vaccine offers prospects of eliminating 90% of cervical cancer,47,48 together with an important proportion of other anogenital cancers as well as oropharyngeal cancer.
It also needs to be acknowledged that by identifying and treating precursor lesions, screening has had a preventive effect on cervix and colorectal cancers, and early detection has reduced the mortality from breast, prostate and lung cancer.
Prospects for the future of therapeutic cancer prevention
Our successes to date in therapeutic cancer prevention have been based on repurposing drugs originally developed for other uses, i.e., endocrine agents used for breast cancer treatment, taking advantage of the fact that effects on the contralateral breast provide an opportunity to assess preventive effects. For aspirin, most of the trials were for cardiovascular disease prevention, and long-term follow-up provided clear evidence for an anti-cancer effect as well. Given the expense of running large prevention trials and developing new agents from scratch, it seems likely that future preventive agents will be identified from agents used for other indications and repurposed for cancer prevention. Programmes to actively explore opportunities to do this will be essential for expanding our portfolio of useful agents to prevent cancer. This is probably best achieved by mining general practice databases and large cohorts such as the UK Biobank. Promising agents include metformin, bisphosphonates, vitamin D and some dietary elements such as curcumin and sulforaphane.49
It is clear that a major obstacle in establishing the role of therapeutic cancer prevention is achieving wider acceptance by the profession and the general population. The cardiologists have been very successful in this respect; one aspect of their success has been to label risk factors such as high cholesterol or high blood pressure as diseases in their own right and thus worthy of treatment. Such factors are in short supply for cancer, and even in breast cancer, where risk assessment is the most developed, the only known marker is breast density (discussed above). We need to find ways to make preventive therapy more widely discussed and offered, and adding a prevention component to the breast cancer screening, and eventually other screening programmes, is one promising avenue. Aspirin has suffered from the fact that many authorities and societies that have made recommendations on preventive use reviewed it at a time before the benefits on cancer were known, and only when considered for cardiovascular disease prevention alone, where the general population the risks associated with GI bleeding were similar to the benefits. This has changed dramatically with the discovery of a major effect on cancer incidence, which dominates any effect on cardiovascular disease, but sadly the older recommendations are still widely believed. One approach to establishing aspirin as a more widespread preventive agent would be to get NICE to review the evidence and hopefully make a recommendation for aspirin use in the general population.
Dr Sam Smith has made a major effort to understand the reluctance of general practitioners to recommend preventive therapy for cancer.50 His surveys have indicated that GPs are prepared to continue to prescribe preventive medicine such as tamoxifen or aromatase inhibitors to women at high risk of breast cancer, provided therapy is initiated in specialist centres and then referred to the general practitioner for continued use. This work and continuing studies in this area are essential if we are to achieve a change of opinion in the medical profession, which is key to widespread acceptance.50
Conflict of interest
The author’s institution has received funding or material without cost for research studies from AstraZeneca, Aventis Pharma, Myriad Genetics, Bayer, Qiagen, Beckton Dickinson, Hologic, Abbott, Genera Biosystems, Roche, Trovagene and Cepheid. J.C. has received honoraria or consulting fees from AstraZeneca, Myriad Genetics, Bayer, Roche, Qiagen, Becton Dickinson, Roche, Trovagene, Merck, Atossa Genetics and Cancer Prevention Pharmaceuticals.
References
- 1.Beatson CT. On treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:162–165. doi: 10.1016/S0140-6736(01)72384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IARC. IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans, Sex Hormones (II), Lyon. 21, (1979). [PubMed]
- 3.Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/S0140-6736(03)14596-5. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–1059. doi: 10.1016/S0140-6736(97)08233-0. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandt PA, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur. J. Cancer. 1976;12:419–424. doi: 10.1016/0014-2964(76)90030-X. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Baum M. Tamoxifen and contralateral breast cancer (letter) Lancet. 1985;ii:282. doi: 10.1016/S0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. doi: 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Wang DY, Bulbrook RD. The prevention of breast cancer. Lancet. 1986;ii:83–86. doi: 10.1016/S0140-6736(86)90729-4. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 1998;90:1371–1387. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/S0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 13.Powles T, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Lancet. 1998;352:93–97. doi: 10.1016/S0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 15.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuzick J, Wickerham L, Powles T. Differing perspectives on breast cancer chemoprevention. JAMA Oncol. 2016;2:276–277. doi: 10.1001/jamaoncol.2015.4406. [DOI] [PubMed] [Google Scholar]
- 18.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 19.Vogel VG, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev. Res (Phila.) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzick J. Aromatase inhibitors for breast cancer prevention. J. Clin. Oncol. 2005;23:1636–1643. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 22.Goss PE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 23.Cuzick J, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J. Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 24.Ko KL, et al. Adjuvant tamoxifen-induced mammographic breast density reduction as a predictor for recurrence in estrogen receptor-positive premenopausal breast cancer patients. Breast Cancer Res. Treat. 2013;142:559–567. doi: 10.1007/s10549-013-2726-4. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy. J. Natl Cancer Inst. 2015;107:2246–56. [Google Scholar]
- 26.Nyante SJ, et al. Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer in postmenopausal patients with breast cancer. J. Clin. Oncol. 2013;31:2249–2256. doi: 10.1200/JCO.2012.44.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engmann NJ, et al. Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors. Cancer Epidemiol. Biomark. Prev. Jun. 2017;26:930–937. doi: 10.1158/1055-9965.EPI-16-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012;14:R102. doi: 10.1186/bcr3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 30.Brentnall AR, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2015;17:147. doi: 10.1186/s13058-015-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzick J, et al. Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized tamoxifen prevention trials. J. Clin. Oncol. 2017;35:743–750. doi: 10.1200/JCO.2016.69.8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans DG, et al. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: a case-control study. J. Med. Genet. Feb. 2017;54:111–113. doi: 10.1136/jmedgenet-2016-104125. [DOI] [PubMed] [Google Scholar]
- 33.Vachon CM, et al. The contributions of breast density and common genetic variation to breast cancer risk. J. Natl Cancer Inst. 2015;107:dju397. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans DG, et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br. J. Cancer. 2016;114:1045–1052. doi: 10.1038/bjc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly LS, et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer. 2014;110:1681–1687. doi: 10.1038/bjc.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J. Surg. Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 37.Cuzick J, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490–499. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuzick J, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann. Oncol. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothwell PM, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–34. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 42.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann. Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 43.Chubak J, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016;164:814–825. doi: 10.7326/M15-2117. [DOI] [PubMed] [Google Scholar]
- 44.Ali R, Toh HC, Chia WK, ASCOLT Trial Investigators. The utility of Aspirin in Dukes C and High Risk Dukes B Colorectal cancer--the ASCOLT study: study protocol for a randomized controlled trial. Trials Dec. 2011;14:261. doi: 10.1186/1745-6215-12-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coyle C, et al. ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials. 2016;51:56–64. doi: 10.1016/j.cct.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat. Rev. Clin. Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 47.Joura EA, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 48.Kavanagh K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect. Dis. 2017;17(1293):1302. doi: 10.1016/S1473-3099(17)30468-1. [DOI] [PubMed] [Google Scholar]
- 49.Cuzick J. Preventive therapy for cancer. Lancet Oncol. 2017;18:e472–e482. doi: 10.1016/S1470-2045(17)30536-3. [DOI] [PubMed] [Google Scholar]
- 50.Smith SG, et al. Prescribing tamoxifen in primary care for the prevention of breast cancer: a national online survey of GPs’ attitudes. Br. J. Gen. Pract. 2017;67:e414–e427. doi: 10.3399/bjgp17X689377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuzick J, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baron JA, et al. randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 53.Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 54.Benamouzig R, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/S0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 55.Smith SG, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann. Oncol. 2016;27:575–590. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]