This phase 2 study investigated eribulin in previously treated Japanese patients with advanced/metastatic soft tissue sarcoma. Eribulin showed clinical activity together with a manageable safety profile.
Keywords: eribulin, soft tissue sarcoma, survival, phase 2 trial, Japan
Abstract
Objective
Eribulin, a microtubule dynamics inhibitor, is approved for the treatment of patients with breast cancer and soft tissue sarcoma. We investigated the efficacy and safety of eribulin in Japanese patients with soft tissue sarcoma.
Methods
This open-label, multicenter, nonrandomized, Phase 2 study enrolled Japanese patients with measurable, advanced/metastatic soft tissue sarcoma of high/intermediate grade and ≥1 prior chemotherapy for advanced disease. Patients received eribulin mesilate 1.4 mg/m2 intravenously over 2–5 minutes on Days 1 and 8 of a 21-day cycle. The primary endpoint was progression-free rate at 12 weeks. Secondary endpoints included overall survival, progression-free survival and safety. Efficacy analyses were stratified by histology (liposarcoma or leiomyosarcoma, and other subtypes).
Results
Overall, 52 patients were enrolled and 51 patients were treated. Patients with liposarcoma/leiomyosarcoma (n = 35) had similar characteristics to those with other subtypes (n = 16), except for a higher proportion of women (63% vs 38%, respectively) and patients with Eastern Cooperative Oncology Group performance status 0 (57% vs 44%). Progression-free rate at 12 weeks was 60% in liposarcoma/leiomyosarcoma patients, 31% in other subtypes and 51% overall. Median progression-free survival was 5.5 months in liposarcoma/leiomyosarcoma patients, 2.0 months in other subtypes and 4.1 months overall. Median overall survival was 17.0 months in liposarcoma/leiomyosarcoma patients, 7.6 months in other subtypes and 13.2 months overall. The most common Grade 3–4 adverse events were neutropenia (86%), leukopenia (75%), lymphopenia (33%), anemia (14%) and febrile neutropenia (8%).
Conclusion
Eribulin showed clinical activity with a manageable safety profile in previously treated Japanese patients with advanced/metastatic soft tissue sarcoma.
Introduction
Sarcomas are rare solid tumors associated with substantial morbidity and mortality. The yearly incidence of soft tissue sarcomas (STSs) is estimated at 3–5 per 100,000 (1, 2). STS is a heterogeneous group of tumors, including over 50 histological subtypes with varying outcomes in terms of chemosensitivity and survival (3). Chemotherapy is the most commonly used treatment option in patients with advanced, metastatic or inoperable STS. Anthracycline-based chemotherapy has been used as first-line chemotherapy. Ifosfamide (4, 5), pazopanib (6) and trabectedin (7, 8) are therapeutic options after the failure of first-line chemotherapy in Japan, but the prognosis remains poor (9, 10).
Eribulin mesilate is a fully synthetic, optimized analog of halichondrin B, originally isolated from the marine sponge Halichondria okadai. Eribulin is a microtubule dynamics inhibitor that induces irreversible mitotic blockade by binding with high affinity to tubulin at the plus (growing) ends of microtubules, with minimal effect on microtubule shortening (11–13). Eribulin is approved as monotherapy for the treatment of inoperable and recurrent breast cancer in Japan.
The efficacy and safety of eribulin in sarcoma have been demonstrated in a Phase 2 study conducted by the European Organisation for Research and Treatment of Cancer (EORTC) enrolling patients with progressive or high-grade STS, including liposarcoma (LPS; also known as adipocytic sarcoma), leiomyosarcoma (LMS), synovial sarcoma and other sarcomas. The progression-free survival (PFS) at 12 weeks was 32% for LMS and 47% for LPS, suggesting that further investigations with eribulin are warranted in these common STS subtypes (14). Based on these results, a randomized Phase 3 study (NCT01327885) compared eribulin with dacarbazine in previously treated patients with advanced LPS/LMS (15). In that study, the primary endpoint of OS was significantly longer for eribulin compared with dacarbazine (median OS: 13.5 vs 11.5 months; hazard ratio [HR]: 0.77 [95% confidence interval (CI): 0.62–0.95]; P = 0.0169). The adverse events (AEs) were consistent with the safety profile reported from the previous findings, and eribulin had a manageable tolerability (15). Based on the results of this pivotal Phase 3 trial, eribulin received approval as monotherapy for the treatment of STS in Japan and unresectable or metastatic LPS in the USA and Europe. However, because Japanese patients were not included in these sarcoma studies, the data for this population remain lacking. The current Phase 2 study evaluated the efficacy and tolerability of eribulin in Japanese patients with previously treated advanced or metastatic STS, including LPS, LMS and other defined subtypes.
Material and methods
Study design
In this open-label, multicenter, nonrandomized, Phase 2 study, patients received eribulin mesilate 1.4 mg/m2 (equivalent to eribulin 1.23 mg/m2 [expressed as free base]), administered intravenously over 2–5 minutes on Days 1 and 8 of a 21-day cycle until disease progression, intolerance or withdrawal of consent. In case of toxicity, treatment was delayed or the eribulin mesilate dose was reduced to 1.1 or 0.7 mg/m2 (equivalent to eribulin 0.97 or 0.62 mg/m2, respectively, [expressed as free base]; Online Supplementary Table S1). Re-escalation was not permitted.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and was approved by the relevant Institutional Review Boards of all participating institutions. All patients provided written informed consent.
Patients
Eligible patients aged 20 years and greater had histologically or cytologically confirmed, measurable, advanced or metastatic STS of high or intermediate grade; had received ≥1 standard chemotherapy for advanced disease (anthracycline, ifosfamide or combination); had documented disease progression within 6 months before study enrollment; had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and had adequate bone marrow, renal and liver function. Ineligible histologies included embryonal rhabdomyosarcoma, chondrosarcoma, osteosarcoma, Ewing sarcoma/primitive neuroectodermal tumor, gastrointestinal stromal tumor, dermatofibrosarcoma protuberans, inflammatory myofibroblastic tumor, neuroblastoma, malignant mesothelioma and mixed mesodermal tumors of the uterus. In addition to standard exclusion criteria, patients were ineligible if they had significant cardiovascular impairment, pre-existing peripheral neuropathy exceeding Grade 2 or had any toxicity > Grade 1 (except for peripheral neuropathy or alopecia) related to prior anticancer therapy.
Study assessments
Tumor response was evaluated every 6 weeks according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) (16) until progressive disease was confirmed and at week 12. Tumor assessment was performed by an independent review committee. Responses were confirmed at least 4 weeks after the first observation of complete response (CR) or partial response (PR).
AEs were graded according to the Common Terminology Criteria for Adverse Events (version 4.0). AEs that emerged during treatment (from the first dose of study drug up to 30 days after the last dose) were recorded. Serious AEs that occurred on study and up until 30 days after the last dose of the study drug were recorded, regardless of their relationship to study treatment.
Statistical analysis
The primary endpoint was the progression-free rate at 12 weeks after start of therapy (PFR12wks) and is presented with exact CIs using the binomial distribution. PFR12wks is the recommended endpoint for Phase 2 studies of STSs (17) and has been used in other previous studies (14, 18). Secondary endpoints included PFS, OS, objective response rate (ORR; comprising CR or PR of best overall response) and safety.
The primary analysis set for efficacy and safety evaluations consisted of all patients who received ≥ 1 dose of study drug. Efficacy analyses were conducted by disease subtype (LPS or LMS [LPS/LMS] and other subtypes [OTH]), and were performed secondarily for all patients combined. No formal comparisons between strata were planned.
PFR12wks was defined as the proportion of patients with PFS (“success”) measured as a binary variable based on the tumor response assessed at Week 12 after the start of treatment. Eribulin treatment was considered a “success” if a radiological evaluation ≥12 weeks after start of therapy indicated stable disease or objective response; all other outcomes were classified as treatment failure.
Statistical analyses were performed with SAS (version 9.3). The planned sample size was approximately 35 patients with LPS/LMS, with ≥5 patients for each histological subtype together with 16–20 patients with OTH for a total of 51–55 patients, to allow adequately powered analysis based on the 1-sample binomial distribution. The required number of patients with LPS/LMS was determined to ensure 80% power to reject the threshold PFR12wks; P0 of ≤20% if the expected PFR12wks; P1 of ≥40% is true, with a one-sided Type I error rate (α) of 0.05 (17). The number of patients with OTH was determined based on the P0 of ≤15% and the P1 = 35–40%, with a one-sided α of <0.1. Accrual of 16 patients provided a power of 71–83%. Synovial sarcoma, pleomorphic malignant fibrous histiocytoma and rhabdomyosarcoma were mainly enrolled as OTH sarcomas in this study. These histological subtypes are generally higher grade malignancy and have poorer prognosis, compared with LPS/LMS. Therefore, we set P0 = 15% and P1 = 35–40% conservatively as not to underestimate the efficacy for the OTH group.
This study was registered with ClinicalTrials.gov number NCT01458249. The first patient was enrolled on 14 November 2011, and the cutoff date for analysis was 14 November 2014.
Results
Patients
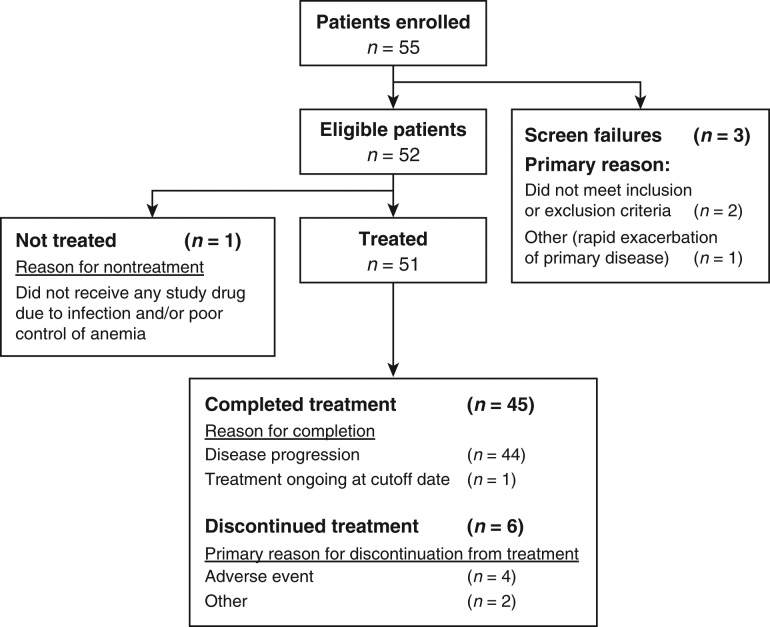
Fifty-two eligible Japanese patients were enrolled at 13 sites in Japan between November 2011 and December 2012—51 of whom received treatment and were included in all analyses. One patient did not receive treatment because of infection and poor control of anemia (Fig. 1). Patients had a median age of 52 years (range: 28–73), and 28 (55%) were female (Table 1). Time since first diagnosis varied widely but was most commonly 2–5 years (in 47% of patients). Patients in the LPS/LMS stratum (n = 35) had similar baseline characteristics to those with OTH (n = 16) except for a higher proportion of women (63% vs 38%; respectively), a higher proportion of patients with an ECOG PS of 0 (57% vs 44%), and differences in the primary disease site (Table 1). Patients had received a median of 2 (range: 1–7) prior chemotherapy regimens for advanced/metastatic disease; all patients had received prior anthracycline.
Figure 1.
Patient disposition and primary reason for discontinuation.
Table 1.
Patient and disease characteristicsa
| Parameter | Liposarcoma or leiomyosarcoma (n = 35) | Other histologies (n = 16) | All patients (n = 51) |
|---|---|---|---|
| Median age, years (range) | 50 (29–73) | 54 (28–71) | 52 (28–73) |
| Sex, n (%) | |||
| Female | 22 (63) | 6 (38) | 28 (55) |
| Male | 13 (37) | 10 (63) | 23 (45) |
| ECOG PS, n (%) | |||
| 0 | 20 (57) | 7 (44) | 27 (53) |
| 1 | 15 (43) | 9 (56) | 24 (47) |
| Median time since initial diagnosis, years (range) | 3 (0–10) | 2 (0–7) | 3 (0–10) |
| Site of primary lesion, n (%) | |||
| Appendix | 0 | 1 (6) | 1 (2.0) |
| Connective and soft tissue | 13 (37) | 9 (56) | 22 (43) |
| Retroperitoneum and peritoneum | 14 (40) | 1 (6) | 15 (29) |
| Uterusc | 7 (20) | 2 (13) | 9 (18) |
| Mediastinum | 1 (3) | 1 (6) | 2 (4) |
| Large intestine (excluding appendix) | 0 | 1 (6) | 1 (2) |
| Nasal cavity | 0 | 1 (6) | 1 (2) |
| Disease histology | |||
| Liposarcomab | 16 (46) | 0 | 16 (31) |
| Leiomyosarcoma | 19 (54) | 0 | 19 (37) |
| Alveolar soft part sarcoma | 0 | 1 (6) | 1 (2) |
| Endometrial stromal sarcoma | 0 | 2 (13) | 2 (4) |
| Fibrosarcoma | 0 | 2 (13) | 2 (4) |
| MFH | 0 | 3 (19) | 3 (6) |
| MPNST | 0 | 1 (6) | 1 (2) |
| Rhabdomyosarcoma | 0 | 2 (13) | 2 (4) |
| Solitary fibrous tumor | 0 | 2 (13) | 2 (4) |
| Synovial sarcoma | 0 | 3 (19) | 3 (6) |
| Tumor grade, n (%) | |||
| Intermediate | 9 (26) | 4 (25) | 13 (26) |
| High | 26 (74) | 12 (75) | 38 (75) |
| Median number of prior chemotherapy regimens for advanced/metastatic disease (range) | 2 (1–7) | 2 (1–6) | 2 (1–7) |
| Drug classd | |||
| Anthracycline | 35 (100) | 16 (100) | 51 (100) |
| Ifosfamide | 25 (71) | 11 (69) | 36 (71) |
| Docetaxel | 15 (43) | 7 (44) | 22 (43) |
| Gemcitabine | 15 (43) | 6 (38) | 21 (41) |
| Etoposide | 5 (14) | 2 (13) | 7 (14) |
| Dacarbazine | 5 (14) | 1 (6) | 6 (12) |
| Platinum agent | 5 (14) | 1 (6) | 6 (12) |
| TNM classification | |||
| T classification | |||
| Tx | 31 (89) | 13 (81) | 44 (86) |
| T0 | 0 | 0 | 0 |
| T1:T1a | 0 | 0 | 0 |
| T1:T1b | 1 (3) | 1 (6) | 2 (4) |
| T2:T2a | 0 | 0 | 0 |
| T2:T2b | 3 (9) | 2 (13) | 5 (10) |
| N classification | |||
| Nx | 7 (20) | 6 (38) | 13 (26) |
| N0 | 26 (74) | 7 (44) | 33 (65) |
| N1 | 2 (6) | 3 (19) | 5 (10) |
| M classification | |||
| Mx | 0 | 1 (6) | 1 (2) |
| M0 | 0 | 0 | 0 |
| M1 | 35 (100) | 15 (94) | 50 (98) |
an = 51; full analysis set.
bMyxoid liposarcoma (n = 9), dedifferentiated liposarcoma (n = 6) and well differentiated liposarcoma (n = 1).
cIncludes corpus uteri (n = 7) and uterus not otherwise specified (n = 2).
dThe most common drug classes/agents are shown.
Data are number (in percentage) unless otherwise stated. Percentages may not total 100 because of rounding.Full analysis set included all patients who received at least one dose of study drug.ECOG PS, Eastern Cooperative Oncology Group performance status; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; TNM, tumor, node, metastasis.
Study drug exposure
The median number of eribulin cycles administered was four (range: 1–49). Patients in the LPS/LMS and OTH strata received a median of 7 and 2.5 cycles, respectively. Almost all patients of both strata discontinued treatment because of disease progression (LPS/LMS, 30/35 patients, 85.7%; OTH: 14/16 patients, 87.5%). The median relative dose intensity was virtually identical in both strata (88.0% and 88.5%, respectively). Eribulin drug delays and dose reductions each occurred in 16 (31%) patients, and dose interruptions occurred in 19 (37%) patients. There were no major protocol deviations.
Efficacy
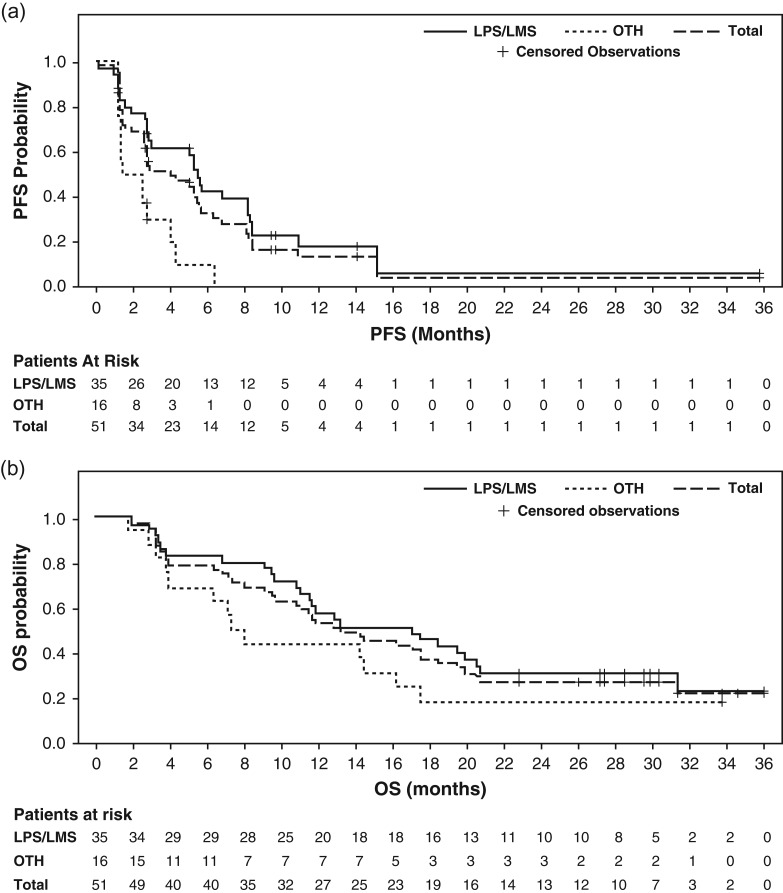
PFR12wks in the LPS/LMS stratum was 60% (21/35 patients; 95% CI: 42–76%), 31% in OTH (5/16 patients; 95% CI: 11–59%) and 51% (26/51 patients; 95% CI: 37–65%) in all patients. The median PFS was 5.5 months (95% CI: 2.8–8.2) in the LPS/LMS stratum, 2.0 months (95% CI: 1.2–4.1) in OTH and 4.1 months (95% CI: 2.6–5.6) overall (Table 2 and Fig. 2A). In an exploratory analysis by disease subtype, PFR12wks was 81% (13/16 patients; 95% CI: 54–96%) in LPS, and 42% (8/19 patients; 95% CI: 20–67%) in LMS; corresponding values for median PFS were 6.8 months (95% CI: 5.1–8.4) and 2.9 months (95% CI: 1.3–8.2), respectively (Online Supplementary Table S2). A total of seven patients (44%) with LPS and six patients (32%) with LMS exceeded 6 months for PFS (Online Supplementary Figures S1 and S2). PFR12wks was achieved by seven of nine patients (77.8%) with myxoid LPS and five of six patients (83.3%) with dedifferentiated LPS. One patient with well differentiated LPS also achieved PFR12wks. In the OTH category, two patients with endometrial stromal sarcoma, one patient with synovial sarcoma, one patient with solitary fibrous tumor and one patient with fibrosarcoma achieved PFR12wks (Online Supplementary Figure S3).
Table 2.
Efficacy outcomes following eribulin treatmenta
| Outcome | Liposarcoma or leiomyosarcoma (n = 35) | Other histologies (n = 16) | All patients (n = 51) |
|---|---|---|---|
| PFR12wks, n (%), [95% CI] | 21 (60) [42–76] | 5 (31) [11–59] | 26 (51) [37–65] |
| PFR12wks, n (%), [90% CI]b | 21 (60) [48–100] | 5 (31) [16–100] | 26 (51) [41–100] |
| Median PFS, months (95% CI) | 5.5 (2.8–8.2) | 2.0 (1.2–4.1) | 4.1 (2.6–5.6) |
| Median OS, months (95% CI) | 17.0 (11.0–20.5) | 7.6 (3.8–16.1) | 13.2 (9.5–18.3) |
| OS rate at % (95% CI): | |||
| 6 months | 83 (66–92) | 69 (41–86) | 78 (64–87) |
| 12 months | 57 (39–72) | 44 (20–66) | 53 (39–66) |
| 18 months | 46 (29–61) | 19 (5–40) | 37 (24–50) |
| 24 months | 31 (17–47) | 19 (5–40) | 28 (16–40) |
| Best overall response, n (%) | |||
| ORR (95% CI) | 0 (0–10) | 0 (0–21) | 0 (0–7) |
| Complete response | 0 | 0 | 0 |
| Partial response | 0 | 0 | 0 |
| Stable disease | 28 (80) | 8 (50) | 36 (71) |
| ≥11 weeks | 26 (74) | 8 (50) | 34 (67) |
| Progressive disease | 7 (20) | 8 (50) | 15 (29) |
an = 51; full analysis set.
bOne-sided 90% CI calculated using the exact method of binomial distribution.
Full analysis set included all patients who received at least one dose of study drug.CI, confidence interval; PFR12wks, progression-free rate at 12 weeks; PFS, progression-free survival; ORR, objective response rate (complete or partial response); OS, overall survival.
Figure 2.
Kaplan–Meier plots of (a) progression-free survival and (b) overall survival following eribulin treatment. LMS, leiomyosarcoma; LPS, liposarcoma; OS, overall survival; OTH, other soft tissue sarcoma subtypes; PFS, progression-free survival.
In total, 38 of 51 (75%) patients had died at database cutoff date (25/35 [71%] patients in the LPS/LMS stratum, 13/16 [81%] patients with OTH). The median OS was 17.0 months in the LPS/LMS stratum (95% CI: 11.0–20.5), 7.6 months in OTH (95% CI: 3.8–16.1) and 13.2 months (95% CI: 9.5–18.3) in total (Table 2, Fig. 2B, and Online Supplementary Table S2). No CRs or PRs were observed, giving an ORR of 0% (Table 2 and Online Supplementary Table S2). However, tumor shrinkage was observed in 18 patients (15 of them in the LPS/LMS stratum). Thirty-four (67%) patients achieved stable disease for ≥11 weeks, including 26 (74%) in the LPS/LMS stratum and 8 (50%) with OTH (Table 2).
Safety and tolerability
All treated patients experienced ≥1 AE (Table 3). Of these, the most common AEs (occurring in ≥30% of patients) were leukopenia (100% of patients), neutropenia (98%), lymphopenia (78%), anemia (47%), cancer pain (45%), nausea and pyrexia (41% each), malaise (39%), and constipation and peripheral neuropathy (31% each). All treated patients experienced ≥1 treatment-related AE. The most frequent Grade 3 or 4 treatment-related AEs (data not shown) were neutropenia (86% of patients), leukopenia (75%), lymphopenia (31%), anemia (12%) and febrile neutropenia (8%). Granulocyte-colony stimulating factor (G-CSF) was not used prophylactically in this study. Serious AEs were reported in 15 (29%) patients and were treatment-related in 5 (10%) patients, with events including febrile neutropenia, streptococcal infection, infectious pleural effusion, pulmonary embolism, hepatic hemorrhage and tumor hemorrhage. One fatal cardiac failure during the study was considered unrelated to study drug but was attributed to prior doxorubicin treatment; otherwise, there were no treatment-related deaths (or Grade 5 AEs). Four (8%) patients experienced AEs that led to withdrawal of eribulin, including two events that were probably treatment-related (infectious pleural effusion and interstitial lung disease). The eribulin dose was reduced because of neutropenia in 13 (26%) patients. Overall, 16 (31%) patients had treatment-related AEs necessitating dose reduction. New or worsened Grade 1–2 peripheral neuropathy developed in 16 (31%) patients with a median time to onset of 27 weeks. Neuropathy was the reason for dose reduction in one patient after 216 days on study.
Table 3.
Most frequent adverse events in patients treated with eribulin, and the respective proportions of those patients with Grade 3 or higher severity
| Adverse event, n (%) | In ≥10% of patients | |
|---|---|---|
| All grades | Grade ≥3 | |
| Any adverse event | 51 (100) | 49 (96) |
| Hematological | ||
| Anemia | 24 (47) | 7 (14) |
| Leukopenia | 51 (100) | 38 (75) |
| Lymphopenia | 40 (78) | 17 (33) |
| Neutropenia | 50 (98) | 44 (86) |
| Gastrointestinal | ||
| Constipation | 16 (31) | 0 |
| Diarrhea | 7 (14) | 0 |
| Nausea | 21 (41) | 0 |
| Stomatitis | 13 (26) | 0 |
| General and administration site | ||
| Fatigue | 9 (18) | 0 |
| Malaise | 20 (39) | 0 |
| Edema peripheral | 5 (10) | 0 |
| Pyrexia | 21 (41) | 1 (2) |
| Hepatobiliary | ||
| Hepatic function abnormal | 5 (10) | 0 |
| Infections | ||
| Nasopharyngitis | 11 (22) | 0 |
| Upper respiratory tract infection | 11 (22) | 0 |
| Investigations | ||
| ALT elevation | 14 (28) | 3 (6) |
| AST elevation | 13 (26) | 2 (4) |
| ALP elevation | 5 (10) | 0 |
| CPK elevation | 8 (16) | 0 |
| LDH elevation | 11 (22) | 0 |
| CRP elevation | 6 (12) | 0 |
| Metabolism and nutrition | ||
| Appetite decrease | 12 (24) | 0 |
| Hypertriglyceridemia | 5 (10) | 1 (2) |
| Hypoalbuminemia | 10 (20) | 2 (4) |
| Hypokalemia | 6 (12) | 3 (6) |
| Hypophosphatemia | 8 (16) | 5 (10) |
| Musculoskeletal and connective tissue | ||
| Arthralgia | 5 (10) | 0 |
| Back pain | 7 (14) | 0 |
| Neoplasms benign, malignant and unspecifiedb | ||
| Cancer pain | 23 (45) | 3 (6) |
| Nervous system | ||
| Dysgeusia | 12 (24) | 0 |
| Headache | 6 (12) | 0 |
| Peripheral neuropathy | 16 (31) | 0 |
| Psychiatric | ||
| Insomnia | 5 (10) | 0 |
| Renal and urinary | ||
| Proteinuria | 5 (10) | 0 |
| Respiratory, thoracic and mediastinal | ||
| Cough | 6 (12) | 0 |
| Oropharyngeal pain | 5 (10) | 0 |
| Skin and subcutaneous tissue | ||
| Alopecia | 14 (28) | NA |
| Rash | 6 (12) | 0 |
an = 51; safety analysis set.
bIncluding cysts and polyps.
Safety analysis set included all patients who received at least one dose of the study drug and had at least one post-baseline safety evaluation. Italics indicate adverse events that occurred in at least 30% of patients treated with eribulin.ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatinine phosphokinase; CRP, C-reactive protein; LDH, lactate dehydrogenase; NA, not applicable.
Discussion
This Phase 2 study in Japanese patients with advanced/metastatic STS investigated the efficacy and safety of eribulin monotherapy. The patient population enrolled was representative of the target population of patients with previously treated advanced/metastatic STS. The data in this study indicate clinical activity of eribulin in these patients.
The primary efficacy endpoint findings were promising, with a PFR12wks of 60% in patients with LPS/LMS; specifically, 81% in patients with LPS and 42% in patients with LMS. The PFR12wks in patients with OTH was 31%, and 51% in the overall population. The secondary efficacy endpoints support the primary data, with median PFS and OS of 5.5 and 17.0 months, respectively, in patients with LPS/LMS. While the relatively small number of patients in this study and its nonrandomized nature may be perceived as potential limitations, these outcomes are comparable with, if not more favorable than, the previous EORTC Phase 2 study with eribulin where PFR12wks was achieved in 47% (95% CI: 29–65%) of patients with LPS and 32% (95% CI: 18–49%) with LMS (14). We have also compared our results with those from the pivotal Phase 3 study of eribulin (NCT01327885) in 452 previously treated patients with intermediate- or high-grade advanced LPS/LMS (15). In that study, the primary endpoint of OS was met, with a 2-month difference observed in favor of eribulin compared with dacarbazine in the overall population (median OS: 13.5 vs 11.5 months; HR: 0.77 [95% CI: 0.62–0.95]; P = 0.0169). In the eribulin arm, the median OS in patients with LPS was 15.6 months, compared with 12.7 months in patients with LMS. PFR12wks in that study was 33% with eribulin and 29% with dacarbazine, while the median PFS was 2.6 months in both treatment arms (15).
The efficacy of eribulin for each STS subtype were reported as exploratory in this study. For LPS, PFR12wks was 77.8% in patients with myxoid LPS and 83.3% in patients with dedifferentiated LPS. Although dedifferentiated LPS has shown generally poor prognosis and lower responses to chemotherapy compared with myxoid LPS (19), eribulin, interestingly, showed a high PFR12wks for this subtype. Additionally, PFR12wks was achieved in various subtypes in OTH (endometrial stromal sarcoma [2/2 patients], synovial sarcoma [1/3 patients], solitary fibrous tumor [1/2 patients] and fibrosarcoma [1/2 patients]). Specifically, more than 20% tumor shrinkage was shown, and PFS exceeded 6 months in synovial sarcoma. Eribulin is considered as active drug for various subtypes of STS.
Eribulin had a manageable safety profile in this study, with no new or unexpected safety findings. Neutropenia was a common AE but was short-lasting and reversible. The incidence of Grade 3–4 neutropenia was higher in this study compared with Phase 2 study (86% vs 52%) (14). Patients in our study may have been more heavily pretreated, having received up to seven (median 2) prior regimens for advanced/metastatic disease, whereas the number of prior regimens was not reported in the previous Phase 2 study (14). Alternatively, interstudy differences may be attributable to the different ethnicities of patients involved in these studies, including population-related pharmacogenomic differences (20,21). Neutropenia was managed by dose modifications and G-CSF (administered in 49% of patients overall). Grade 3–4 treatment-related febrile neutropenia occurred in 6–8% of patients in both studies. Severe nonhematological AEs were uncommon.
In conclusion, data from this Phase 2 study show clinical activity of eribulin, based on PFR12wks, PFS and OS, with a manageable safety profile in previously treated Japanese patients with advanced/metastatic STS.
Supplementary Material
Acknowledgements
The authors would like to thank all of the patients, as well as the investigators and their teams, who participated in this study. Editorial assistance was provided by Oxford PharmaGenesis Inc., and was funded by Eisai.
Conflict of interest statement
Yoichi Naito received honoraria and research funding from Eisai Co., Ltd.; Hideo Morioka received honoraria from Daiichi-Sankyo Co., Ltd.; Kenichi Saito is an employee of Eisai Co., Ltd.; Shun Asami is an employee of Eisai Co., Ltd.; Kazuo Isu received honoraria from Eisai Co., Ltd.; Yasuo Yazawa received research funding from Eisai Co., Ltd.; Akira Kawai, Nobuhito Araki, Toshifumi Ozaki, Hideshi Sugiura, and Akihiko Matsumine, have nothing to disclose.
Supplementary data
Supplementary data are available at Japanese Journal of Clinical Oncology online.
Funding
Eisai Co., Ltd., Japan.
ClinicalTrials.gov registration number
References
- 1. Mastrangelo G, Coindre JM, Ducimetière F, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer 2012;118:5339–48. [DOI] [PubMed] [Google Scholar]
- 2. Stiller CA, Trama A, Serraino D, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 2013;49:684–95. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Soft Tissue Sarcoma. Version 1.2015.
- 4. Le Cesne A, Antoine E, Spielmann M, et al. High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol 1995;13:1600–8. [DOI] [PubMed] [Google Scholar]
- 5. Martin-Liberal J, Alam S, Constantinidou A, et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma 2013;2013:868973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- 7. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawai A, Araki N, Sugiura H, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study. Lancet Oncol 2015;16:406–16. [DOI] [PubMed] [Google Scholar]
- 9. ESMO/European Sarcoma Network Working Group Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii102–12. [DOI] [PubMed] [Google Scholar]
- 10. Schöffski P, Cornillie J, Wozniak A, Li H, Hompes D.. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat 2014;37:355–62. [DOI] [PubMed] [Google Scholar]
- 11. Smith JA, Wilson L, Azarenko O, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 2010;49:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res 2011;71:496–505. [DOI] [PubMed] [Google Scholar]
- 13. Dybdal-Hargreaves NF, Risinger AL, Mooberry SL. Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent. Clin Cancer Res 2015;21:2445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol 2011;12:1045–52. [DOI] [PubMed] [Google Scholar]
- 15. Schöffski P, Chawla S, Maki R, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–37. [DOI] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 17. Van Glabbeke M, Verweij J, Judson I, Nielsen OS. EORTC Soft Tissue and Bone Sarcoma Group . Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 2002;38:543–9. [DOI] [PubMed] [Google Scholar]
- 18. Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–32. [DOI] [PubMed] [Google Scholar]
- 19. Jones RL, Fisher C, Al-Muderis O, Judson IR. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853–60. [DOI] [PubMed] [Google Scholar]
- 20. Gandara DR, Kawaguchi T, Crowley J, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol 2009;27:3540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lara PN Jr., Chansky K, Shibata T, et al. Common arm comparative outcomes analysis of phase 3 trials of cisplatin + irinotecan versus cisplatin + etoposide in extensive stage small cell lung cancer: final patient-level results from Japan Clinical Oncology Group 9511 and Southwest Oncology Group 0124. Cancer 2010;116:5710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.