Abstract
Phylogenetic and neurobiological theories suggest that inter-individual differences in high frequency heart rate variability (HF-HRV) are associated with inter-individual differences in social behavior and social cognition. To test these theories, we investigated whether individuals with high and low HF-HRV would show different preferences for cooperative behavior in social contexts. We recorded resting state HF-HRV in 84 healthy individuals before they completed the Social Value Orientation task, a well-established measure of cooperative preferences. HF-HRV was derived from short-term (300 s) and ultra-short-term (60 s, 120 s) recordings of participants’ heart rate to determine the robustness of possible findings. Irrespective of recording length, we found a sex-dependent association between inter-individual differences in HF-HRV and inter-individual differences in social value orientation: The preference for cooperation was more pronounced among individuals with high as compared low HF-HRV, albeit only in male and not in female participants. These findings suggest that males with high HF-HRV are more inclined to engage in cooperative behavior than males with low HF-HRV.
Introduction
Across industrialized and non-industrialized societies, humans show a remarkable and distinct suite of cooperative behavior1–4. Humans often cooperate with one another, even if cooperation is costly for the self and beneficial for the other5,6. Cooperation usually emerges during interactions with well-known individuals because cooperative behavior is more expected, reinforced and rewarded among well-known than among less known individuals7–11. However, cooperation also occurs among unknown individuals, which do not necessarily expect, reinforce or reward cooperative behavior7–11. Individuals may, thus, have a preference for cooperation, independent of considerations regarding the expectancy, reinforcement or reward of cooperation, which is driving their behavior during social interactions12. As an individual’s preference for cooperation appears to affect cooperative behavior in various contexts13–19, there is a growing interest for biomarkers indicating whether an individual is more likely to cooperate or defect during social interactions.
Vagally mediated heart rate variability (vmHRV), a measure of parasympathetic induced changes in heart rate [HR]20, has been suggested to be a promising biomarker for an individual’s behavior in social contexts21,22. An individual’s social behavior is orchestrated by a network of prefrontal and para-limbic brain regions that are relevant for a plethora of social processes23, implying that functional and structural changes in these brain regions are associated with changes in social cognition and social interaction. As functional and structural changes in these brain regions are also associated with changes in vmHRV22,24,25, changes in vmHRV reflect changes in an individual’s social behavior. Inter-individual differences in vmHRV have, therefore, been suggested to indicate inter-individual differences in social cognition and social interaction21,22. Accordingly, there are marked differences in social cognition and social interaction between individuals that show differences in vmHRV21,22. For instance, individuals with high vmHRV are more likely to recognize facial expressions of others26–28, to regulate emotional responses towards others29–31 and to establish cooperative relationships with others32–34 than individuals with low vmHRV. The difference in cooperative behavior between individuals with high and low vmHRV may be due to different preferences for cooperation32,33,35, suggesting a more pronounced preference for and display of cooperation among individuals with high than low vmHRV.
To investigate whether inter-individual differences in vmHRV would be associated with inter-individual differences in cooperation, we assessed vmHRV and cooperative preferences in a relatively large sample of participants. Inter-individual differences in vmHRV were determined on basis of short-term (300 s) and ultra-short-term (120 s, 60 s) resting state recordings of participants’ HR. Whereas the use of short-term measures of vmHRV is recommended and well established20, the use of ultra-short-term measures of vmHRV is less established because it is currently unknown whether these measures are similarly associated with inter-individual differences in social cognition and social interaction as short-term measures. Using short-term and ultra-short-term measures of vmHRV allowed us to investigate whether the association between inter-individual differences in vmHRV and inter-individual differences in cooperative preferences would be invariant across recording conditions. The use of these measures, thus, helped us to assess the robustness of the association between inter-individual differences in vmHRV and inter-individual differences in cooperative preferences. Inter-individual differences in cooperative preferences were determined with the Social Value Orientation task [SVO]36, an established resource allocation task that differentiates between cooperative and non-cooperative allocations on basis of participants’ choices over a continuum of self/other payoff allocations (see Fig. 1). The SVO differs markedly from other resource allocation tasks, including those that have been used in comparable studies32,33,35, because participants’ cooperative or non-cooperative allocations are not affected by strategic considerations36. Participants’ allocations on the SVO, thus, represent an unbiased measure of their preference for cooperation. Using the SVO36, we expected to find the preference for cooperative allocations to be more pronounced among participants with high than low vmHRV. We also expected that inter-individual differences in cooperative preferences would be mediated by participants’ sex because male and female participants perform differently on resource allocation tasks that measure inter-individual differences in cooperation37,38.
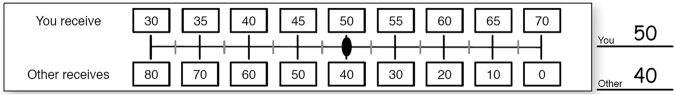
Figure 1.
Example of a continuum of self/other pay off allocations that are used in the Social Value Orientation Task [SVO]36.
Method
Participants
Prior to participant recruitment, we performed a power analysis to determine the number of participants that we needed to detect a meaningful association between inter-individual differences in vmHRV and inter-individual differences in cooperative preferences. Due to a lack of previous studies investigating the association of inter-individual differences in vmHRV with inter-individual differences in cooperative preferences, we turned to studies that investigated the association of inter-individual differences in vmHRV with inter-individual differences in cooperative behavior32,33. As these studies revealed medium-to-large sized associations (r = 0.35, f = 0.37)32,33, we expected to find an association of similar size in our study. G*Power39 indicated that we had to recruit 82 participants to be able to detect such an association in dimensional and categorical analyses [α = 0.05, 1-β = 80, r = 0.35, f = 0.35]. Slightly over-recruiting, we included 84 participants, 41 males and 43 females, in our study. In order to be included in the study, participants had to be aged between 18 and 35 years and to be native speakers. Participants with mental disorders and participants who were in psychotherapeutic treatment were excluded from the study. Of the 84 participants, 8 participants did not provide valid data due to equipment dysfunction (n = 1) or misunderstanding of the task instructions (n = 7). Consequently, the data of 76 participants, 39 males and 37 females, were considered in the analyses (see Table 1).
Table 1.
Participant characteristics.
| Male participants | Female participants | Test statistic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | F(1,74)/F(1,59) | p | η p 2 | |
| Age | 26.49 | 4.58 | 18 | 35 | 25.81 | 3.61 | 20 | 34 | 0.508 | 0.478 | 0.007 |
| Heart rate variability | |||||||||||
| Log-HF-HRV-300 | 2.71 | 0.54 | 1.23 | 3.75 | 2.79 | 0.41 | 1.71 | 3.65 | 0.528 | 0.470 | 0.007 |
| Log-HF-HRV-120 | 2.76 | 0.59 | 1.17 | 4.12 | 2.82 | 0.46 | 1.93 | 3.82 | 0.260 | 0.611 | 0.004 |
| Log-HF-HRV-60 | 2.85 | 0.60 | 1.20 | 4.03 | 2.86 | 0.42 | 1.95 | 4.12 | 0.007 | 0.936 | 0.000 |
| Social value orientation | |||||||||||
| SVO anglea | 32.83 | 11.05 | 0.00 | 61.39 | 32.00 | 10.89 | −3.81 | 50.83 | 0.108 | 0.743 | 0.000 |
| IA indexb | 0.31 | 0.32 | 0.05 | 1.00 | 0.11 | 0.11 | 0.00 | 0.43 | 10.370 | 0.002** | 0.149 |
Note. Log-HF-HRV-300 = log-transformed high frequency heart rate variability derived from short-term HR recordings (300 s), Log-HF-HRV-120 = log-transformed high frequency heart rate variability derived from ultra-short-term HR recordings (120 s), Log-HF-HRV-60 = log-transformed high frequency heart rate variability derived from ultra-short-term HR recordings (60 s), SVO angle = Social Value Orientation angle36, IA Index = Inequality Aversion Index36.
aData on SVOA angle was available for 76 participants, 37 females and 39 males.
bData on IA index was only available for 61 participants, 30 females and 31 males because these were the only participants that met the conditions for the determination of the IA index36.
**p ≤ 0.01.
All participants provided written-informed consent before taking part in the study and were fully debriefed after completion of the study. The study was approved by the ethics committee of the University of Rostock and carried out in accordance with the Declaration of Helsinki.
Procedure
After arrival at the laboratory, participants were asked to use the bathroom to control for the effects of bladder filling and gastric distension on vmHRV40. Participants were then seated in a comfortable chair and prepared for a resting state recording of their HR. In line with recent recommendations41, participants were instructed to sit still, to breathe spontaneously and to keep their eyes open during the HR recording. Immediately after receiving the instructions, participants’ HR was recorded for a total duration of 5 min (300 s). Thereafter, participants completed the SVO task36.
Heart rate variability
HR was continuously recorded with the RS800 (Polar Electro Oy, Kempele, Finland), a mobile HR monitor that has been shown to record participants’ HR as accurate as conventional HR monitors42,43. The RS800 comprises a two-lead chest belt system for data recording (sample rate: 1000 Hz) and a wrist watch for data storage. Device specific software (Polar ProTrainer 5; Polar Electro Oy, Kempele, Finland) was used to transfer the recorded data from the wrist watch to a computer for data processing with Kubios HRV 2.244. Following established guidelines20, the recorded data was detrended (smoothn priors: λ = 500), visually inspected and, whenever necessary, artifact corrected with adaptive filtering (cubic spline interpolation). Overall, less than 5% of participants’ data had to be corrected for artifacts, indicating the reliability and validity of the HR recording. After artifact correction, the recorded data was subjected to a spectral analysis (Fast Fourier Transformation; Welch’s periodogram: 256 s window with 50% overlap) to determine HF-HRV (0.15–0.4 Hz) as outlined in established guidelines20. HF-HRV was determined for different recording lengths45, including a short-term recording (300 s from the start of the recording session, HF-HRV-300) and two ultra-short-term recordings (60 s from the start of the recording session, HF-HRV-60; 120 s from the start of the recording session, HF-HRV-120). In contrast to other HRV measures, HF-HRV is a robust measure of changes in HR that are mediated by the vagus nerve20.
Social Value Orientation
The SVO task, an established resource allocation task36, comprised 6 primary and 9 secondary items with a choice over a defined continuum of self/other payoff allocations (see Fig. 1). Participants had to choose the allocation that reflected their most preferred joint payoffs for themselves and another participant whose identity remained anonymous throughout the study. Participants’ choices on the primary items were used to determine an index of participants’ social value orientation, the SVO angle36. Higher angular degrees on the SVO angle indicated participants’ preference for cooperative allocations (i.e., pro-social allocations, allocations with higher payoffs for the other than for the self) as compared to non-cooperative allocations (i.e., pro-self allocations, allocations with lower payoffs for the other than for the self). To further describe participants’ preference for cooperative allocations, participants’ choices on the secondary items were used to compute another index of participants’ social value orientation, the inequality-aversion index [IA index36. An index of 1 indicated that participants’ preference for cooperative allocations was driven by the motivation of joint maximization (i.e., maximizing payoffs for both, the self and the other), whereas an index of 0 indicated that participants’ preference for cooperative allocations was driven by the motivation of inequality aversion (i.e., minimizing differences between payoffs for the self and payoffs for the other).
Statistical Analysis
SPSS 22 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Preliminary analyses were performed to investigate the characteristics of male and female participants. To this end, analyses of variances (ANOVAs) were used to compute differences in age (years), social value orientation (SVO angel, IA index) and HF-HRV (HF-HRV-300, HF-HRV-120, HF-HRV-60) between male and female participants. Main analyses were performed to investigate the association between inter-individual differences in HF-HRV and inter-individual differences in social value orientation among male and female participants. These analyses comprised categorical and dimensional analyses. For the categorical analyses, two-way ANOVAs (Sex × Group) were used to compute differences in social value orientation (SVO angle, IA index) between male and female participants with high and low HF-HRV on basis of a median-split. For the dimensional analyses, correlations between HF-HRV and social value orientation (SVO angle, IA index) were computed, separately for male and female participants. In addition, Fisher’s z-transformation46 was used to compare the respective correlation coefficients with one another. To assess the robustness of the categorical and dimensional analyses, the analyses were performed for HF-HRV measures that were derived from short-term (HF-HRV-300) and ultra-short-term (HF-HRV-120, HF-HRV-60) recordings of participants’ HR. Correspondence between short-term and ultra-short-term measures of HF-HRV was determined on basis of intra-class correlations (ICC: absolute agreement, two-way ANOVA47). Prior to all analyses, HF-HRV was log transformed (log 10) to account for deviations from normality distribution. The significance level for all analyses was set at p ≤ 0.05. In addition to the significance level (p), effect size measures (ηp2, r and q) were determined to facilitate the interpretation of (marginally) significant findings48.
Results
Participant characteristics
A one-way ANOVA revealed no age differences between male and female participants [F(1,74) = 0.508, p = 0.478, ηp2 = 0.007; see Table 1]. A series of further one-way ANOVAs also revealed no differences in HF-HRV between male and female participants, regardless whether HF-HRV was derived from short-term or ultra-short-term recordings of participants’ HR [F ≤ 0.528, p ≥ 0.470, ηp2 ≤ 0.004; see Table 1]. Another series of one-way ANOVAs indicated differences in IA index [F(1,59) = 10.370, p = 0.002, ηp2 = 0.149; see Table 1] but not in SVO angle [F(1,74) = 0.180, p = 0.743, ηp2 = 0.000; see Table 1] between male and female participants. Female participants showed a lower IA index than male participants.
Short-term measures of heart rate variability and measures of social value orientation
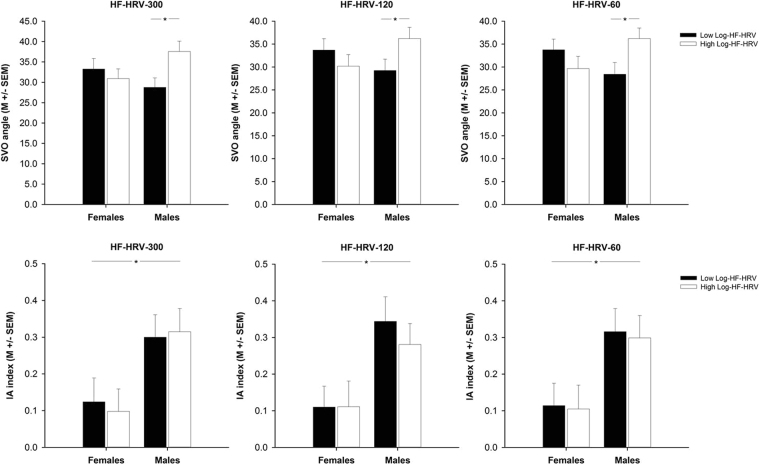
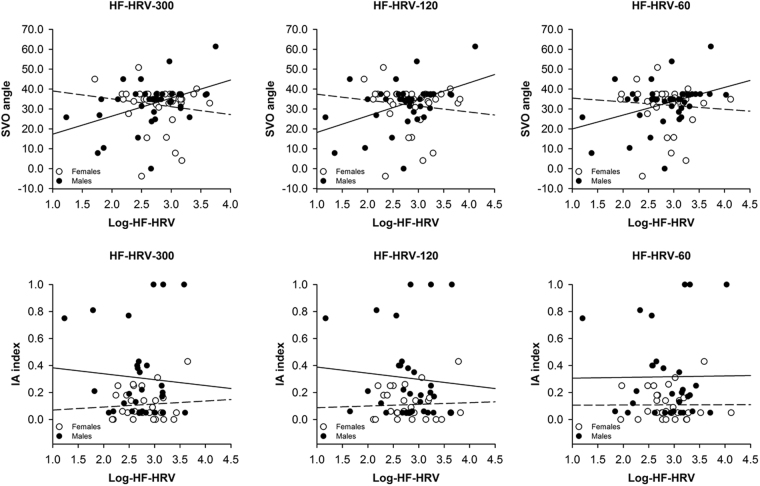
A two-way ANOVA (Sex × Group) indicated sex-specific differences in SVO angle between male and female participants with low and high HF-HRV [all effects of sex or group: F ≤ 1.752, p ≥ 0.190, ηp2 ≤ 0.024; interaction of sex and group: F(1,72) = 5.219, p = 0.025, ηp2 = 0.068; see Table S1 and Fig. 2]. Follow-up one-way ANOVAs revealed differences in SVO angle between male participants with high and low HF-HRV [F(1,37) = 7.167, p = 0.011, ηp2 = 0.162; see Fig. 2] but not between female participants with high and low HF-HRV [F(1,35) = 0.420, p = 0.521, ηp2 = 0.012; see Fig. 2]. SVO angle was higher in male participants with high HF-HRV than in male participants with low HF-HRV. Accordingly, there was a correlation between HF-HRV and SVO angle in male [r(39) = 0.349, p = 0.030; see Table S2 and Fig. 3] but not in female [r(37) = −0.195, p = 0.247; see Table S2 and Fig. 3] participants as indicated by a series of correlation analyses. A direct comparison of the respective correlation coefficients confirmed that HF-HRV was positively correlated with SVO angle in male but not in female participants [z = 2.349, p = 0.019, q = 0.562; see Fig. 3].
Figure 2.
Barplots demonstrating differences in social value orientation (SVO angle, IA index) between participants with high (white bars) and low (black bars) log-transformed high-frequency heart rate variability (Log-HF-HRV) that was derived from short-term (300 s) or ultra-short-term (120 s, 60 s) recordings of male and female participants’ heart rate. Bars represent mean values (M) and error bars represent standard error of mean values (SE M). *p ≤ 0.05.
Figure 3.
Scatterplots with lines of best fit demonstrating correlations between social value orientation (SVO angle, IA index) and log-transformed high frequency heart rate variability (Log-HF-HRV) that was derived from short-term (300 s) or ultra-short-term (120 s, 60 s) recordings of male (black circles, solid lines) and female (white circles, dotted lines) participants’ heart rate.
A two-way ANOVA (Sex × Group) indicated sex-specific differences in IA index between male and female participants that did not depend on participants’ HF-HRV [effect of sex: F(1,57) = 9.948, p = 0.003, ηp2 = 0.149; all other effects and interactions of sex or group: F ≤ 0.105, p ≥ 0.747, ηp2 ≤ 0.002; see Table S1 and Fig. 2]. Female participants showed a lower IA index than male participants. A series of correlation analyses revealed no correlations between HF-HRV and IA Index in male or female participants [r ≤ −0.090, p ≥ 0.634; see Table S2 and Fig. 3].
Ultra-short-term measures of heart rate variability and measures of social value orientation
A series of two-way ANOVAs (Sex × Group) confirmed the aforementioned sex-specific differences in SVO angle between participants with low and high HF-HRV [all effects involving sex or group: F(1,72) ≤ 0.573, p ≥ 0.452, ηp2 ≤ 0.008; all interactions of sex and group: F(1,72) ≥ 4.527, p ≤ 0.037, ηp2 ≥ 0.059; see Table S1 and Fig. 2]. Follow-up one-way ANOVAs revealed, again, a higher SVO angle in male participants with high HF-HRV than in male participants with low HF-HRV [F(1,37) ≥ 4.219, p ≤ 0.047, ηp2 ≥ 0.102; see Fig. 1] and a similar SVO angle in female participants with high and low HF-HRV [F(1,35) ≤ 1.270, p ≥ 0.267, ηp2 ≤ 0.035; see Fig. 1]. Correlation analyses confirmed the aforementioned pattern of correlations between HF-HRV and SVO angle: There was, again, a positive correlation between HF-HRV and SVO angle in male [r(39) ≥ 0.333, p ≤ 0.038; see Table S2 and Fig. 3] but not in female [r(37) ≤ −0.256, p ≥ 0.126; see Table S2 and Fig. 3] participants as indicated by a direct comparison of the respective correlation coefficients [z ≥ 2.309, p ≤ 0.021, q ≥ 0.552; see Table S2 and Fig. 3].
A two-way ANOVA (Sex × Group) confirmed the aforementioned sex-specific differences in IA index between male and female participants that were independent of participants’ HF-HRV [all effects of sex: F(1,57) ≥ 10.060, p ≤ 0.002, ηp2 ≥ 0.150; all other effects and interactions of sex or group: F(1,57) ≤ 0.260, p ≥ 0.612, ηp2 ≤ 0.005; see Table S1 and Fig. 2]. Female participants showed, again, a lower IA index than male participants. A series of correlation analyses confirmed that there were no correlations between HF-HRV and IA Index in male or female participants [all r ≤ −0.119, all p ≥ 0.524; see Table S2 and Fig. 3].
Short-term and ultra-short-term measures of heart rate variability
A series of intra-class correlation analyses was performed to investigate the correspondence between HF-HRV measures that were derived from short-term and ultra-short term recordings of participants’ HR. According to these analyses, there was a high correspondence between the different HF-HRV measures among male as well as female participants [all ICC ≥ 0.878; see Table 2).
Table 2.
Intra-class correlations between short-term and ultra-short-term measures of heart rate variability.
| Female participants | Male participants | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| Log-HF-HRV-300 vs.Log-HF-HRV-120 | 0.946 | [0.896, 0.972] | 0.961 | [0.926, 0.980] |
| Log-HF-HRV-300 vs. Log-HF-HRV-60 | 0.878 | [0.764, 0.937] | 0.931 | [0.829, 0.968] |
| Log-HF-HRV-120 vs. Log-HF-HRV-60 | 0.974 | [0.935, 0.988] | 0.949 | [0.901, 0.974] |
Note. ICC = intra-class correlations, 95% CI = 95% confidence interval, Log-HF-HRV-300 = log-transformed high frequency heart rate variability derived from short-term HR recordings (300 s), Log-HF-HRV-120 = log-transformed high frequency heart rate variability derived from ultra-short-term HR recordings (120 s), Log-HF-HRV-60 = log-transformed high frequency heart rate variability derived from ultra-short-term HR recordings (60 s).
Discussion
In the present study, we investigated whether inter-individual differences in vmHRV would be associated with inter-individual differences regarding the preference for cooperation. We found a sex-dependent association between inter-individual differences in vmHRV and inter-individual differences in cooperative preferences. Male participants with high vmHRV showed more cooperative preferences than male participants with low vmHRV. Female participants with low and high vmHRV, on the contrary, did not differ in their cooperative preferences. Considering that inter-individual differences in cooperative preferences as well as inter-individual differences in vmHRV were more pronounced among male than female participants (see Table 1 and Fig. 3 for a range of the respective values that was larger in male than female participants), it was more likely that an association between inter-individual differences in cooperative preferences and inter-individual differences in vmHRV emerged in analyses involving male rather than female participants. However, this association only emerged in analyses of participants’ SVO angle but not in analyses of participants’ IA index. Analyses of participants’ IA index are generally more complicated than analyses of participants’ SVO angle because the determination of the IA index depends on more conditions than the determination of the SVO angle36. As some participants did not meet the conditions for these types of analyses, fewer participants were considered in the analyses of the IA index than in the analyses of the SVOA angle. Differences in statistical power may, thus, have accounted for the inconsistency of findings. The findings of the analyses regarding participants’ IA index should, therefore, be treated with caution, until replicated and extended in future studies. For this reason, we centered our discussion on the findings that emerged in the analyses of participants’ SVO angle rather than on the findings that emerged in the analyses of participants’ IA index. In this respect, it is important to note that it did not matter whether short-term or ultra-short-term measures of vmHRV were considered in these analyses. Moreover, short-term and ultra-short-term measure of vmHRV were substantially associated with one another, replicating and extending findings of a previous study whose focus was not on frequency domain measures of vmHRV as in the present study but on time domain measures of vmHRV45. Taken together, these findings suggest an a robust and substantial association between inter-individual differences in vmHRV and inter-individual differences regarding cooperative preferences in male as compared to female participants.
Inter-individual differences in vmHRV are associated with various aspects of social behavior, which may help to understand why inter-individual differences in vmHRV account for inter-individual differences in cooperative preferences and cooperative behavior. As individuals with high vmHRV are more successful in regulating emotional responses towards others than individuals with low vmHRV29–31, they may be less stressed and more relaxed during social interactions49–51. Due to the absence of feelings of stress, individuals with high vmHRV may be more motivated to engage in cooperative behavior than individuals with low vmHRV32,33, which may be reflected in the respective differences regarding individuals’ preference for cooperation. Moreover, by displaying cooperative behavior32,33, individuals with high vmHRV may have less difficulties to initiate and maintain social relationship than individuals with low vmHRV49,50. This may explain why individuals with high vmHRV report more feelings of connectedness and comfort in social contexts than individuals with low vmHRV34,49–51. Of note, individuals suffering from mental disorders that are characterized by discomfort in social contexts and impairments in social interactions often display lower vmHRV than healthy individuals52,53. For instance, depressed individuals, which are known to display lower vmHRV than non-depressed individuals54,55, have marked difficulties in sustaining cooperative relationships56. Inter-individual differences in vmHRV may, thus, account for inter-individual differences regarding the preference for and display of cooperative behavior in healthy as well as mentally-disordered individuals, indicating that vmHRV may indeed be a biomarker for social behavior21,22. Future studies investigating the association between inter-individual differences in vmHRV and inter-individual differences in cooperation in healthy as well as in mentally-disordered individuals may help to determine whether vmHRV qualifies as biomarker for social behavior.
The neurobiological mechanisms mediating the association between inter-individual differences in vmHRV and inter-individual differences in cooperation may be best elucidated by comparing the neurobiological mechanisms underlying changes in vmHRV with the neurobiological mechanisms underlying changes in cooperation. Changes in cooperative preferences and cooperative behavior are mediated by a network of prefrontal, para-limbic and meso-limbic brain regions57,58. Of these brain regions, prefrontal and para-limbic ones are of particular relevance because functional and structural changes in these brain regions lead to profound changes regarding the preference for59–62 and the display of 63–69 cooperative behavior. Prefrontal and para-limbic brain regions are also part of a network of brain regions that mediate changes in vmHRV22,24,25. As functional70 and structural71 changes in these brain regions are closely associated with changes in vmHRV, changes in vmHRV may reflect changes in cooperative preferences and cooperative behavior that are due to functional and structural changes in the aforementioned network of brain regions. It may, thus, be conceivable that the preference for and the display of cooperative behavior is more pronounced among individuals with high than low vmHRV because individuals with high HRV are more efficient in recruiting the relevant brain regions driving cooperation in social contexts. In this regard, it is noteworthy that individuals suffering from mental disorders that are associated with deficits in social behavior show alterations in vmHRV52,53 as well as alterations in networks of brain regions that are centered on prefrontal and para-limbic brain regions72. Inter-individual differences in vmHRV may, thus, indicate inter-individual differences regarding the engagement of brain regions that mediate cooperative preferences and cooperative behavior in healthy as well as mentally-disordered individuals, implying once more that vmHRV may function as a biomarker for social behavior21,22. Future studies should consider behavioral and neural measures in their analyses on the association between inter-individual differences in vmHRV and inter-individual differences in cooperation to determine whether vmHRV has the potential to work as a biomarker for social behavior.
The present study does not only help to elucidate the neurobiological mechanisms underlying cooperation in social contexts, but also suggest some methodological modifications that may be helpful for further studies investigating the association of inter-individual differences in vmHRV with inter-individual differences in cooperative preferences and cooperative behavior. First of all, inter-individual differences in cooperation do not necessarily have to be assessed with complex tasks, like, for example, a re-iterated trust game10. Simpler tasks, such as the SVO, which has excellent psychometric properties36, may be sufficient to differentiate between cooperative and non-cooperative individuals. The SVO does not require real or simulated interactions between participants, indicating that the administration of the SVO is less time- and resource-consuming than the administration of more complex tasks36. Second, it may be sufficient to assess inter-individual difference in vmHRV on basis of ultra-short-term instead of short-term HR recordings. There was not only a remarkable correspondence between ultra-short-term and short-term masures of vmHRV regarding the measurement of inter-individual differences in vmHRV but also regarding the measurement of the association between inter-individual differences in vmHRV and inter-individual differences in cooperative preferences, replicating and extending the findings of a previous study45. Ultra-short-term measures of vmHRV, thus, represent reliable and valid alternatives to short-term measures of vmHRV, indicating a need to revise current guidelines that recommend the use of short-term measures over the use of ultra-short term measures20. Using ultra-short-term instead of short-term measures may save time and resources during the assessment of vmHRV, which has been considered as a simple measure regarding the engagement of prefrontal and para-limbic brain regions during the regulation of social processes21,22. Combining ultra-short-term measures of vmHRV, such as HF-HRV-120, with short measures of cooperation, such as the SVO, may, thus, be interesting for researchers that need to investigate the neurobiological basis of inter-individual differences in cooperation in a time- and resource-efficient manner. In particular, researchers investigating biomarkers for social behavior in large-scale studies, such as genome-wide association studies, may benefit from the combined use of the aforementioned measures when time and resources are scarce.
Overall, the findings of the present study indicate that inter-individual differences in vmHRV are associated with inter-individual differences in cooperative preferences, albeit only in males and not in females. These findings are consistent with those of previous studies that revealed an association between inter-individual differences in vmHRV and inter-individual differences in cooperative behavior. Taken together, these findings support phylogenetic and neurobiological theories that suggest that vmHRV may work as a biomarker for various social processes21,22, including those that are related to cooperative preferences and cooperative behavior.
Electronic supplementary material
Acknowledgements
The authors would like to thank Nils Seitz for research assistance. Funding for this study was supported by an Open Access Publishing grant that was provided by the German Research Foundation (DFG) and the University of Rostock. AL was supported by a grant provided by the German Research Foundation (DFG; LI 2517/2-1). The funding source had no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author Contributions
A.L., A.M.M. and M.W. designed the study. A.M.M. and R.J. collected the data. A.L., M.W. and R.P. analyzed the data. A.L. wrote the manuscript. A.O.H., A.M.M., M.W., R.J. and R.P. contributed to writing, reviewing and editing of the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25739-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexander Lischke, Email: alexander.lischke@uni-greifswald.de.
Matthias Weippert, Email: matthias.weippert@uni-rostock.de.
References
- 1.Henrich J, et al. In search of homo economicus: behavioral experiments in 15 small-scale societies. Am Econ Rev. 2001;91(2):73–78. doi: 10.1257/aer.91.2.73. [DOI] [Google Scholar]
- 2.Henrich J, et al. Markets, religion, community size, and the evolution of fairness and punishment. Science. 2010;327(5972):1480–1484. doi: 10.1126/science.1182238. [DOI] [PubMed] [Google Scholar]
- 3.Henrich J, et al. Costly punishment across human societies. Science. 2006;312(5781):1767–70. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- 4.Roth, A. E., Prasnikar, V., Okuno-Fujiwara, M. & Zamir, S. Bargaining and market behavior in Jerusalem, Ljubljana, Pittsburgh, and Tokyo: An experimental study. Am Econ Rev 1068–1095 (1991).
- 5.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–91. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 6.Fehr E, Fischbacher U, Gachter S. Strong reciprocity, human cooperation, and the enforcement of social norms. Hum Nat. 2002;13(1):1–25. doi: 10.1007/s12110-002-1012-7. [DOI] [PubMed] [Google Scholar]
- 7.Fehr E, Gachter S. Altruistic punishment in humans. Nature. 2002;415(6868):137–40. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- 8.Wedekind C, Milinski M. Cooperation through image scoring in humans. Science. 2000;288(5467):850–2. doi: 10.1126/science.288.5467.850. [DOI] [PubMed] [Google Scholar]
- 9.Guth W, Schmittberger R, Schwarze B. An experimental-analysis of ultimatum bargaining. J Econ Behav Organ. 1982;3(4):367–388. doi: 10.1016/0167-2681(82)90011-7. [DOI] [Google Scholar]
- 10.Berg J, Dickhaut J, McCabe KA. Trust, reciprocity, and social-history. Games Econ Behav. 1995;10:122–142. doi: 10.1006/game.1995.1027. [DOI] [Google Scholar]
- 11.Kahneman, D., Knetsch, J. L. & Thaler, R. H. Fairness and the assumptions of economics. J Bus S285–S300 (1986).
- 12.Messick DM, Mcclintock CG. Motivational bases of choice in experimental games. J Exp Soc Psychol. 1968;4(1):1–25. doi: 10.1016/0022-1031(68)90046-2. [DOI] [Google Scholar]
- 13.Smeesters D, Warlop L, Van Avermaet E, Corneille O, Yzerbyt V. Do not prime hawks with doves: the interplay of construct activation and consistency of social value orientation on cooperative behavior. J Pers Soc Psychol. 2003;84(5):972–87. doi: 10.1037/0022-3514.84.5.972. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk E, De Cremer D, Handgraaf MJJ. Social value orientations and the strategic use of fairness in ultimatum bargaining. J Exp Soc Psychol. 2004;40(6):697–707. doi: 10.1016/j.jesp.2004.03.002. [DOI] [Google Scholar]
- 15.Kanagaretnam K, Mestelman S, Nainar K, Shehata M. The impact of social value orientation and risk attitudes on trust and reciprocity. J Econ Psychol. 2009;30(3):368–380. doi: 10.1016/j.joep.2008.12.003. [DOI] [Google Scholar]
- 16.Karagonlar G, Kuhlman DM. The role of Social Value Orientation in response to an unfair offer in the Ultimatum Game. Organ Behav Hum Dec. 2013;120(2):228–239. doi: 10.1016/j.obhdp.2012.07.006. [DOI] [Google Scholar]
- 17.Van Lange PAM, Bekkers R, Schuyt TNM, Van Vugt M. From games to giving: Social value orientation predicts donations to noble causes. Basic Appl Soc Psych. 2007;29(4):375–384. doi: 10.1080/01973530701665223. [DOI] [Google Scholar]
- 18.De Cremer D, Van Lange PAM. Why prosocials exhibit greater cooperation than proselfs: The roles of social responsibility and reciprocity. Eur J Pers. 2001;15:S5–S18. doi: 10.1002/per.418. [DOI] [Google Scholar]
- 19.DeDreu CKW, Vanlange PAM. The impact of social value orientations on negotiator cognition and behavior. Pers Soc Psychol Bull. 1995;21(11):1178–1188. doi: 10.1177/01461672952111006. [DOI] [Google Scholar]
- 20.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R, Thayer JF, Khalsa SS, Lane RD. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev. 2017;75:274–296. doi: 10.1016/j.neubiorev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Quintana DS, Guastella AJ, Outhred T, Hickie IB, Kemp AH. Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. Int J Psychophysiol. 2012;86(2):168–72. doi: 10.1016/j.ijpsycho.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Lischke A, Lemke D, Neubert J, Hamm AO, Lotze M. Inter-individual differences in heart rate variability are associated with inter-individual differences in mind-reading. Sci Rep. 2017;7(1):11557. doi: 10.1038/s41598-017-11290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendt, J., Weymar, M., Junge, M., Hamm, A.O. & Lischke, A. Heartfelt memories: Cardiac vagal tone correlates with increased memory for untrustworthy faces. Emotion (2018). [DOI] [PubMed]
- 28.Geisler FCM, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biol Psychol. 2013;93(2):279–86. doi: 10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Geisler FCM, Vennewald N, Kubiak T, Weber H. The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Pers Individ Dif. 2010;49(7):723–728. doi: 10.1016/j.paid.2010.06.015. [DOI] [Google Scholar]
- 30.Williams DP, et al. Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Front Psychol. 2015;6:261. doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beffara B, Bret AG, Vermeulen N, Mermillod M. Resting high frequency heart rate variability selectively predicts cooperative behavior. Physiol Behav. 2016;164(Pt A):417–28. doi: 10.1016/j.physbeh.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Dunn BD, Evans D, Makarova D, White J, Clark L. Gut feelings and the reaction to perceived inequity: the interplay between bodily responses, regulation, and perception shapes the rejection of unfair offers on the ultimatum game. Cogn Affect Behav Neurosci. 2012;12(3):419–29. doi: 10.3758/s13415-012-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kogan A, Gruber J, Shallcross AJ, Ford BQ, Mauss IB. Too much of a good thing? Cardiac vagal tone’s nonlinear relationship with well-being. Emotion. 2013;13(4):599–604. doi: 10.1037/a0032725. [DOI] [PubMed] [Google Scholar]
- 34.Kogan A, et al. Vagal activity is quadratically related to prosocial traits, prosocial emotions, and observer perceptions of prosociality. J Pers Soc Psychol. 2014;107(6):1051–1063. doi: 10.1037/a0037509. [DOI] [PubMed] [Google Scholar]
- 35.Task Force of the European Society of Cardiology, Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J17 354–381 (1996). [PubMed]
- 36.Murphy RO, Ackermann KA, Handgraaf MJJ. Measuring social value orientation. Judgm Decis Mak. 2011;6:771–81. [Google Scholar]
- 37.Balliet D, Li NP, Macfarlan SJ, Van Vugt M. Sex differences in cooperation: a meta-analytic review of social dilemmas. Psychol Bull. 2011;137(6):881–909. doi: 10.1037/a0025354. [DOI] [PubMed] [Google Scholar]
- 38.Croson R, Gneezy U. Gender Differences in Preferences. J Econ Lit. 2009;47(2):448–474. doi: 10.1257/jel.47.2.448. [DOI] [Google Scholar]
- 39.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 40.Quintana DS, Heathers JA. Considerations in the assessment of heart rate variability in biobehavioral research. Front Psychol. 2014;5:805. doi: 10.3389/fpsyg.2014.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintana DS, Alvares GA, Heathers JA. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. 2016;6:e803. doi: 10.1038/tp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weippert M, et al. Comparison of three mobile devices for measuring R-R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur J Appl Physiol. 2010;109(4):779–86. doi: 10.1007/s00421-010-1415-9. [DOI] [PubMed] [Google Scholar]
- 43.Quintana DS, Heathers JA, Kemp AH. On the validity of using the Polar RS800 heart rate monitor for heart rate variability research. Eur J Appl Physiol. 2012;112(12):4179–80. doi: 10.1007/s00421-012-2453-2. [DOI] [PubMed] [Google Scholar]
- 44.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Munoz ML, et al. Validity of (ultra-)short recordings for heart rate variability measurements. PLoS One. 2015;10(9):e0138921. doi: 10.1371/journal.pone.0138921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- 47.Fleiss, J. L., Levin, B. & Paik, M. C. Statistical methods for rates and proportions. New York: John Wiley & Sons (2013).
- 48.Cohen, J. Statistical power analysis for the behavioral sciences. New York: Earlbaum (1988).
- 49.Kok BE, Fredrickson BL. Upward spirals of the heart: autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biol Psychol. 2010;85(3):432–436. doi: 10.1016/j.biopsycho.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oveis C, et al. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265–70. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- 51.Lischke, A. et al. Heart rate variability is associated with psychosocial stress in distinct social domains. J Psychosom Res (2018). [DOI] [PubMed]
- 52.Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41(2):89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol. 2015;98(2):338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Kemp AH, et al. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One. 2012;7(2):e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pulcu E, et al. Social-economical decision making in current and remitted major depression. Psychol Med. 2015;45(6):1301–13. doi: 10.1017/S0033291714002414. [DOI] [PubMed] [Google Scholar]
- 57.Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- 58.Ruff CC, Fehr E. The neurobiology of rewards and values in social decision making. Nat Rev Neurosci. 2014;15(8):549–62. doi: 10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- 59.Haruno M, Kimura M, Frith CD. Activity in the nucleus accumbens and amygdala underlies individual differences in prosocial and individualistic economic choices. J Cogn Neurosci. 2014;26(8):1861–70. doi: 10.1162/jocn_a_00589. [DOI] [PubMed] [Google Scholar]
- 60.Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nat Neurosci. 2010;13(2):160–1. doi: 10.1038/nn.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fermin AS, et al. Representation of economic preferences in the structure and function of the amygdala and prefrontal cortex. Sci Rep. 2016;6:20982. doi: 10.1038/srep20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emonds G, Declerck CH, Boone C, Seurinck R, Achten R. Establishing cooperation in a mixed-motive social dilemma. An fMRI study investigating the role of social value orientation and dispositional trust. Soc Neurosci. 2014;9(1):10–22. doi: 10.1080/17470919.2013.858080. [DOI] [PubMed] [Google Scholar]
- 63.Rilling JK, et al. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/S0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 64.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 65.De Quervain DJ, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 66.Krueger F, et al. Neural correlates of trust. Proc Natl Acad Sci USA. 2007;104(50):20084–9. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27(4):951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheele D, et al. Amygdala lesion profoundly alters altruistic punishment. Biol Psychiatry. 2012;72(3):e5–7. doi: 10.1016/j.biopsych.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 69.Koscik TR, Tranel D. The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia. 2011;49(4):602–611. doi: 10.1016/j.neuropsychologia.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakaki M, et al. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkelmann T, et al. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct Funct. 2017;222(2):1061–1068. doi: 10.1007/s00429-016-1185-1. [DOI] [PubMed] [Google Scholar]
- 72.Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9–24. doi: 10.1002/da.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.