Abstract
Aging is associated with several biological, physiological, cellular and histological changes. In the present study, we investigated the effect of aging on different signaling pathways, including antioxidant system, apoptosis and immune status. Several natural products were used to ameliorate and block aging-related changes. Melatonin and turmeric have been known to ameliorate and decrease aging-related changes. However, the exact mechanism(s) of their action is not fully understood. In the present study, we tried to uncover the regulatory mechanism(s) by which melatonin and turmeric work against aging. We found that aging differentially regulated blood serum immunoglobulins; increased IgA and decreased IgE. Furthermore, all the serum cytokines investigated (TNF-α, IFN-γ, IL-6 and IL-8) were highly increased by aging. In addition, the antioxidant upstream regulators; DJ-1 and NRF2 were markedly repressed with aging in thymus tissues. We also found that aging induced apoptosis promoting genes p53 and Bax mRNA in thymus tissues. Finally, we found clear histological changes in thymus and spleen tissues. Administration of either melatonin or tumeric clearly ameliorated and blocked to some extinct the effect of aging. Altogether, aging was associated with downregulation of antioxidant regulators; DJ-1 and NRF2, promoted apoptosis and induced changes in the immune status. Furthermore, melatonin and tumeric markedly reversed the action of aging through activating DJ-1/NRF2 signaling pathway and inhibiting p53/Bax apoptotic pathway.
Keywords: Aging, melatonin, tumeric, DJ-1, NRF2
Introduction
Aging is a time-dependent natural physiological process; including several biological and physiological changes. One of the most widely accepted hypotheses of aging is the increase of free radical or oxidative stress [1]. The free-radical theory of aging is based upon the use of antioxidants to diminish the age-related changes and several reports indicated that antioxidants can inhibit and block oxidative damage to improve the quality of life [1,2]. There is an increasing evidence proposed that various naturally-occurring antioxidants had ameliorative effect on aging-related changes. Therefore, in the current study, the ameliorative effects of melatonin and turmeric against aging-induced changes were investigated and evaluated.
It is stated that, melatonin has its own antioxidative action in addition to intensifying the endogenous antioxidant activities, which in turn exert a powerful antioxidative action [3]. Furthermore, melatonin is reported to have beneficial effects in rats against aging [4]. In addition, curcumin (the active ingredient of turmeric) was suggested as a potent antioxidant and antiinflammatory candidate having multifaceted therapeutic actions against several age-related diseases [5].
NRF2 is a transcription factor and is considered as an antioxidants’ master regulator because it regulates the expression of a wide panel of antioxidant genes [6,7]. In addition, it is stated that NRF2 is lined to aging [8,9]. NRF2 is known as a downstream target for DJ-1 protein [10]. Another study showed that NRF2 could be regulated also independent of DJ-1 protein [11]. DJ-1/PARK7 is initially identified as an oncogene; associated with cancer and male infertility [12]. DJ-1 is reported to be involved in various biological processes; including the regulation of ROS levels and induction of apoptosis [13]. DJ-1 is therefore, a signaling molecule responsive to cellular redox state, which exerts antioxidant functions [14]. Therefore, DJ-1 protects cells against apoptosis triggered by increased level of ROS through protecting mitochondrial integrity by attenuating the transcription of antioxidant genes [15]. Recent studies linked DJ-1 expression fluctuations with aging [16,17].
During the present research, the effect of aging in rats (Rattus norvgicus) on the regulation of selected immunoglobulins (IgA and IgE), aging-related cytokines (TNF-α, IFN-γ, IL-6 and IL-8) in blood serum and antioxidant master upstream signaling molecules such as DJ-1 and NRF2 and apoptosis-inducing proteins in the thymus tissues as well as the histological changes in thymus and spleen tissues were investigated. Next, the ameliorative effects of two natural products (melatonin and tumeric) and their mechanisms against aging were investigated.
Materials and methods
Animals and housing condition
Young adult male albino rats (3-4 months), (Rattus norvgicus), weighing from 95-120 g, and twenty three aged male albino rats (16-18 months), weighing from 350-400 g were obtained from the breeding colony of the Ministry of Health (Helwan-Egypt). Upon arrival, the animals were weighed; group housed (three animals per cage) and kept in a well-ventilated animal facility under normal laboratory conditions. They were acclimatized for one week prior to experimentation. Food and water were available ad libitum throughout the study. All procedures were in accordance with institutional guidelines and follow the Guide for Care and Use of Laboratory Animals.
Chemicals and plant materials
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) was purchased from Sigma Chemical Co. (St. Louis, Mo, USA). It was prepared in ethanol due to its instability in non-sterile solutions. Briefly, melatonin was dissolved in 0.5 ml of 100% ethanol and diluted with phosphate-buffered saline (PBS) to a final concentration of 10 mg melatonin/1 ml of 0.5% ethanolic PBS [18]. The bottles of melatonin solution were covered with aluminum foil; and kept in a refrigerator; fresh solutions were prepared every two days.
Turmeric
The turmeric (Curcuma longa) was obtained, in the powder form available commercially, from a local market in El-Minia city, Egypt. The powdered turmeric was mixed with laboratory diet at a concentration of 2% w/w.
Experimental design
After one week of acclimatization, a group of young adult rats at 3-4-months of age (n = 6) was assigned as normal control adult rats [normal adult (N. adult)]; they were provided with normal diet and water ad libitum throughout the course of the study. Aged rats (16-18 months-old) were randomly divided into four experimental groups.
The first group of aged animals was considered as normal control aged group (N. aged group; n = 6) in which animals received laboratory diet, and they did not receive any substances or undergo any experimental manipulation.
The second group of animals [aged+melatonin (aged+Mel) Group; n = 5] was treated orally with melatonin (10 mg/kg) five times a week for 90 days in the late afternoon (4.00-6.00 pm).
The third group of rats (n = 6) was used as vehicle control for the aged+Mel group, and received 0.5% ethanolic phosphate-buffered saline by oral intubation in the late afternoon.
The fourth group of rats [aged+turmeric (aged+Tum) Group; n = 6] was fed powdered tumeric mixed with normal laboratory diet at a concentration of 2% w/w for 90 consecutive days. The selection of this dose was based on a previous research study [19].
The design of all experimental groups is summarized in Table 1. The experiments were continued for 90 days and all animals were weighed weekly.
Table 1.
The scheme of the experimental design
| Groups | No. of rats | Dose | Route | Time | Scarifying time |
|---|---|---|---|---|---|
| N. adult | 6 | …………… | …… | …… | After 90 days |
| N. aged | 6 | …………… | …… | …… | After 90 days |
| Aged+Mel | 5 | 10 mg/kg.b.wt | Oral | Five times a week for 90 days | After 90 days |
| Vehicle control | 6 | 0.5% ethanolic + PBS | Oral | Five times a week for 90 days | After 90 days |
| Aged+Tum | 6 | 2% w/w | Mixed with diet | For 90 days | After 90 days |
Note: N, normal; Mel, melatonin; Tum, turmeric.
Blood and tissue sampling
At the end of the experiment, the animals were decapitated under deep ether anesthesia at the morning time. Blood samples were collected and allowed to clot at room temperature. After that, they were centrifuged for 30 min at 4000 rpm. Serum aliquots were extracted and stored in microcentrifuge tubes and kept at -80°C until assayed.
After blood sampling, the thymus and spleen were immediately removed from each animal. Samples of thymus and spleen were fixed in neutral formalin solution (10%), dehydrated and embedded in parablast (m.p.54-56; Sigma). Five-micrometer thick sections were prepared and stained with Haematoxylin and Eosin (H&E) using standard procedures for general histological architecture. Moreover, samples of thymus were kept in -80°C until be used for RNA extraction for real time PCR analyses.
Histological analysis and pathological scoring system
Sections of thymus and spleen were examined with light microscopy and separately scored using a semiquantitative scoring system.
Thymus: As shown in Table 2, subjective analysis of the degree of thymic involution was based on various parameters known to change with increasing age. Thymus sections were then semiquantitatively and qualitatively graded for the presence of thymic involution using a scale from 0 to 3 as follows: 0 = absent; 1 = mild; 2 = moderate; 3 = severe.
Table 2.
Histopathological scoring system of the thymus
| Score | Degree of lymphocytic depletion | Count of pyknotic nuclei in the cortex | Adipose tissue infiltration | C/M boundaries | C/M ratio | IL septae Thickening |
|---|---|---|---|---|---|---|
| (0) | Nil (---) | None | None (---) | Distinct | > 1:1 | Nil (----) |
| (1) | Mild (+) | 1-3 | Mild (+) | Distinct | 1:1 | Mild (+) |
| (2) | Moderate (++) | 4-10 | Moderate (++) | Slightly indistinct | < 1:1 | Moderate (++) |
| (3) | Marked (+++) | > 10 | Marked (+++) | Indistinct | < 1:1 | Marked (+++) |
Note: C/M, cortex to medullary; IL, interlobular.
Spleen: Sections of spleen were graded for the presence of lymphocytic depletion, and a score from 0 to 4 was attributed as: 0 (none), 1 (minimal), 2 (mild), 3 (moderate) and 4 (severe).
Immunological studies
Determination of Immunoglobulin-A (IgA) and Immunoglobulin-E (IgE)
Total Immunoglobulin (Ig) A and E in blood serum samples were measured from all the experimental groups by Enzyme-Linked Immuosorbent Assay (ELISA) kits (CellTrend GmbH, Lukenwalde, Germany; Cat No. 52400, 52600, respectively) Then, the results were calculated and expressed as nanograms per milliliter (ng/ml).
Determination of cytokines Tumor Necrosis Factor-alfa (TNF-α), Interferon-γ (IFN-γ), interleukin-6 (IL-6), and interleukin-8 (IL-8)
The levels of TNF-α, IFN-γ, IL-6, and IL-8 in the blood serum were determined by enzyme immunoassay using ELISA kits according to the manufacturer’s recommendations (Koma Biotech Inc; Cat. No. K0331196, ScienCell Research Laboratories; Cat. No. EK0374, Bioo Scientific; Cat. No. 2202, and Cusabio Biotech Co; Cat. No. CSB-E07273r, respectively). The principal method for all the immunoassay was the same for all cytokines. The results are expressed as picograms per milliliter (pg/ml).
Gene expression analyses
The expression levels of the indicated genes were investigated in thymus tissues of rats from the all experimental groups using quantitative real-time PCR (RT-PCR). The procedure of analysis was performed as previously described [11]. Total RNA was extracted with Qiazol (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Five µg of extracted RNA was reverse transcribed into cDNA using the first strand cDNA synthesis kit (Applied Biosystems, Fosters city, CA, USA), and the resulting cDNA was diluted 10-fold and kept at -20°C. The real time RT-PCR primers were designed by the primer Express 1.5 software (Applied Biosystems) as follows: GAPDH forward, 5’-ATCTTCTTGTGCAGTGCCAGC-’3 and GAPDH reverse, 5’-GAAGGCAGCCCTGGTAACC-’3 and Bax forward, 5’-TCATGAAGACAGGGGCCTTT-’3 and Bax reverse, 5’-CTGCAGCTCCATGTTGTTGT-’3 and NRF2 forward, 5’-TGTCAGCTACTCCCAGGTTG-’3 and NRF2 reverse, 5’-ATCAGGGGTGGTGAAGACTG-’3 and DJ-1 forward, 5’-GGAGCAGAGGAGATGGAGAC-’3 and DJ-1 reverse, 5’-TCACCAAAGCCGACTCAGAT-’3 and TNF-α forward, 5’-CGTCGTAGCAAACCACCAAG-’3 and TNF-α reverse, 5’-GAGGCTGACTTTCTCCTGGT-’3 and P53 forward, 5’-CTCCTCTCCCCAGCAAAAGA-’3 and P53 reverse, 5’-GTAGACTGGCCCTTCTTGGT-’3. QPCR was carried out using 7500 fast (Applied Biosystems, USA). All samples were amplified in triplicate in a 96-well plate using the following cycling conditions: 10 minutes at 95°C, and 40 cycles at 95°C for 15 seconds followed by one minute at 60°C.
Statistical analysis
The data were presented as mean ± standard error. P-value > 0.05 was considered statistically significant (using the SPSS program, version 16.0). Statistical investigations were performed using ANOVA (analysis of variance) followed by Post-Hoc test for multiple comparisons. Calculations of real time PCR results were performed by determining the values of Δcycle threshold (ΔCt) using the endogenous control (GAPDH) for normalization. Then 2-ΔΔCt for each treatment was calculated and statistical analysis for data were performed as previously described [20].
Results
Attenuation of aging-induced changes in immunoglobulin-A (IgA) and immunoglobulin-E (IgE) by melatonin and Turmeric administration
The current data showed the effect of aging on serum IgA and IgE in rats. Results showed that aging significantly increased (P < 0.01) serum IgA value as shown in normal aged rats (104.45±0.736) when compared to normal adult rats (88.1±4.47). Interestingly, melatonin or turmeric administration to normal aged rats for 12 weeks (aged+Mel or aged+Tum groups) resulted in a significant decrease (P < 0.01) in serum IgA levels when compared with normal aged rats and restored them to their normal level (Figure 1A). On the other hand, the serum IgE level was significantly decreased (P < 0.01) in the normal aged group when compared to normal adult rats. However, treatment of aged rats with melatonin or turmeric for 12 weeks (aged+Mel and aged+Tum groups) markedly increased (P < 0.01) the serum IgE and restored its low level as in normal adult rats (Figure 1B).
Figure 1.
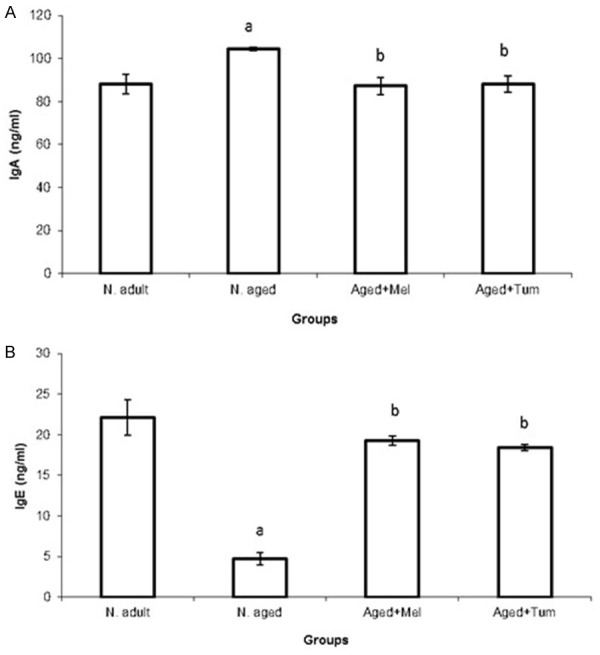
Serum levels of immunoglobulin-A (IgA) (A) and immunoglobulin-E (IgE) (B) in normal adult rats, normal aged and normal aged treated with either melatonin or turmeric for 12 weeks. The level of IgA and IgE were investigated in blood serum of rats as indicated in materials and methods section. Data then were calculated and statistical analyses were performed. N = normal; Mel = melatonin; Tum = turmeric. A = P < 0.05 vs. normal adult animals, b = P < 0.05 vs. normal aged rats.
Attenuation of aging-induced increases in serum cytokines by melatonin and turmeric
The modulatory effect of melatonin and turmeric on the changes induced by aging in serum cytokines; Tumor Necrosis Factor-alpha (TNF-α), Interferon-gamma (IFN-γ), Interleukin-6 (IL-6) and Interleukin-8 (IL-8) were evaluated. The present data showed that, the levels of Tumor Necrosis Factor-alpha (TNF-α), Interferon-gamma (IFN-γ), Interleukin-6 (IL-6) and Interleukin-8 (IL-8) were significantly increased (P < 0.01) in serum of normal aged rats when compared with those of normal adult rats (Figure 2). In contrast, melatonin or turmeric administration to normal aged rats for 12 weeks (aged+Mel or aged+Tum groups, respectively) resulted in a significant decrease (P < 0.01) in their levels toward normal adult levels, but their values were still significantly higher than those of normal adult rats (Figure 2).
Figure 2.
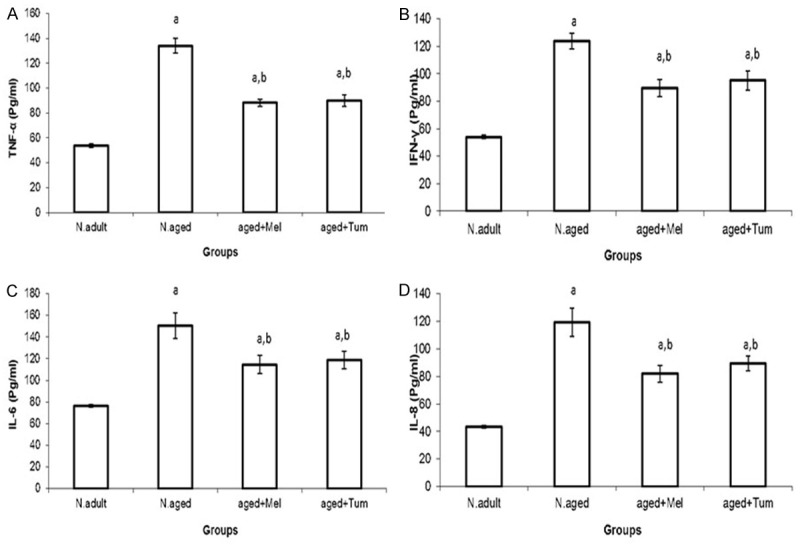
Serum levels of Tumor Necrosis Factor-alpha (TNF-α) (A), interferon gamma (IFN-γ) (B), interleukon 6 (IL-6) (C), and interleukon 8 (IL-8) (D) in normal adult rats, normal aged, and normal aged treated with either melatonin or turmeric for 12 weeks as indicated in methods section. N = normal; Mel = melatonin; Tum = turmeric. A = P < 0.01 vs. normal adult animals, b = P < 0.01 vs. normal aged rats.
Modulatory effects of melatonin and turmeric on aging-induced changes in apoptosis-related genes
To determine the age-related changes in gene expression of DJ-1, NRF2, p53, Bax and TNF-α in thymus, gene expression analyses for the indicated genes were investigated using quantitative real time PCR (Figures 3, 4). The mRNA levels of DJ-1 (Figure 3A) and NRF2 (Figure 3B) genes were highly expressed in thymus tissues of normal adult rats. On aging, the DJ-1 and NRF2 mRNA level were significantly repressed (P < 0.01) when compared with the normal adult rats. Interestingly, treatment the aged rats with melatonin or turmeric for 12 weeks (aged+Mel or aged+Tum) increased markedly the mRNA expression levels of both DJ-1 and NRF2 (Figure 3A, 3B).
Figure 3.
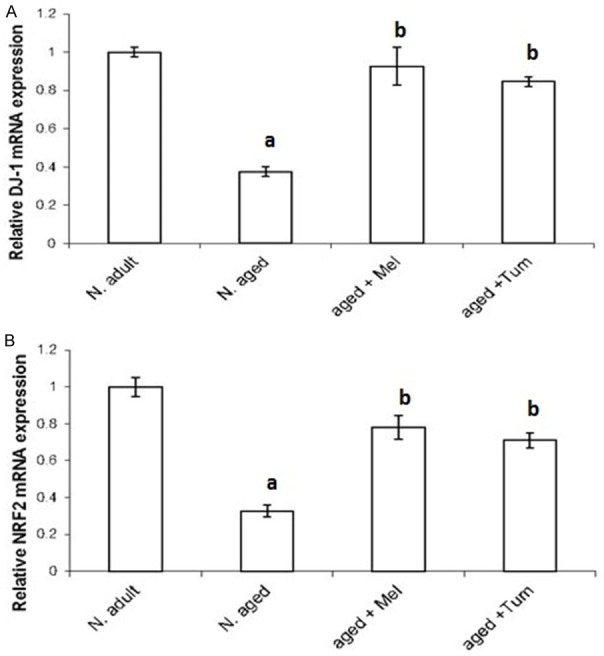
Age-related changes in mRNA expression levels of DJ-1 and NRF2 in thymus tissue. DJ-1 (A) and NRF2 (B) mRNA expression levels in thymus tissues of normal adult rats, normal aged and normal aged treated with either melatonin or turmeric for 12 weeks were investigated using real time quantitative PCR. Data were then statistically analyzed as indicated in methods section. N = normal; Mel = melatonin; Tum = turmeric. A = P < 0.05 vs. normal adult animals. B = P < 0.05 vs. normal aged rats.
Figure 4.
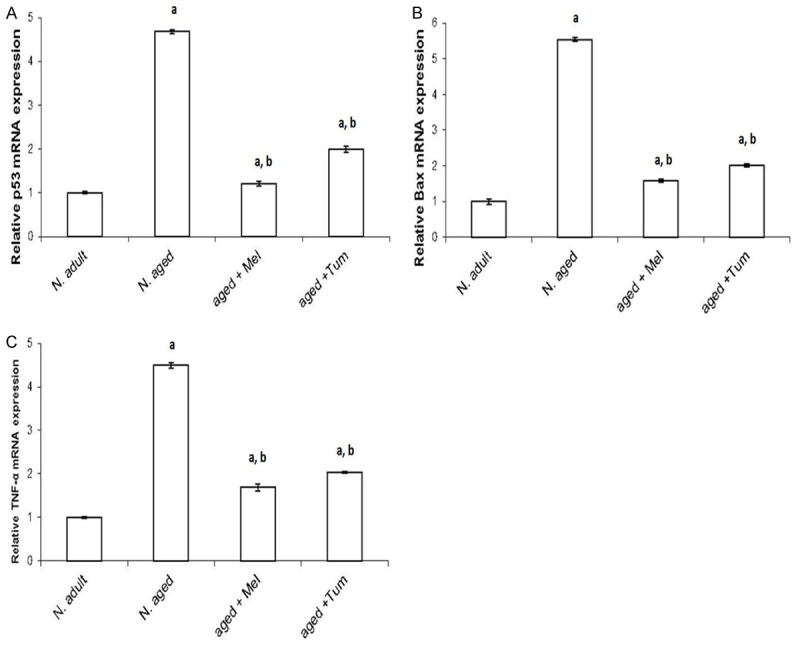
Age-related changes in p53, Bax and TNF-α mRNA expression levels. The expression levels of p53 (A), Bax (B) and TNF-α (C) mRNA in thymus tissue of normal adult rats, normal aged and normal aged treated with either melatonin or turmeric were investigated using real time quantitative PCR and statistical significance were then analyzed. N = normal; Mel = melatonin; Tum = turmeric. A = P < 0.05 vs. normal adult; b = P < 0.05 vs. normal aged rats.
On the other hand, basal mRNA level of p53 (Figure 4A), Bax (Figure 4B) and TNF-α (Figure 4C) were low expressed in thymus tissues of normal adult rats. The p53, Bax and TNF-α mRNA expression levels were significantly elevated (P < 0.01) in thymus tissues of normal aged rats (N. aged group), compared with the normal adult control rats (N. adult group). The enhanced expressions of p53, Bax and TNF-α by aging were significantly (P < 0.01), and then diminished in aged animals treated with either melatonin or turmeric for 12 weeks (aged+Mel or aged+Tum groups) (Figure 4A-C respectively). However, their values after treatment with either melatonin or turmeric didn’t reach their basal levels in control normal adult rats (Figure 4).
Aging induced cellular morphological changes in rat thymus and the ameliorative effect of melatonin and turmeric
In normal control adult rats, the thymus gland is a bi-lobed organ covered with a thin connective tissue capsule. Each lobe is partly divided by connective tissue septa into incomplete lobules. The thymic lobules comprise a darkly stained peripheral cortex and a lightly stained central medulla. The cortex consists mainly of small and densely packed lymphocytes (Figure 5A). The medulla consists of a large number of reticulo-epithelial cells (Figure 5A), in addition to a few fully mature lymphocytes, macrophages and occasional Hassall’s corpuscles.
Figure 5.
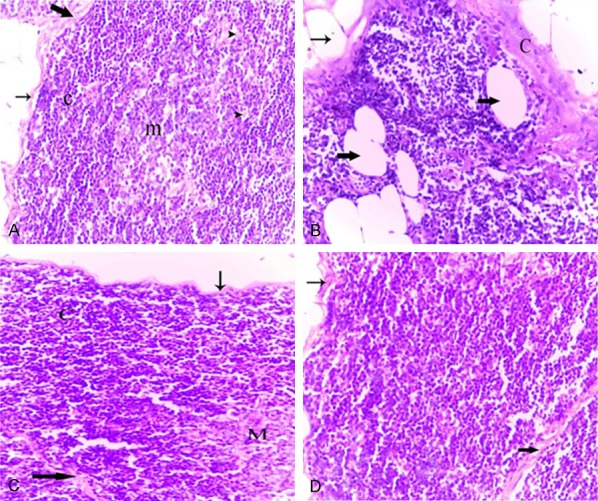
Photomicrographs of thymus tissues of normal adult rat (A), normal aged (B), normal aged treated with either melatonin (C) or turmeric (D). (A) Thymus tissues of normal adult rats showing a dark stained cortex (c) with densely backed lymphocytes and light stained medulla (m) with lymphocytes and macrophages (short arrows) as well as epithelial cells (irregular arrows). Note the thin capsule (thin arrow) and the thin interlobular septum (thick arrow). (B) Thymus tissues of normal aged rats showing the lymphocytes depletion of the cortex. Note the thickened capsule (C) and increased numbers of adipocytes infiltered the capsular (thin arrow) and extended into the cortex (thick arrows). (C) Thymus of a normal aged rat treated with melatonin showing increased lymphocytic population in the cortical (C) and medullary (M) regions. Note the thin capsule (thin arrow) and interlobular septum (thick arrow), as well as the absence of adipocytes. (D) Thymus of a normal aged rat treated with turmeric showing the histological improvement in the thymic tissue. Note: the cortical (C) and medullary (M) regions. (H&E, 400X).
Thymus of aged rats showed marked thymic involution compared with those obtained from the normal adult rats. The capsule and inter lobular septa became slightly thickened. The cortex showed obvious lymphocytic depletion with the appearance of numerous shrunken, necrotic as well as apoptotic lymphocytes (Figure 5B). The cortical reticuloepithelial cells were clearly identified due to the reduction in the numbers of cortical lymphocytes (Figure 5B). Such alterations in the cortical cellularity resulted in a great loss of demarcation between cortex and medulla. In addition, increased numbers of adipocytes infiltered the capsular and septal areas, and extended into the cortex (Figure 5B). In the medulla, the epithelial component appeared more prominent due to the decrease in the density of medullary lymphocytes. A variety of epithelial cells were arranged in the cortex or in the medulla as prominent tubular structures lined by cuboidal epithelium.
Melatonin or turmeric administration resulted in obvious alterations in the cellular density and cellular compositions of the thymus of normal aged rats. However, the histological appearance of the aged thymus under these various treatment conditions was similar. Specifically, melatonin or turmeric administration to normal aged rats for 12 weeks (aged+Mel or aged+Tum groups, respectively) produced a marked improvement in aged thymic tissue. The density of the cortical lymphocytes was apparently increased, and the number of necrotic thymocytes seemed to be decreased compared to that seen in the thymus of normal aged rats (Figure 5C, 5D). Also, the thickened interlobular septa as well as the thickened capsule appeared thinner than those observed in the thymus of normal aged rats (Figure 5C, 5D). Moreover, it has been noticed that no adipocytes were found in all examined thymus sections of these groups.
Aging induced cellular morphological changes in rat spleen and the ameliorative effect of melatonin and turmeric
The spleen of adult rats showed normal histological architecture composed of two distinct compartments, the red pulp and the white pulp (Figure 6A, 6B); trabeculae of smooth muscles and fibroelastic tissue were also seen in the splenic parenchyma. The red pulp was consisted of a meshwork of splenic cords, which contained various types of cells, and venous sinuses (Figure 6A). The white pulp was composed of periarteriolar lymphoid sheath (PALS), lymphoid follicles, and prominent marginal zone located between the white and red pulp (Figure 6B). The follicle sometimes had germinal centers which occasionally contained apoptotic cells and tingible body macrophages engulfing apoptotic bodies (Figure 6B). Furthermore, a variety of pigments were detected in the spleen of control rats. Particularly, deposits were seen throughout the cytoplasm of macrophages in the red pulp, and sometimes in the white pulp as well.
Figure 6.
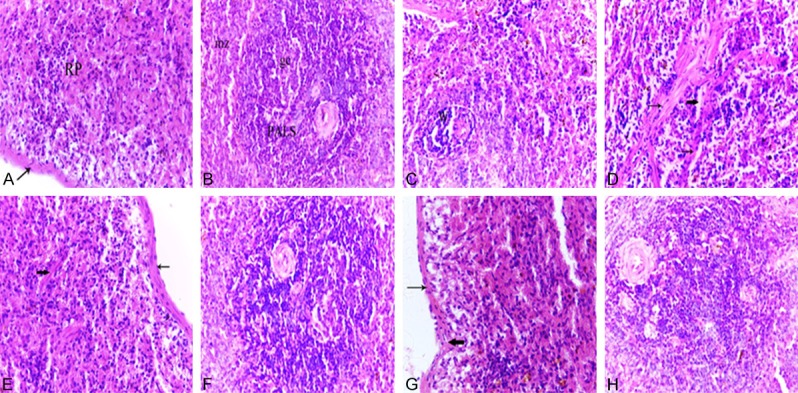
Photomicrographs of spleen tissues of a normal adult rat (A, B), normal aged (C, D), normal aged treated with either melatonin (E, F) or turmeric (G, H). (A) Spleen of an adult rat showing the splenic red pulp (RP). Note the less-thickened capsule (arrow). (B) Spleen of an adult rat showing the PALS and marginal zones (mz). Note the presence of follicles containing prominent germinal centers (gc) with tangible body macrophages which appeared with cytoplasmic engulfed apoptotic debris. (C) Spleen of a normal aged rat revealed the decreased cellularity of both white pulp and red pulp areas. Note the lymphocytic depletion of the white pulp (W) and the marginal zone is hardly defined. (D) Spleen of a normal aged rat showing the lymphocytic depletion of the white pulp (W) with shrinkage and apoptotic cells. Note the tangible body macrophages with cytoplasmic engulfed apoptotic bodies. (H&E, 400X). (E) Spleen of a normal aged rat treated with melatonin showing the increased lymphocytic population of the splenic red pulp. Note the less-thickened capsule (thin arrow) and trabeculae (thick arrow). (F) Spleen of a normal aged rat treated with melatonin showing a marked improvement where the lymphocytic population of the white pulp was increased. (G) Spleen of a normal aged rat treated with turmeric showing the increased lymphocytic population of the splenic red pulp. Note the less-thickened capsule (thin arrow) and trabeculae (thick arrow) and the decrease number of pigments. (H) Spleen of a normal aged rat treated with turmeric showing apparent increase in the lymphocyte population of white pulp. (H&E, 400X).
The histological architecture of spleen sections from normal aged rats showed striking alterations compared to those of adult animals. Specifically, all spleen sections of aged rats revealed an obvious decrease in the cellularity of both white pulp and red pulp areas compared with normal adult rats. The white pulp areas showed lymphocytic depletion with the appearance of numerous shrunken and necrotic lymphocytes (Figure 6C). The PALS of the white pulp showed marginal zones with dilated marginal sinusoids. Additionally, many apoptotic cells and tangible body macrophages with cytoplasmic-engulfed apoptotic bodies were seen in the white pulp. Pigment-laden macrophages were commonly observed in the red pulp and white pulp (Figure 6C and 6D). Also, pronounced dilatation and congestion of splenic blood vessels and sinuses were found (Figure 6D), accompanied by an increase in hemosiderin pigments (hemosiderosis). The capsule and trabeculae appeared more thickened compared to those of adult animals (Figure 6D).
Melatonin or turmeric administration for 12 weeks exerted prominent ameliorative effects on the histological alterations induced by aging in the spleen tissue. In specific, the white pulp became easily defined with increased lymphocytic population (Figure 6F and 6H). The marginal zone became well-differentiated (Figure 6F and 6H). The splenic cords of the red pulp exhibited an obvious increase in the cellular population, specifically lymphocytes (Figure 6E and 6G). The hemosiderin and pigment-laden macrophages were reduced all over the splenic tissue (Figure 6E-H). Furthermore, the thickness of the trabeculae as well as the capsule appeared clearly decreased compared to that observed in the spleen of normal aged rats (Figure 6E and 6G).
Histopathological scores of thymus and spleen
The thymus and spleen histopathological scores were higher (P < 0.01) in normal aged rats than in normal adult rats (Tables 3, 4). In contrast, melatonin or turmeric administration to normal aged rats for 12 weeks (aged+Mel or aged+Tum groups, respectively) caused a significant decrease (P < 0.01) in the histopathological scores, when compared to aged rats (Tables 3, 4). However, there were no significant differences in the thymic or splenic histopathology scores between aged+Mel or aged+Tum groups and the normal adult animals (Tables 3, 4).
Table 3.
Histopathological scores of the thymus
The histopathological alterations were graded according to parameters previously described in the Materials and Methods section. Data represent means ± SEM. N = normal; Mel = melatonin; Tum = turmeric.
P < 0.01 vs. normal adult animals.
P < 0.01 vs. normal aged rats.
Table 4.
Histopathological scores of the spleen
The histopathological alterations were graded as described in the Materialsand Methods section. Data represent means ± SEM. N = normal; Mel = melatonin; Tum = turmeric.
P < 0.01 vs. normal adult animals.
P < 0.01 vs. normal aged rats.
Discussion
Ample data suggested that melatonin is implicated in aging regulation [21-23]. It could be observed that melatonin declined with age, and this reduction in the level of melatonin is an important indicator for the increased oxidative stress by aging [21]. Furthermore, turmeric is reported also to be involved in aging regulation [5,24]. In the present research, the effect of aging on selected immunoglobulins (IgA and IgE), some cytokines (TNF-α, IFN-γ, IL-6 and IL-8), antioxidant markers (NRF2 and DJ-1), and apoptotic markers (p53 and Bax) in addition to the histological changes accompanied with aging were investigated in rats. In addition, the ameliorative effect of melatonin and turmeric against aging was investigated.
In the present work, IgA was significantly increased in serum of normal aged rats with a concurrent decrease in IgE level when compared to that of normal adult rats. These findings are in agreement with those reported that aging resulted in elevation in the levels of serum IgA and IgG [25]. Total serum IgE levels increased progressively from the age of 1 month to the age of 5 months and then declined after the age of one-year [26]. Such observations were also reported that serum IgE levels and antigen-specific IgE production both decreased with age [27]. Currently, we found that administration of both melatonin and turmeric decreased IgA and increased IgE serum level in aged rats, and this is consistent with the findings that melatonin and IgA were correlatively expressed [28]. Also, consistently with the current results, it is stated that melatonin administration inhibited the serum IgE levels in the model mice [29]. Meanwhile, we found that curcumin uncovered aging-induced immunoglobulin A and E changes. On the other hand, it could be also found that curcumin elevated immunoglobulin-A in rats [30] and inhibited IgE in Guinea pigs [31]. Also, another study showed that curcumin had no effect on the immune status [32]. Melatonin is a potent therapeutic candidate regulating immune function in aged individuals [22].
In the present study, the levels of Tumor Necrosis Factor-alpha (TNF-α), Interferon-gamma (IFN-γ), Interleukin-6 (IL-6) and Interleukin-8 (IL-8) were significantly increased in serum of normal aged rats compared to that recorded in normal adult rats. These findings were supported by the findings that, aging is associated with increased levels of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β [33]. Moreover, our findings indicated that melatonin significantly decreased TNF-α, IFN-γ, IL-6 and IL-8 when compared to normal aged rats without melatonin supplementation. In this respect, melatonin was found to modulate the inflammatory status of through aging [34]. Similarly, turmeric administration to normal aged rats decreased TNF-α, IFN-γ, IL-6 and IL-8 when compared to normal aged rats. These anti-inflammatory effects of turmeric were supported by finding that, curcumin counteracted the pro-inflammatory status [5].
Regarding the effect of aging on the antioxidant master regulator NRF2 and its upstream signaling DJ-1 protein; current data showed that both DJ-1 and NRF2 mRNA levels were significantly declined with aging. In this respect, it is stated that DJ-1 is implicated in oxidative stress and aging regulation [35,36]. Furthermore, NRF2 is suggested as a potential therapeutic candidate against oxidative stress and aging [6,8]. Consistent with the present data, it is stated that decline of NRF2 caused age-related features [9]. The tumor suppressor p53 was correlatively expressed with aging [37,38]. Consistently, here we found that p53 mRNA expression level increased with aging in thymus tissues. Also, current data is consistent with the finding that the expression of the proapoptotic Bax protein was increased with aging [39]. Consistent with our data, it could be found that melatonin reversed the effect of aging on p53 and Bax expression [40]. Consistently, other studies showed that turmeric treatment decreased p53 and Bax expression levels [41,42].
The thymus gland is a primary lymphoid organ responsible for the differentiation and maturation of T lymphocytes and is termed the “the immunological clock of aging” [43]. The thymus gradually decreases its capacity to generate immunocompetent T cells and becomes minimally functional gradually with aging; undergoes dramatic alterations in its size, morphology and cell composition, a process termed “age-associated thymic involution” [44]. The present observations showed that, thymus of aged rats showed a marked thymic involution compared to normal adult rats. The data presented herein confirm the earlier reports which stated that thymus of old rats showed decreased cortical lymphocytes with apparent of epithelial cells, increased fibrovascular tissues, loss of cortical and medullary boundaries, augmented levels of apoptosis [44,45]. The appearance of numerous shrunken, necrotic as well as apoptotic lymphocytes in the thymus of aged rats is associated with a concurrent increase in the level of TNF-α in serum of normal aged rats. Furthermore, the present data also revealed an increased number of adipocytes infiltered the capsular and septal areas, and extended into the cortex of thymus of aged rats. These results supported data from other studies, which demonstrated that thymus is the major immune organ that is largely replaced with fat during aging [46].
It is well established that the pineal gland through its principal hormone melatonin affects lymphoid tissue size, structures and function [47]. In the present work, administration of melatonin to normal aged rats produced a marked improvement in aged thymic tissue such as increased the density of the cortical lymphocytes, decreased the number of necrotic thymocytes and absence of adipocytes when compared to that seen in the thymus of normal aged rats. These results are consistent with the finding that, melatonin prevented age-related thymic involution through regulation of thymocyte apoptosis [48]. The adipose tissue is also an important target for the action of melatonin. In this issue, it was demonstrated that melatonin reverted age-related changes caused by adiposity [49]. This is the first report to the available knowledge, showing that turmeric has beneficial effects on thymus and splenic tissues abnormalities in aged rats. Similar to melatonin treatment, turmeric administration to normal aged rats for 12 weeks produced a marked improvement in aged thymic tissue. In addition, curcumin may suppress preadipocyte differentiation and thus reduce the number of adipocytes and fat content of adipose tissue [50]. Thus, the current data support the suggestion that aging is a chronic inflammatory process with a shift towards a proinflammatory cytokine profile in tissues. This suggestion is further confirmed by current immunological results in which the levels of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-8) were significantly increased in serum of normal aged rats compared to that recorded in serum of normal adult rats.
Histopathological examination showed a loss of the cellularity of both the white and red pulps in the spleen of aged rats compared to those from controls. These alterations were associated with the appearance of numerous shrunken and necrotic lymphocytes and congestion of the splenic sinusoids. The present findings are consistent with numerous studies that confirm the occurrence of condition of immunosuppression in aging [45,51]. In this context, normal aging has been shown to induce noticeable alterations in the splenic architecture including the presence of thickly dyed karyopyknosis and many apoptotic cells as well as congested splenic sinus and increased macrophages. Similar results were obtained by different authors who observed that with the development of aging, there is a substantial reduction in lymphocytes of splenic white pulp and huge increasing macrophages [45,51]. Furthermore, in the present study, splenic sections of aged rats treated with melatonin showed a marked improvement in the histological picture, where the white pulp became easily defined with increased lymphocytic population. The marginal zone became well-differentiated. The hemosiderin pigments were reduced in the red pulp compared to that seen in the spleen of normal aged rats. These results are in agreement with the finding that melatonin has a potential role in maintaining the function and activity of splenic tissue in old rats [45].
Conclusively, the present study showed that aging is implicated with changes of several signaling cascades such as immunoglobulins, cyokines, and antioxidants- and apoptosis-related proteins. These changes could be reversed using melatonin and tumeric administration. These data suggest melatonin and tumeric as potent anti-aging candidates through activating the antioxidant upsetream regulators; DJ-1 and NRF2 and inhibiting apoptosis-inducing proteins p53 and Bax.
Disclosure of conflict of interest
None.
References
- 1.Harman D. Free radical involvement in aging. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Free radical theory of aging: an update. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 4.Mauriz JL, Molpeceres V, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J Pineal Res. 2007;42:222–230. doi: 10.1111/j.1600-079X.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010;7:1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petri S, Korner S, Kiaei M. Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurol Res Int. 2012;2012:878030. doi: 10.1155/2012/878030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan LI, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail IA, Abdel Shakor AB, Hong SH. DJ-1 protects breast cancer cells against 2’-Benzoyloxycinnamaldehyde-induced oxidative stress independent of Nrf2. J Cell Physiol. 2015;230:2262–9. doi: 10.1002/jcp.24957. [DOI] [PubMed] [Google Scholar]
- 12.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 13.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong N, Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum Mol Genet. 2008;17:3357–67. doi: 10.1093/hmg/ddn230. [DOI] [PubMed] [Google Scholar]
- 15.Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. 2013;2013:836760. doi: 10.1155/2013/836760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohm MR, Melkonyan H, Thanos S. Life-time expression of the proteins peroxiredoxin, beta-synuclein, PARK7/DJ-1, and stathmin in the primary visual and primary somatosensory cortices in rats. Front Neuroanat. 2015;9:16. doi: 10.3389/fnana.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–61. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Hafez AM. Antigenotixic activity of melatonin and selenium against genetic damage induced by paraquat. Australian Journal of Basic and Applied Sciences. 2009;3:2130–2143. [Google Scholar]
- 19.Sadeek EA, El-Razek FH. The chemo-protective effect of turmeric, chili, cloves and cardamom on correcting iron overload-induced liver injury, oxidative stress and serum lipid profile in rat models. Journal of American Science. 2010;6:7. [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Reiter RJ, Craft CM, Johnson JE Jr, King TS, Richardson BA, Vaughan GM, Vaughan MK. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology. 1981;109:1295–1297. doi: 10.1210/endo-109-4-1295. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC. Melatonin, immune function and aging. Immun Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turek FW, Zee P, Van Reeth O. Melatonin and aging. Adv Exp Med Biol. 1999;460:435–440. doi: 10.1007/0-306-46814-x_52. [DOI] [PubMed] [Google Scholar]
- 24.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 26.Pauwels R, Bazin H, Platteau B, Van der Straeten M. The effect of age on IgE production in rats. Immunology. 1979;36:145. [PMC free article] [PubMed] [Google Scholar]
- 27.Stoy PJ, Roitman-Johnson B, Walsh G, Gleich GJ, Mendell N, Yunis E, Blumenthal MN. Aging and serum immunoglobulin E levels, immediate skin tests, and RAST. J Allergy Clin Immunol. 1981;68:421–426. doi: 10.1016/0091-6749(81)90195-0. [DOI] [PubMed] [Google Scholar]
- 28.Park SJ, Tokura H. Bright light exposure during the daytime affects circadian rhythms of urinary melatonin and salivary immunoglobulin A. Chronobiol Int. 1999;16:359–71. doi: 10.3109/07420529909116864. [DOI] [PubMed] [Google Scholar]
- 29.Park G, Lee SH, Oh DS, Kim YU. Melatonin inhibits neuronal dysfunction-associated with neuroinflammation by atopic psychological stress in NC/Nga atopic-like mouse models. J Pineal Res. 2017:63. doi: 10.1111/jpi.12420. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki Y, Han Y, Kayahara M, Watanabe T, Arishige H, Kato N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:68–71. doi: 10.3177/jnsv.56.68. [DOI] [PubMed] [Google Scholar]
- 31.Thakare VN, Osama MM, Naik SR. Therapeutic potential of curcumin in experimentally induced allergic rhinitis in guinea pigs. Int Immunopharmacol. 2013;17:18–25. doi: 10.1016/j.intimp.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Ilsley SE, Miller HM, Kamel C. Effects of dietary quillaja saponin and curcumin on the performance and immune status of weaned piglets. J Anim Sci. 2005;83:82–8. doi: 10.2527/2005.83182x. [DOI] [PubMed] [Google Scholar]
- 33.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Puig Á, Rancan L, Paredes SD, Carrasco A, Escames G, Vara E, Tresguerres JA. Melatonin decreases the expression of inflammation and apoptosis markers in the lung of a senescence-accelerated mice model. Exp Gerontol. 2016;75:1–7. doi: 10.1016/j.exger.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Bonilha VL, Bell BA, Rayborn ME, Samuels IS, King A, Hollyfield JG, Xie C, Cai H. Absence of DJ-1 causes age-related retinal abnormalities in association with increased oxidative stress. Free Radic Biol Med. 2017;104:226–237. doi: 10.1016/j.freeradbiomed.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biteau B, Jasper H. It’s all about balance: p53 and aging. Aging (Albany NY) 2009;1:884. doi: 10.18632/aging.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2:471. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapasi AA, Singhal PC. Aging splenocyte and thymocyte apoptosis is associated with enhanced expression of p53, bax, and caspase-3. Mol Cell Biol Res Commun. 1999;1:78–81. doi: 10.1006/mcbr.1999.0106. [DOI] [PubMed] [Google Scholar]
- 40.Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernández C, Soria-Valles C, De Gonzalo-Calvo D, Tolivia D, Pallás M, Camins A, Rodríguez-Colunga MJ, Coto-Montes A. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J Pineal Res. 2009;46:106–14. doi: 10.1111/j.1600-079X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee B, Chakraborty S, Ghosh D, Raha S, Sen PC, Jana K. Benzo(a)pyrene induced p53 mediated male germ cell apoptosis: Synergistic protective effects of curcumin and resveratrol. Front Pharmacol. 2016;7:245. doi: 10.3389/fphar.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Yan Y, Cao Y, Yang Y, Zhao Q, Jing R, Hu J, Bao J. Potential therapeutic and protective effect of curcumin against stroke in the male albino stroke-induced model rats. Life Sci. 2017 doi: 10.1016/j.lfs.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmunity Reviews. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 45.El-Sokkary GH, Reiier RJ, Abdel-Ghaffar SK. Melatonin supplementation restores cellular proliferation and DNA synthesis in the splenic and thymic lymphocytes of old rats. Neuroendocrinol Lett. 2003;24:215–223. [PubMed] [Google Scholar]
- 46.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson RJ, Demas GE. Role of melatonin in mediating seasonal energetic and immunologic adaptations. Brain Res Bull. 1997;44:423–430. doi: 10.1016/s0361-9230(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 48.Provinciali M, Di Stefano G, Bulian D, Tibaldi A, Fabris N. Effect of melatonin and pineal grafting on thymocyte apoptosis in aging mice. Mech Ageing Dev. 1996;90:1–19. doi: 10.1016/0047-6374(96)01746-0. [DOI] [PubMed] [Google Scholar]
- 49.Grad BR, Rozencwaig R. The role of melatonin and serotonin in aging: update. Psychoneuroendocrinology. 1993;18:283–295. doi: 10.1016/0306-4530(93)90025-g. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7:467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]


