Dear Editor,
Thymic epithelial cells (TECs) orchestrate the differentiation of haematopoietic precursors into functional and self-tolerant T cells. TECs are surprisingly dynamic, with a high proliferative rate (~8-10% per day) capable of replacing the entire compartment in approximately 2 weeks [1, 2]. These findings imply similarly high rates of TEC death during homeostasis, yet the mechanisms and impact of cell death processes upon age-related thymic involution are unknown. We recently found that loss of the pro-survival BCL-2 family member, MCL-1, provoked abnormal TEC death, and thymic atrophy [3]. However, the identification of this requirement for TEC survival does not inform the physiological death processes governing their homeostasis. Therefore, we employed conditional genetic loss-of-function approaches to disable specific cell death modalities, to determine how TECs die under homeostatic conditions.
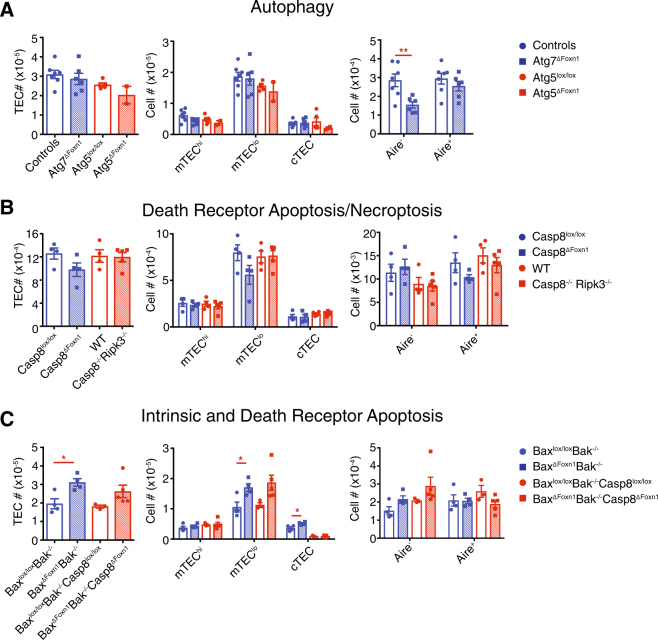
Unlike most tissues, TECs constitutively undergo macro-autophagy under resting conditions [4]. To establish whether TEC homeostasis is controlled by this process, we generated mice with TEC-specific ablation of the key autophagy genes, Atg5 or Atg7. Atg5ΔFoxn1, and Atg7ΔFoxn1 mice had normal thymic cellularity, TEC numbers, cortical TEC (cTEC), and mTEC subset composition (Fig. 1a, Figure S1A). AIRE− cells were reduced in Atg7ΔFoxn1 mice (Fig. 1a), implying a pro-survival role for autophagy in this subset; however, overall these data suggest that autophagy does not induce TEC death.
Fig. 1.
Loss of apoptosis, autophagy or necroptosis does not grossly alter thymic epithelial cell homeostasis. a–c Overall numbers of TECs, TEC subsets, and AIRE+ or AIRE− cells were determined by flow cytometry in mice with deletion of genes critical for autophagy (a), the death receptor pathway of apoptosis and necroptosis (b) or the intrinsic and death receptor pathways of apoptosis (c). TECs were classified into: mTEChigh (Ly51−UEA-1+MHCIIhigh or Ly51−MHCIIhigh), mTEClow (Ly51−UEA-1+MHCIIlow or Ly51−MHCIIlow), and cTECs (Ly51+UEA-1-MHCII+ or Ly51+MHCII+). “Controls” group in autophagy panel includes Atg7lox/lox and Foxn1+/+Atg7lox/+ mice (no difference observed between these groups). Data are representative for at least two independent experiments (except data for Atg5 which was a single experiment) with n ≥ 2/group. Graphical data (a) are pooled from two experiments for Atg7ΔFoxn1 only. Each point represents an individual mouse and graph bars indicate mean ± SEM. Groups were compared with a Student’s t-test (two sided, unpaired); *p < 0.05; **p < 0.01
RNA sequencing data from TEC subsets [3] revealed expression of mediators of the death receptor pathway of apoptosis (e.g. FAS, TRAIL-R, FADD, and caspase-8). Therefore, we deleted an essential transducer of this pathway, caspase-8, specifically in TECs (Western blotting revealed residual amounts of caspase-8 remained in TEC (Figure S1B)). However, we did not observe increased TEC numbers in Casp8ΔFoxn1 compared to Casp8lox/lox mice (Fig. 1b), suggesting that death receptor signaling is not critical for the death of TECs. However, caspase-8 can also serve a pro-survival role by antagonizing RIPK3/MLKL-driven necroptosis, for example, following engagement of TNFR1. To address whether necroptosis obscured an accumulation of TECs that would otherwise be detected in the absence of caspase-8-mediated, death receptor-induced apoptosis, we assayed the thymic phenotype in Casp8−/−Ripk3−/− mice (prior to the onset of lymphadenopathy and systemic autoimmunity) where necroptosis and death receptor-mediated apoptosis are both disabled. These mice exhibited a normal thymus and TEC compartment, suggesting that neither death receptor nor necroptosis pathways mediate TEC death (Fig. 1b, Figure S1A).
We recently found that the loss of the pro-survival BCL-2 family member, MCL-1, provoked abnormal TEC death, and thymic atrophy [3]. However, this finding does not necessarily imply that the intrinsic pathway of apoptosis normally controls TEC homeostasis. To test whether the intrinsic pathway of apoptosis is required for physiological TEC death under homeostatic conditions, we removed the essential effectors of this pathway, BAX and BAK, only in TECs by creating BaxΔFoxn1Bak−/− mice (Figure S1B). We did not observe any gross changes in thymic cellularity, cTEC, mTEChigh or expression of AIRE in these mice compared to their respective controls; however, there was a specific increase in MHCIIlow mTEC (mTEClow) in the BaxΔFoxn1Bak−/− mice (Fig. 1c, Figure S1A). This phenotype was not exacerbated by the additional absence of caspase-8 in BaxΔFoxn1Bak−/−Casp8ΔFoxn1 mice (Fig. 1c, Figure S1A), indicating that only the intrinsic pathway of apoptosis-mediated substantial TEC death in young thymi. To determine whether BAX/BAK-mediated apoptosis affected thymic involution, we analysed 1.5-year-old BaxΔFoxn1Bak−/− mice and found that while mTEClow numbers remained substantially increased, overall thymic cellularity was unaffected (Figure S1C). Collectively, these findings indicate that the intrinsic pathway of apoptosis promotes the death of mTEClow during thymic homeostasis. This wave of apoptosis may accompany the differentiation of mature mTEChigh AIRE+ TECs transitioning back into the MHCIIlow subset [5, 6].
Electronic supplementary material
Acknowledgements
We gratefully acknowledge the Gray, Strasser and Herold labs for valuable feedback. We thank the WEHI Flow Cytometry Laboratory for technical assistance; B Helbert, K Mackwell, C Young, C Hall for mouse genotyping; G Siciliano, K Humphreys, S O’Connor and H Marks for animal husbandry; S Korsmeyer for Baxlox/loxBak−/−, R Hakem for Casp8lox/lox, GA Holländer for Foxn1Cre and J Silke for Casp8−/−Ripk3−/− mice. This work was supported by grants GNT0637353, GNT1049724 and GNT1121325 and Career Development Fellowship-2 1090236 (for D.H.D.G.), 1016701 and Senior Principal Research Fellow [SPRF] Fellowship 1020363 (for A.S) from the Australian National Health and Medical Research Council and MIRS and MIFRS (for R.J.) from the University of Melbourne.
Author Contributions
Conceptualization, R.J., A.S. and D.H.D.G.; Methodology, R.J, A.S. and D.H.D.G.; Investigation, R.J., G.D., I.T. and D.H.D.G.; Resources, J.D.M., A.S., D.H.D.G.; Writing–Original draft, R.J. and D.H.D.G.; Writing–Review and Editing, R.J., J.D.M., G.D., A.S. and D.H.D.G.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1038/s41418-018-0093-8) contains supplementary material, which is available to authorized users.
References
- 1.Gabler J, et al. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol. 2007;37:3363–72. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 2.Gray D, et al. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–8. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain R, et al. A critical epithelial survival axis regulated by MCL-1 maintains thymic function in mice. Blood. 2017;130:2504–15. doi: 10.1182/blood-2017-03-771576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nedjic J, et al. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 5.Metzger TC, et al. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep. 2013;5:166–79. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, et al. xxxxxxxxx. Front Immunol. 2012;3:19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.