Abstract
Background
Clinical guidelines recommend monitoring of blood pressure at home using an automatic blood pressure device for the management of hypertension. Devices are not often calibrated against direct blood pressure measures, leaving health care providers and patients with less reliable information than is possible with current technology. Rigorous assessments of medical devices are necessary for establishing clinical utility.
Objective
The purpose of our study was 2-fold: (1) to assess the validity and perform iterative calibration of indirect blood pressure measurements by a noninvasive wrist cuff blood pressure device in direct comparison with simultaneously recorded peripheral and central intra-arterial blood pressure measurements and (2) to assess the validity of the measurements thereafter of the noninvasive wrist cuff blood pressure device in comparison with measurements by a noninvasive upper arm blood pressure device to the Canadian hypertension guidelines.
Methods
The cloud-based blood pressure algorithms for an oscillometric wrist cuff device were iteratively calibrated to direct pressure measures in 20 consented patient participants. We then assessed measurement validity of the device, using Bland-Altman analysis during routine cardiovascular catheterization.
Results
The precalibrated absolute mean difference between direct intra-arterial to wrist cuff pressure measurements were 10.8 (SD 9.7) for systolic and 16.1 (SD 6.3) for diastolic. The postcalibrated absolute mean difference was 7.2 (SD 5.1) for systolic and 4.3 (SD 3.3) for diastolic pressures. This is an improvement in accuracy of 33% systolic and 73% diastolic with a 48% reduction in the variability for both measures. Furthermore, the wrist cuff device demonstrated similar sensitivity in measuring high blood pressure compared with the direct intra-arterial method. The device, when calibrated to direct aortic pressures, demonstrated the potential to reduce a treatment gap in high blood pressure measurements.
Conclusions
The systolic pressure measurements of the wrist cuff have been iteratively calibrated using gold standard central (ascending aortic) pressure. This improves the accuracy of the indirect measures and potentially reduces the treatment gap. Devices that undergo auscultatory (indirect) calibration for licensing can be greatly improved by additional iterative calibration via intra-arterial (direct) measures of blood pressure. Further clinical trials with repeated use of the device over time are needed to assess the reliability of the device in accordance with current and evolving guidelines for informed decision making in the management of hypertension.
Trial Registration
ClinicalTrials.gov NCT03015363; https://clinicaltrials.gov/ct2/show/NCT03015363 (Archived by WebCite at http://www.webcitation.org/6xPZgseYS)
Keywords: patient self-management, diastolic hypertension, telemonitoring, vital signs, smartphone applications
Introduction
Hypertension is a global health problem affecting over a billion people [1]. If uncontrolled, it is a major risk factor for stroke, myocardial infarction, and kidney failure; it remains the leading cause of cardiovascular morbidity and mortality [2,3]. Diagnosis and management of hypertension relies intensively on the indirect measurement of blood pressure in outpatient settings.
Manual auscultatory and automated oscillometry (ie, clinical and patient blood pressure monitoring methods) are common means for diagnosing hypertension and guiding appropriate medical management. Current guidelines (Canadian Hypertension Education Program, CHEP; American Society of Hypertension; International Society of Hypertension; European Society of Hypertension, ESH) recommend confirmation of hypertension (ie, blood pressure ≥135/85) with ambulatory or home blood pressure monitoring [4-6] . In recent years, there has been a dramatic growth in automated devices and increased use of mobile health apps [7]. However, there is limited information about their accuracy or precision, which creates a risk for inappropriate therapy or a treatment gap [8,9]. Clinically, the term treatment gap refers to hypertensive patients who are left untreated because of underestimated noninvasive blood pressure readings, which could be a health risk for the patient.
Automated clinical oscillometric or consumer-level devices have been generally compared with manual auscultatory measurements [10-12], while studies using invasive blood pressure measurements for validation or calibration are increasing [13-15]. Regulatory agencies (eg, Health Canada; Food and Drug Administration, FDA) license devices without mandatory independent third-party, peer-reviewed assessment of the validity of measurements or calibration standards. The principal directive of regulatory agencies for these sphygmomanometer devices (Health Canada and FDA Class II) is to ensure physical safety and personal data security [16,17] rather than guarantee accuracy and precision for clinical diagnostic purposes. The minimal requirements prescribed by Health Canada are not the minimal requirements of a clinician.
Increasingly, automated devices are being assessed against direct intra-arterial standards for clinical certainty, yet, most still do not report the uncertainty of the single blood pressure measurement. Comparing direct and indirect blood pressure measures simultaneously ensures that intraphysiological variability can be accounted for in the measures. The difference between 2 methods can then be validated with multiple measures within a patient population. There is a growing need for more accurate devices to measure blood pressure [18] to better diagnose and manage hypertension according to clinical guidelines. This could be accomplished by calibration to simultaneous direct measures in addition to auscultatory calibration using indirect measurements to reduce the treatment gap in hypertension. Generally, consumer devices, clinical devices, and the true invasive blood pressure measures in healthy and hypertensive patients should be in agreement with each other. Our objective was to assess the validity of indirect measures of blood pressure by a wrist-worn blood pressure device in direct comparison with simultaneously recorded gold standard intra-arterial blood pressure measures for the purpose of iterative device calibration.
Methods
Recruitment and Screening
The clinical protocol was approved by the Horizon Health Network Research Ethics Board, and the study is registered with the National Institute of Health Clinical Trials Registry database (NCT 03015363). The patient participant inclusion/exclusion criteria were: (1) a referral from the patient’s attending cardiologist to undergo a first-time nonemergent diagnostic cardiac catheterization procedure for clinically valid indications; (2) participants aged ≥19 years; (3) wrist circumference should be in the range of 13.5 to 23 cm; (3) participant should be willing to voluntarily sign the study-specific informed consent form; and (4) participant should have no previous percutaneous coronary intervention, coronary artery bypass graft, abdominal aortic aneurysm, peripheral vascular disease, aortic stenosis, arrhythmia, tremors (before or during procedure), or carotid bruits. In accordance with the Association for the Advancement of Medical Instrumentation (AAMI) standards for clinical investigation using reference-based invasive blood pressure monitoring [19], we undertook a 2-day protocol (Figure 1).
Figure 1.
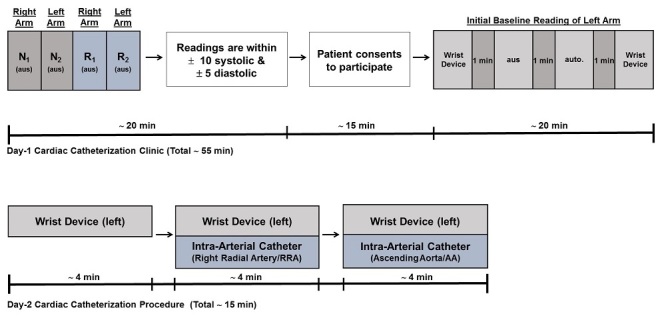
Procedural overview: timelines for patient recruitment and data collection. Day 1-Timeline intake of patients for inclusion screening followed by consent and initial data collection. N and R are 2 trained investigators measuring blood pressure twice using the auscultatory method (aus); initial baseline readings used an automatic upper arm cuff (auto.). Day 2-Timeline for simultaneous invasive and noninvasive blood pressure measurements.
Blood Pressure Measurements
Logistically, the wrist cuff cannot be applied to the same arm that is being cannulated for radial pressure. Thus, patients were screened for bilateral upper arm auscultatory blood pressure equality (day 1; within ±10 mm Hg systolic and ±5 mm Hg diastolic pressure; see Multimedia Appendix 1) by 2 separate blinded readings performed using an upper arm aneroid sphygmomanometer (Welch Allyn Canada Ltd, Mississauga, ON) by 2 health professionals (Figure 1). This ensured that all patients did not have undiagnosed peripheral vascular disease to cause arm inequality in blood pressure. All eligible participating patients voluntarily provided documented informed consent. After obtaining the consents, 4 blood pressure readings were taken approximately 1 min apart to establish a baseline (Figure 1); 3 different devices were used for general observational comparison, according to standard procedures or manufacturers’ instructions: (1) an oscillometric wrist cuff device (Cloud Diagnostics Inc, PULSEWAVE Health Monitor, Kitchener ON), (2) an upper arm oscillometric device (Welch Allyn Canada Ltd, Mississauga, ON), (3) and an upper arm aneroid sphygmomanometer (Welch Allyn Canada Ltd, Mississauga, ON). The pulse pressure waveform recorded by the wrist cuff device was digitized at 100 Hz and stored securely on a cloud-based server for subsequent analysis.
On the day of the cardiac catheterization procedure (Day 2), patients were given 1mg of midazolam and 50 µg of fentanyl for presedation ; blood pressure readings were taken as indicated in Figure 1 with the patient in the supine position. A total of 10 readings were taken with 2 measurements for each method (Figure 1); the first set of 2 wrist cuff measures were taken for patient conditioning and were not subsequently used for comparison in this study. The second set of 2 wrist cuff measures were taken while simultaneously recording 2 intra-arterial pressures at the right radial artery. The third set of 2 wrist cuff measures were taken while simultaneously recording 2 intra-arterial pressures at the ascending aorta. Each measurement was treated independently throughout this study (N=160 intra-arterial measurements in total for recalibration; 20 participants). Digital records of previously monitored data in a clinical setting using indirect, double-observer auscultatory measurements were secured for the purpose of clinical comparative analysis (N=375 measurements; 97 participants).
Intra-arterial pressure was measured with a fluid-filled 5 or 6 French gauge catheter (Cordis AVANTI+) attached to a pressure transducer (NAMIC, Navilyst Medical, Marlborough, MA, USA), which is consistent with the recent recommendation by the ARTERY Society task force [20]. The pressure transducer was zeroed before the start of the catheterization procedure. Intra-arterial pressure was recorded with hemodynamic software (MAC-LAB, General Electric Company). To avoid verapamil or heparin-induced changes in vascular tone, both drugs were administered as per standard of care procedure after pressure readings were complete.
Calculated device values of systolic and diastolic pressures were initially derived from the Cloud Diagnostics Inc application reports (original auscultatory calibration). The intra-arterial dataset was split into a training set and a testing set using the jackknife technique, as originally described [21]. The jackknife technique is a power data analysis tool suitable for small original data samples. In a dataset of N readings, the jackknife iterative processing can be described as the systematic resampling of a single reading from the entire dataset to be used as the testing set and the rest N−1 readings are used as the training set. This is repeated N times and during the ith iteration, the ith reading is chosen as the testing set and the rest N-1 readings are chosen as the training set. During each iteration the training set is used to obtain calibration coefficients, which are then used on the testing set. After making algorithm adjustment, new systolic and diastolic pressures were obtained directly from the engineers at Cloud Diagnostics Inc.
Statistical Analysis and Data Reporting
Data analysis was performed via Bland-Altman (Tukey mean difference) plot analysis [22,23] to assess the accuracy of the wrist cuff relative to intra-arterial measures. Systolic and diastolic validity was set a priori as any 2 measures being within 10 and 5 mm Hg, respectively (based on a normally distributed relevant clinical population) [24,25] (See Multimedia Appendix 2). The absolute mean difference and SD of all Bland-Altman plots are reported as the bias [20,26,27] (Also, see Multimedia Appendix 3). A one-tailed Fisher exact test was used to determine the sensitivity of the device in assessing measures of hypertension versus normotension.
Results
Blood Pressure Device Calibration
Here, we used intra-arterial blood pressure measurements in comparison with the noninvasive wrist cuff method (Figure 2).
Figure 2.
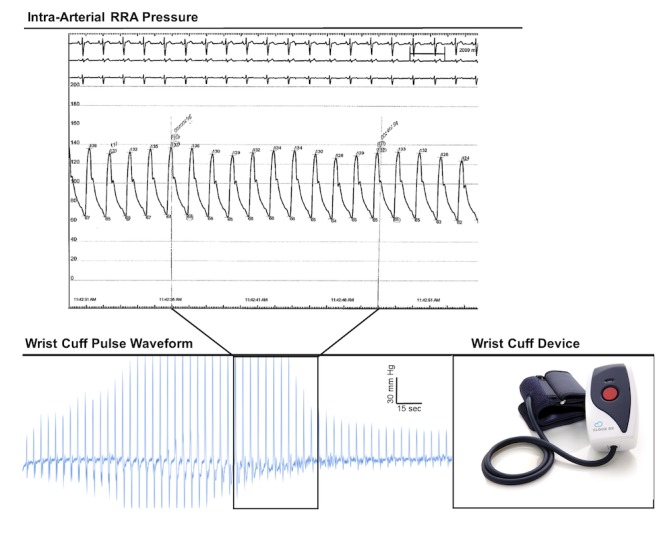
Physiological tracings. Representative hemodynamic tracing from the intra-arterial pressure catheter with electrocardiogram (ECG). Representative pulse waveform report from the wrist-cuff blood pressure device.
A total of 74 potentially eligible patients were screened, of which 37 consented, 3 withdrew, and 14 failed to meet the criteria during the (day 2) procedure. A summary of the patients’ characteristics that completed all aspects of this study is presented in Table 1.
Table 1.
Patient participant characteristics.
| Characteristics | Patient participants (N=20) | |
| Sex (male/female) | 15/5 | |
| Age in years, mean (SD); range | 62.0 (SD 9.0); 43-77 | |
| BMIa, mean (SD); range | 30.6 (SD 5.7); 21-45.4 | |
| Central aortic pressure (mm Hg) |
|
|
|
|
Systolic, mean (SD); range | 133.4 (SD 22.0); 96.7-179.3 |
|
|
Diastolic, mean (SD); range | 67.4 (SD 8.7); 50.6-85.1 |
| Peripheral arterial pressure (mm Hg) |
|
|
|
|
Systolic, mean (SD); range | 145.7 (SD 20.2); 113.0-184.8 |
|
|
Diastolic, mean (SD); range | 66.2 (SD 9.1); 49.4-84.8 |
| Wrist circumference in cm (left), range | 15.5-21.5 | |
| Smoking, % (Y/N) | 15 (3/17) | |
| Diabetes, % (Y/N) | 35 (7/13) | |
| Statin, % (Y/N) | 70 (14/6) | |
| Hypertension, % (Y/N) | 75 (15/5) | |
aBMI: body mass index.
The mean direct right radial arterial systolic pressure was 145.7 (SD 20.2) mm Hg, and the mean direct ascending aorta systolic pressure was 133.4 (SD 22.0) mm Hg. This illustrates the real physiological difference of 14.4 (SD 10.3), with P<.001, as a result of pressure augmentation [28]. There was no physiological difference in direct right radial arterial diastolic pressure and direct ascending aorta diastolic pressure (66.2 [SD 9.1] mm Hg vs 67.4 [SD 8.7] mm Hg), respectively; absolute mean difference of 5.1 (SD 3.6), P=.23).
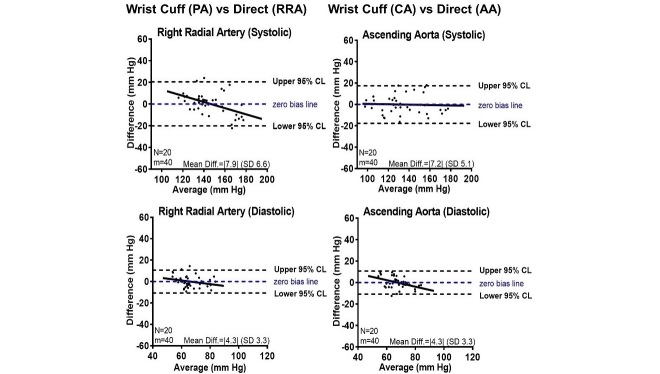
Initially, the absolute mean difference of the wrist cuff compared with direct systolic measures using the original auscultatory calibration settings was 10.8 (SD 9.7), with P<.001, while the absolute mean difference of the diastolic measures was 16.1 (SD 6.3), with P<.001 (See Multimedia Appendix 4). Next, we adjusted the algorithm using the intra-arterial blood pressure datasets. First, we applied an iterative calibration of the wrist cuff to radial artery pressures, and the absolute mean difference of the systolic and diastolic measures was 7.9 (SD 6.6), with P=.87 and 4.3 (SD 3.3), with P>.99, respectively (Figure 3). However, we noted a negative slope trend line that may suggest an attenuated pressure when intraradial systolic pressure is greater than 150 mm Hg. Then, we calibrated the wrist cuff to ascending aortic pressures, and the absolute mean difference of the systolic and diastolic measures was 7.2 (SD 5.1), with P=.97 and 4.3 (SD 3.3), with P=.98, respectively (Figure 2), with a near 0 systolic pressure trend.
Figure 3.
Direct intra-arterial blood pressure agreement with indirect wrist cuff measures. Bland-Altman plot analyses of pressure measurement agreement with: systolic and diastolic blood pressure wrist cuff measurements after peripheral algorithm (PA) adjustment and direct right radial artery (RRA) blood pressure measurements (N=20; mean=80), and systolic and diastolic blood pressure wrist cuff measurements after central algorithm (CA) adjustment and direct ascending aorta (AA) blood pressure measurements (N=20; mean=80).
To further assess the value of the central algorithm independently of our calibration dataset, we sourced an arms-length dataset of double-observer auscultatory blood pressure measures from Cloud Diagnostics Inc (375 measurements; 97 participants). The average absolute mean difference of the initial algorithm markedly improved with the central pressure calibrated algorithm in both the systolic and diastolic pressures (7.5 [SD 7.3] vs 6.1 [SD 4.7] and 18.0 [SD 7.6] vs 9.8 [SD 6.0], respectively; see Multimedia Appendix 5). This is an improvement in accuracy of 20% with 38% less variability for systolic measures and 46% more accuracy with 19% less variability for diastolic measures using an independent dataset. Therefore, central pressure calibration improved the accuracy and reliability of the wrist-cuff device comparisons to direct pressures and upper arm cuff measures, which is relevant to clinical practice guidelines.
Blood Pressure Variability Assessment
To demonstrate measurement variability, a representative illustration of instantaneous intra-arterial pressures that were recorded every 10 s over a period of 4 min is shown in Figure 4. Measurements varied by approximately 20 and 10 mm Hg for systolic and diastolic pressures, respectively.
Figure 4.
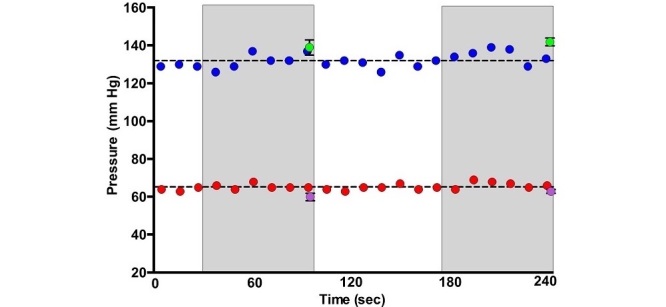
Simultaneous recordings of direct and indirect blood pressures. Representative illustration of instantaneous intra-arterial blood pressures every 10 s over 4 min (blue=systolic; red=diastolic) along with 2 simultaneous readings of wrist cuff blood pressure (green=systolic; purple=diastolic) with SD. Wrist cuff blood pressure is acquired over approximately 1 min (gray boxes).
Subsequently, all invasive measures having SD 1 were plotted (Figure 5). Direct pressure analysis (both radial and aortic) illustrates pressure augmentation of peripheral blood pressure (Figure 5). Several measures have an SD that crosses the clinical threshold for diagnosing hypertension based on the CHEP guidelines [4] or the Systolic Blood Pressure Intervention Trial (SPRINT) [29]. Similar results were obtained by plotting the indirect measures (Figure 5). Taken together, in the absence of intraphysiological variability reporting (Figure 5), the wrist cuff produces diagnostically similar data to direct arterial pressure with a lower treatment gap risk than a noncalibrated indirect measure, such as the upper arm measures used in this study. Approximately 75% (15/20) of the patients in this study were already diagnosed with hypertension. According to direct intra-aortic and indirect wrist cuff measures, approximately 40% (Figure 5) were above the blood pressure target provided by the CHEP guidelines. When measures were plotted as being either hypertension positive or negative, the centrally-calibrated wrist cuff measures were on par with direct aortic measures using either CHEP- [4] or SPRINT-based [29] thresholds (Figure 5), whereas there was a significant difference between direct aortic pressure measures and calibrated wrist cuff measures compared with the upper arm cuff (P=.04; see Figure 5).
Figure 5.
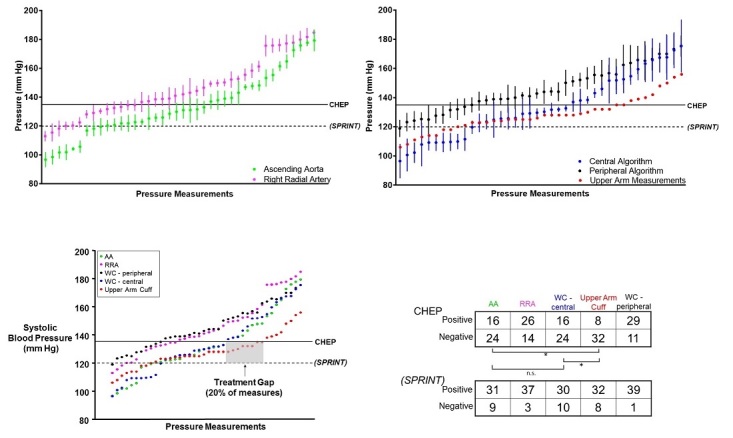
Relevant threshold comparison of peripheral and central pressures, directly and indirectly. Direct intra-arterial systolic pressure measurements from the right radial artery (magenta) and ascending aorta (green). Indirect wrist cuff systolic pressure measurements using the peripheral algorithm (black) and central algorithm (blue); upper arm measurements included with no possibility of a measure of uncertainty. All course of mean systolic measures (without SD) with guideline and trial target thresholds. Note: gray box shows upper arm measures at risk of treatment gap based on Canadian Hypertension Education Program (CHEP) and Systolic Blood Pressure Intervention Trial (SPRINT) thresholds.
Discussion
Principal Findings
In this study, we assessed the validity of indirect measures of a novel wrist cuff blood pressure device in direct comparison with simultaneously recorded gold standard intra-arterial pressures. This study shows that a calibrated wrist cuff blood pressure device is in agreement with the precision and accuracy of intra-arterial pressure measurements in an in-patient setting. To our knowledge, this study is the first to test and incorporate iterative calibration of a commercially available blood pressure device and also the first to show measurement uncertainty in the device output. This study shows that an automated upper arm device when compared with other indirect methods was less accurate than the gold standard calibration used here; this could reduce the risk of a treatment gap. A calibrated wrist cuff device, if used properly, is capable of producing measures that are clinically accurate for the management of systolic hypertension in accordance with the guidelines for home blood pressure monitoring. In our center, we identified a clinical need to use wrist cuff devices for patients in intensive care (eg, minimize sleep disturbance) or with physical limitations to upper arm cuff use (eg, frailty, obesity). To achieve broad clinical reliability, additional use and monitoring of the device, such as in clinical trials, is warranted.
Clinical Relevance
This wrist cuff device offers several advantages in comparison with many upper arm devices for the measurement and medical management of hypertension. A common complaint among patients and front-line health care providers, especially when performing repetitive blood pressure monitoring, is that they experience pain with upper arm compression [30]. Frail patients are more likely to become physically intolerant to multiple daily measures with an arm cuff. Furthermore, these devices, when activated in hospitals at night, disrupt essential circadian sleep quality [31]. Obese, senior, and frail patients most often experience cuff malposition issues, either from obese, conical upper arms or from mobility or dexterity issues for self-fastening and positioning [30]. Patients with conical or obese arms and the frail elderly are key target demographics in need of hypertension identification and management.
Individual blood pressure measures from this wrist cuff device report the degree of uncertainty in each measure. While several mean blood pressure measurements were below the threshold for hypertension based on the CHEP guidelines [4] or the SPRINT trial target [29], they were within 1 SD of the threshold. This poses the question as to whether these patients would benefit from more aggressive therapeutic intervention. Future work should assess the frequency by which this occurs in an outpatient setting using a longitudinal study.
Calibration Issues With Indirect Oscillometric Devices: Peer Review, Interdependence on Therapeutics, and the Level of Uncertainty
The variability between radial and aortic pressures is a function of differences in compliance, vasoactivity, and pressure augmentation, which become more variable with aging and disease [25,28,32]. Thus, noninvasive blood pressure can be calibrated to either radial artery or aortic pressure. Specifically, a universal protocol that recommends intra-arterial pressure as a reference standard to validate noninvasive blood pressure is currently being developed via collaboration of the AAMI and the ESH [20]. While this wrist cuff blood pressure device was calibrated to aortic pressures [20,33-37] in this study, we also presented wrist cuff blood pressure data calibrated to either radial artery or aortic pressures for scientific interest. This natural intraphysiological variability from the mean (Figure 4, dashed line) is derived from variability of neuro-endocrine stimulation, arterial tone, heart rhythm, cardiac output, and respiration. Thus, the correct and accurate way to calculate blood pressure should include intrinsic measures of variance [38] (measure of uncertainty or SD). This was achieved for the first time with a blood pressure device that reported an SD for each measure.
Blood pressure devices are almost exclusively calibrated to manual auscultatory measurements [10-12,17]. In addition to the calibration inconsistency among blood pressure devices as previously described [18], unreliable measurements among automated blood pressure devices are common [39,40]. Most protocols for device comparison are focused on variability within a population rather than variability of blood pressure measures within an individual. Most automated devices currently do not report the uncertainty of the blood pressure measure. We also observed that upper arm measures were not in agreement with aortic pressures and could produce a treatment gap of approximately 20%. The need to align blood pressure measures with the medical management of hypertension is of paramount importance [41]. Additionally, many (medical or life) insurers have a vested financial interest in the reliability of blood pressure as an index of health in determining premiums and eligibility. Fundamentally, the management of hypertension and blood pressure devices are interdependent, yet a barrier to achieving optimal disease management is, at least in part, related to the current lack of information about their precision, accuracy, and level of uncertainty. Indeed, systematic review of noninvasive blood pressure devices reportedly meeting the engineering standards of either the AAMI, ESH, or British Hypertension Society protocols [19,42,43] are inconsistently adhered to and are not always in agreement [18]. Often, incongruent variables are reported in the Bland-Altman analyses that invariably lead to a mean difference of 0 with increasing n-values, which is statistically unacceptable [27], whereas clauses in the protocol can allow for removal of potentially relevant measures (ie, 12/8 rule) [44]. Furthermore, engineering and clinical standards are not comparable—the former is concerned with device reproducibility, while the latter is concerned with interpatient and intrapatient variability. Regulatory agencies (eg, Health Canada, FDA) that license these devices are responsible only for aspects relating to product safety and the comparability to other market devices and not for determining the validity of the measurements, [16,17]. More efforts are required to advance device quality and functionality using a patient-centered approach to accommodate the interdependencies between blood pressure devices and (clinical trial-approved) therapeutics for the management of hypertension [8,9]. A universal protocol that is clinically practical and can consistently determine device validity, including when challenged by a direct pressure analysis, is currently being developed and estimated to be released in 2018 [20].
Study Limitations and the Impact of Precise and Accurate Blood Pressure Measures on Current Clinical Guidelines
Further calibration studies using this device should include special populations such as pediatric, obese, or frail patients, or those with an underlying arrhythmia or peripheral vascular disease. Also, patients with peripheral movement artifacts (eg, tremors) represent an incremental challenge to wrist cuff devices that would be less pronounced for upper arm cuff devices. Finally, more work is required to determine whether diastolic pressure and diagnoses using indirect diastolic pressure measures are required to be accurate to direct central pressures.
To diagnose hypertension according to guidelines, we must be confident with device measures [45,46]. It is encouraging that large clinical trials have demonstrated improved outcomes in populations achieving even 1 mm Hg reductions in blood pressure, yet devices have no provision for this single digit resolution for an individual patient. Guidelines tend to utilize 5 mm Hg increments and we are not aware of any device that has this resolution without applying variability filtering [44]. Indeed, intrapatient variability of blood pressure within a short period of time can vary by increments of >10 mm Hg, notwithstanding intraday variability of 25 mm Hg or more. This not only necessitates serial averaging of blood pressure measures but also compounds the standard error in the mean of measures. A greater awareness of uncertainty could help to establish criteria of acceptability in a measure. Now, the question, given the recent results of the SPRINT trial [29], whether the lowering of the blood pressure target was achieved by having patients at 120 mm Hg or the consequence of greater certainty in patient catchment below the existing 135 mm Hg threshold will have to be faced. Future clinical trials should report the devices used for blood pressure analysis and their level of uncertainty. This will provide an opportunity to factor in-device uncertainty as guidelines are further refined.
Conclusions
The wrist cuff calibrated here to the gold standard—the central (ascending aortic) pressure—is an accurate device that can be used in accordance with guidelines for informed decision making in the management of systolic hypertension.
Acknowledgments
The following funding sources were pertinent to this study: New Brunswick Health Research Foundation and Health+Life Sciences New Brunswick Inc (KRB and SL) and The Natural Sciences and Engineering Research Council of Canada (KRB and JAS). JAS is a New Investigator with the Heart and Stroke Foundation of Canada. Devices and technical support were provided in-kind by Cloud Diagnostics Inc. Cloud Diagnostics Inc contracted Health+Life Sciences New Brunswick Inc to fund, at arms-length, this study with partnership funding provided by the New Brunswick Health Research Foundation. The authors are grateful to Dr Fong, Dr Hubacek, Dr Barry, and Dr Paddock for their support in recruitment and data collection and Alexandra Yip and Dr Stoica for helpful discussions. We are also appreciative of the patients, nurses, and nurse associates of The New Brunswick Heart Centre.
Abbreviations
- AAMI
Association for the Advancement of Medical Instrumentation
- CHEP
Canadian Hypertension Education Program
- ESH
European Society of Hypertension
- FDA
Food and Drug Administration
- SPRINT
Systolic Blood Pressure Intervention Trial
Assessment of bilateral blood pressure equality.
Normality analysis of acquired BP.
Correlational, actual indirect-direct error trend, paired simultaneous, and normalized % ratio difference comparative analysis of measured direct and indirect data BP.
Device initial algorithm vs intra-arterial pressure measures.
Device initial and central algorithms vs auscultatory pressure measures.
Footnotes
Conflicts of Interest: None declared.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017 Dec 7;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)31919-5 .S0140-6736(16)31919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y, Khatibzadeh S, Khoo J, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd HK, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van DR, van DA, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. http://europepmc.org/abstract/MED/23245609 .S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1.S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 4.Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB, Nerenberg K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GVR, Gryn SE, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Sivapalan P, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NRC, Moe GW, Howlett JG, Boulanger J, Prebtani A, Kline G, Leiter LA, Jones C, Côté A, Woo V, Kaczorowski J, Trudeau L, Tsuyuki RT, Hiremath S, Drouin D, Lavoie KL, Hamet P, Grégoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM, Hypertension Canada Hypertension Canada's 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017 May;33(5):557–76. doi: 10.1016/j.cjca.2017.03.005.S0828-282X(17)30110-1 [DOI] [PubMed] [Google Scholar]
- 5.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet J, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014 Jan;32(1):3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 6.Authors/Task Force Members. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen M, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM, Additional Contributor: Simone Binno (Italy) Document Reviewers. De Backer G, Roffi M, Aboyans V, Bachl N, Bueno H, Carerj S, Cho L, Cox J, De Sutter J, Egidi G, Fisher M, Fitzsimons D, Franco OH, Guenoun M, Jennings C, Jug B, Kirchhof P, Kotseva K, Lip GY, Mach F, Mancia G, Bermudo FM, Mezzani A, Niessner A, Ponikowski P, Rauch B, Rydén L, Stauder A, Turc G, Wiklund O, Windecker S, Zamorano JL. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2016 Jul;23(11):NP1–96. doi: 10.1177/2047487316653709.2047487316653709 [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov DV. Medical internet of things and big data in healthcare. Healthc Inform Res. 2016 Jul;22(3):156–63. doi: 10.4258/hir.2016.22.3.156. http://www.e-hir.org/journal/viewJournal.html?year=2016&vol=022&num=03&page=156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell NR, Gelfer M, Stergiou GS, Alpert BS, Myers MG, Rakotz MK, Padwal R, Schutte AE, O'Brien E, Lackland DT, Niebylski ML, Nilsson PM, Redburn KA, Zhang X, Burrell L, Horiuchi M, Poulter NR, Prabhakaran D, Ramirez AJ, Schiffrin EL, Touyz RM, Wang J, Weber MA. A call to regulate manufacture and marketing of blood pressure devices and cuffs: a position statement from the World Hypertension League, International Society of Hypertension and Supporting Hypertension Organizations. J Clin Hypertens (Greenwich) 2016 May;18(5):378–80. doi: 10.1111/jch.12782. doi: 10.1111/jch.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez NG, Cohen IG, Kesselheim AS. FDA regulation of mobile health technologies. N Engl J Med. 2014 Jul 24;371(4):372–9. doi: 10.1056/NEJMhle1403384. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch C, Krüger R, Saito K, Yamashita S, Sawanoi Y, Beime B, Bramlage P. Comparison of the Omron RS6 wrist blood pressure monitor with the positioning sensor on or off with a standard mercury sphygmomanometer. Blood Press Monit. 2014 Oct;19(5):306–13. doi: 10.1097/MBP.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 11.Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007 Aug;12(4):233–42. doi: 10.1097/MBP.0b013e32813fa386.00126097-200708000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Yoshika M, Yokoi T. Validation of Omron RS8, RS6, and RS3 home blood pressure monitoring devices, in accordance with the European Society of Hypertension International Protocol revision 2010. Vasc Health Risk Manag. 2013;9:265–72. doi: 10.2147/VHRM.S44569. doi: 10.2147/VHRM.S44569.vhrm-9-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alihanoglu YI, Kayrak M, Ulgen MS, Yazici M, Yazici M, Yilmaz R, Demir K, Dogan Y, Sizer M, Ozhan H, Koc F, Bodur S. The impact of central blood pressure levels on the relationship between oscillometric and central blood pressure measurements: a multicenter invasive study. J Clin Hypertens (Greenwich) 2013 Sep;15(9):681–6. doi: 10.1111/jch.12166. doi: 10.1111/jch.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mireles SA, Jaffe RA, Drover DR, Brock-Utne JG. A poor correlation exists between oscillometric and radial arterial blood pressure as measured by the Philips MP90 monitor. J Clin Monit Comput. 2009 Jun;23(3):169–74. doi: 10.1007/s10877-009-9178-8. [DOI] [PubMed] [Google Scholar]
- 15.Umana E, Ahmed W, Fraley MA, Alpert MA. Comparison of oscillometric and intraarterial systolic and diastolic blood pressures in lean, overweight, and obese patients. Angiology. 2006;57(1):41–5. doi: 10.1177/000331970605700106. [DOI] [PubMed] [Google Scholar]
- 16.Minister of Justice of Canada. 2016. Mar 16, Consolidation: medical device regulations, SOR/98-282 http://laws-lois.justice.gc.ca/
- 17.U.S. Department of Health and Human Services Food and Drug Administration. 2014. The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications [510(k)] http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM284443.pdf .
- 18.Wan Y, Heneghan C, Stevens R, McManus RJ, Ward A, Perera R, Thompson M, Tarassenko L, Mant D. Determining which automatic digital blood pressure device performs adequately: a systematic review. J Hum Hypertens. 2010 Jul;24(7):431–8. doi: 10.1038/jhh.2010.37. http://europepmc.org/abstract/MED/20376077 .jhh201037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association for the Advancement of Medical Instrumentation. 2013. ANSI/AAMI/ISO 81060-2 http://www.aami.org/
- 20.Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, Boutouyrie P, Chen CH, Chowienczyk P, Cockcroft JR, Cruickshank JK, Ferreira I, Ghiadoni L, Hughes A, Jankowski P, Laurent S, McDonnell BJ, McEniery C, Millasseau SC, Papaioannou TG, Parati G, Park JB, Protogerou AD, Roman MJ, Schillaci G, Segers P, Stergiou GS, Tomiyama H, Townsend RR, Van Bortel LM, Wang J, Wassertheurer S, Weber T, Wilkinson IB, Vlachopoulos C. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017 Oct 1;38(37):2805–12. doi: 10.1093/eurheartj/ehw632.2964710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller RG. The jackknife--a review. Biometrika. 1974;61(1):1–15. [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–10.S0140-6736(86)90837-8 [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995 Oct 21;346(8982):1085–7. doi: 10.1016/s0140-6736(95)91748-9.S0140-6736(95)91748-9 [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983 Jul;53(1):96–104. doi: 10.1161/01.res.53.1.96. http://circres.ahajournals.org/cgi/pmidlookup?view=long&pmid=6861300 . [DOI] [PubMed] [Google Scholar]
- 25.Avolio AP, Xu K, Butlin M. Application of cardiovascular models in comparative physiology and blood pressure variability. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:217–20. doi: 10.1109/EMBC.2013.6609476. [DOI] [PubMed] [Google Scholar]
- 26.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb) 2015;25(2):141–51. doi: 10.11613/BM.2015.015. http://www.biochemia-medica.com/2015/25/141 .bm-25-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewitte K, Fierens C, Stöckl D, Thienpont LM. Application of the Bland-Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem. 2002 May;48(5):799–801. http://www.clinchem.org/cgi/pmidlookup?view=long&pmid=11978620 . [PubMed] [Google Scholar]
- 28.Munir S, Guilcher A, Kamalesh T, Clapp B, Redwood S, Marber M, Chowienczyk P. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008 Jan;51(1):112–8. doi: 10.1161/HYPERTENSIONAHA.107.096016. http://hyper.ahajournals.org/cgi/pmidlookup?view=long&pmid=17998476 .HYPERTENSIONAHA.107.096016 [DOI] [PubMed] [Google Scholar]
- 29.SPRINT Research Group. Wright Jr JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff Jr DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015 Nov 26;373(22):2103–16. doi: 10.1056/NEJMoa1511939. http://europepmc.org/abstract/MED/26551272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boubouchairopoulou N, Kollias A, Chiu B, Chen B, Lagou S, Anestis P, Stergiou GS. A novel cuffless device for self-measurement of blood pressure: concept, performance and clinical validation. J Hum Hypertens. 2017 Jul;31(7):479–82. doi: 10.1038/jhh.2016.101.jhh2016101 [DOI] [PubMed] [Google Scholar]
- 31.Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013 Sep 9;173(16):1554–5. doi: 10.1001/jamainternmed.2013.7791. http://europepmc.org/abstract/MED/23817602 .1705722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995 Jan;88(1):103–9. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- 33.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension. 2016 Jan;67(1):183–90. doi: 10.1161/HYPERTENSIONAHA.115.06066. http://hyper.ahajournals.org/cgi/pmidlookup?view=long&pmid=26597821 .HYPERTENSIONAHA.115.06066 [DOI] [PubMed] [Google Scholar]
- 34.Stergiou GS, Parati G, Vlachopoulos C, Achimastos A, Andreadis E, Asmar R, Avolio A, Benetos A, Bilo G, Boubouchairopoulou N, Boutouyrie P, Castiglioni P, de la Sierra A, Dolan E, Head G, Imai Y, Kario K, Kollias A, Kotsis V, Manios E, McManus R, Mengden T, Mihailidou A, Myers M, Niiranen T, Ochoa JE, Ohkubo T, Omboni S, Padfield P, Palatini P, Papaioannou T, Protogerou A, Redon J, Verdecchia P, Wang J, Zanchetti A, Mancia G, O'Brien E. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions - position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2016 Sep;34(9):1665–77. doi: 10.1097/HJH.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 35.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M, CAFE Investigators. Anglo-Scandinavian Cardiac Outcomes Trial Investigators. CAFE Steering Committee and Writing Committee Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006 Mar 7;113(9):1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=16476843 .CIRCULATIONAHA.105.595496 [DOI] [PubMed] [Google Scholar]
- 36.Miyashita H. Clinical assessment of central blood pressure. Curr Hypertens Rev. 2012 May;8(2):80–90. doi: 10.2174/157340212800840708. http://europepmc.org/abstract/MED/22866025 .CHR-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014 Jul;35(26):1719–25. doi: 10.1093/eurheartj/eht565. http://eurheartj.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=24459197 .eht565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989 Nov 1;14(5):1139–48. doi: 10.1016/0735-1097(89)90408-7. https://linkinghub.elsevier.com/retrieve/pii/0735-1097(89)90408-7 .0735-1097(89)90408-7 [DOI] [PubMed] [Google Scholar]
- 39.Stergiou GS, Lourida P, Tzamouranis D, Baibas NM. Unreliable oscillometric blood pressure measurement: prevalence, repeatability and characteristics of the phenomenon. J Hum Hypertens. 2009 Dec;23(12):794–800. doi: 10.1038/jhh.2009.20.jhh200920 [DOI] [PubMed] [Google Scholar]
- 40.Koohi I, Batkin I, Groza VZ, Shirmohammadi S, Dajani HR, Ahmad S. Metrological characterization of a method for blood pressure estimation based on arterial lumen area model. IEEE Trans Instrum Meas. 2017 Apr;66(4):734–45. doi: 10.1109/TIM.2017.2657978. [DOI] [Google Scholar]
- 41.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015 Feb;9(2):130–6. doi: 10.1016/j.jash.2014.12.001.S1933-1711(14)00899-7 [DOI] [PubMed] [Google Scholar]
- 42.O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A, Working Group on Blood Pressure Monitoring of the European Society of Hypertension European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010 Feb;15(1):23–38. doi: 10.1097/MBP.0b013e3283360e98.00126097-201002000-00004 [DOI] [PubMed] [Google Scholar]
- 43.O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, Bland M, Coats A, Atkins N. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(suppl 2):S43–62. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Padwal RS, McLean D, Ringrose J. Should patients with higher blood pressure variability be excluded from validation studies? An assessment of the '12/8' rule. Blood Press Monit. 2017 Apr;22(2):112–4. doi: 10.1097/MBP.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 45.Myers MG. Limitations of home blood pressure monitoring in clinical practice. Can J Cardiol. 2015 May;31(5):583–4. doi: 10.1016/j.cjca.2015.03.008.S0828-282X(15)00197-X [DOI] [PubMed] [Google Scholar]
- 46.Jones DW, Weatherly L, Hall JE. SPRINT: what remains unanswered and where do we go from here? Hypertension. 2016 Feb;67(2):261–2. doi: 10.1161/HYPERTENSIONAHA.115.06723. http://hyper.ahajournals.org/cgi/pmidlookup?view=long&pmid=26553235 .HYPERTENSIONAHA.115.06723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of bilateral blood pressure equality.
Normality analysis of acquired BP.
Correlational, actual indirect-direct error trend, paired simultaneous, and normalized % ratio difference comparative analysis of measured direct and indirect data BP.
Device initial algorithm vs intra-arterial pressure measures.
Device initial and central algorithms vs auscultatory pressure measures.