Abstract
Interactions between bacteria and colon cancer cells influence the transcription of the host cell. Yet is it undetermined whether the bacteria itself or the communication between the host and bacteria is responsible for the genomic changes in the eukaryotic cell. Now, we have investigated the genomic and epigenetic consequences of co-culturing colorectal carcinoma cells with membrane vesicles from pathogenic bacteria Vibrio cholerae and non-pathogenic commensal bacteria Escherichia coli. Our study reveals that membrane vesicles from pathogenic and commensal bacteria have a global impact on the gene expression of colon-carcinoma cells. The changes in gene expression correlate positively with both epigenetic changes and chromatin accessibility of promoters at transcription start sites of genes induced by both types of membrane vesicles. Moreover, we have demonstrated that membrane vesicles obtained only from V. cholerae induced the expression of genes associated with epithelial cell differentiation. Altogether, our study suggests that the observed genomic changes in host cells might be due to specific components of membrane vesicles and do not require communication by direct contact with the bacteria.
Introduction
During growth, both Gram-negative and Gram-positive bacteria release membrane vesicles (MVs) from the bacterial surface. MVs secretion can occur in different environments including aquatic environments, during formation of biofilms and in the infected host1. They are spherical membranous particles with 20–300 nm diameters, and through their formation may entrap common or specific bacterial components such us periplasmic components, lipopolysaccharides (LPS), peptidoglycan, phospholipids, nucleic acids, proteins, ion metabolites, enzymes, and specific bacterial components2–4. These MVs need to be considered in many contexts of bacterial interactions with the host environment, where they may be involved in extracellular signalling. Moreover, MVs serve as long distance vehicles of multifunctional bacterial cargos including several toxins and immune modulators5–17. MVs may use several different pathways to internalize into the host cells, including endocytosis as well as fusion with the eukaryotic plasma membrane18. Previously, we reported that the cytolethal distending toxin (CDT), a genotoxin associated with MVs from Aggregatibacter actinomycetemcomitans, was internalized in both HeLa cells and human gingival fibroblasts (HGF) via a mechanism of MVs fusion with lipid rafts in the plasma membrane10. The exact intracellular fate of MVs and how they interact with intracellular organelles still remains unclear. MVs-associated factors may cause very distinct and biologically relevant effects in the target cells such as: (1) empowering bacteria to subvert both the host immune and microbiome-associated defence systems, (2) preventing disease development or (3) mediating the anti-inflammatory and intestinal barrier protection5,19–21.
Epigenetic modifications in eukaryotes contribute to modulate gene transcription and they may include DNA methylation, histone modifications and modulation of long non-coding RNA and microRNA expression22. DNA methylation is mainly associated with transcriptional gene repression, whereas histone modifications are correlated with either transcriptional gene activation or repression. Moreover, the state of chromatin compaction affects gene transcription. Previous studies have shown that bacterial pathogens are able to modulate the host transcriptional profile through epigenetic modifications. For instance, changes in DNA methylation and histone acetylation of host cells have been identified in response to infections of several types of bacteria such as Helicobacter pylori23,24, uropathogenic Escherichia coli25, Porphyromonas gingivalis24, Fusobacterium nucleatum26, Listeria monocytogenes24,27,28, Legionella pneumophila29 or commensal Bacteroides vulgatus30. Interestingly, some studies have identified that the intestinal microbiome alters the miRNA expression of the gut as a mechanism to maintain the intestinal symbiotic system31–34. Moreover, short-chain fatty acids produced by intestinal microbiota are known to act as a histone deacetylase inhibitors35, which suggests that commensal bacteria might also induce epigenetic changes into the gut microbiota. However, the mechanism by which bacteria might induce epigenetic changes of the colon tissue is not fully elucidated. Previously, it was proposed that commensal bacteria regulate intestinal inflammation through DNA methylation of the TLR4 gene36. Furthermore, an epidemiological study suggested that an oral bacterium, Fusobacterium nucleatum, might contribute to epigenetic changes in colon carcinoma tissue37. Yet, it is not well understood how the communication between bacteria and host occurs, or whether any released component from bacteria might be responsible of such genomic effects. To our knowledge, it is unknown whether bacterial MVs can also target host cell epigenetics as an alternative to the presence of whole bacteria. In this study, we aimed to investigate the genomic and epigenetic consequences of co-culturing HCT8 colorectal carcinoma cells with MVs isolated from pathogenic and non-pathogenic commensal bacterial strains, Vibrio cholerae strain C6706 and Escherichia coli K-12 strain MC1061, respectively.
Results
In this study we examined the impact of MVs isolated from V. cholerae and E. coli on HCT8 cells from human ileocecal colorectal adenocarcinoma. MVs were isolated from the supernatants of bacteria grown to late-stationary phase and visualised with Transmission Electron Microscopy (Fig. 1A, upper panel). Then, HCT8 cells were co-cultured with MVs from E. coli or V. cholerae or mock-treated for 5 hours. After incubation, cells were collected to further determine changes in gene expression (by RNA sequencing), histone mark modification of active promoters (by H3K4me3 ChIP sequencing) and chromatin accessibility (by FAIRE sequencing) (Fig. 1A, lower panel).
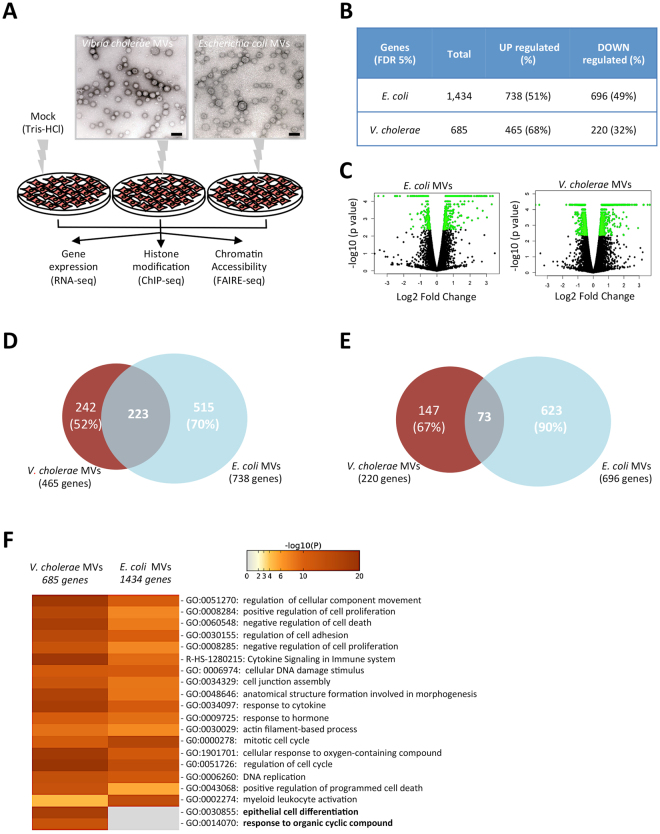
Figure 1.
Bacterial membrane vesicles (MVs) target the gene transcription of HCT8 colorectal carcinoma cell line. (A) HCT8 cells were co-cultured with MVs from E. coli, V. cholerae or mock-treated (control) for 5 hours. The setup of the study with the following methods is shown: RNA sequencing, ChIP sequencing (H3K4me3) and FAIRE sequencing (nucleosome-free DNA), Bars; 200 nm. (B) Table of RNA transcripts significantly regulated by MVs from E. coli or V. cholerae compared to mock. (C) Volcano plots of differentially regulated host cell genes by MVs from E. coli or V. cholerae compared to mock. (D) Venn Diagram showing the overlap of upregulated genes between HCT8 cells co-cultured with MVs from E. coli and V. cholerae. (E) Venn Diagram showing the overlap of downregulated genes between HCT8 cells co-cultured with MVs from E. coli and V. cholerae. (F) Gene ontology enriched terms for genes differentially regulated in HCT8 cells co-cultured with MVs from E. coli or V. cholerae.
MVs isolated from E. coli and V. cholerae affect the gene expression of HCT8 cells
To determine the effect of MVs on colon carcinoma cells, we first assessed the influence of E. coli MVs and V. cholerae MVs on global gene expression in HCT8 cells. In order to identify the primary impact of both MVs on host cell gene expression, we used an early incubation time point of 5 hours. To determine differential regulation of transcripts by specific bacterial MVs we isolated RNA from MVs-treated cells and performed RNA sequencing (Fig. 1A). We identified a total of 1,434 and 685 genes differentially regulated by E. coli MVs and V. cholerae MVs, respectively (Fig. 1B). We considered 2-fold changes in gene expression as differential regulation when cells treated with MVs were compared to untreated cells. Around 51% (738 out of 1,434) of the genes affected by E. coli MVs were significantly upregulated at least two-fold when compared to control cells (Fig. 1B and left panel of Fig. 1C). Moreover, we observed that around 68% (465 out of 685) of the genes affected by V. cholerae MVs treatment were significantly upregulated at least two-fold when compared to control cells (Fig. 1B and right panel of Fig. 1C). The comparison of gene transcripts significantly upregulated by both types of MVs revealed a substantial overlap and included 223 genes (Fig. 1D). Furthermore, the analysis of the downregulated genes revealed a more limited overlap (73 genes) between those affected by V. cholerae and E. coli MVs (Fig. 1E). By contrast, we identified that around 70% of the upregulated and 90% of the downregulated genes affected by E. coli MVs were not significantly affected by V. cholerae MVs, whereas only 52% of the upregulated and 67% of the downregulated genes affected by V. cholerae MVs were not significantly affected by E. coli MVs (Fig. 1D,E). The results of this analysis indicated that the MVs of the two species of bacteria induced differential gene expression in HCT8 cells. However, when we performed quantification of the global expression of genes regulated by treatment of cells with either of the two species of MVs we identified less differential gene expression. The analysis indicated that the significantly upregulated genes in the cells treated with E. coli MVs could also be upregulated by V. cholerae MVs and vice versa. The quantification of the global expression indicated that genes significantly downregulated by E. coli MVs were not downregulated by V. cholerae MVs and vice versa. (Supplementary Fig. S1). Altogether, these results suggested that both E. coli and V. cholerae MVs could stimulate a similar set of gene transcripts. Furthermore, we investigated how the genes regulated by both MVs relate to cell-specific functions. We performed pathway analysis with the distinct subset of genes modulated by E. coli MVs or V. cholerae MVs treatment (1,434 and 685 genes, respectively). The results revealed that the genes regulated by both types of MVs showed enrichment of common GO terms. Moreover, the GO term analysis revealed epithelial cell differentiation and response to organic cyclic compound as GO terms specifically enriched for treatment with V. cholerae MVs (Fig. 1F). The changes in gene expression were confirmed by realtime PCR after exposing HCT8 cells to different concentrations of MVs. We identified that gene transcripts enriched at GO term epithelial cell differentiation were exclusively regulated by V. cholerae MVs (Supplementary Fig. 2A). Moreover, the expression of gene transcripts commonly upregulated by both MVs was validated by realtime PCR (Supplementary Fig. 2B). Furthermore, we aimed to validate whether the selective role of the pathogenic MVs might be observed in other cancer cell lines. Hence, we exposed MCF-7 breast cancer cells to different concentrations of MVs from V. cholerae and determined the expression of genes enriched towards epithelial cell differentiation (Supplementary Fig. 2C). The exposure of breast cancer cells to different concentrations of MVs from V. cholerae did not increase the expression of the cell differentiation genes investigated as was observed in colon cancer cells, which suggested a tissue-specific role of the pathogenic bacteria.
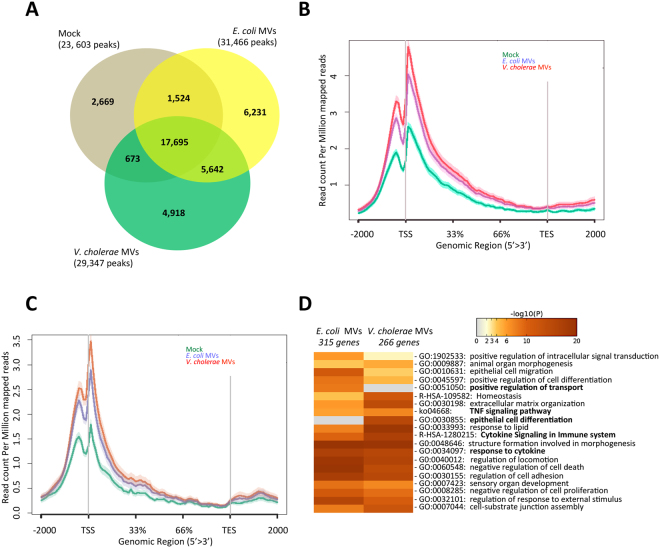
To determine whether MVs have an impact on the epigenetics of colon carcinoma cells, we assessed the H3K4me3 interaction at genome-wide level by ChIP sequencing. H3K4me3 is a histone mark modification at the transcription start sites (TSS) that correlates positively with active transcription. We performed H3K4me3 ChIP sequencing of the HCT8 cells, and their binding interactions were called using MACS38, where mock and MVs-treated HCT8 cells were analysed. We found 31,466 H3K4me3 binding events with E. coli MVs treatment, 29,347 with V. cholerae MVs treatment and 23,603 with mock treatment (Fig. 2A). The comparison of binding events revealed a substantial overlap in H3K4me3 binding sites among the three conditions (75% for mock: 17,695 out of 23,603; 56% for E. coli MVs: 17,695 out of 31,466; and 60% for V. cholerae MVs: 17,695 out of 29,347). Subsequently, we investigated whether the upregulation of gene transcripts, due to the co-culture with MVs from E. coli or V. cholerae, correlated with an increase of histone mark H3K4me3 at their TSS. Hence, we analysed the distribution of reads from H3K4me3 Chip-sequencing experiments in a window covering the transcription start sites (TSS) and the termination end sites (TES) at genes that were upregulated after MV treatment. We observed that both pathogenic and non-pathogenic MVs increased the signal with H3K4me3 around the TSS of genes (relative to control treated cells) that were upregulated by E. coli MVs (Fig. 2B). In the same regard, we identified in both types of MVs an increase of H3K4me3 signal around the TSS of genes upregulated by V. cholerae MVs (Fig. 2C). These results were in agreement with the expression data (Fig. 1) and supported that the MVs from both pathogenic and non-pathogenic bacteria have a positive impact on the activation of gene transcripts in HCT8 cells. Next, we performed a pathway analysis within the MVs-regulated genes associated with H3K4me3 peaks. This analysis showed enrichment of common GO terms. Moreover, the analysis revealed a specific gene signature associated with positive regulation of transport in case of E. coli MVs treatment and epithelial cell differentiation in case of V. cholerae MVs treatment (Fig. 2D), which confirmed our results from Fig. 1D. All together, these results supported the idea that MVs from pathogenic V. cholerae might have an impact on the set of genes involved in differentiation of colon carcinoma cells.
Figure 2.
MVs influence the epigenetic mark H3K4me3 at promoters and TSS of gene transcripts in HCT8 colorectal cancer cell line. (A) Venn Diagram showing the overlap of H3K4me3 peaks of HCT8 cells treated with MVs from E. coli, V. cholerae or mock-treated cells. (B) H3K4me3 signal at transcription start sites (TSS) of upregulated genes from HCT8 cells co-cultured with E. coli MVs (738 genes). (C) H3K4me3 signal at TSS of upregulated genes from HCT8 cells co-cultured with V. cholerae MVs (465 genes). (D) Gene ontology enriched terms for upregulated genes and H3K4me3 binding in HCT8 cells co-cultured with MVs from E. coli or V. cholerae.
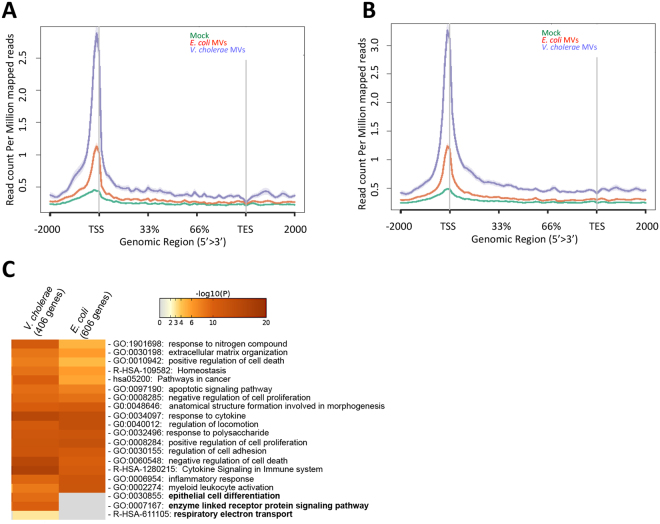
Previously, it has been reported that transcriptional activation correlates with an increase of nucleosome-free DNA39, which is an event that facilitates the binding of basic transcriptional machinery and activity of RNA polymerase. Therefore, we aimed to investigate whether MVs might influence the DNA-accessibility at the TSS of MVs-targeted genes identified at Fig. 1. We performed formaldehyde-assisted isolation of regulatory elements (FAIRE) coupled with high-throughput sequencing to identify euchromatic regions of the genome40. FAIRE is a method used for determining open chromatin regions (nucleosome-free DNA) that are accessible to transcription factors and the basal transcriptional machinery. The DNA accessibility of a promoter or TSS correlates with active gene transcription. Hence, HCT8 cells were treated with E. coli MVs, with V. cholerae MVs or mock-treated for 5 h, as indicated in Fig. 1A. Furthermore, nucleosome-free DNA was isolated and sequenced. Finally, we examined the FAIRE signal (nucleosome-free DNA) at genes upregulated by MVs from V. cholerae (Fig. 3A) and by MVs from E. coli (Fig. 3B). MVs from both bacterial species were able to open the chromatin at TSS of genes leading to upregulation of target genes. Interestingly, the effect of V. cholerae MVs on the chromatin at TSS was more pronounced compared to that of E. coli MVs. Next, we performed pathway analysis of upregulated genes that contained FAIRE signal. The results revealed that the upregulated genes from both types of bacterial MVs were significantly enriched for the majority of the terms. Interestingly, this analysis revealed that a few pathways were exclusively enriched for V. cholerae MVs (Fig. 3C). Among others, the term of epithelial cell differentiation was one of the most significant pathways identified, which confirmed additionally our findings with the histone mark H3K4me3 (from Fig. 2D).
Figure 3.
Influence of MVs on the opening of chromatin at promoters and TSS of upregulated gene transcripts in HCT8 colorectal cancer cell line. (A) FAIRE signal (nucleosome free-DNA) at TSS of upregulated genes (465 genes) in HCT8 cells co-cultured with MVs from V. cholerae. (B) FAIRE signal at TSS of upregulated genes (738 genes) in HCT8 cells co-cultured with MVs from E. coli. (C) GO enriched terms of genes upregulated and with FAIRE signal in HCT8 cells co-cultured with MVs from E. coli or V. cholerae.
Differential effects of MVs isolated from pathogenic and non-pathogenic bacteria on the target genes
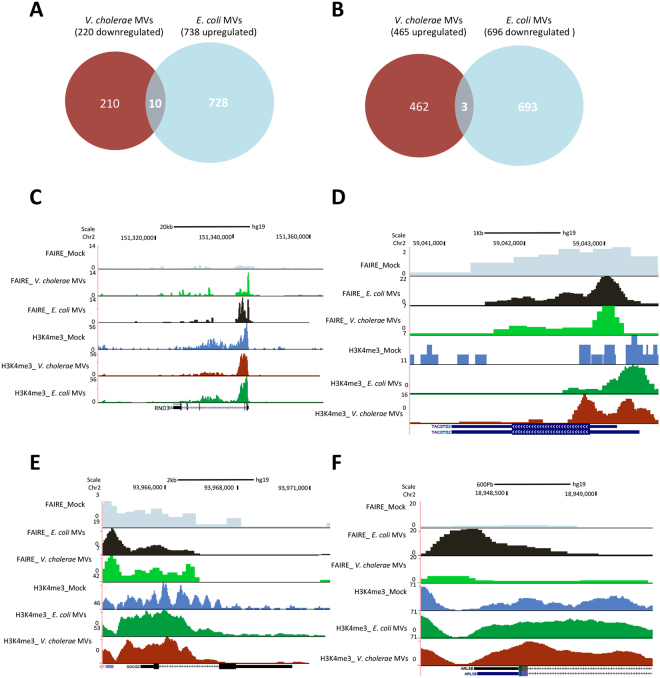
Our results suggested that a high number of gene transcripts, significantly regulated in colorectal carcinoma cell line, were commonly induced after both MV treatments. Interestingly, a subset of genes associated with epithelial cell differentiation were consistently upregulated exclusively by V. cholerae MVs. Next, we aimed to determine whether V. cholerae MVs could differentially regulate the gene transcription compared to E. coli MVs and vice versa. For this analysis, we compared the gene transcripts upregulated by E. coli MVs with the gene transcripts downregulated by V. cholerae MVs (Fig. 4A). The results revealed that a very low number of genes upregulated by E. coli vesicles were in fact downregulated by V. cholerae vesicles (1.3%, 10 out of 738 genes). Moreover, we compared the gene transcripts upregulated by V. cholerae MVs with the gene transcripts downregulated by E. coli MVs (Fig. 4B). We also observed that a very low number of genes upregulated by V. cholerae MVs were in fact downregulated by E. coli MVs (0,6%, 3 out of 465 genes). Despite the fact that a few of these genes are differentially regulated by V. cholerae MVs and E. coli MVs, we could identify some genes, which are important in the development of colon cancer. For instance, RND3 (Fig. 4C), TACSTD2 (Fig. 4D) and SOCS2 (Fig. 4E) were examples of genes upregulated by E. coli MVs and downregulated by V. cholerae MVs. Interestingly, V. cholerae MVs increased the chromatin accessibility at the TSS of the target genes, whereas the E. coli MVs did not have such effect (Fig. 4C–E). Finally, we also identified the gene ARL5b (Fig. 4F) to be upregulated by V. cholerae MVs and downregulated by E. coli MVs. In agreement with the gene expression data, an increase of the histone mark H3K4me3 signal was observed at the TSS of ARL5b gene in cells treated with the V. cholerae MVs when compared to E. coli MVs treatment or mock (Fig. 4F).
Figure 4.
Influence of MVs on differentially regulated gene transcripts in HCT8 colorectal cancer cell line. (A) Venn Diagram showing the overlap of genes upregulated by E. coli MVs and downregulated by V. cholerae MVs. (B) Venn Diagram showing the overlap of genes downregulated by E. coli MVs and upregulated by V. cholerae MVs. (C–F) Examples of gene transcripts regulated in opposite way: (C) RND3 (E. coli up and V. cholerae down), (D) TACSTD2 (E. coli up and V. cholerae down), (E) SOCS2 (E. coli up and V. cholerae down) and (F) ARL5B (E. coli down and V. cholerae up).
Discussion
This study has demonstrated that MVs from pathogenic and commensal bacteria have a significant and global impact on the gene expression of colon carcinoma cells. Interestingly, the co-culture of HCT8 cells with both pathogenic and non-pathogenic MVs increased significantly the transcription of common targets genes, which suggests a nonspecific effect of MVs. Importantly, the changes in gene expression in our study correlated with both epigenetic changes and chromatin accessibility of promoters and TSS of genes induced by both pathogenic and non-pathogenic MVs. The changes in gene expression observed when bacteria are present might be due to any of the components of MVs rather than to direct contact between bacteria and the host cell, as suggested previously25,27. The release of MVs is a commonly occurring process when bacteria adapt to different environments both in vivo and in vitro. For example, Salmonella Typhimurium releases MVs during its intracellular growth in epithelial cells and macrophages41. Moreover, MVs produced by Helicobacter pylori, a causal organism of gastric ulcers, release a virulence factor inside the gastric cells42. Another example with pathogenic bacteria is related with Neisseria meningitides, which has been demonstrated to release MVs containing the endotoxin LPS in blood43.
Mechanistically, most viral or bacterial infections induce host DNA methylation indirectly via chronic inflammation, however recent studies have indicated that some viruses have direct epigenetic effects at gene transcripts of host cells23,44–46. Our study reveals that genes enriched with the GO term cellular immunity were identified among the genes commonly upregulated and enriched with H3K4me3 signals by both MVs in colon cancer cells. This supports that MVs might directly control the transcription of the host cells and probably their response to the immune system. However, the bacterial components that contribute to the increase of transcription in the host cell and their interplay with the immune function are important questions still unresolved. Previously, it was reported that bacterial LPS cause inflammation by means of activating p38 MAPK pathway and the nuclear transcription factor–κB (NF-κB)26,47,48. Furthermore, chronic inflammation causes epigenetic modifications leading to carcinogenesis, as described in the case of H. pylori49–51. In response, the host cell activates the TGF-β1 pathway to counteract the bacteria-induced NF-κB recruitment to the Il-6 promoter, which causes a reduction of histone acetylation/phosphorylation of the promoter30,48.
Our study also demonstrates that MVs from pathogenic bacteria V. cholerae have a selective impact on gene transcripts associated with epithelial cellular differentiation in colon cancer cells. Interestingly, MVs from V. cholerae increased the expression of genes that have a key role in cellular differentiation. For instance, the expression of the nuclear receptor for Vitamin D (VDR) genes were selectively upregulated by MVs from V. cholerae in colon carcinoma cells. In fact, the expression and the activation of VDR promotes the differentiation of colon carcinoma cells52. Hence, the increased expression of VDR might induce the expression of genes that promote differentiation and the formation of adherens junctions (AJ). The core complex of AJ is formed of transmembrane cell-cell adhesion molecules cadherins and adaptor proteins. Clustering of these molecules at junctions regulates cellular responses, with crucial effects on the physiology and on the epithelial cell differentiation53. Our study also reveals that MVs from V. cholerae induces the expression TJP1 (also know how ZO-1) and its protein product is a component of the adherens junctions. Interestingly, the decrease in ZO-1 expression reduces human trophoblast cell-cell fusion and differentiation54. Altogether, our results support the hypothesis that components specific to V. cholerae MVs would be responsible for the regulation of gene transcripts related to the differentiation of colon carcinoma cells. Interestingly, it has been reported that the major virulence factor of V. cholerae, cholera toxin (CT), suppresses carcinogenesis in a mouse model of inflammation-driven sporadic colon cancer55. Now, our study adds a value to the emerging notion that MVs produced by V. cholerae might impact the transcription of selective genes and, therefore, might play a role in colon cancer differentiation. Recent studies have also shown that protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS-mediated intrinsic pathway and inhibited tumour growth in mice model56,57. Bacterial pathogens can exploit several eukaryotic signalling pathways during an infection to disrupt host-signalling pathways for bacterial survival and replication. They have evolved specific effector proteins to hijack host cell signalling, including MAPK signalling, G-protein signalling, signals controlling cytoskeletal dynamics, innate immune responses, and epigenetic modifications for their own benefit58. Future studies should aim to identify the specific MVs-associated factors from V. cholerae responsible for modulation of host cell selective gene expression. Another challenge is to understand how these factors work together to orchestrate a successful infection by bacterial pathogens. Finally, the results of this study also reveal that the impact of the pathogenic MVs might be tissue specific. Whereas in this study we have investigated a human ileocecal adenocarcinoma cell line, the results might in the future be validated in additional colon-rectal carcinoma cells.
Materials and Methods
Bacterial strains, growth conditions and membrane vesicles isolation
Bacterial strains used in this study were: E. coli K-12 strain MC106117 and V. cholerae C6706 strain (O1 El Tor, Inaba, SmR)9. Strains were grown at 37 °C in either Luria–Bertani (LB) broth or on LB agar for 16 hours. MVs were isolated from bacterial culture supernatants, as described earlier16,17. Briefly, cultures were centrifuged and filtered to remove bacteria. Further, supernatants were ultracentrifuged at 100,000 × g for 2 h at 4 °C, pellets were washed and re-suspended in 20 mM Tris-HCl. The MVs concentration was estimated using the Bicinchoninic Acid (BCA) Assay kit (Thermo Scientific Pierce, Rockford, IL)5.
Transmission electron microscopy (TEM)
Negative staining of isolated MVs was performed as described earlier16.
Cell culture and infection conditions
Human ileocecal colorectal adenocarcinoma (HCT8) and breast adenocarcinoma (MCF-7) cells were cultured in RPMI 1640 (GIBCO) and DMEM medium respectively, supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin at 37 °C in a humid 5% CO2 atmosphere. Cells were seeded 16 h prior the infection, medium was changed and MVs or control (20 mM Tris-HCl) were incubated with HCT8 cells (600 μg MVs per 106 HCT8 cells) for 5 hours. Cells were collected for further RNA or chromatin isolation.
Chromatin Immunoprecipitation (ChIP)
Targeted genomic regions were identified by using the cross-linking (X)-ChIP protocol as described previously40. After the MV infection, cells were fixed 10 min with 1% formaldehyde and then quenched with glycine (125 mM). Chromatin was incubated overnight at 4 °C with Chip grade H3K4me3 antibody (5 μg, ab8580, Abcam) and equal amounts of Protein A&G Agarose Beads (Life technologies). Library preparation for sequencing was done following the instructions of TruSeq DNA sample preparation kit from Illumina or MicroPlex Library preparation kit from Diagenode.
Formaldehyde-Assisted Isolation of Regulatory Elements (FAIRE)
After the MV infection, cells were fixed 10 min with 1% formaldehyde and then quenched with glycine (125 mM). FAIRE experiment was performed as previously described40. DNA was fragmented using Bioruptor sonicator (Diagenode). Non-chromatinized DNA was isolated by phenol-chloroform extraction followed by reverse cross-link at 65 °C overnight. Purified DNA fragments were processed with MicroPlex Library preparation kit from Diagenode.
ChIP and FAIRE sequencing data Analyses
Reads generated by the genome analyzer were aligned against the human genome using Bowtie 2 software (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml) with default parameters. Peak calling was performed by MACS38 and HOMER version 4.7.2.
RNA isolation and quality control
After the infection, HCT8 cells in triplicates were used for RNA extraction. Total RNA was isolated with the total RNA isolation kit according to the manufacturer’s protocol (RNeasy Mini Kit, Qiagen). NanoDrop 2000 assessed RNA yield. Sequencing libraries were prepared from 1 μg total RNA using the TruSeq stranded mRNA library preparation kit (Illumina Inc) including poly-A selection and sequenced at the SNP&SEQ Technology Platform (Uppsala) in HiSeq2500 rapid mode.
The RNAseq analysis was done by the National Bioinformatics Infrastructure Sweden (http://www.scilifelab.se/platforms/bioinformatics/, www.nbis.se). Reads were aligned to the transcriptome (hg19, UCSC database by Illumina) using Tophat2. Assembly of transcripts and differential gene expression analysis were performed using Cufflinks/Cutdiff.
Pathway analysis
Functional interpretation of differentially expressed genes and their association to the host cell pathways for each of the MVs treatments were done using Metascape software. For each treatment, the affected genes were analyzed separately for up- and downregulated genes. We identified canonical pathways that were enriched or overrepresented, with the significance of the association between the signature and the canonical pathway measured in two ways: (1) A ratio of the number of genes from the signature that map to the pathway divided by the total number of genes that map to the canonical pathway calculated; (2) A right-sided Fisher’s exact test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone.
Electronic supplementary material
Acknowledgements
We thank Sergiu Netotea from National Bioinformatics Infrastructure Sweden for RNAseqanalysis.
Author Contributions
A.H. and S.N.W. conceived all the experiments. S.V., S.W., M.D. conducted all the experiments, and computational analyses were conducted by S.G. A.H. and S.N.W. wrote the manuscript with the help from the other authors. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Svitlana Vdovikova and Siv Gilfillan contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25308-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sun Nyunt Wai, Email: sun.nyunt.wai@umu.se.
Antoni Hurtado, Email: a.h.rodriguez@ncmm.uio.no.
References
- 1.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deatherage BL, et al. Biogenesis of bacterial membrane vesicles. Mol Microbiol. 2009;72:1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jan AT. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front Microbiol. 2017;8:1053. doi: 10.3389/fmicb.2017.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vdovikova S, et al. A Novel Role of Listeria monocytogenes Membrane Vesicles in Inhibition of Autophagy and Cell Death. Front Cell Infect Microbiol. 2017;7:154. doi: 10.3389/fcimb.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aung KM, et al. Naturally Occurring IgG Antibodies Provide Innate Protection against Vibrio cholerae Bacteremia by Recognition of the Outer Membrane Protein U. J Innate Immun. 2016;8:269–283. doi: 10.1159/000443646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsalobre C, et al. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol Microbiol. 2006;59:99–112. doi: 10.1111/j.1365-2958.2005.04938.x. [DOI] [PubMed] [Google Scholar]
- 8.Bielig H, et al. NOD-like receptor activation by outer membrane vesicles from Vibrio cholerae non-O1 non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect Immun. 2011;79:1418–1427. doi: 10.1128/IAI.00754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rompikuntal PK, et al. Outer Membrane Vesicle-Mediated Export of Processed PrtV Protease from Vibrio cholerae. PLoS One. 2015;10:e0134098. doi: 10.1371/journal.pone.0134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rompikuntal PK, et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80:31–42. doi: 10.1128/IAI.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duperthuy M, et al. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 2013;9:e1003620. doi: 10.1371/journal.ppat.1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elluri S, et al. Outer Membrane Vesicles Mediate Transport of Biologically Active Vibrio cholerae Cytolysin (VCC) from V. cholerae Strains. PLoS One. 2014;9:e106731. doi: 10.1371/journal.pone.0106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galka F, et al. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun. 2008;76:1825–1836. doi: 10.1128/IAI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouokam JC, et al. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect Immun. 2006;74:2022–2030. doi: 10.1128/IAI.74.4.2022-2030.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindmark B, et al. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009;9:220. doi: 10.1186/1471-2180-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Membrane vesicle-mediated release of bacterial RNA. Sci Rep. 2015;5:15329. doi: 10.1038/srep15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wai SN, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/S0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 18.O’Donoghue EJ, Krachler AM. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016;18:1508–1517. doi: 10.1111/cmi.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fábrega MJ, et al. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole J, Morris P, Dickman MJ, Dockrell DH. The therapeutic potential of epigenetic manipulation during infectious diseases. Pharmacol Ther. 2016;167:85–99. doi: 10.1016/j.pharmthera.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori N, Ushijima T. Epigenetic impact of infection on carcinogenesis: mechanisms and applications. Genome Med. 2016;8:10. doi: 10.1186/s13073-016-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med. 2012;2:a010272. doi: 10.1101/cshperspect.a010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolg C, et al. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab Invest. 2011;91:825–836. doi: 10.1038/labinvest.2010.197. [DOI] [PubMed] [Google Scholar]
- 26.Martins MD, et al. Epigenetic Modifications of Histones in Periodontal Disease. J Dent Res. 2016;95:215–222. doi: 10.1177/0022034515611876. [DOI] [PubMed] [Google Scholar]
- 27.Schmeck B, et al. Intracellular bacteria differentially regulated endothelial cytokine release by MAPK-dependent histone modification. J Immunol. 2005;175:2843–2850. doi: 10.4049/jimmunol.175.5.2843. [DOI] [PubMed] [Google Scholar]
- 28.Opitz B, et al. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176:484–490. doi: 10.4049/jimmunol.176.1.484. [DOI] [PubMed] [Google Scholar]
- 29.Schmeck B, et al. Histone acetylation and flagellin are essential for Legionella pneumophila-induced cytokine expression. J Immunol. 2008;181:940–947. doi: 10.4049/jimmunol.181.2.940. [DOI] [PubMed] [Google Scholar]
- 30.Haller D, et al. Transforming growth factor-beta 1 inhibits non-pathogenic Gram negative bacteria-induced NF-kappa B recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–23860. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- 31.Cougnoux A, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014;63:1932–1942. doi: 10.1136/gutjnl-2013-305257. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4:409–419. doi: 10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S, et al. Salmonella Engages Host MicroRNAs To Modulate SUMOylation: a New Arsenal for Intracellular Survival. Mol Cell Biol. 2015;35:2932–2946. doi: 10.1128/MCB.00397-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickertsson JA, Rasmussen LJ, Friis-Hansen L. Enterococcus faecalis Infection and Reactive Oxygen Species Down-Regulates the miR-17-92 Cluster in Gastric Adenocarcinoma Cell Culture. Genes (Basel) 2014;5:726–738. doi: 10.3390/genes5030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, et al. Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem. 2011;286:35755–35762. doi: 10.1074/jbc.M111.271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara T, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–1318. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radman-Livaja M, Rando OJ. Nucleosome positioning: how is it established, and why does it matter? Dev Biol. 2010;339:258–266. doi: 10.1016/j.ydbio.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilfillan S, Fiorito E, Hurtado A. Functional genomic methods to study estrogen receptor activity. J Mammary Gland Biol Neoplasia. 2012;17:147–153. doi: 10.1007/s10911-012-9254-4. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-del Portillo F, Stein MA, Finlay BB. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect Immun. 1997;65:24–34. doi: 10.1128/iai.65.1.24-34.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Necchi V, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 43.Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with “blebbing” meningococcus. Lancet. 2002;360:1741. doi: 10.1016/S0140-6736(02)11721-1. [DOI] [PubMed] [Google Scholar]
- 44.Balakrishnan, L. & Milavetz, B. Epigenetic Regulation of Viral Biological Processes. Viruses9, 10.3390/v9110346 (2017). [DOI] [PMC free article] [PubMed]
- 45.Zhang Y, et al. Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation. Oncotarget. 2017;8:96323–96339. doi: 10.18632/oncotarget.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsusaka K, Funata S, Fukayama M, Kaneda A. DNA methylation in gastric cancer, related to Helicobacter pylori and Epstein-Barr virus. World J Gastroenterol. 2014;20:3916–3926. doi: 10.3748/wjg.v20.i14.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Santa F, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamon MA, Cossart P. Histone modifications and chromatin remodeling during bacterial infections. Cell Host Microbe. 2008;4:100–109. doi: 10.1016/j.chom.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Nardone G, Compare D, De Colibus P, de Nucci G, Rocco A. Helicobacter pylori and epigenetic mechanisms underlying gastric carcinogenesis. Dig Dis. 2007;25:225–229. doi: 10.1159/000103890. [DOI] [PubMed] [Google Scholar]
- 50.Nishizawa T, Suzuki HG. Carcinogenesis and Underlying Molecular Mechanisms: Helicobacter pylori and Novel Targeted Therapy. Biomed Res Int. 2015;2015:794378. doi: 10.1155/2015/794378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepulveda AR. Helicobacter, Inflammation, andGastric Cancer. Curr Pathobiol Rep. 2013;1:9–18. doi: 10.1007/s40139-013-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer HG, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amini R, Chartier NT, Labbe JC. Syncytium biogenesis: It’s all about maintaining good connections. Worm. 2015;4:e992665. doi: 10.4161/21624054.2014.992665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pidoux G, et al. ZO-1 is involved in trophoblastic cell differentiation in human placenta. Am J Physiol Cell Physiol. 2010;298:C1517–1526. doi: 10.1152/ajpcell.00484.2008. [DOI] [PubMed] [Google Scholar]
- 55.Doulberis M, et al. Cholera-toxin suppresses carcinogenesis in a mouse model of inflammation-driven sporadic colon cancer. Carcinogenesis. 2015;36:280–290. doi: 10.1093/carcin/bgu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray T, Pal A. PAR-1 mediated apoptosis of breast cancer cells by V. cholerae hemagglutinin protease. Apoptosis. 2016;21:609–620. doi: 10.1007/s10495-016-1229-2. [DOI] [PubMed] [Google Scholar]
- 57.Ray T, Chakrabarti MK, Pal A. Hemagglutinin protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS mediated intrinsic pathway and regresses tumor growth in mice model. Apoptosis. 2016;21:143–154. doi: 10.1007/s10495-015-1194-1. [DOI] [PubMed] [Google Scholar]
- 58.Alto NM, Orth K. Subversion of cell signaling by pathogens. Cold Spring Harb Perspect Biol. 2012;4:a006114. doi: 10.1101/cshperspect.a006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.