Abstract
Aging has been targeted by genetic and dietary manipulation and by drugs in order to increase lifespan and health span in numerous models. Metformin, which has demonstrated protective effects against several age-related diseases in humans, will be tested in the TAME (Targeting Aging with Metformin) trial, as the initial step in the development of increasingly effective next-generation drugs.
Introduction
Over the past decades, remarkable progress has occurred in the science of aging in model organisms. Studies have demonstrated that genetic pathways modulate healthy lifespan in diverse species across great evolutionary distance and established that aging-related pathways constitute a target for intervention (Barzilai et al., 2012; Longo et al., 2015). Lifespan has been verifiably modulated by genetic, pharmacologic, and dietary interventions in multiple model systems.
With support from an R24 grant from the NIA (J. Kirkland, N.B., S. Austad), we gathered gerontologists with expertise in the biology of aging and in clinical geriatrics to discuss ways to target aging in humans. This effort resulted in the design of the study “Targeting Aging with Metformin” (TAME). This trial has been under reviews through several funding mechanisms and has received planning funding from the American Federation of Aging Research. An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging. The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect healthcare delivery and costs. If TAME demonstrates that metformin modulates aging and its diseases, beyond an isolated impact on diabetes, it would pave the way for development of next-generation drugs that directly target the biology of aging. Here, we summarize the major reasons why metformin was chosen to initiate this research.
Targeting Health Span
Interventions that target aging pathways are capable of dramatically extending lifespan and, most importantly, health span, the period of life during which an individual is fully functional and free of chronic illness. There is overwhelming evidence that single gene mutations in nutrient-sensing pathways, such as insulin/insulin-like growth factor (IGF) signaling (Bartke et al., 2001) or the mechanistic target of rapamycin (mTOR) signaling pathways, extend lifespan and health span in invertebrates. More importantly, these pathways have been evaluated in mammalian models, in which health span and lifespan have been extended by genetic manipulation or drugs (Johnson et al., 2013). This raises hope for new interventions, including drugs that slow the aging process and slow the appearance of age-related disease by modulating conserved pathways of aging, as further discussed and developed in recent reviews (de Cabo et al., 2014; Fontana and Partridge, 2015; Fontana et al., 2010).
Interventions to Prolong Lifespan
Recognizing that aging can be targeted, the NIH developed the NIA Interventions Testing Program (ITP). The ITP tests diets, drugs, or other interventions to see if they prevent disease and extend lifespan in genetically heterogeneous (outbred) mice (http://www.nia.nih.gov/research/dab/interventions-testing-program-itp). This program is conducted at multiple centers in order to control for laboratory-specific environmental differences, and testing is done in both male and female animals (Miller et al., 2007; Nadon et al., 2008). Major findings of the ITP include that nordihydroguaiaretic acid and aspirin each increase lifespan of male mice (Strong et al., 2008) and acarbose and 17-α-Estradiol extend mouse lifespan preferentially in males (Harrison et al., 2014). Studies of rapamycin (an mTOR inhibitor) have established the most compelling evidence for targeting aging. When rapamycin is administered late in life, it extends lifespan (Harrison et al., 2009; Miller et al., 2011), slows aging in a dose-dependent manner, shows differential effects by sex (Wilkinson et al., 2012), and is synergistic with metformin.
Metformin Modulates the Biology of Aging and Health Span in Model Organisms
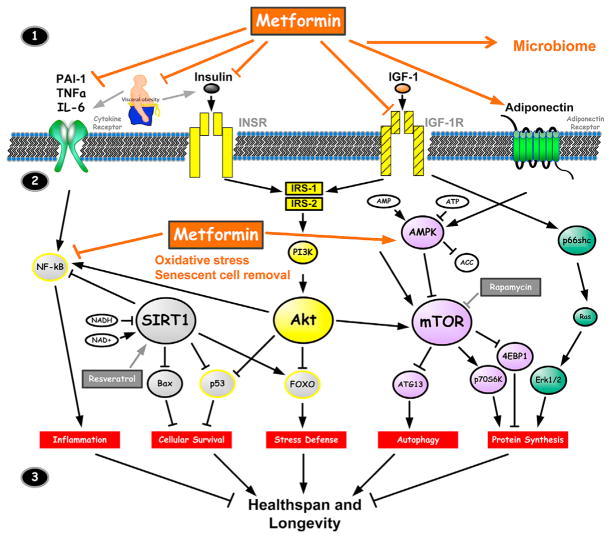
Metformin is a drug approved to treat diabetes but appears to target a number of aging-related mechanisms. Some mechanisms are relevant to glucose metabolism, but with respect to aging these may not be the most important ones. Metformin’s multiple aging-relevant actions at the cellular and organismal levels are depicted in Figure 1. Specifically for aging, metformin leads to decreased insulin levels, decreased IGF-1 signaling (Liu et al., 2011), inhibition of mTOR (Kickstein et al., 2010; Nair et al., 2014; Pérez-Revuelta et al., 2014), inhibition of mitochondrial complex 1 in the electron transport chain and reduction of endogenous production of reactive oxygen species (ROS) (Batandier et al., 2006; Bridges et al., 2014; Zheng et al., 2012), activation of AMP-activated kinase (AMPK) (Cho et al., 2015; Duca et al., 2015; Foretz et al., 2010; Lien et al., 2014; Lu et al., 2015; Zheng et al., 2012), and reduction in DNA damage (Algire et al., 2012). Metformin favorably influences metabolic and cellular processes closely associated with the development of age-related conditions, such as inflammation (Saisho, 2015), autophagy (Song et al., 2015; Xie et al., 2011), and cellular senescence (Jadhav et al., 2013; Moiseeva et al., 2013). In C. elegans metformin extends lifespan by several possible mechanisms including the alteration of the microbiome, specifically by changing microbial folate and methionine metabolism (Cabreiro et al., 2013). To date, there is no evidence for such effects in humans. Also, other investigators have suggested additional mechanisms for metformin actions (De Haes et al., 2014; Onken and Driscoll, 2010) supporting widely pleotropic effects.
Figure 1. Metformin Targets Multiple Pathways of Aging.
The figure depicts schematically the current consensus within the biology of aging community as to pathways that are important in order to target aging and indicates at which points metformin has been shown to have effects (see text). Key take-away: outside of the cell (1, top), metformin has been shown to affect the receptors for cytokines, insulin, IGF-1, and adiponectin, all pathways that are activated with aging and, when modulated, are associated with longevity. (1) Intracellular (2, middle) metformin inhibits the inflammatory pathway and activates AMPK, increasing inhibition of mTOR, which seems to be a major target to modulate aging. Through some of these mechanisms, it also modulates oxidative stress and removes senescent cells (the mitochondrial pathways are not shown, and the mechanisms by which metformin induces senescent cell removal remain unclear). (2) These processes jointly (3, bottom) affect inflammation, cellular survival, stress defense, autophagy, and protein synthesis, which are major biological outcomes associated with aging/longevity. Adapted from Barzilai et al. (2012).
It is currently unclear whether metformin has multiple effects on multiple pathways or whether its observed effects reflect downstream consequences of a primary action on a single mechanism of aging. For example, an attractive explanation suggests (Foretz et al., 2014) that the primary action of metformin is to inhibit mitochondrial complex 1. This inhibition may have multiple downstream effects, but importantly, it would lead to a change in the AMP/ATP ratio, which then activates AMPK. This activation maybe relevant to metformin’s known effect on hepatic glucose production (through decreased gluconeogenesis), but it also may suppress lipid synthesis and exert insulin-sensitizing effects, resulting in decreased plasma insulin levels and decreased mTOR activity. However, it is also possible that the singular effect of metformin has not yet been identified, and therefore metformin’s mechanisms of action are worth further investigation.
Beyond these cellular processes, there is a growing body of evidence that metformin can delay aging and increase healthy lifespan in vivo, specifically in nematodes and several rodent strains by adding metformin to the diet (Anisimov et al., 2008, 2011; Cabreiro et al., 2013; De Haes et al., 2014). It increases mean lifespan in female outbred mice by ~40% (Anisimov et al., 2008). When started early in life, mean lifespan was increased by 14%, but with initiation at older ages, this effect declined (Anisimov et al., 2011). Metformin delays the onset of carcinoma and extends lifespan by a mean of 8% in a breast cancer model (Anisimov et al., 2010), and extends lifespan by ~20% in a model of Huntington’s disease (Ma et al., 2007) only in males. A more recent study (Martin-Montalvo et al., 2013) demonstrated that metformin increased lifespan by 4%–6% in different mouse breeds. The effects on health span indices such as time on rotarod, distance on treadmill, open field tests, cataract index, oral glucose tolerance tests, insulin tolerance, and cognitive function (Allard et al., 2016) were improved by ~30%. As expected in these studies, metformin also increased AMPK activity and increased antioxidant protection, resulting in reductions in both chronic inflammation and accumulation of oxidative damage (Martin-Montalvo et al., 2013), all of which may contribute to health span and lifespan seen in animal models.
Not all studies have shown similar effects of metformin on life or health span. Feeding metformin to Drosophila resulted in a robust activation of AMPK and reduced lipid stores, but did not increase lifespan (Slack et al., 2012). One possibility is that the dose of metformin in this study was toxic. The dose of 1 mM is well above the comparable dose range in humans, and indeed doses higher than this increased mortality. This is also the case in mammals. When using a 10-fold increase in the dose that showed benefit in mice, mortality increased (Martin-Montalvo et al., 2013). Smith et al. (2010) did not demonstrate increased lifespan in metformin-treated rats, although the high dose used (~15 times the dose used in humans) may have been toxic. Additionally, the investigators used caloric restriction as a positive control and failed to observe the expected increased lifespan.
Human Studies of Metformin that Target Age-Related Diseases
If metformin can target and delay aging, its administration should be associated with fewer age-related diseases in general, rather than merely the decreased incidence of a single disease. Data from several randomized clinical trials and multiple observational studies provide evidence for such an effect, which would not be expected from glucose lowering alone.
Clinical Trials
The Diabetes Prevention Program (DPP)
The DPP was a randomized trial in U.S. adults at high risk for T2DM by virtue of obesity and impaired glucose tolerance (Knowler et al., 2002). Over 3,000 subjects were randomly assigned to placebo, metformin (850 mg twice daily), or a lifestyle-modification program. Metformin reduced the incidence of T2DM by 31% compared to placebo over a mean follow-up of ~3 years and was effective in all age categories in preventing diabetes, defined by HbA1C level, including the ~20% who were age 60 or older at baseline (Knowler et al., 2015). Further, metformin treatment was associated with improvement in cardiovascular disease (CVD) risk factors (Goldberg et al., 2013; Haffner et al., 2005) and subclinical atherosclerosis (coronary artery calcium) in male participants (Goldberg et al., 2015).
The United Kingdom Prospective Diabetes Study
Patients with T2DM allocated to metformin compared with conventional treatment had risk reduction of ~20% (p = 0.032) for CVD and 42% (p = 0.017) for diabetes-related death (UKPDS Group, 1998). This evidence from UKPDS provides rationale for metformin’s designation as first-line therapy for most patients with T2DM.
Other Trials
In the HOME trial of insulin-treated T2DM patients, addition of metformin resulted in 40% reduction (compared with placebo) in a CVD composite after 4 years of follow-up (Kooy et al., 2009). In non-diabetic subjects, the GIPS III study (Lexis et al., 2014) failed to demonstrate the benefit of short-term metformin treatment (4 months) on left ventricular ejection fraction, major adverse cardiovascular events, and mortality in post-myocardial infarction patients, and the CAMERA trial (Preiss et al., 2014) showed no effect of metformin (18 months) on carotid intimal medial thickness.
Observational Studies
The majority of observational data support metformin benefit in CVD, but residual bias and confounding cannot be ruled out (e.g., most studies have been conducted in patients with diabetes and include an active comparator, which could itself be cardio-toxic). Metformin’s potential CVD benefits—particularly in the area of primary prevention—remain an active area of research, including an ongoing randomized trial in the UK (The Glucose Lowering In Non-diabetic hyperglycaemia Trial, GLINT, http://www.isrctn.com/ISRCTN34875079; Anfossi et al., 2010; Whittington et al., 2013).
Observational Studies Suggest Metformin Decreases Cancer Incidence
Several epidemiologic studies have shown that metformin use is associated with reduced cancer incidence and mortality (Landman et al., 2010; Lee et al., 2011; Libby et al., 2009; Monami et al., 2011; Tseng, 2012). While one meta-analysis (Stevens et al., 2012) did not show that metformin prevents cancer, a more thorough analysis that included more data and accounted for heterogeneous comparators showed that overall cancer incidence was reduced by 31% and cancer mortality by 34% (Gandini et al., 2014). There is also evidence from studies performed both in vitro and in vivo of metformin’s role in attenuating tumorigenesis (Anisimov and Bartke, 2013; Karnevi et al., 2013; Liu et al., 2011; Quinn et al., 2013; Salani et al., 2012; Tosca et al., 2010). The mechanisms proposed relate to reduced insulin levels, improved insulin action, decreased IGF-1 signaling, and activation of AMPK. Numerous ongoing studies are testing the effect of metformin as adjuvant cancer therapy, with a recently published trial showing negative results in advanced pancreatic cancer (Kordes et al., 2015). Although no trials yet have reported effects of chronic treatment on cancer prevention, studies in early-stage cancer or pre-malignancy suggest this may be fruitful (DeCensi et al., 2015).
Association of Metformin with Better Cognitive Function
Emerging evidence suggests that metformin may preserve cognitive function. In the Singapore Longitudinal Aging Study, metformin use was associated with a 51% reduced risk of cognitive impairment (defined by modified Mini-Mental Status Exam score % 23), which remained robust to adjustment for vascular and non-vascular risk factors. Further, the lowest risk was seen in those with longer-term (> 6 years) metformin use (Ng et al., 2014). A large observational study of metformin-treated T2DM patients reported lower rates of dementia than in those treated with other diabetes medications (Cheng et al., 2014). One study suggested that T2DM patients treated with metformin had increased risk for poor cognitive performance (Moore et al., 2013); however, it had a number of methodological flaws (Alagiakrishnan et al., 2013) and has not been replicated. In one small clinical trial, T2DM patients with depression (n = 58) were treated with metformin or placebo for 24 weeks (Guo et al., 2014). The metformin group showed improved cognitive performance and reduced depressive symptoms, concurrent with improved glycemic control. In an unpublished trial, non-diabetic subjects (n = 80) with mild cognitive impairment showed significant improvements in some cognitive domains after 12 months of metformin treatment (Luchsinger et al., 2016). No definitive trials have been conducted.
Association of Metformin with Decreased Mortality
A recent study (Bannister et al., 2014) used retrospective observational data from the UK Clinical Practice Research Datalink. Patients with T2DM who were treated with metformin or sulphonylurea (SU) monotherapy were compared to separate age- and sex-matched control groups without diabetes. SU-treated patients had lower survival than both matched non-diabetic controls and metformin-treated diabetic patients. Surprisingly, metformin-treated diabetic patients had survival rates similar to (and, among those age > 70, even better than) their matched non-diabetic control group, despite the fact that the diabetic patients were more obese and had greater co-morbidities at baseline. Mortality benefits have also been described in other observational studies and long-term follow-up of the UKPDS cohort, which showed 36% reduction in all-cause mortality in the metformin treatment group (p = 0.011) (UKPDS Group, 1998). Not all studies have been positive—for example, an analysis from the Medicare Current Beneficiary Survey showed only a non-statistically significant survival benefit for metformin-treated patients (Tinetti et al., 2015).
Considerations in Designing Human Metformin Trials
Dosing
While metformin can beprescribed at dosages of up to 2,250 mg/day, no further effects of decreasing glucose are noted after 1,600–1,700 mg/day. After a single oral dose, metformin is rapidly distributed to many tissues following partial absorption by the small intestine, but the luminal concentration in the gastrointestinal tract remains high. After a single 1.5 g dose, the peak plasma concentration of 18 mM occurs in 3 hr, with a mean plasma half-life of about 20 hr (Foretz et al., 2014). It is suggested, however, that an equivalent dose for mice would be up to 10-fold higher. Studies on biodistribution of metformin in mice showed accumulation mainly in the gastrointestinal tract, kidney, and liver.
Safety
Metformin has been used with an excellent safety record for over 60 years. Side effects are monitored closely within clinical trials, and the safety of met-formin use in DPP/DPPOS was reported on in 2012, when over 18,000 patients-years of follow-up had accrued, and by which time ~20% of the cohort was age 70 or older (mean age ~64). There were no cases of lactic acidosis or significant hypoglycemia (Diabetes Prevention Program Research Group, 2012). Mild anemia occurred in ~12% of metformin-treated participants versus ~8% in the placebo group (p = 0.04). Vitamin B12 deficiency occurred in ~7% of metformin group versus 5% in placebo group after 13 years; risk of B12 deficiency increases with duration of use but was not greater in older compared with younger subjects in DPPOS (Lalau et al., 1990). Further, the risk of lactic acidosis appears to be related to renal function, not age per se, and is currently considered to be very low (Aroda et al., 2016).
In the TAME study, we plan to enroll 3,000 subjects, ages 65–79, in ~14 centers across the U.S. Rather than study the effects of metformin on each separate condition, we will measure time to a new occurrence of a composite outcome that includes cardiovascular events, cancer, dementia, and mortality. TAME will also assess important functional and geriatric end points.
If successful, TAME will mark a paradigm shift, moving from treating each medical condition to targeting aging per se. We expect this to facilitate the development of even better pharmacologic approaches that will ultimately reduce healthcare costs related to aging.
Acknowledgments
The American Federation for Aging Research has supported the TAME initiative. This work was supported by the Nathan Shock Center of Excellence for the Biology of Aging (P30AG038072, N.B.), the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation for Medical Research) (N.B.), grant 1R24AG044396 from the National Institute on Aging (NIA) (PI, Kirkland; co-PI, N.B.), and grant P30 AG021332 from the NIA to the Wake Forest Older Americans Independence Center (S.B.K.).
References
- Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med. 2013;16:277–286. [PubMed] [Google Scholar]
- Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, Viollet B, Ferbeyre G, Pollak MN. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R. Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res. 2016;301:1–9. doi: 10.1016/j.bbr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi G, Russo I, Bonomo K, Trovati M. The cardiovascular effects of metformin: further reasons to consider an old drug as a cornerstone in the therapy of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2010;8:327–337. doi: 10.2174/157016110791112359. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany, NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroda VR, Edelstein S, Goldberg R, Knowler WC, Marcovina S, Orchard T, Bray GA, Schade D, Temprosa M, White NH, et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101:1754–1761. doi: 10.1210/jc.2015-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16:1165–1173. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Bio-membr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci. 2014;69:1299–1305. doi: 10.1093/gerona/glu073. [DOI] [PubMed] [Google Scholar]
- Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, Yu KS, Cho JY. Anti-hyperglycemic mechanism of metformin occurs via the AMPK/LXRα/POMC pathway. Sci Rep. 2015;5:8145. doi: 10.1038/srep08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci USA. 2014;111:E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, Lazzeroni M, Vingiani A, Gentilini O, Petrera M, Viale G, et al. Effect of Metformin on Breast Ductal Carcinoma In Situ Proliferation in a Randomized Presurgical Trial. Cancer Prev Res (Phila) 2015;8:888–894. doi: 10.1158/1940-6207.CAPR-15-0048. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, Arakaki R, Watson K, Horton E, Barrett-Connor E. Lifestyle and metformin treatment favorably influence lipo-protein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013;98:3989–3998. doi: 10.1210/jc.2013-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Temprosa M, Aroda VR, Barrett-Connor EL, Budoff M, Crandall J, Dabelea D, Horton ES, Mather KJ, Orchard TJ, et al. Effect of long-term interventions in the Diabetes Prevention Program (DPP) and its Outcome Study on coronary artery calcium (CAC) Diabetes. 2015;64(Supplement 1A):LB5. doi: 10.1161/CIRCULATIONAHA.116.025483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav KS, Dungan CM, Williamson DL. Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech Ageing Dev. 2013;134:548–559. doi: 10.1016/j.mad.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnevi E, Said K, Andersson R, Rosendahl AH. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer. 2013;13:235. doi: 10.1186/1471-2407-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Köhler A, Glossmann H, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Eldelstein SL, Goldberg RB, Ackermann RT, Crandall JP, Florez JC, Fowler SE, Herman WH, Horton ES, Kahn SE, et al. Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care. 2015;38:51–58. doi: 10.2337/dc14-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy A, de Jager J, Lehert P, Bets D, Wulffelé MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- Lalau JD, Vermersch A, Hary L, Andrejak M, Isnard F, Quichaud J. Type 2 diabetes in the elderly: an assessment of metformin (metformin in the elderly) Int J Clin Pharmacol Ther Toxicol. 1990;28:329–332. [PubMed] [Google Scholar]
- Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexis CP, van der Horst IC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AF, van der Werf HW, Schurer RA, Pundziute G, Tan ES, et al. GIPS-III Investigators. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA. 2014;311:1526–1535. doi: 10.1001/jama.2014.3315. [DOI] [PubMed] [Google Scholar]
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien F, Berthier A, Bouchaert E, Gheeraert C, Alexandre J, Porez G, Prawitt J, Dehondt H, Ploton M, Colin S, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Shi J, Li M, Gui B, Fu R, Yao G, Duan Z, Lv Z, Yang Y, Chen Z, et al. Activation of AMPK by metformin inhibits TGF-β-induced collagen production in mouse renal fibroblasts. Life Sci. 2015;127:59–65. doi: 10.1016/j.lfs.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Bagiella E. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J Alzheimers Dis. 2016;51:501–514. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN, Ferbeyre G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, Melani C, Vitale V, Romano D, Barchielli A, Marchionni N, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–131. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, et al. AIBL Investigators. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, Peralba JM, Harrison DE. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) 2008;30:187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41:61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, Di Monte DA. Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis. 2014;5:e1209. doi: 10.1038/cddis.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, Fisher M, Packard CJ, Sattar N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- Quinn BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, Hollander MC, Gills JJ, Dennis PA. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila) 2013;6:801–810. doi: 10.1158/1940-6207.CAPR-13-0058-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr Metab Immune Disord Drug Targets. 2015;15:196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- Salani B, Maffioli S, Hamoudane M, Parodi A, Ravera S, Passalacqua M, Alama A, Nhiri M, Cordera R, Maggi D. Caveolin-1 is essential for metformin inhibitory effect on IGF1 action in non-small-cell lung cancer cells. FASEB J. 2012;26:788–798. doi: 10.1096/fj.11-192088. [DOI] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS ONE. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Jr, Elam CF, Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65:468–474. doi: 10.1093/gerona/glq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, Crowe FL, Farmer AJ, Harrison S, Hirst JA, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–2603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, McAvay G, Trentalange M, Cohen AB, Allore HG. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: population based cohort study. BMJ. 2015;351:h4984. doi: 10.1136/bmj.h4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosca L, Ramé C, Chabrolle C, Tesseraud S, Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–418. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409–416. doi: 10.1530/EJE-12-0369. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther. 2013;27:5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]