Abstract
Cyclic nucleotide-gated (cng) non-selective cation channels have been cloned from a number of animal systems. These channels are characterized by direct gating upon cAMP or cGMP binding to the intracellular portion of the channel protein, which leads to an increase in channel conductance. Animal cng channels are involved in signal transduction systems; they translate stimulus-induced changes in cytosolic cyclic nucleotide into altered cell membrane potential and/or cation flux as part of a signal cascade pathway. Putative plant homologs of animal cng channels have been identified. However, functional characterization (i.e. demonstration of cyclic-nucleotide-dependent ion currents) of a plant cng channel has not yet been accomplished. We report the cloning and first functional characterization of a plant member of this family of ion channels. The Arabidopsis cDNA AtCNGC2 encodes a polypeptide with deduced homology to the α-subunit of animal channels, and facilitates cyclic nucleotide-dependent cation currents upon expression in a number of heterologous systems. AtCNGC2 expression in a yeast mutant lacking a low-affinity K+ uptake system complements growth inhibition only when lipophilic cyclic nucleotides are present in the culture medium. Voltage clamp analysis indicates that Xenopus laevis oocytes injected with AtCNGC2 cRNA demonstrate cyclic-nucleotide-dependent, inward-rectifying K+ currents. Human embryonic kidney cells (HEK293) transfected with AtCNGC2 cDNA demonstrate increased permeability to Ca2+ only in the presence of lipophilic cyclic nucleotides. The evidence presented here supports the functional classification of AtCNGC2 as a cyclic-nucleotide-gated cation channel, and presents the first direct evidence (to our knowledge) identifying a plant member of this ion channel family.
Cyclic nucleotides (cAMP and cGMP) are important (secondary) signaling molecules in both eukaryote and prokaryote cells (Reggiani, 1997). They are typically involved in sensing extracellular stimuli and the transduction of the signal into altered metabolic responses (Zagotta and Siegelbaum, 1996; Reggiani, 1997). Cyclic nucleotide involvement in sensory perception, at least in animal systems, often occurs through the action of cell-membrane-localized cyclic nucleotide-gated (cng), non-selective cation channel proteins (Zagotta and Siegelbaum, 1996). Cng channels involved in light (i.e. in rod and cone cells), taste (gustatory receptors), and smell (olfactory receptors) perception and in chemotaxis (in sperm) have been recently cloned from a variety of animal systems (Goulding et al., 1992; Bonigk et al., 1993; Weyand et al., 1994; Misaka et al., 1997). These cDNAs encode pore-forming (i.e. α) subunits of channel proteins that facilitate the conductance of cations (typically K+, Ca2+, and Na+) across cell membranes upon the direct binding of cAMP or cGMP to the intracellular portion of the polypeptide (Zagotta and Siegelbaum, 1996).
Cng cation channel α-subunits share some sequence homology and secondary structure similarity with α-subunits of animal voltage-gated outward-rectifying K+-selective ion channel (Shaker) proteins. Like Shaker α-subunits, cng-gated cation channels cloned to date from animal systems have six membrane-spanning regions and a P (pore) region, with intracellular hydrophilic N and C termini (Zagotta and Siegelbaum, 1996). The pore region of (both animal and plant) K+-selective voltage-gated channels retains a highly conserved signature pore sequence that determines K+ selectivity (Heginbotham et al., 1994; Ketchum and Slayman, 1996). The pore region of cng-gated channels (which, as noted above, do not display K+ selectivity) retains some but not all of the K+ channel signature pore sequence.
Cng channels are defined functionally as ligand-gated channels that are activated by ligand (cyclic nucleotide) binding to the channel protein. Conductance facilitated by some plant (Hoshi, 1995) and animal (Bruggemann et al., 1993) voltage-gated K+ channels is also affected by cyclic nucleotide, but in a different manner. In this case, the rectified conductance of channels is activated by voltage, but direct binding of cyclic nucleotide to the protein modulates the voltage:current relationship. Plant homologs (KAT1, AKT1, and KST1) of animal Shaker K+ channels that have been cloned and functionally characterized have cyclic-nucleotide-binding sites (Anderson et al., 1992; Sentenac et al., 1992; Muller-Rober et al., 1995). However, these channels are structurally and functionally distinct from animal cng channels. Binding of cyclic nucleotide to this class of channels results in a reduction of current at a given voltage, but voltage is the primary determinant of conductance (Hoshi, 1995).
Cytosolic cyclic nucleotides are also known to modulate the conductance of other classes of K+-selective channels, but in an indirect fashion (Zagotta and Siegelbaum, 1996). In this third category of cyclic nucleotide regulation of channel function, the effect is mediated by cyclic-nucleotide-dependent protein kinase phosphorylation of the ion channel. Cyclic nucleotide binding to protein kinase allows for kinase-dependent phosphorylation of some K+ channels; cyclic-nucleotide-dependent phosphorylation modulates conductance of the channel (Rudy et al., 1991; Wang and Giebisch, 1991). Assmann and co-workers (Li et al., 1994) have demonstrated that K+ currents across some native plant cell membranes are modulated by cAMP-dependent protein kinase phosphorylation of the channel. Preliminary reports (Kamasani et al., 1997) indicated that induced currents upon expression of the plant K+ channel KAT1 in X. laevis oocytes are inhibited by kinase-dependent phosphorylation.
Understanding the molecular basis for regulation of cation transport across animal cell membranes has been facilitated by the cloning and functional characterization of cDNAs encoding ion channels representing many different protein families. This work has led to a rather complex picture of diverse channel families with different functions (i.e. involvement in action potentials, maintenance of membrane potential, ion transport, and sensory signal transduction, etc.) in animal systems. Our understanding of the involvement of ion channels in plant cell function is just beginning, and will be advanced by the ongoing discovery of new classes of plant ion channels and the subsequent characterization of the mechanisms regulating ion conductance facilitated by these transport proteins. cDNAs have been cloned from plants which encode inward-rectifying (Schachtman et al., 1992; Sentenac et al., 1992; Muller-Rober et al., 1995) and outward-rectifying (Czempinski et al., 1997; Gaymard et al., 1998) voltage-gated K+ channels. However, no cDNA has yet been cloned from plants that encodes a protein functionally demonstrated to be a member of the cng ion channel family of proteins. The objective of the work described in this report was to undertake this effort.
MATERIALS AND METHODS
Isolation of AtCNGC2
The Arabidopsis expressed sequence tag (EST) database (dbEST) was screened using an animal cng channel sequence (the chick cone photoreceptor; GenBank accession no. X89598). An EST (stock no. 38D12T7; GenBank accession nos. T04542 and T13368) encoding a partial-length putative cng channel was obtained from the DNA Stock Center at the Arabidopsis Biological Resource Center (The Ohio State University, Columbus). A cDNA library CD 4-7 obtained from the Arabidopsis Biological Resource Center (D'Alessio et al., 1992) was screened using the EST 38D12T7 sequence as a probe. Several positive clones were identified. One clone was sequenced to completion utilizing a DNA sequencing system (Silver Sequence, Promega, Madison, WI) and was shown to encode a full-length putative plant cng cation channel called AtCNGC2. The plasmid construct is labeled pZL-cngc.
All standard molecular biology procedures for library screening, subcloning, and DNA sequencing were performed essentially as described in Ausubel et al. (1987). All enzymes were obtained from Gibco-BRL (Cleveland) unless otherwise noted.
Computational Analysis
The Expressed Sequence Tag (EST) database (dbEST) and the non-redundant (NR) database at GenBank were screened using BLAST software through the Internet (Madden et al., 1996). Other sequence databases utilized include: The Institute for Genomic Research (TIGR) and the University of Minnesota-EST Arabidopsis database.
DNA sequence analyses were undertaken using the Genetics Computer Group (GCG) Version 9.1 software package (Madison, WI) run on an open VMS workstation at The University of Connecticut Biotechnology Center (Telnet address: clone3.mcb.uconn.edu). The computer-assisted hydropathy plots were done using the program RAOARGOSfrom the PC/Gene Computational Software Package (IntelliGenetics, University of Geneva) under a DOS environment. The sequence alignment analysis was undertaken using Clustal W 1.7 software (Higgins and Sharp, 1988) running under the University of Connecticut UNIX workstation.
Expression of cDNA and Synthesis of cRNA Encoding AtCNGC2
The cDNA encoding AtCNGC2 was subcloned into the yeast (Saccharomyces cerevisiae) expression vector pYES2 (Invitrogen, Carlsbad, CA). The resultant plasmid, labeled pYES-cngc, was used for the yeast transformation and subsequent complementation experiments. The plasmid construct pZL-cngc, containing AtCNGC2, was used to generate full-length sense RNA encoding methylated, capped runoff transcripts following the protocol and procedures outlined in the Epicentre AmpliScribe T7 High Yield Transcription Kit manual (Epicentre Technologies, Madison, WI). The resultant purified sample was used directly for injection into oocytes (50 nL/oocyte containing 50 ng cRNA). The cDNA encoding AtCNGC2 was subcloned into the mammalian expression vector pcDNA3 (Invitrogen). The resultant plasmid, labeled pcDNA3-cngc, was used for mammalian cell transformation and subsequent expression studies.
All standard molecular biology procedures for DNA manipulation, cDNA expression, and cRNA synthesis were performed essentially as described in Ausubel et al. (1987) unless otherwise noted. All enzymes were obtained from Gibco-BRL unless otherwise noted.
Yeast Complementation Studies
pYES-cngc and pYES2 (empty cassette) were transformed into the K+-uptake deficient Saccharomyces cerevisiae yeast mutant strain CY162 (MATα ura3–52, trk1Δ, his3Δ200, his4–15, and trk2Δ::pck64 provided by Dr. L. Kochian, Cornell University, Ithaca, NY) following the lithium acetate transformation protocol precisely as described in Ausubel et al. (1987). Positive transformants were selected by uracil prototrophy on synthetic minimal media (SMM) (i.e. YNB agar without amino acids [BIO 101, Vista, CA] with the addition of CSM-URA [BIO 101], 100 mm KCl, and 2% [w/v] dextrose). The resultant plates were incubated at 30°C for 4 d and positive colonies isolated for subsequent experiments.
To test for complementation of the trk1Δ and trk 2Δ mutation, yeast colonies (transformed with pYES-cngc or pYES2) were transferred to fresh SMM plates supplemented with 2 mm KCl (substituting 2% [w/v] Gal and 0.5% [w/v] Suc for the dextrose). The transformed yeast cultures were also plated on SMM agar (2 mm KCl, Gal, and Suc) with the addition of 10 μm dibutyryl-cAMP or dibutyryl-cGMP (Sigma, St. Louis). Complementation of K+ uptake in the yeast mutant was also assayed in liquid medium. Single colonies were isolated from appropriate plates and transferred to a liquid culture consisting of a modification of the synthetic minimal media described above (YNB formulation substituting NaI for KI and NaH2PO4 for KH2PO4, with the addition of 0.1 mm KCl). Growth rates were monitored by determining A595 of the developing cultures after 24 h. Absorbance measurements of the developing cultures were converted to protein concentrations from a standard curve generated using a Bradford protein assay kit (Sigma).
Functional Expression in Oocytes
Standard methods (Very et al., 1995) were used to express AtCNGC2 cRNA in X. laevis oocytes. For each experiment, 50 nL of water containing 50 ng of cRNA encoding AtCNGC2 (50 nL of water for controls) was injected into stage 5 X. laevis oocytes prepared and cultured by standard methods. Two-electrode voltage clamp recordings in the whole-cell configuration were performed 5 d after injection utilizing an amplifier (GeneClamp 500, Axon Instruments, Foster, CA). Voltage stimuli were generated and currents were recorded using pClamp 6.04 software (Axon Instruments) in a bath solution (similar to that used for recording KAT1 currents from oocytes; Schachtman et at., 1992) containing 96 mm KCl, 1.8 mm CaCl2, 1.8 mm MgCl2, and 10 mm HEPES-KOH, pH 7.5. Current recordings were filtered at 2 kHz. Leak currents were measured without cNMP and were subtracted from each trace (Baumann et al., 1994; Eismann et al., 1994; Crary et al., 1998). After leak currents were measured, oocytes were perfused with 10 μm dibutyryl-cNMP for 30 min prior to obtaining a second series of voltage clamp recordings (Ehold = −60 mV, step voltages between +80 and −160 mV in 20-mV increments). The recording chamber was perfused at a rate of 2 mL/min with bath solutions with or without ligand. Pipettes were pulled from KIMAX-51 capillaries (KIMBLE Products, Vineland, NJ).
Functional Expression in HEK293 Cells
pcDNA3-cngc was co-transfected with CD8 into the human embryonic kidney cell line HEK293 (American Type Tissue Culture Collection, Rockville, MD) following an electroporation and selectivity method described in Jurman et al. (1994). The cNMP-induced rise in [Ca2+]i was measured in individual cells by fluorescence spectroscopy using the Ca2+ sensitive dye FURA-2. The components associated with the photometry system included an inverted microscope (Nikon, Tokyo), a computerized MAC2000 modular system, and a 150-W Xenon lamp (Ludl Electronic Products, New York). This system allows for single cell measurements by directing 100% of the emitted light to a photometer. The dye was excited at 340 and 380 λ with emission intensity (510 λ) measured in volts. A Ca2+ imaging calibration kit with FURA-2 pentasodium salt (Molecular Probes, Eugene, OR) was used to calibrate the system, and the ratio of the two signals (340/380) was plotted as a function of [Ca2+]i according to the formula described by Grynkiewicz et al. (1985).
Fluorescence imaging of cNMP-induced Ca2+ entry into AtCNGC2 transfected and control cells (non-transfected) was performed according to the method described by Baumann et al. (1994) with the following modifications. Cells were loaded with the Ca2+-sensitive fluorescent dye FURA-2 by incubation in loading buffer (120 mm NaCl, 3 mm KCl, 1 mm CaCl2, 3 mm MgCl2, 50 mm Glc, 5 mm NaOH, and 10 mm HEPES, pH 7.4) with 2 μm FURA-2/AM (Molecular Probes) for 30 min at 37°C (under 5% CO2). The experimental protocol used by Baumann et al. (1994) to demonstrate functional expression of the Drosophila retinal cng was followed to evaluate AtCNGC2 currents in this system. Dye-loaded AtCNGC2-transfected and non-transfected control cells were incubated for 30 min with 50 μm dibutyryl-cNMP in loading buffer (except that the [Mg2+] was 10 mm); this high level of divalent cation blocks cNMP-activated Ca2+ entry (Baumann et al., 1994). [Ca2+]i of HEK293 cells was monitored (cells were exposed to cNMP and Ca2+ throughout the time course of the experiment) in the presence of high [Mg2+] (blocking cNMP-activated Ca2+ currents), after perfusion in Mg2+-free loading buffer (removing the Mg2+ block of cng channel currents), and then after the block was restored by perfusion again with loading buffer containing high [Mg2+].
RESULTS AND DISCUSSION
The amino acid sequence of an animal cng ion channel (the chick cone photoreceptor; GenBank accession no. X89598) was used to search the Arabidopsis EST database. Several homologous EST clones were identified. One clone (GenBank accession no. T04542) encoding a 1.2-kb insert was used to screen an Arabidopsis cDNA library (CD4–7, Ohio State University Arabidopsis Resource Center). A full-length cDNA clone corresponding to the EST probe was identified. The isolated cDNA, AtCNGC2 (GenBank accession no. AF067798), is 2,374 bp in length and harbors an open reading frame of 2,178 bp encoding a polypeptide of 726 amino acids with a predicted molecular mass of 83.3 kD. The presence of the gene encoding AtCNGC2 in the Arabidopsis genome was confirmed by Southern-blot analysis (data not shown). Another group submitted an identical sequence to the database (accession no. Y16328, Köhler and Neuhaus, 1998).
The deduced amino acid sequence encoded by AtCNGC2 (Fig. 1) was aligned with cng channels cloned from several plant (AtCNGC1 a second putative Arabidopsis cng clone, and HvCBT1, a barley homolog) and animal (CRET and BOLF) species. A computer-assisted hydropathy plot (results not shown) of the deduced AtCNGC2 polypeptide indicated the presences of six putative transmembrane domains. This is consistent with animal cng channels and plant inward-rectifying K+ channels (for review, see Zagotta and Siegelbaum, 1996; Maathuis et al., 1997). In addition, sequence analysis indicates that AtCNGC2 contains a potential pore-forming region with lower hydrophobicity (relative to the membrane spanning domains) between S5 and S6, as well as putative cyclic nucleotide and calmodulin-binding domains.
Figure 1.
Multiple amino acid sequence alignment of AtCNGC2 with several other animal and (putative) plant cng channel proteins. The amino acid sequences were aligned using the Clustal W 1.7 multiple-alignment program (Higgins and Sharp, 1988). Identical, strongly, and weakly conserved amino acid residues are denoted with asterisks (*), colons (:), and periods (.), respectively, based on the Gonnet Pam250 scoring matrix as described in the documentation provided with Clustal W 1.7. Proposed domains corresponding to the plant sequences are overlined and denoted as follows: S1 to S6 indicate the putative transmembrane domain, P indicates the pore region, CNBD indicates the cyclic-nucleotide binding domain, and CaMBS (double-overlined) indicates the calmodulin binding site as determined utilizing programs from the PC/Gene computational software package (IntelliGenetics, University of Geneva) as well as data presented in several manuscripts cited in the results section. The corresponding regions displayed in the two animal sequences are underlined and similarly annotated. GenBank accession numbers corresponding to the various peptides are as follows: AtCNGC1 from Arabidopsis (Y16327); HvCBT1 from barley (AJ002610); CRET from a chick retinal channel (X89598); and BOLF from a bovine olfactory channel (X55010).
The six putative transmembrane segments (S1–S6), the pore (P) region, the cyclic nucleotide-binding domain (CN), and the calmodulin-binding domain (CaM) of AtCNGC2 are shown in Figure 1. AtCNGC2 shows a relatively low overall sequence identity (22% or less) with the animal cng channels shown in Figure 1; however, the displayed sequences share significant homology in several positions restricted predominately to the C terminus. The most conserved regions are the S6 transmembrane domain, the pore region, and the cyclic nucleotide binding domain. As expected, AtCNGC2 shows greater overall identity with the putative plant cng channels (46% for AtCNGC1; 32% for HvCBT1). Moreover, the structural domains between the plant cng channels share a relatively high degree of homology. The alignment between plant and animal membrane spanning domains upstream from the pore region is significantly hampered by the fact that plant cng channels include about 50 more amino acids between S5 and S6.
The putative cyclic-nucleotide-binding domain located in AtCNGC2 has structural features consistent with corresponding domains in animals cng channels (compare with Ludwig et al., 1990; Bonigk et al., 1993). A key amino acid residue associated with nucleotide binding is present as a conservative substitution in AtCNGC2. Specifically, the Asn at position D600 replaces a Glu residue in the other plant and animal sequences. Other invariant residues are distinguished with asterisks in Figure 1. Although the overall sequence identity between plant and animal CN-binding domains is low, their homology to the animal sequences is sufficient to distinguish this region as a cyclic-nucleotide-binding site.
The denoted S4 domain for AtCNGC2, commonly described as the voltage-sensing region in voltage-gated K+ channels, contains a number of evenly spaced basic residues indicative of that motif (Jan and Jan, 1992). However, the plant and animal cng channels have fewer positively charged residues in this domain.
The putative pore region for AtCNGC2 shows significant homology to both the animal and other putative plant cng channel sequences. GYGD, the consensus sequence for K+ channels, is not present in the animal cng channels. Animal cng channels contain an acidic Glu residue shown to play a critical role in binding both monovalent and divalent cations (Eismann et al., 1994) in this region. AtCNGC1 and HvCBT1 share a GQNL in this position. The neutral Gln is characteristic of (animal) nonspecific cation channels (Kerr and Sansom, 1995). AtCNGC2 displays an ANDL (amino acid residue positions 415–418) at this aligned region; the acidic Asp distinguishes it from the other plant channels.
A putative CaM binding domain for AtCNGC2 located within the proposed CN binding site is identified in Figure 1. The proposed site contains two major hydrophobic (Y647 and Y660) anchors separated by 12 amino acid residues. It also includes two minor evenly spaced hydrophobic (A650 and L654) residues and a positively charged conserved Arg (R659). This region is able to form a basic amphiphilic α-helix indicative of known CaM binding sites (O'Neil and DeGrado, 1990; Ikura et al., 1992). These features are loosely conserved in the barley sequence and the other Arabidopsis sequence shown in Figure 1.
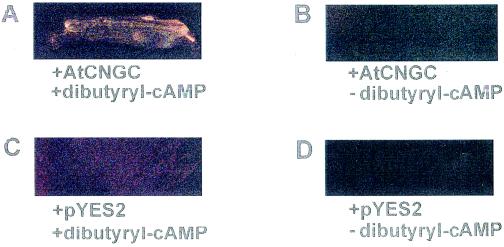
One manner in which the functional characterization of AtCNGC2 was undertaken involved heterologous expression in the K+-uptake-deficient yeast (S. cerevisiae) mutant CY162 (Gaber et al., 1988; Ko and Gaber, 1991). The Trk1 and Trk2 K+ transporter deletions in this mutant are lethal at low external [K+]; complementation of this mutation (i.e. growth at low K+) has been used to demonstrate function of a number of cloned plant K+ transporters (Anderson et al., 1992; Sentenac et al., 1992; Schachtman and Schroeder, 1994; Quintero and Blatt 1997; Schachtman et al., 1997; Fu and Luan, 1998). In the series of experiments shown in Figure 2 and Table I, the ability of the AtCNGC2 translation product to facilitate K+ transport (i.e. K+ uptake into the yeast mutant) was evaluated by monitoring growth of the yeast at low external [K+]. The CY162 yeast did not grow on solid medium when transfected with the empty plasmid (pYES2) either in the presence or absence of the lipophilic cyclic nucleotide analog dibutyryl-cAMP (Fig. 2). Transfection with AtCNGC2 alone also did not complement the K+ uptake mutation. However, when lipophilic cAMP was supplied to the growth medium, transfection with AtCNGC2 did allow for growth of the mutant yeast at low external [K+].
Figure 2.
Complementation (evaluated as growth on solid media in the presence of lipophilic cAMP) of K+-uptake mutation in CY162 yeast by transfection with AtCNGC2. Yeast was transformed with either plasmid containing AtCNGC2 (A and B) or empty pYES2 plasmid (C and D), and grown on solid medium containing 2 mm KCl in the presence (A and C) or absence (B and D) of 10 μm dibutyryl-cAMP. At high (100 mm) KCl, yeast transformed with either the empty plasmid or AtCNGC2 grew well in the absence or presence of cyclic nucleotide (data not shown). Pictures were taken after growth for 7 d at 30°C. This experiment was repeated twice.
Table I.
Transfection with AtCNGC2 enhances growth in liquid culture of the K+-uptake-deficient yeast mutant in the presence of (lipophilic) cyclic nucleotide. CY162 yeast transfected with either pYES2 (control) or pYES-cngc were grown in liquid cultures containing 0.1 mm KCl as the sole K+ source in the absence or presence of 10 μm lipophilic (dibutyryl) analogs of cyclic nucleotides. Treatment means (shown ±se) represent four independent cultures grown under each experimental condition. Results of four independent experiments are shown.
| Experiment | Growth
|
|||
|---|---|---|---|---|
| Treatment | Control | +AtCNGC2 | % Increase | |
| μg protein/mL | ||||
| 1 | +cAMP | 3.23 ± 0.46 | 22.4 ± 0.80 | 593 |
| 1 | −cAMP | 1.67 ± 0.38 | 2.06 ± 0.44 | 23 |
| 2 | +cAMP | 3.16 ± 0.75 | 18.6 ± 0.91 | 488 |
| 3 | +cAMP | 3.49 ± 0.71 | 21.2 ± 1.01 | 507 |
| 4 | +cAMP | 2.10 ± 0.42 | 21.5 ± 1.25 | 923 |
| 4 | +cGMP | 4.66 ± 0.62 | 19.2 ± 0.55 | 312 |
The addition of (non-lipophilic) cAMP to the solid growth medium did not facilitate growth of the mutant yeast transfected with either AtCNGC2 or the empty plasmid (data not shown), suggesting that the cAMP binding domain of AtCNGC2 is cytosolic; animal cng channels have been shown to have this protein architecture (Zagotta and Siegelbaum, 1996). We observed that on solid medium, growth of the mutant yeast transfected with AtCNGC2 was less robust than growth that occurred when other (e.g. KAT1, data not shown) plant K+ transporters were used to complement the K+ uptake mutations of this yeast strain. Additional CY162 yeast complementation experiments were undertaken using liquid culture conditions (Table I). In liquid culture with low (i.e. the same as in the solid growth medium; 2 mm) [K+], we found that the CY162 yeast strain grew well without transfection, as did yeast transformed with AtCNGC2 (data not shown).
Further experiments were undertaken with liquid culture made up with synthetic medium formulated such that the sole K+ source provided 0.1 mm K+. Even under these conditions, the yeast mutant transfected with the empty plasmid (i.e. control) displayed a basal level of growth in liquid culture (Table I). In the absence of dibutyryl-CNMP, the mutant yeast transfected with either the empty plasmid or AtCNGC2 displayed the same level of basal growth (experiment 1 in Table I). However, as shown in four independent experiments with this liquid medium, transfection of the yeast with AtCNGC2 resulted in a significant increase in the growth rate in the presence of dibutyryl-cAMP (Table I). Growth of the yeast transfected with AtCNGC2 was found to be increased by either (lipophilic) cAMP or cGMP, indicating that AtCNGC2 is responsive to either of these cyclic nucleotides (experiment 4 in Table I).
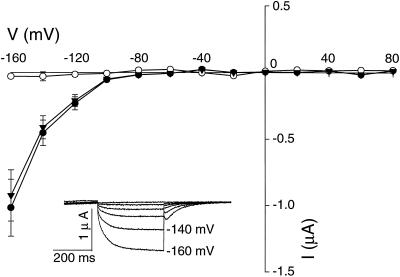
Further functional studies of AtCNGC2 were undertaken by expression of this putative plant cng channel in X. laevis oocytes. Voltage clamp studies were performed on control (water-injected) oocytes and oocytes expressing AtCNGC2 (Fig. 3). No K+ currents were observed in control oocytes in the absence (data not shown) or presence of cyclic nucleotides (cNMP). In contrast to control oocytes, the addition of dibutyryl-cNMP (10 μm cAMP or cGMP) to the recording bath solution resulted in cNMP-dependent K+ currents. Moreover, depolarizing voltages resulted in no current with these oocytes, confirming that AtCNGC2 is an inwardly rectified cNMP gated ion channel.
Figure 3.
Voltage clamp analysis of cyclic nucleotide-activated currents in oocytes expressing AtCNGC2. Recordings were made at −60 mV holding potential and command potentials between +80 and −160 mV (in 20-mV increments) on water-injected oocytes in the presence of 10 μm dibutyryl-cAMP (○; n = 6), and on oocytes injected with AtCNGC2 cRNA in the presence of 10 μm dibutyryl-cAMP (●; n = 10) and 10 μm dibutyryl-cGMP (▾; n = 9). In the main body of the figure, current:voltage relationships (current values are shown ±se) are portrayed. In the inset, representative time-dependent currents are shown for an oocyte expressing AtCNGC2 in the presence of dibutyryl-cAMP with command potentials precisely as described above.
As pointed out by Assmann (1995), the level of cyclic nucleotide used to invoke a physiological response by plant proteins is an important consideration, due to the extremely low levels (up to approximately 1.5 μm; but the localized concentration may be greater in specific cell types or cell compartments) of cyclic nucleotides thought to be present in plant cells. It should be noted that the level of (lipophilic) cyclic nucleotide used in these experiments, and those shown in Figure 2 and Table I (i.e. 10 μm) is substantially lower than that used to characterize animal cng channels upon expression in oocytes (e.g. 0.2 mm, Kaupp et al., 1989; 2 mm, Yao et al., 1995). The concentration of lipophilic cyclic nucleotide used in our experiments was also at or below that used in other studies with plant cells to elicit ion currents across native membranes (Kurosaki et al., 1994; Kurosaki 1997; Volotovski et al., 1998) as well as numerous other physiological responses (Ichikawa et al., 1997; Reggiani, 1997, and refs. therein).
We cannot know the exact cNMP level in the oocyte cytosol that is maintained in the presence of 10 μm lipophilic cAMP/cGMP in the recording bath solution. The activation by cNMP of AtCNGC2 currents can be best evaluated using a detached cell, patch/voltage clamp configuration for current recordings from oocyte membranes. We are currently setting up such a system to continue this line of investigation. Monitoring cyclic nucleotide activation of AtCNGC2 currents in this configuration would also allow for the evaluation of calmodulin/Ca2+ interaction with cyclic nucleotide gating of AtCNGC2 currents. In addition, patch clamp analyses would provide confirmation that gating of AtCNGC2 currents by cyclic nucleotide is mediated by direct binding of the ligand to the channel.
Functional expression of AtCNGC2 was also performed in the human embryonic kidney cell line HEK293 using fluorescence spectroscopy. Animal cng channels are known to be both permeable to and blocked by divalent cations to different extents depending on their role in various signal transduction pathways (Zagotta and Siegelbaum, 1996). Baumann et al. (1994) used an experimental protocol making use of this effect in their functional characterization of a Drosophila cng channel. They found that high external [Mg2+] blocked inward Ca2+ currents through this cng channel. We followed a similar strategy in our functional expression of AtCNGC2 in HEK293 cells. Ca2+ permeability (i.e. increased [Ca2+]i) of AtCNGC2 transfected cells was observed only in the presence of dibutyryl-cAMP or dibutyryl-cGMP (Fig. 4).
Figure 4.
Cytosolic Ca2+ rise in HEK293 cells expressing AtCNGC2. A, Dibutyryl-cAMP- or dibutyryl-cGMP-activated Ca2+ influx are depicted in top two panels, respectively. The bottom panel shows the change in cytosolic [Ca2+] of non-transfected (CK) cells in the presence of cyclic nucleotide. The closed arrows designate the transition from high [Mg2+] (10 mm) to Mg2+-free perfusion buffer (the absence of Mg2+ intiates Ca2+ influx); open arrows designate the transition from Mg2+-free buffer to high-[Mg2+] buffer. B, Histogram representing peak cytosolic Ca2+ values after the addition of dibutyryl cNMP (D-cAMP n = 6; D-GMP n = 7) or membrane-impermeable cNMP (cAMP n = 5; cGMP n = 4) in AtCNGC2-transfected cells and non-transfected cells (CK) (treated with dibutyryl-cAMP; n = 7).
Upon removal of external Mg2+, [Ca2+]i of HEK293 cells expressing AtCNGC2 rose in the presence of either dibutyryl-cAMP (Fig. 4A, top panel) or dibutyryl-cGMP (Fig. 4A, middle panel); no Ca2+ rise was observed in AtCNGC2 transfected cells in the absence of cNMP in the perfusion bath (Fig. 4A, bottom panel). As shown in Figure 4A, replacement of the Mg2+ block prevented further Ca2+ entry, and the action of an endogenous HEK293 cell Ca-ATPase efflux system (Baumann et al., 1994) reduced [Ca2+]i back down close to basal levels (Fig. 4A, top and middle panels). The results of a series of such experiments are summarized in Figure 4B. Similar to results (not shown) obtained when AtCNGC2 was functionally expressed in yeast and oocytes, external cNMP (i.e. cAMP and cGMP, in contrast to the lipophilic analogs) did not activate AtCNGC2 currents in HEK293 cells (Fig. 4B). Inward Ca2+ rise was not observed when HEK293 cells (non-transfected) were exposed to lipophilic cNMP (Fig. 4B). Preliminary experiments examining ion selectivity of AtCNGC2 expressed in oocytes using voltage clamp analysis (not shown) also indicated that AtCNGC2 is permeable to other monovalent cations (except, interestingly, Na+); such selectivity is not typically observed in studies of cloned animal cng channels (Zagotta and Siegelbaum, 1996).
In summary, sequence analysis indicates that AtCNGC2 shows homology to animal cng channels. Our functional analyses of AtCNGC2 in a yeast mutant, X. laevis oocytes, and cultured HEK293 cells indicate that AtCNGC2 is an inwardly rectified ion channel that conducts a number of cations and is activated by internal but not external cAMP and cGMP, and that high external divalent cations block channel conductance. Sequence analysis also identified a calmodulin binding site on AtCNGC2. These are all properties shared with cloned animal cng channels. Some evidence is present in the published literature that is consistent with the presence of cng channels in plants. cNMP-dependent inward Ca2+ and K+ flux has been observed across the plant cell plasmalemma (Kurosaki et al., 1994; Kurosaki, 1997; Volotovski et al., 1998). However, these studies of native membranes did not identify a specific transport protein as mediating ion conductance, and, further, could not rule out ion channels other than members of the cng channel family.
In addition, a number of studies have recently reported the cloning of plant cDNAs encoding polypeptides with sequence homology to animal cng channels. Köhler and Neuhaus (1998) reported the sequence of AtCNGC2 and another putative Arabidopsis cng channel (AtCNGC1 in Fig. 1) in the Plant Gene Register (accession nos. Y16328 and Y16327, respectively). Schuurink et al. (1998) cloned a barley cDNA (HvCBT1 in Fig. 1) encoding a putative cng channel. However, ion transport functions of the translation products of these plant cDNAs were not reported. HvCBT1 was found not to complement growth of the K+ uptake yeast mutant used in our work, although the barley protein was shown to bind calmodulin (Schuurink et al., 1998). Thus, the results presented here, including demonstration of cyclic-nucleotide-dependent cation transport by AtCNGC2 in three different heterologous expression systems, to our knowledge represent the first functional characterization of a cloned plant member of the cng family of ion channels.
The cytosolic secondary messengers cAMP, cGMP, calmodulin, and Ca2+, in addition to inward K+ and Ca2+ currents across the plasmalemma, are well known to be involved in numerous signal transduction pathways in plants. Our studies suggest that AtCNGC2 function in planta may be related to some of these signal transduction cascades. Further functional analysis of AtCNGC2 in heterologous expression systems focusing on the interactive effects these secondary messengers have on AtCNGC2 channel currents may provide an excellent context to extend our understanding of the molecular basis for at least some signaling pathways in plants.
ACKNOWLEDGMENTS
The pursuit of knowledge can be akin to drinking from a “magic” bottle of wine; the more you pour, the more that is left in the bottle. This work is fondly dedicated to the memories of two colleagues who, as plant physiologists, drank with gusto from that bottle during their too-short careers, and left us all richer and wiser from their efforts: Dr. Bruce Wasserman (Rutgers University, New Brunswick, NJ) and Dr. Richard Crain (University of Connecticut). We wish to thank Dr. Leon Kochian (Cornell University) for providing the CY162 yeast strain and the KAT1 cDNA, and Xiao Zhang for initial work involving the screening of libraries that led to the cloning of the AtCNGC2 cDNA.
NOTE ADDED IN PROOF
After submission of this manuscript, a paper appeared in print that reported the characterization of AtCNGC2, AtCNGC1, as well as sequences encoding several other (putative) Arabidopsis cng channels (Köhler et al., 1999). Functional characterization (i.e. cyclic nucleotide-dependent cation flux) of these cDNAs was not reported, although the translation products of AtCNGC1 and AtCNGC2 were demonstrated to bind calmodulin.
Footnotes
This material is based on work supported by the National Science Foundation (grant nos. MCB–9513921 and BIR–9512977) and by the Department of Energy (grant no. DE–FG02–95ER20202). This is Storrs Agricultural Experiment Station publication no. 1,886.
LITERATURE CITED
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Cyclic AMP as a second messenger in higher plants: status and future prospects. Plant Physiol. 1995;108:885–889. doi: 10.1104/pp.108.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1987. [Google Scholar]
- Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. Primary structure and functional expression of a Drosophilacyclic nucleotide-gated channel present in eyes and antennae. EMBO. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigk W, Altenhofen W, Muller F, Dose A, Illing M, Molday RS, Kaupp UB. Rod and cone photoreceptor cells express distinct genes for cGMP-gated channels. Neuron. 1993;10:865–877. doi: 10.1016/0896-6273(93)90202-3. [DOI] [PubMed] [Google Scholar]
- Bruggemann A, Pardo LA, Stuhmer W, Pongs O. Ether-a-go-go encodes a voltage-gated channel permeable to K+ and Ca2+and modulated by cAMP. Nature. 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- Crary JI, Gordon SE, Zimmerman AL. Perfusion system components release agents that distort functional properties of rod cyclic nucleotide-gated ion channels. Vis Neurosci. 1998;15:1189–1193. doi: 10.1017/s0952523898156134. [DOI] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Ehrhardt T, Muller-Rober B. New structure and function in plant K+ channels: CKO1, an outward rectifier with a steep Ca2+dependency. EMBO J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio JM, Bebee R, Hartley JL, Noon MC, Polayes D. Lambda ziplox: automatic subcloning of cDNA. Focus. 1992;14:76–79. [Google Scholar]
- Eismann E, Muller F, Heinemann SH, Kaupp UB. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+blockage, and ionic selectivity. Proc Natl Acad Sci USA. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H-H, Luan S. AtKUP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber RF, Styles CA, Fink GR. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud J-B, Sentenac H. Identification and disruption of a plant Shaker-like outward channel involved in K+release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Ngai J, Kramer RH, Colicos S, Axel R, Siegelbaum SA, Chess A. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992;8:45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Heginbotham L, Tatiana ZO, MacKinnon R. Mutations in the K+channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DG, Sharp PM. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Phyisol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Suzuki Y, Czaja I, Schommer C, LeBnick A, Schell J, Walden R. Identification and role of adenylyl cyclase in auxin signalling in higher plants. Nature. 1997;390:698–701. doi: 10.1038/37810. [DOI] [PubMed] [Google Scholar]
- Ikura M, Clore GM, Gronenborn AM, Zhu G, Klee CB, Bax A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992;256:632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Structural elements involved in specific K+channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Jurman ME, Boland LM, Liu Y, Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. BioTechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- Kamasani UR, Zhang X, Lawton M, Berkowitz GA. Ca-dependent protein kinase modulates activity of the K channel KAT1. Plant Physiol. 1997;114:S-980. [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, Cook NJ, Kangawa K, Matsuo H, Hirose T, Miyata T, Numa S. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kerr ID, Sansom MSP. Cation selectivity in ion channels. Nature. 1995;373:112. doi: 10.1038/373112a0. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Slayman CW. Isolation of an ion channel gene from Arabidopsis thaliana using the H5 signature sequence from voltage-dependent K+channels. FEBS Lett. 1996;378:19–26. doi: 10.1016/0014-5793(95)01417-9. [DOI] [PubMed] [Google Scholar]
- Ko CH, Gaber RF. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Merkle T, Neuhaus G. Characterization of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis. Plant J. 1999;18:97–104. doi: 10.1046/j.1365-313x.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Köhler C, Neuhaus G. Cloning and partial characterization of two putative cyclic nucleotide-regulated ion channels from Arabidopsis thaliana, designated CNGC1 ( Y16327), CNGC2 ( Y16328) (PGR 98-062) Plant Physiol. 1998;116:1604. [Google Scholar]
- Kurosaki F. Role of inward K+ channel located at carrot plasma membrane in signal cross-talking of cAMP with Ca2+cascade. FEBS Lett. 1997;408:115–119. doi: 10.1016/s0014-5793(97)00403-1. [DOI] [PubMed] [Google Scholar]
- Kurosaki F, Kaburaki H, Nishi A. Involvement of plasma membrane-located calmodulin in the response decay of cyclic nucleotide-gated cation channel of cultured carrot cells. FEBS Lett. 1994;340:193–196. doi: 10.1016/0014-5793(94)80136-3. [DOI] [PubMed] [Google Scholar]
- Li W, Luan S, Schreiber SL, Assmann SM. Cyclic AMP stimulates K+ channel activity in mesophyll cells of Vicia fabaL. Plant Physiol. 1994;106:957–961. doi: 10.1104/pp.106.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Margalit T, Eismann E, Lancet D, Kaupp UB. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Lett. 1990;270:24–29. doi: 10.1016/0014-5793(90)81226-e. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI. Roles of higher plant K+channels. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden TL, Tatusov RL, Zhang J. Application of network BLAST server. Methods Enzymol. 1996;266:131–141. doi: 10.1016/s0076-6879(96)66011-x. [DOI] [PubMed] [Google Scholar]
- Misaka T, Kusakabe Y, Emori Y, Gonoi T, Arai S, Abe K. Taste buds have a cyclic nucleotide-activated channel, CNGgust. J Biol Chem. 1997;272:22623–22629. doi: 10.1074/jbc.272.36.22623. [DOI] [PubMed] [Google Scholar]
- Muller-Rober B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil KT, DeGrado WF. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Quintero FJ, Blatt MR. A new family of K+ transporters from Arabidopsisthat are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- Reggiani R. Alteration of levels of cyclic nucleotides in response to anaerobiosis in rice seedlings. Plant Cell Physiol. 1997;38:740–742. [Google Scholar]
- Rudy B, Kentros C, Vega-Saenz de Miera E. Families of potassium channel genes in mammals: toward an understanding of the molecular basis of potassium channel diversity. Mol Cell Neurosci. 1991;2:89–102. doi: 10.1016/1044-7431(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Kumar R, Schroeder JI, Marsh EL. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc Natl Acad Sci USA. 1997;94:11079–11084. doi: 10.1073/pnas.94.20.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the ArabidopsisKAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schuurink RC, Shartzer SF, Fath A, Jones RL. Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc Natl Acad Sci USA. 1998;95:1944–1949. doi: 10.1073/pnas.95.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Very A-A, Gaymard F, Bosseux C, Sentenac H, Thibaud J-B. Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 1995;7:321–332. doi: 10.1046/j.1365-313x.1995.7020321.x. [DOI] [PubMed] [Google Scholar]
- Volotovski ID, Sokolovsky SG, Molchan OV, Knight MR. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998;117:1023–1030. doi: 10.1104/pp.117.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Giebisch G. Dual modulation of renal ATP-sensitive K+channel by protein kinase A and C. Proc Natl Acad Sci USA. 1991;88:9722–9725. doi: 10.1073/pnas.88.21.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand I, Godde M, Frings S, Weiner J, Muller F, Altenhofen W, Hatt H, Kaupp UB. Cloning and functional expression of a cyclic-nucleotide gated channel from mammalian sperm. Nature. 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- Yao X, Segal AS, Welling P, Zhang X, McNicholas CM, Engel D, Boulpaep EL, Desir GV. Primary structure and functional expression of a cGMP-gated potassium channel. Proc Natl Acad Sci USA. 1995;92:11711–11715. doi: 10.1073/pnas.92.25.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]