Abstract
Leaf photosynthetic capacity is mainly constrained by nitrogen (N) and phosphorus (P). Little attention has been given to the photosynthetic capacity of mature forests with high calcium (Ca) and magnesium (Mg) in the Karst critical zone. We measured light-saturated net photosynthesis (Asat), photosynthetic capacity (maximum carboxylation rate [Vcmax], and maximum electron transport rate [Jmax]) as well as leaf nutrient contents (N, P, Ca, Mg, potassium [K], and sodium [Na]), leaf mass per area (LMA), and leaf thickness (LT) in 63 dominant plants in a mature subtropical forest in the Karst critical zone in southwestern China. Compared with global data, plants showed higher Asat for a given level of P. Vcmax and Jmax were mainly co-regulated by N, P, Mg, and LT. The ratios of Vcmax to N or P, and Jmax to N or P were significantly positively related to Mg. We speculate that the photosynthetic capacity of Karst plants can be modified by Mg because Mg can enhance photosynthetic N and P use efficiency.
Introduction
The highly sensitive Karst Critical Zones (CZs) account for about 12% of the global terrestrial land area1, with more than 54 × 104 km2 distributed in southwestern China2. The Critical Zone (CZ) is defined by the US National Research3 as “a heterogeneous, near surface environment in which complex interactions involving rock, soil, water, air and living organisms regulate the natural habitat and determine availability of life sustaining resources.” Compared with other CZs, Karst CZs were developed on limestone, and are characterized by shallow and heterogeneous soils with higher calcium (Ca) and magnesium (Mg) contents than those of other soils, and substantial leaching4,5. Further, these soils exhibit lower nitrogen (N) and phosphorus (P) storage than non-Karst CZs soils, and have limited plant productivity4–7. Plants use different leaf economic strategies to adapt to low nutrient availability8,9. Understanding how nutrients constrain photosynthetic capacity of mature forests in Karst CZs is a prerequisite for evaluating gross primary production and predicting the carbon cycle in these areas. The maximum carboxylation rate (Vcmax) and maximum electron transport rate (Jmax) are proxies for photosynthetic capacity.
Leaf N and P are both essential nutrients involved in photosynthetic capacity. Photosynthetic capacity is usually positively related to leaf N because a large portion of N is invested in photosynthetic machinery8,10,11. Consequently, N-deficiency could reduce carboxylation capacity and electron transport rates12. In addition to leaf N, leaf P is one of the most important component of chemical compounds which are closely related to photosynthesis13,14. Consequently, P-deficiency can reduce light-use efficiency, electron transport rates15,16, enzyme activity in the Calvin cycle, regeneration of ribulose bisphosphate (RuBP)17, and the fraction of leaf N allocated to photosynthetic machinery18.
It is widely accepted that photosynthetic capacity at global scale is mainly controlled by leaf N and P11,19,20 concentrations which depend on soil nutrient status21. Reich and Oleksyn22 demonstrated that global patterns of leaf N to P ratios increase toward low latitudes and with mean temperature. Photosynthetic capacity was mainly constrained by N in temperate ecosystems12,23, and by P rather than N in subtropical and tropical ecosystems24,25. However, many previous studies reported that single-nutrient limitations or N and P co-limitation were widespread, and N and P co-limitation was more common of the two, especially in tropical ecosystems26–29.These studies highlighted the importance of synergistic interactions between N and P in regulating plant growth. Domingues et al.30 reported that N and P co-limited photosynthetic capacity in West Africa woodlands. Niinemets et al.31 observed that plant primary productivity in Karst grasslands (calcareous meadows) in temperate regions was co-limited by N and P due to low N and P availabilities in soil. Therefore, photosynthetic capacity in a mature subtropical Karst forest in southwestern China was expected to be co-limited by N and P.
Traditionally, primary productivity was predicted using linear relationships between photosynthetic capacity and leaf N32. However, this relationship can be modified by P with increasing P limitation20. On the basis of a cross-biome analysis of the impact of P limitation on the relationship between Asat and N, Reich et al.33 found that the slope of Asat-N, used as an indicator of photosynthetic N use efficiency, was higher in the Arctic and temperate ecosystems at 1.59 and 1.48, respectively, than in tropical and subtropical ecosystems at 1.23 and 1.10, respectively. In a meta-analysis of global-scale data, Kattge et al.10 found that the slope of Vcmax-N was flatter in tropical biomes, and the uncertainty in the relationship between Vcmax and leaf N was larger than that in other biomes. In addition, the uncertainty between Vcmax and leaf N can be decreased when considering P limitation on photosynthesis in tropical biomes30,34. Up till now, P limitation on photosynthetic capacity is an ongoing area of research in tropical forests34. However, research has yet to focus on the role of leaf P in photosynthetic capacity in a mature subtropical Karst forest, where N and P storage are limiting in soils.
In addition to N and P, other leaf mineral nutrients can modify tune the photosynthetic capacity35,36. Previous experiments under controlled conditions demonstrated that photosynthetic capacity can be tuned by Ca, Mg, potassium [K], and sodium [Na]. Ca ions (Ca2+) provide the terminal acceptor and regulate photosynthetic electron flow37, while Mg (Mg2+) and K (K+) ions have been implicated as light-harvesting counter-ions in thylakoids, and have opposing effects38. Battie-Laclau et al.38 evaluated the limitations of K and Na on Asat in Eucalyptus grandis, and showed that photosynthetic capacity may be improved by supplying these two elements. However, to our knowledge, only one group has reported that Asat was significantly and positively associated with N, P, K, Ca, and Mg, and that in five sapling tree species in the central Amazon rainforest under natural conditions39.
Soil quantities and storage of nutrients in Karst were much lower than those in non-Karst ecosystems due to shallow Karst soils5,6,40. However, Ca and Mg contents in Karst soils were higher than those in non-Karst soils41. The particular characteristics of Karst soils give us a unique opportunity to investigate from the point view of plant growth and economics how leaf N, P, and mineral nutrients regulate mass-based photosynthetic capacity. In this study, we selected a mature subtropical forest in the Karst CZ in southwestern China, and measured CO2 response curves of 63 C3 dominant plant species and their corresponding leaf traits (N, P, K, Ca, Mg, Na, leaf mass per area (LMA), and leaf thickness [LT]). The objective of this study was to determine whether: (1) leaf N and P co-limited photosynthetic capacity, (2) leaf mineral nutrients tune the photosynthetic capacity and if so, (3) how leaf mineral nutrients modified the relationship of photosynthetic capacity to N and photosynthetic capacity to P.
Results
Comparison of light-saturated net photosynthesis with the global data set
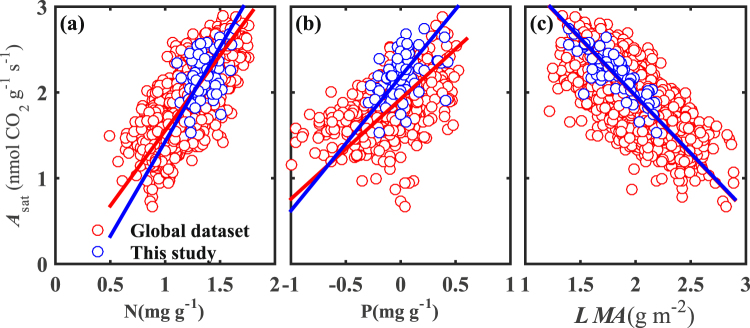
We compared the relationships of Asat to leaf N, P, and LMA in this study with those in the global data set (Fig. 1). The averaged value of Asat was 200.84 ± 116.63 nmol CO2 g−1 s−1, and ranged from 33.81 to 562.03 nmol CO2 g−1 s−1 (see Supplementary Table S1); this was within the normal range of the global dataset (4.65 to 778.41 nmol CO2 g−1 s−1)19.
Figure 1.
The relationships of leaf light-saturated net photosynthesis (Asat) to (a) leaf nitrogen (N), (b) phosphorus (P), and (c) leaf mass per area (LMA). Both axes are in log10 scale.
Compared to global data set19, plants showed a higher Asat for a given leaf P level in the mature subtropical forest, i.e. high photosynthetic P use efficiency. The slope of Asat-N in a standardized major axis fit was slightly but not significantly steeper (P = 0.333), while the intercept was slightly smaller than that in the global data set (P = 0.06; Fig. 1a). The slope of Asat-P was significantly steeper (P < 0.05), and the intercept was significantly larger than that in global data set (P < 0.05; Fig. 1b). The slope (P = 0.24) and intercept (P = 0.70) of Asat-LMA of two data sets were not significantly different (Fig. 1c).
Relationships of Vcmax and Jmax with leaf traits
We disentangled the contributions of leaf traits to photosynthetic capacity using path analysis. The Pearson correlation analysis showed that the photosynthetic capacity (Vcmax, and Jmax) was positively related to leaf N, P, Mg K, and Na, and negatively related to LT (P < 0.05) (see Table S6, Figs S1–S3). Leaf N, P, Mg, and LT were selected using a multiple stepwise regression method (P < 0.1) as significant independent variables (see Table S4). Pearson correlation analysis showed that leaf N was positively related to P, negatively to LT (P < 0.05), and not related to leaf Mg (P > 0.05) (see Table S5). Leaf P was not related to either leaf Mg or LT (P > 0.05). Leaf Mg was not related to LT (P > 0.05). These results indicated that leaf N, P, Mg, and LT had the potential to alter photosynthetic capacity directly, and leaf N was correlated with leaf P and LT.
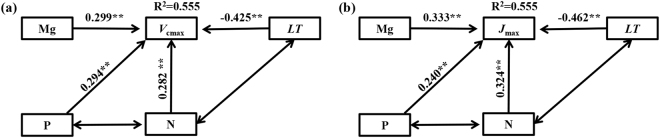
The causal relationships and relative contributions of leaf N, P, Mg, and LT to Vcmax and Jmax were presented in Fig. 2. The models explain 55.5% and 55.5% of the variation in Vcmax and Jmax, respectively. The total contribution of leaf N, P, Mg, and LT to Vcmax was 0.282, 0.294, 0.299, and −0.425, and to Jmax, it was 0.324, 0.240, 0.333, and −0.462, respectively. These results indicated that photosynthetic capacity was influenced by leaf N, P, Mg, and LT.
Figure 2.
The direct and indirect causality of leaf nitrogen (N), magnesium (Mg), and leaf thickness (LT) on (a) maximum carboxylation rate (Vcmax) and (b) maximum electron transport rate (Jmax). One way arrow indicates causality relationship between two variables; Two-way arrows represent correlated relationship between two variables. **P < 0.05, *P < 0.1. Results of model fitting: (a) χ2 = 0.486, d.f. = 4, P = 0.746, AIC = 23.944; (b) χ2 = 0.486, d.f. = 4, P = 0.746, AIC = 23.944.
Relationships of photosynthetic N and P use efficiency to leaf traits
As a whole, photosynthetic N and P use efficiencies were promoted by leaf Mg but limited by LT (Fig. 3). The effect of Mg on photosynthetic N use efficiency was similar to that of photosynthetic P use efficiency. The effect of LT on photosynthetic P use efficiency was less than that on photosynthetic N use efficiency. No relationship was found between leaf Mg and LT (P > 0.05) (see Table S5).
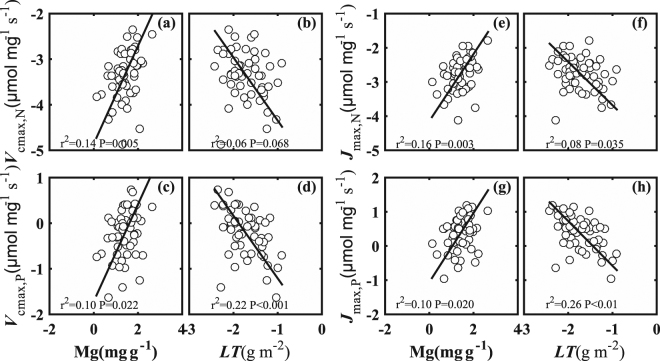
Figure 3.
Log-log plots of the ratio of maximum carboxylation rate (Vcmax) to leaf nitrogen (N) (Vcmax,N) in relation to (a) leaf magnesium (Mg) and (b) leaf thickness (LT). Log-log plots of the ratio of Vcmax to P (Vcmax,P) in relation to (c) Mg and (d) LMA. Log-log plots of the ratio of maximum electron transport rate (Jmax) to N (Jmax,N) in relation to (e) Mg and (f) LT. Log-log plots of the ratio of Jmax to P (Jmax,P) in relation to (g) Mg and (h) LT.
The effect of Mg on photosynthetic N use efficiency was similar to photosynthetic P use efficiency. The Vcmax,N (R2 = 0.14, P < 0.05), Vcmax,P (R2 = 0.10, P < 0.05), Jmax,N (R2 = 0.16, P < 0.05), and Jmax,P (R2 = 0.10, P < 0.05) were positively related to Mg. The slopes of Vcmax,N-Mg (1.07) and Vcmax,P-Mg (1.06) were larger than those of Jmax,N-Mg (0.98) and Jmax,P-Mg (1.01). These results showed that the photosynthetic N and P use efficiency was positively correlated with leaf Mg.
The effect of LT on photosynthetic P use efficiency was less than that on photosynthetic N use efficiency. The Vcmax,N (R2 = 0.06, P = 0.068), Vcmax,P (R2 = 0.22, P < 0.05), Jmax,N (R2 = 0.08, P < 0.05), and Jmax,P (R2 = 0.26, P < 0.05) showed a significant negative relationship with LT. The slopes of Vcmax,N-LT (−1.38) and Vcmax,P-LT (−1.37) were smaller than those of Jmax,N-LT (−1.27) and Jmax,P-LT (−1.31).
Discussion
Argument for mass-based vs. area-based photosynthetic capacity
The ‘leaf economic spectrum’ of traits has been described by Wright et al.11, who demonstrated that the mass-based photosynthetic capacity was positively related to mass-based leaf N and P content, and negatively related to LMA and leaf lifespan. Recently, the biological significance of the ‘leaf economic spectrum’ has become the focus of the debate. Lloyd et al.42 and Osnas et al.43 suggested that these correlations were driven by the variation in LMA, which determined the ratio of structural to metabolic components of the leaves. They thought that the photosynthetic parameters and the associated leaf nutrient traits should be expressed from the viewpoint of photosynthetic physiology on an area-basis. However, Westoby et al.44 and Poorter et al.45 emphasized the critical role of mass-based photosynthetic parameters and the corresponding leaf nutrient traits in plant growth and economics. They thought the mass-based leaf trait was a way to express the difference among species in costs and returns per unit investment.
In this study, we mainly investigated how leaf N, P, and mineral nutrients regulated mass-based photosynthetic capacity from the viewpoint of plant growth and economics. In addition, we also presented the relationship between area-based photosynthetic capacity and associated leaf traits in the Supplementary Tables S1 an S2, and discussed it below, where relevant.
Leaf N and P co-limited photosynthetic capacity
Leaf N and P are generally the major growth-limiting nutrients for plant communities when key physiological processes are considered46. The averaged leaf N content in this study was 23.39 ± 6.72 mg g−1 (see Table S7), larger than that reported by Reich & Oleksyn22 for 2151 plant species (20.1 mg g−1), and by Maire et al.19 for 1658 plant species (19.49 ± 9.30 mg g−1). The averaged leaf P content in this study was 1.11 ± 0.50 mg g−1 (see Table S7), 37% lower than the global average reported by Reich & Oleksyn22 for 923 plant species (1.77 mg g−1), and nearly identical to that reported by Maire et al.19 for 522 plant species (1.03 ± 0.65 mg g−1). Note that the data set of leaf P in Fig. 1b was reported by Maire et al.19, and leaf P was associated with Asat. The averaged leaf N:P in this study was 23.34 ± 7.81, indicating P limitation22.
The importance of synergistic interactions between N and P in regulating plant growth has been reported in many previous studies26–29. Consistent with Karst grassland31 and West Africa woodlands30, photosynthetic capacity was co-limited by N and P in this study (Fig. 2). The seemingly contradictory results can be explained by leaf economy and the differences in allocation strategies of leaf N and P.
There was a trade-off between leaf N allocation to metabolic N and structural N as means of adaptation to the limited nutrient conditions47,48. When nutrient availability was low, the fraction of leaf N partitioned to cell walls was greater, thereby LMA was high and rates of photosynthesis decreased49,50. The range of variation in LMA (24.73–154.61 g m−2) in this study was larger than that for subtropical non-Karst forest (37.08–142.32 g m−2)51. LT was negatively related to leaf N and photosynthetic capacity (see Tables S6). In addition, photosynthesis and its N use efficiency increased with a decrease in N allocation to leaf non-photosynthesis8,47. In this study, Asat and photosynthetic capacity were negatively related to LT (see Table S6). Photosynthetic N use efficiency (slope of Asat-N) in this study was higher than that in other tropical ecosystems33. On the other hand, no relationship was found between area-based photosynthetic capacity and the associated leaf N (see Table S3). These results may indicate that a trade-off existed between leaf N allocation into metabolic and structural N in this mature Karst forest.
However, there was no apparent trade-off between leaf P allocation into metabolic P and structural P9. Leaf P was preferentially allocated to photosynthetic cells in P-limited conditions52. The fraction of P in structural tissues was one order of magnitude lower than that of N53. With decreasing soil P availability, the LMA of tropical trees increased, and leaf P content decreased; however, tropical trees can maintain high photosynthetic P use efficiency without increasing P allocation into structural tissues9. In this study, mass-based leaf P was not related to LT (P > 0.05) (see Table S5), and positively related to mass-based photosynthetic capacity (P < 0.05) (see Table S6). Photosynthetic P use efficiency in Karst plants was higher than that in other tropical ecosystems (Fig. 1b). In addition, area-based photosynthetic capacity was weakly related to leaf P (P < 0.05) (see Table S3). Based on the results mentioned above, we suggested that no trade-off existed between leaf P allocation into metabolic P and structural P in this mature Karst forest.
It is commonly assumed that leaf N:P ratio is often used as a proxy for nutrient limitation. Leaf N:P ratio of <14 indicates that N is the limiting factor, while >16 that P is limiting22. However, these ratios differ when applied to different ecosystems. For example, productivity of desert shrublands was limited by P at N:P of 5–1054, while productivity of invasive species was limited by N at N:P >4055. Productivity of Karst grassland was co-limited by N and P at N:P of 5.6–7.531. The different leaf N and P allocation strategies was the main reason for the high N:P in this mature Karst forest.
Leaf Mg tuned photosynthetic capacity
The contribution of leaf Mg to Vcmax and Jmax was 0.299 and 0.333, respectively (Fig. 2), and photosynthetic N and P use efficiencies were positively related to Mg (Fig. 3). This was consistent with the results reported by Mendes & Marenco39 for tropical saplings. However, photosynthetic capacity of West Africa woodlands was not related to Mg, Ca, K etc.30. The averaged leaf Mg content was 4.61 ± 2.39 mg g−1, which was higher than that in non-Karst tropical and temperate forests56. We speculate that the photosynthetic capacity might be tuned by leaf Mg via enhancing photosynthetic N and P efficiency; a possible mechanism for this may involve the key role which leaf Mg plays in photosynthesis57.
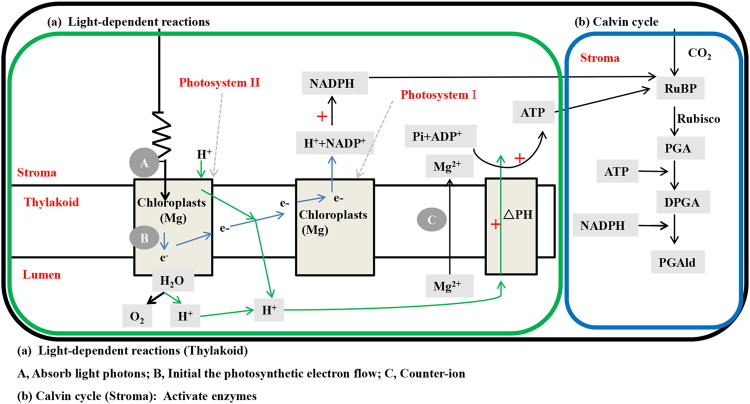
During the light-dependent reactions and the Calvin-cycle stages of photosynthesis, Mg is involved in three key biochemical processes (Fig. 4). First, as a light-dependent reaction, the chlorophyll molecule, which is composed of a central Mg ion surrounded by a group of atoms, is catalyzed by Mg56,58 (Fig. 4a). Neuhaus et al.59 and Jezek et al.60 have reported that Mg fertilizer can increase the concentration of chlorophyll, thus enhancing light harvesting efficiency61 and electron transport rates38,62,63; then, formation rates of nicotinamide adenine dinucleotide phosphate (NADPH) can also increase because NADP+ is the terminal acceptor of electron transport64.
Figure 4.
Roles of Mg in photosynthetic processes: (a) light-dependent reactions (A, absorb light photons59–61; B, initial the photosynthetic electron flow62,63; C, Counter-ion64,66) and (b) Calvin cycle stages (Part 2: active enzymes66–68). The blue arrows indicate the electron flow between photosystem II and photosystem I. The moving of hydrogen ions (H+) is indicated by the green arrows. The red “+” represents the positive effect of Mg on biochemical and physiological processes. ATP, adenosine triphosphate; ADP, adenosine diphosphate; NADPH, nicotinamide adenine dinucleotide phosphate; RuBP, ribulose 1,5-biphosphate; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; Ribulose bisphosphate carboxylase oxygenase; PGA, 3-phosphoglyceric acid; DPGA, 1,3-diphosphoglycerate; PGAld, glyceraladehyde-3-phosphate. Figure 4a was modified from Alexander N. Tikhonov64. Republished with permission of Springer Science and Bus Media B V, from Photosynthesis Research, Alexander N. Tikhonov, Volume 116, issue 2–3, pp 511–534, 2013; permission conveyed through Copyright Clearance Center, Inc.
Second, Mg can also promote the synthesis of adenosine triphosphate (ATP)65 (Fig. 4a). During electron transport, protons are pumped from the stroma into the thylakoid lumen, thus generating a proton (H+) gradient57,66 driving the synthesis of ATP38. When protons are pumped into the thylakoid lumen, Mg2+ is transported into the stroma from the lumen as a counter-ion64. The stimulating role of Mg in the H+ pump has been confirmed by Kana and Govindjee38.
Third, Mg is a cofactor and allosteric modulator for enzymes, and regulates the Calvin cycle by activating many enzymes66 (Fig. 4b). For example, ribulose-1,5-bisphosphate carboxylase (Rubisco) was activated when incubated with CO2 and Mg2+ 67. Also, Pradel et al.68 showed that the concentration of fructose 1,6-bisphosphatase increased with increasing Mg.
However, these results reported by previous studies were obtained under controlled conditions using low Mg supply. Considering the important role of Mg in photosynthesis in low-nutrient ecosystems39, there is an urgent need to explore how leaf Mg tunes photosynthetic capacity under natural conditions, especially in nutrient-poor soils.
Conclusions
Our results revealed that the photosynthetic capacity of Karst plants was co-constrained by N, P, Mg, and LT. Our analysis indicated that nutrient interactions were complex in biochemical and physiological processes. We propose that the accurate prediction of Vcmax and Jmax in a mature subtropical forest with high Ca and Mg should take into consideration not only the role of N and P but also of other mineral nutrients.
Methods
Site information
This research was conducted in a mature subtropical forest (26°14′48″N, 105°45′51″E; elevation, 1460 m) located in Puding County, Guizhou Province, in a Karst critical zone in southwestern China. The climate is subtropical monsoonal, with a mean annual precipitation of 1255 mm and a mean annual air temperature of 15.1 °C69.
Soils in this region were mainly formed by limestone and dolomite70. In this study, the (total and available) soil N and P content (see Table S7) was similar to that of other Karst soils in southwestern China71, but higher than that of non-Karst soils72. However, soil quantities (16.04~61.89 kg m−2) and nutrient storage (see Supplementary Table S7) were much lower than those of non-Karst ecosystem5,6, because of the shallow and heterogeneous soil layer (2–50 cm)73–76.
Vegetation type is a mature mixed evergreen and broad-leaved deciduous forest which is remarkably different from the non-karst forest in this region (subtropical evergreen broad-leaved)5. The dominant species include Itea yunnanensis Franch, Carpinus pubescens Burk., and Lithocarpus confinis Huang et al. (see Supplementary Table S1). Mean content of leaf N, P, Ca, Mg, Na, and K can be found in Table S7. The aboveground carbon stock in mature Karst forest in southwestern China was lower (70.3–142.2 Mg ha−1) than that in subtropical evergreen broad-leaved forests growing in non-Karst regions4. Further, the aboveground carbon stock in this study was higher than that of mature Karst forest in Mexico7, and lower than that of mature Mediterranean forest in Italy77; these differences were probably due to different thicknesses of the soil, and the amount of precipitation.
Gas exchange measurements
Leaf gas exchange was measured from July to August, 2016 using a portable photosynthesis system. This system consisted of an infrared gas analyzer (Li-Cor 6400; Li-Cor Bio Sciences, Lincoln, NE), an artificial light source (6400–02B red/blue LED light source; Li-Cor Bio Sciences), a CO2 injection system with pure CO2, and a CO2 absorbent system with a buffer bottle which supplied stable air flow without CO2. Three individuals per species were collected and measured, with a total of 189 individuals from 63 dominant species (see Supplementary Table S1) in the mature forest. Branches with sun leaves were excised from the upper part of the crown using a lopper (6 m), and immediately stored in a bucket; later, branches were snipped under water with shears to maintain xylem water continuity30. Prior to gas exchange measurements, branches were kept at 25°C for 30 min; then, a fully-expanded, mature leaf was induced for 30 minutes at a saturating light density (1500 μmol m−2 s−1).
The CO2 response curves (A-Ci curve) of light-saturated photosynthesis were determined following procedural guidelines78. In brief, CO2 concentrations inside the chamber varied from 50 to 1800 μmol mol−1(400, 300, 200, 100, 50, 400, 600, 800, 1200, 1400, 1600 and 1800 μmol mol−1). CO2 concentrations were controlled by a CO2 injector system. Photosynthetic photon flux density was set to 1500 μmol m−2 s−1, which was controlled by an artificial light source. The leaf temperature was controlled by the conditioning the block temperature to 25 °C, and the vapor pressure deficit was maintained at ambient condition. Flow rate in the cuvette was set to 500 mL min−1. The cuvette was sealed with plasticine to prevent leakage.
Leaf trait analyses
Immediately after the measurements of leaf gas exchange, leaf area (m−2), fresh mass (mg), and LT (mm) were measured. After that, leaves were oven dried at 40 °C for 48 h, and dry mass (mg) was determined. The LMA (g m−2) was calculated by dividing the corresponding dry mass by leaf area. Thereafter, dried leaves were ground to a powder for nutrient analysis. Mass-based leaf carbon (C) and N contents (mg g−1) were determined by elemental analysis (EURO EA CHNSO Analyser; HEKAtech GmbH, Wegberg, Germany). Mass-based leaf P, Ca, Mg, K, and Na contents (mg g−1) were measured using inductively-coupled plasma-optical emission spectrometry (Optima 5300 DV; Perkin Elmer, Waltham, MA). All auxiliary datasets were presented in Supplementary Table S2; for more related information, see He et al.79.
Response curve analyses
Area-based Asat (μmol CO2 m−2 s−1) under saturating light (1500 μmol m−2 s−1) and CO2 concentration (400 μmol mol−1) was extracted from the A-Ci curves30. Area-based Vcmax and Jmax (μmol CO2 m−2 s−1) were estimated using the Farquhar biochemical model80,81. We used the curve-fitting routine developed by Domingues et al.30. The enzymatic kinetic constants used in the curve-fitting routine were taken from von Caemmerer81. Mesophyll conductance was not estimated, but rather assumed to be infinite. Therefore, Vcmax and Jmax were determined based on the intercellular CO2 concentration. To compare with existing databases, calculated Vcmax and Jmax were standardized to 25 °C82.
Mass-based Asat, Vcmax, and Jmax were calculated by dividing area-based Asat, Vcmax, and Jmax by the corresponding LMA. For photosynthetic N use efficiency (μmol CO2 mg−1 s−1), we defined ratios as Vcmax to N (Vcmax,N), and Jmax to N (Jcmax,N). We defined photosynthetic P use efficiency (μmol CO2 mg−1 s−1) as the ratio of Vcmax to P (Vcmax,P), and of Jmax to P (Jcmax,P).
The relationships between area-based leaf nutrients and photosynthetic capacity are shown in Supplementary Table S3. Area-based leaf nutrients were the product of mass-based leaf nutrient content and LMA.
Statistical analysis
Contributions of leaf traits to Vcmax and Jmax were determined by path analysis83,84. In brief, the advantage of path analysis is to disentangle the causality between variables, and to quantify contributions of independent variables to dependent variable when a prior causal or correlative relationship among variables is known. Path coefficient is a statistic used to represent the causality of the related variables, and is a normalized partial regression coefficient. The contributions of independent variables to dependent variables were represented by path coefficients. A positive value of a path coefficient represented positive contribution, and vice versa. Total contribution of one of the independent variables to the dependent variable was the sum of the direct and indirect path coefficients. The proportion of variance explained was represented by R2. The model had a good fit when 0 ⩽ χ2 ⩽ 2 and 0.05 < P ⩽ 1.
The hypothesized causal relationships between photosynthetic capacity and leaf traits were developed as follows. We assumed that the photosynthetic capacity was regulated by leaf N, P, Ca, Mg, Na, K, and LT. Pearson correlation analysis showed that photosynthetic capacity (Vcmax, and Jmax) was positively related to leaf N, P, Mg, K, and Na, and negatively related to LT (P < 0.05) (see Supplementary Table S6, Figs S1–S3). A stepwise multiple regression analysis was performed to select significant independent variables among leaf traits. Jcmax was co-regulated by leaf N, P, Mg, and LT; however, Vcmax was co-regulated by leaf N, Mg, and LT at the 0.05 level. Vcmax and Jmax were co-regulated by leaf N, P, Mg, and LT at the 0.1 level. In this study, leaf N, P, Mg, and LT were selected as significant independent variables using multiple stepwise regression method (P < 0.1) (see Supplementary Table S4). Pearson correlation analysis showed that leaf N was positively related to P, negatively to LT, and not related to leaf Mg. Leaf P was not related to leaf Mg or to LT (P > 0.05). Leaf Mg was not related to LT (P > 0.05). According to these results, we proposed that leaf N, P, Mg, and LT had potential to alter photosynthetic capacity directly, and leaf N was correlated with leaf P and with LT. Path analyses were performed using AMOs 23.0 (Amos Development CO., Greene, Maine, USA).
Standardized major axis (SMA) regression fit was applied to compare the slope and intercept of Asat-N, Asat-P and Asat-LMA in this study with the global dataset19. The relationships between photosynthetic N and P use efficiency (Vcmax,N, Jcmax,N, Vcmax,P and Jcmax,P) and main contributors of Vcmax and Jmax (Mg and TL) were determined by linear regression of least square method.
Electronic supplementary material
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41571130043, 31470500, and 41671257). All auxiliary dataset were shared from “Functional Trait database of terrestrial ecosystems in China (China_Trait)”. Special thanks to Puding Karst Ecosystem Observation and Research Station, Chinese Academy of Sciences for providing the study with convenient conditions.
Author Contributions
J.W., X.F.W. and X.Y.Z. planed and designed the research. J.W. performed experiments and analyzed data. J.W., X.F.W., X.Y.Z., L.S.G. and Z.D.Y. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25839-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xuefa Wen, Email: wenxf@igsnrr.ac.cn.
Xinyu Zhang, Email: zhangxy@igsnrr.ac.cn.
References
- 1.Xiao SS, Zhang W, Ye YY, Zhao J, Wang KL. Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem. Sci. Rep. 2017;7:41402. doi: 10.1038/srep41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong X, et al. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain. 2018;1:44–50. doi: 10.1038/s41893-017-0004-x. [DOI] [Google Scholar]
- 3.National Research Council (NRC). Basic research opportunities in Earth science. (Washington, 2001).
- 4.Liu CC, et al. Aboveground carbon stock, allocation and sequestration potential during vegetation recovery in the karst region of southwestern China: A case study at a watershed scale. Agr. Ecosyst. Environ. 2016;235:91–100. doi: 10.1016/j.agee.2016.10.003. [DOI] [Google Scholar]
- 5.Liu Y, Liu C, Wang S, Guo K, Yang J. Organic Carbon Storage in Four Ecosystem Types in the Karst Region of Southwestern China (vol 8, e56443, 2013) PloS One. 2014;9:1. doi: 10.1371/journal.pone.0056443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Wang K. Ponderation on the Shortage of Mineral Nutrients in the Soil-Vegetation Ecosystemin Carbonate Rock-distributed Mountain Regions in Southwest China. Earth and Enviroment. 2009;37:337–341. [Google Scholar]
- 7.Aryal DR, De Jong BHJ, Ochoa-Gaona S, Esparza-Olguin L, Mendoza-Vega J. Carbon stocks and changes in tropical secondary forests of southern Mexico. Agr. Ecosyst. Environ. 2014;195:220–230. doi: 10.1016/j.agee.2014.06.005. [DOI] [Google Scholar]
- 8.Guan LL, Wen DZ. More nitrogen partition in structural proteins and decreased photosynthetic nitrogen-use efficiency of Pinus massoniana under in situ polluted stress. J. Plant Res. 2011;124:663–673. doi: 10.1007/s10265-011-0405-2. [DOI] [PubMed] [Google Scholar]
- 9.Hidaka A, Kitayama K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011;99:849–857. doi: 10.1111/j.1365-2745.2011.01805.x. [DOI] [Google Scholar]
- 10.Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Global Change Biol. 2009;15:976–991. doi: 10.1111/j.1365-2486.2008.01744.x. [DOI] [Google Scholar]
- 11.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 12.Hikosaka K. Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J. Plant Res. 2004;117:481–494. doi: 10.1007/s10265-004-0174-2. [DOI] [PubMed] [Google Scholar]
- 13.Bloomfield KJ, Farquhar GD, Lloyd J. Photosynthesis-nitrogen relationships in tropical forest tree species as affected by soil phosphorus availability: a controlled environment study. Funct. Plant Biol. 2014;41:820–832. doi: 10.1071/FP13278. [DOI] [PubMed] [Google Scholar]
- 14.Crous KY, et al. Nitrogen and phosphorus availabilities interact to modulate leaf trait scaling relationships across six plant functional types in a controlled-environment study. New Phytol. 2017;215:992–1008. doi: 10.1111/nph.14591. [DOI] [PubMed] [Google Scholar]
- 15.Conroy JP, Smillie RM, Kuppers M, Bevege DI, Barlow EW. Chlorophyll-alpha fluorescence and photosynthetic and growth-response of pinus-radiata to phosphorus deficiency, drought stress, and high CO2. Plant Physiol. 1986;81:423–429. doi: 10.1104/pp.81.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha HFV, Goncalves JFD, dos Santos UM, Ferreira MJ, Peixoto PHP. Biomass, gas exchange and nutritional aspects of young plants of Balsa Wood (Ochroma pyramidale (Cav. Ex Lamb.) Urb.) subjected to phosphorus fertilization in contrasting irradiance environments. Sci. For. 2016;44:215–230. [Google Scholar]
- 17.Campbell CD, Sage RF. Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.) Plant Cell Environ. 2006;29:844–853. doi: 10.1111/j.1365-3040.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- 18.Warren CR, McGrath JF, Adams MA. Differential effects of N, P and K on photosynthesis and partitioning of N in Pinus pinaster needles. Ann. For. Sci. 2005;62:1–8. doi: 10.1051/forest:2004088. [DOI] [Google Scholar]
- 19.Maire V, et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Global Ecol. Biogeogr. 2015;24:706–717. doi: 10.1111/geb.12296. [DOI] [Google Scholar]
- 20.Walker AP, et al. The relationship of leaf photosynthetic traits - V-cmax and J(max) - to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol. Evol. 2014;4:3218–3235. doi: 10.1002/ece3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich PB, Hobbie SE. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Clim. Change. 2013;3:278–282. doi: 10.1038/nclimate1694. [DOI] [Google Scholar]
- 22.Reich PB, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. P. Natl. Acad. Sci, USA. 2004;101:11001–11006. doi: 10.1073/pnas.0403588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol. Lett. 2014;17:82–91. doi: 10.1111/ele.12211. [DOI] [PubMed] [Google Scholar]
- 24.Ellsworth DS, Crous KY, Lambers H, Cooke J. Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant Cell Environ. 2015;38:1142–1156. doi: 10.1111/pce.12468. [DOI] [PubMed] [Google Scholar]
- 25.Goll DS, et al. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences. 2012;9:3547–3569. doi: 10.5194/bg-9-3547-2012. [DOI] [Google Scholar]
- 26.Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B. Soil parent material-A major driver of plant nutrient limitations in terrestrial ecosystems. Global Change Biol. 2017;23:3808–3824. doi: 10.1111/gcb.13691. [DOI] [PubMed] [Google Scholar]
- 27.Harpole WS, et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011;14:852–862. doi: 10.1111/j.1461-0248.2011.01651.x. [DOI] [PubMed] [Google Scholar]
- 28.Elser JJ, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 29.LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- 30.Domingues TF, et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 2010;33:959–980. doi: 10.1111/j.1365-3040.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- 31.Niinemets U, Kull K. Co-limitation of plant primary productivity by nitrogen and phosphorus in a species-rich wooded meadow on calcareous soils. Acta. Oecol. 2005;28:345–356. doi: 10.1016/j.actao.2005.06.003. [DOI] [Google Scholar]
- 32.Thomas RQ, Brookshire EN, Gerber S. Nitrogen limitation on land: how can it occur in Earth system models? Glob Chang Biol. 2015;21:1777–1793. doi: 10.1111/gcb.12813. [DOI] [PubMed] [Google Scholar]
- 33.Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- 34.Norby RJ, et al. Informing models through empirical relationships between foliar phosphorus, nitrogen and photosynthesis across diverse woody species in tropical forests of Panama. New Phytol. 2017;215:1425–1437. doi: 10.1111/nph.14319. [DOI] [PubMed] [Google Scholar]
- 35.Hohmann-Marriott MF, Blankenship RE. Evolution of Photosynthesis. Annu. Rev. Plant Biol. 2011;62:515–548. doi: 10.1146/annurev-arplant-042110-103811. [DOI] [PubMed] [Google Scholar]
- 36.Townsend AR, Cleveland CC, Houlton BZ, Alden CB, White JWC. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 2011;9:9–17. doi: 10.1890/100047. [DOI] [Google Scholar]
- 37.Hochmal AK, Schulze S, Trompelt K, Hippler M. Calcium-dependent regulation of photosynthesis. Bba-Biomembranes. 2015;1847:993–1003. doi: 10.1016/j.bbabio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Battie-Laclau P, et al. Photosynthetic and anatomical responses of Eucalyptus grandis leaves to potassium and sodium supply in a field experiment. Plant Cell Environ. 2014;37:70–81. doi: 10.1111/pce.12131. [DOI] [PubMed] [Google Scholar]
- 39.Mendes KR, Marenco RA. Photosynthetic traits of tree species in response to leaf nutrient content in the central Amazon. Theor, Exp. Plant Phys. 2015;27:51–59. doi: 10.1007/s40626-015-0031-9. [DOI] [Google Scholar]
- 40.Vargas R, Allen MF, Allen EB. Biomass and carbon accumulation in a fire chronosequence of a seasonally dry tropical forest. Global Change Biol. 2008;14:109–124. [Google Scholar]
- 41.Strom L, Owen AG, Godbold DL, Jones DL. Organic acid behaviour in a calcareous soil implications for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005;37:2046–2054. doi: 10.1016/j.soilbio.2005.03.009. [DOI] [Google Scholar]
- 42.Lloyd J, Bloomfield K, Domingues TF, Farquhar GD. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol. 2013;199:311–321. doi: 10.1111/nph.12281. [DOI] [PubMed] [Google Scholar]
- 43.Osnas JLD, Lichstein JW, Reich PB, Pacala SW. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science. 2013;340:741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- 44.Westoby M, Reich PB, Wright IJ. Understanding ecological variation across species: area-based vs mass-based expression of leaf traits. New Phytol. 2013;199:322–323. doi: 10.1111/nph.12345. [DOI] [PubMed] [Google Scholar]
- 45.Poorter H, Lambers H, Evans JR. Trait correlation networks: a whole-plant perspective on the recently criticized leaf economic spectrum. New Phytol. 2014;201:378–382. doi: 10.1111/nph.12547. [DOI] [PubMed] [Google Scholar]
- 46.Hedin LO. Global organization of terrestrial plant-nutrient interactions. P. Natl. Acad. Sci, USA. 2004;101:10849–10850. doi: 10.1073/pnas.0404222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng YL, et al. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. P. Natl. Acad. Sci, USA. 2009;106:1853–1856. doi: 10.1073/pnas.0808434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hidaka A, Kitayama K. Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. J. Ecol. 2009;97:984–991. doi: 10.1111/j.1365-2745.2009.01540.x. [DOI] [Google Scholar]
- 49.Onoda Y, et al. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017;214:1447–1463. doi: 10.1111/nph.14496. [DOI] [PubMed] [Google Scholar]
- 50.Blanes MC, Vinegla B, Merino J, Carreira JA. Nutritional status of Abies pinsapo forests along a nitrogen deposition gradient: do C/N/P stoichiometric shifts modify photosynthetic nutrient use efficiency? Oecologia. 2013;171:797–808. doi: 10.1007/s00442-012-2454-1. [DOI] [PubMed] [Google Scholar]
- 51.Liu, W. & Leaf N, P. stotichiometry and allocation of main deciduous and evergreen tree species in Tiantong National Forest Park, Zhejiang Province, China. (Esast China Normal University,2014).
- 52.Hayes PE, Clode PL, Oliveira RS. & Lambers, H. Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: An adaptation improving phosphorus-use efficiency. Plant, Cell Environ. 2018 doi: 10.1111/pce.13124. [DOI] [PubMed] [Google Scholar]
- 53.Ji Q. Q. Content and allocation of nitrogen and phosphorus in the leaves of 8 species of trees and shrubs in different successional stage of the evergreen borad-leaved forest and the relations with their photosynthetic abilities in Tiantong National Forest Park, Zhejiang Province, China. (East China Normal University, 2004).
- 54.Drenovsky RE, Richards JH. Critical N: P values: Predicting nutrient deficiencies in desert shrublands. Plant Soil. 2004;259:59–69. doi: 10.1023/B:PLSO.0000020945.09809.3d. [DOI] [Google Scholar]
- 55.Tomassen HBM, Smolders AJP, Limpens J, Lamers LPM, Roelofs JGM. Expansion of invasive species on ombrotrophic bogs: desiccation or high N deposition? J. Appl. Ecol. 2004;41:139–150. doi: 10.1111/j.1365-2664.2004.00870.x. [DOI] [Google Scholar]
- 56.Scott BJ, Robson AD. Changes in the content and from of magnesium in the 1st trifoliate leaf of subterranean clover under altered oe constant root supply. Aust. J. Agric. Res. 1990;41:511–519. doi: 10.1071/AR9900511. [DOI] [Google Scholar]
- 57.Cakmak I, Kirkby EA. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008;133:692–704. doi: 10.1111/j.1399-3054.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 58.Walker CJ, Weinstein JD. Future characterization of the magesium chelatase in isolated developing cucumber chloroplasts-sunstrate-specificitty, regulation, intactness, and atp requirments. Plant Physiol. 1991;95:1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuhaus C, Geilfus CM, Muhling KH. Increasing root and leaf growth and yield in Mg-deficient faba beans (Vicia faba) by MgSO4 foliar fertilization. J. Plant Nutr. Soil Sci. 2014;177:741–747. doi: 10.1002/jpln.201300127. [DOI] [Google Scholar]
- 60.Jezek M, Geilfus CM, Bayer A, Muhling KH. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015;5:1–10. doi: 10.3389/fpls.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kana R. & Govindjee. Role of Ions in the Regulation of Light-Harvesting. Front. Plant Sci. 2016;7:17. doi: 10.3389/fpls.2016.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farhat N, et al. Preferential damaging effects of limited magnesium bioavailability on photosystem I in Sulla carnosa plants. Planta. 2015;241:1189–1206. doi: 10.1007/s00425-015-2248-x. [DOI] [PubMed] [Google Scholar]
- 63.Tang N, Li Y, Chen LS. Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant Nutr. Soil Sci. 2012;175:784–793. doi: 10.1002/jpln.201100329. [DOI] [Google Scholar]
- 64.Tikhonov AN. pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynthesis Res. 2013;116:511–534. doi: 10.1007/s11120-013-9845-y. [DOI] [PubMed] [Google Scholar]
- 65.Shabala S, Pottosin I. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- 66.Shaul O. Magnesium transport and function in plants: the tip of the iceberg. BioMetals. 2002;15:309–323. doi: 10.1023/A:1016091118585. [DOI] [PubMed] [Google Scholar]
- 67.Lorimer GH, Badger MR, Andrews TJ. Actication of ribulose-1,5-bisphosphate carboxylase by Carbon-dioxide and Magnesiu-ions-Equtibria, Kinetics, A suggested mechanism, and physiological implications. Biochemistry. 1976;15:529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- 68.Pradel J, Soulie JM, Buc J, Meunier JC, Ricard J. On the activation of Fructose-1,6-bioshosphatase of Spinach-chloroplasts and the regulation of the Clavin cyscle. Eur. J. Biochem. 1981;113:507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- 69.Zeng C, Liu ZH, Zhao M, Yang R. Hydrologically-driven variations in the karst-related carbon sink fluxes: Insights from high-resolution monitoring of three karst catchments in Southwest China. J. Hydrol. 2016;533:74–90. doi: 10.1016/j.jhydrol.2015.11.049. [DOI] [Google Scholar]
- 70.Zhou, Y. & Luo, M. Influenceing factors of Soil Thickness in Karst Small Watershed. Journal of Mountain Agriculture and Biology36, 1–5 (2017).
- 71.Zhang W, et al. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil. 2015;391:77–91. doi: 10.1007/s11104-015-2406-8. [DOI] [Google Scholar]
- 72.Huang W, et al. Differences in the responses of photosystem I and photosystem II of three tree species Cleistanthus sumatranus, Celtis philippensis and Pistacia weinmannifolia exposed to a prolonged drought in a tropical limestone forest. Tree Physiol. 2013;33:211–220. doi: 10.1093/treephys/tps132. [DOI] [PubMed] [Google Scholar]
- 73.He NP, Yu Q, Wu L, Wang YS, Han XG. Carbon and nitrogen store and storage potential as affected by land-use in a Leymus chinensis grassland of northern China. Soil Biol. Biochem. 2008;40:2952–2959. doi: 10.1016/j.soilbio.2008.08.018. [DOI] [Google Scholar]
- 74.Jobbagy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000;10:423–436. doi: 10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2. [DOI] [Google Scholar]
- 75.Lu XT, Yin JX, Jepsen MR, Tang JW. Ecosystem carbon storage and partitioning in a tropical seasonal forest in Southwestern China. For. Ecol. Manage. 2010;260:1798–1803. doi: 10.1016/j.foreco.2010.08.024. [DOI] [Google Scholar]
- 76.Li ZP, et al. Assessment of soil organic and carbonate carbon storage in China. Geoderma. 2007;138:119–126. doi: 10.1016/j.geoderma.2006.11.007. [DOI] [Google Scholar]
- 77.De Simon G, Alberti G, Delle Vedove G, Zerbi G, Peressotti A. Carbon stocks and net ecosystem production changes with time in two Italian forest chronosequences. Eur. J. For. Res. 2012;131:1297–1311. doi: 10.1007/s10342-012-0599-4. [DOI] [Google Scholar]
- 78.Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003;54:2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- 79.He N, et al. Variation in leaf anatomical traits from tropical to cold-temperate forests and linkage to ecosystem functions. Funct. Ecol. 2018;32:10–19. doi: 10.1111/1365-2435.12934. [DOI] [Google Scholar]
- 80.Farquhar GD, Caemmerer SV, Berry JA. A biochemical-model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 81.von Caemmerer, S. Biochemical Model of Leaf Photosynthesis (SCIRO, 2000).
- 82.Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001;24:253–259. doi: 10.1111/j.1365-3040.2001.00668.x. [DOI] [Google Scholar]
- 83.Schemske DW, Horvitz CC. Plant animal interactions and fruit production in a neotropical herb- A Path-analysis. Ecology. 1988;69:1128–1137. doi: 10.2307/1941267. [DOI] [Google Scholar]
- 84.Huxman TE, Turnipseed AA, Sparks JP, Harley PC, Monson RK. Temperature as a control over ecosystem CO2 fluxes in a high-elevation, subalpine forest. Oecologia. 2003;134:537–546. doi: 10.1007/s00442-002-1131-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.