Abstract
BACKGROUND
Previous studies in isolated perfused rat lungs have revealed that endothelial barrier disruption after intratracheal administration of Pseudomonas aeruginosa (strain 103; PA103) only occurs after accumulation of extracellular mitochondrial DNA (mtDNA) damage-associated molecular patterns (DAMPs) in the perfusate and is suppressed by addition of DNase to the perfusion medium. Herein, we tested the hypothesis that intratracheal DNase—a route of administration readily translatable to patient with ventilator-associated pneumonia (VAP)—also enhances degradation of mtDNA and prevents bacteria-induced lung injury.
METHODS
Intratracheal DNase was administered to isolated rat lungs either before or after intratracheal challenge with PA103 to determine if bacteria-induced mtDNA DAMP-dependent lung injury could be prevented or reversed by enhanced mtDNA degradation. To explore whether this concept is translatable to patients with VAP, consecutive patients suspected of VAP were prospectively enrolled. All patients suspected of VAP received a bronchoalveolar lavage (BAL) with quantitative culture for the diagnosis of VAP. Mitochondrial and nuclear DNAs were measured from the BAL. MtDNA DAMPs (i.e., ND6) were measured from serum at time of suspected diagnosis and at 24 to 48 hours afterward.
RESULTS
Intratracheal PA103 caused significantly increased the vascular filtration coefficient (Kf) and perfusate mtDNA DAMPs. In contrast, lungs pretreated or posttreated with intratracheal DNase were protected from increases in Kf and mtDNA DAMPs. Patients with the diagnosis of VAP had significantly higher mtDNA DAMPs in the BAL (248.70 ± 109.7 vs. 43.91 ± 16.61, p < 0.05, respectively) and in the serum at 24 hours (159.60 ± 77.37 vs. 10.43 ± 4.36, p < 0.05; respectively) when compared with patients that did not have VAP.
CONCLUSION
These findings in isolated perfused rat lungs and a cohort of severely injured patients reveal an association between bacterial pneumonia and accumulation of mtDNA DAMPs in the lung and serum. Furthermore, administration of intratracheal DNase I prevented and reversed pulmonary endothelial dysfunction evoked by PA103.
Keywords: Mitochondrial DNA, DAMPs, Ventilator-associated pneumonia, DNase, dornase α
Ventilator-associated pneumonia (VAP) is a type of health care–associated pneumonia that develops after more than 48 hours of mechanical ventilation. It is a common and serious condition occurring in over 10% of all ventilated patients with the incidence significantly higher in severely injured individuals.1 Importantly, the morbidity associated with VAP is highly variable. Although the virulence of the causative bacterium is likely a factor in the severity of VAP, the mechanism for such variability in the progression of VAP to adult respiratory distress syndrome and multiple organ dysfunction syndrome is not known. One explanation for this variability is that key molecule(s) linking lung bacterial colonization to cellular responses and local and systemic inflammation have not yet been identified. Herein, we propose that the severity of VAP may be due to the accumulation of intra-alveolar mitochondrial (mt) damage-associated molecular patterns (DAMPs) which are known to activate resident and inflammatory cells (e.g., intraalveolar macrophages).
We and others have found that proinflammatory mtDAMPs, including mtDNA fragments and mtDNA-associated proteins, are released into the circulation of severely injured patients where their abundance is associated with the occurrence of multiorgan system failure and survival.2 These fragments of the mitochondrial genome propagate damage from the initial site of injury to distant organs through TLR-mediated activation of inflammatory and resident cells.3 Studies in animal and cell culture models show that mtDNA DAMPs released as a consequence of oxidant stress or bacterial infection cause endothelial injury and pulmonary edema.4–9 Further implicating mtDNA DAMPs in this process, degradation of mtDNA DAMPs completely abrogate endothelial dysfunction in an isolated lung model.4 We therefore sought to test the hypothesis that VAP is associated with accumulation of proinflammatory mtDNA DAMPs that can be abrogated by intratracheal administration of DNase I. A translationally significant aspect of the present study is the delivery of the enzyme by intratracheal administration; decades of clinical use of DNase I administered in this manner to cystic fibrosis patients have unequivocally demonstrated its safety.
METHODS
Isolated Lung Preparation and Assessment of the Vascular Filtration Coefficient
Lungs of adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were isolated and perfused as described previously4,7 according to a protocol approved by the University of South Alabama IACUC (protocol 533850). Briefly, after intraperitoneal injection of sodium pentobarbital, male rats weighing 250 to 300 g were ventilated with a Harvard rodent ventilator (Harvard Apparatus Company, Holliston, MA) using a humidified gas mixture consisting of 21%O2, 5%CO2, and 74%N2 at 60 breaths/min, a tidal volume of 2.5 mL, and a positive end-expiratory pressure of 2.5 cm H2O. Following sternotomy and intracardiac injection of heparin sulfate (100 U), the pulmonary artery and left ventricle were cannulated and the pulmonary circulation perfused at a constant flow rate (0.04 mL/g body weight/min) with physiologic salt solution (in mM: 3.2 CaCl2, 119 NaCl, 4.7 KCl, 1.17MgSO4, 1.18KH2PO4, 22.6NaHCO3, and 5.5 d-glucose) containing 4% bovine serum albumin. The heart and lungs were removed en bloc and suspended in a humidified chamber from a force displacement transducer (Grass FT03; Natus Neurology, Middleton, WI) to record real-time changes in lung weight. In all experiments, zone III conditions were maintained. Pulmonary arterial and venous pressures (Pa and Pv, respectively) were continuously monitored with Cobe pressure transducers (Cobe Laboratories, Lakewood, CO) and a Model 7F Grass polygraph recorder. Pulmonary capillary pressure (Pc) was estimated using the double occlusion method.10 The vascular filtration coefficient (Kf) was calculated and expressed as milliliters per minute per cm H2O per 100 g.10
Experimental Protocols in Isolated Rat Lungs
To determine if pretreatment with DNase I suppressed mtDNA DAMP-dependent lung injury (see Figure, Supplemental Digital Content 1A, http://links.lww.com/TA/A823), isolated rat lungs were instilled via the trachea with DNase I (16 units) or 0.1 mL saline through a 27-gauge needle. Thirty minutes later, a baseline Kf (Kf1) was measured, after which an intratracheal instillation of an LD50 dose of PA103 (5 × 107 colony-forming units) was administered.11 Kf (Kf2) and perfusate mtDNA DAMPs were measured 15 minutes after the instillation of PA103. Saline was instilled into the trachea as a negative control for the bacteria and DNase.
The reversal arm (see Figure, Supplemental Digital Content 1B, http://links.lww.com/TA/A823) was conducted in a similar manner to the prevention arm, except that DNase I or saline was administered after PA103. Briefly, the isolated lungs were perfused and ventilated for 30 minutes, after which Kf (Kf1) was measured immediately before the instillation of PA103. The DNase or saline was administered 15 minutes after instillation of PA103. The Kf (Kf2) and perfusate mtDNA DAMPs were measured after an additional 15minutes, again using saline as a negative control.
Isolation of DNA From Perfusion Medium and Determination of mtDNA Abundance
As previously described,4 approximately 50mL of perfusate was collected after each isolated rat lung experiment and stored at −20°C until further processing. After samples were thawed, 10 mL was centrifuged at 18,000g at 4°C for 20 minutes to pellet cells and organelles. Seven milliliters of the supernatant was then decanted and centrifuged at 100,000g at 4°C for 30 minutes to remove subcellular debris, leaving any free DNA suspended in the supernatant. After this final centrifugation step, DNA was isolated from 1 mL of the supernatant using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions.
Quantitation of mtDNA DAMP Abundance in Perfusion Medium
Quantitative real-time polymerase chain reaction (PCR) was used to detect selected approximately 200-bp sequences of the mitochondrial genome.2 Primers were developed using Beacon Designer software, as shown in Supplemental Digital Content 2 (http://links.lww.com/TA/A823). The mtDNA sequence quantified corresponded to the region encoding cytochrome c oxidase subunit II. Quantitative real-time PCR was done using the HotStart-IT SYBR Green One Step quantitative PCR (qPCR) kit (Affymetrix Inc., Santa Clara, CA) following the manufacturer's instructions. An iCycler with the iQ5 Multicolor Real-Time PCR Detection System (BIO-RAD, Hercules, CA) was used to analyze each sample. The amplification efficiency of each primer set was determined against a calibration curve, which was constructed by performing PCR with serial dilutions of a known concentration of template DNA.
Human Patient Recruitment
Consecutive patients in the surgical trauma intensive care unit at the University of South Alabama Medical Center with suspected VAP were prospectively enrolled. This study was approved by the University of South Alabama Institutional Review Board (protocol 608052-2). All patients suspected of VAP received a bronchoalveolar lavage (BAL) with quantitative culture for the diagnosis of VAP. A quantitative culture with greater than 10,000 CFU/mL was considered positive. Patients with inhalation injury secondary to smoke were excluded.
Isolation of Extracellular mtDNA From Patient Serum
mtDNA DAMPs corresponding to a segment of the ND6 gene were measured in serum at time of BAL and at 24 to 48 hours after BAL. Samples were centrifuged within 1 hour of collection at 1,200g for 25 minutes at 21°C, and 200 µL of the plasma fraction was decanted and processed using the Qiagen DNEasy kit to isolate DNA. qPCR, using USB VeriQuest Fast SYBR Green qPRC Master Mix and the manufacturer's protocol, was applied to quantify approximately 200-bp sequence corresponding to the ND6 mitochondrial genomic region (see Table, Supplemental Digital Content 2, http://links.lww.com/TA/A823).
Isolation and Quantitation of mtDNA in BAL
BAL specimens were centrifuged at 14,000g for 20 minutes at room temperature. Two hundred microliters of the resulting supernatant was then decanted for DNA isolation using the Qiagen DNEasy kit. Additionally, samples were analyzed for quantification of human B2M, indicative of nuclear DNA content. Finally, bacterial 16S was amplified as an alternate indicator of infectious pneumonia. Primers used are listed in Supplemental Digital Content 2 (http://links.lww.com/TA/A823). The quantity of mtDNA DAMPs in the serum and BAL specimens were directly compared to the diagnosis of VAP on quantitative culture.
Statistical Analyses
Data are expressed as means ± SEM. Statistical significance was determined by unpaired t test using Graphpad Prism software (Graphpad Software, La Jolla, CA), with significance defined as p less than 0.05.
RESULTS
Effect of Intratracheal DNase Administered Before or After Induction of Acute Lung Injury by PA103
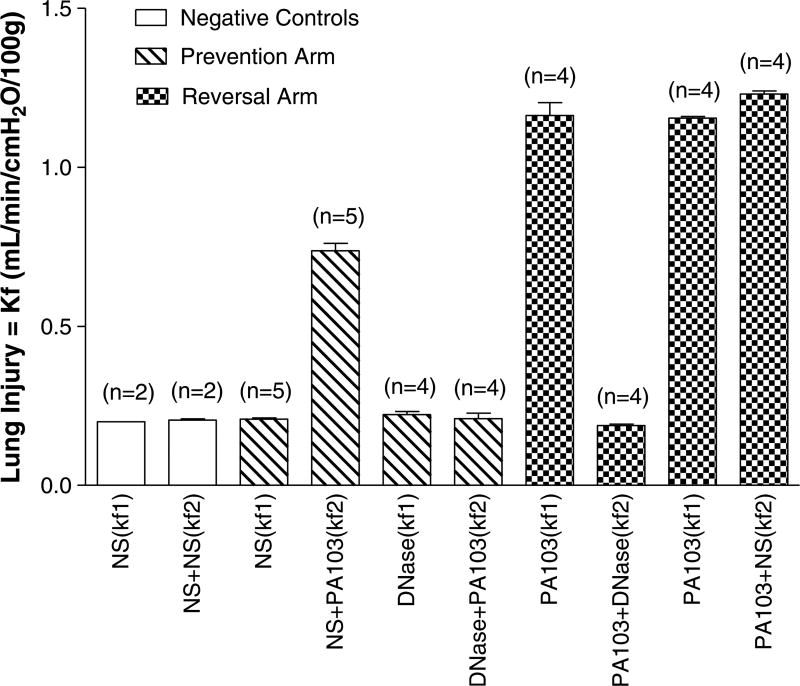
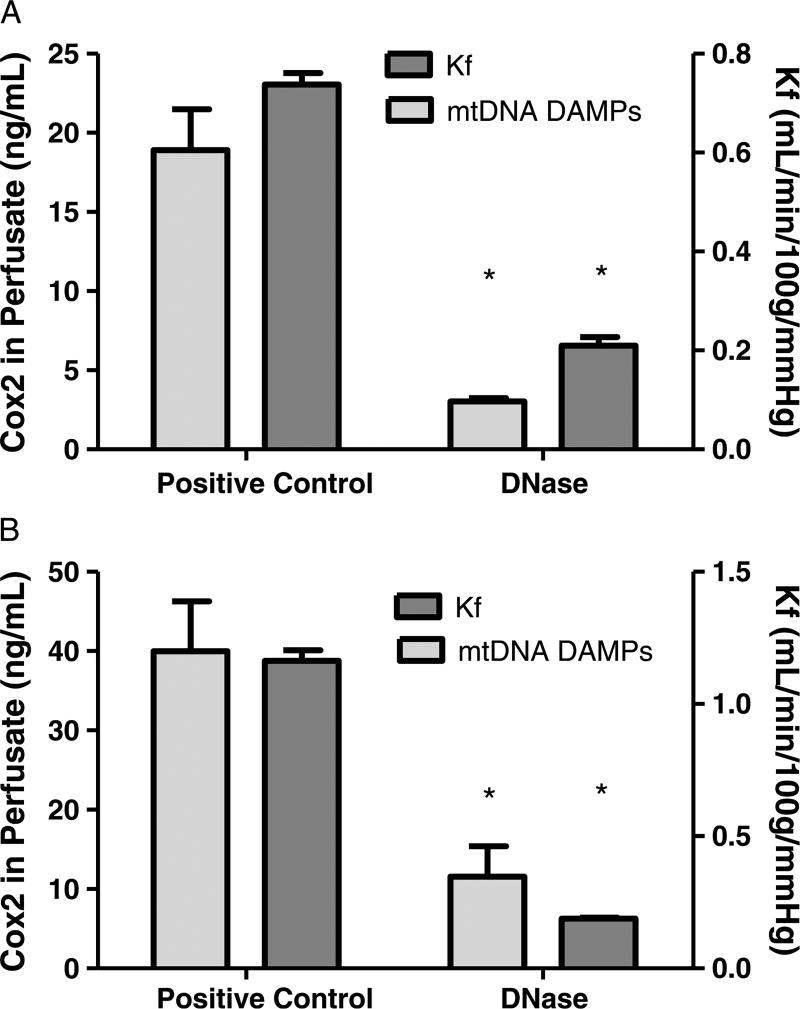
Intratracheal PA103 caused significant increases in the Kf in positive controls compared to both experimental groups receiving intratracheal DNase (prevention, 0.73 ± 0.2 vs. 0.20 ± 0.01; reversal, 1.29 ± 0.05 vs. 0.18 ± 0.003; p < 0.05). The Kf results for the positive and negative control for each experimental group are shown in Figure 1. The increase in Kf was mirrored by accumulation of extracellular mtDNA in the perfusate which was significantly decreased in the prevention (Fig. 2A) and reversal (Fig. 2B) experimental groups containing DNase (prevention, 18.9 ± 2.6 vs. 3.0 ± 0.2; reversal, 39.9 ± 6.3 vs. 11.5 ± 3.8; p < 0.05).
Figure 1.
DNase prevents and reverses pulmonary vascular endothelial barrier injury in a rat model of bacterial pneumonia. The Kfs labeled in this figure correlate with Figure 1. Negative controls: NS (Kf1): NS was given at time zero and Kf was checked after 30 minutes (A). NS + NS (Kf2): NS was given at time zero and again at 30 minutes (A). Prevention Model: NS (Kf1): NS was given at time zero and Kf was checked after 30 minutes (A). NS + PA103 =NS was given at time zero and PA103 at 30minutes (A). DNase (Kf1) = DNase was given at time zero and Kf was checked at 30minutes (A). DNase + PA103 = DNase was given at time zero and PA103 at 30 minutes (A). Reversal Model: PA103 (Kf1): PA103 was given after 15 minutes and Kf was checked 15 minutes later (B). PA103 + DNase (Kf2): PA103 was given at 15minutes and DNase was given at 30minutes (B). PA103 (Kf1): PA103 was given after 15 minutes and Kf was checked 15 minutes later (B). PA103 + NS (Kf2): PA103 was given at 15 minutes and NS was given at 30 minutes (B). NS: normal saline, PA103: Pseudomonas aeruginosa strain 103, Kf: vascular filtration coefficient (i.e., lung injury).
Figure 2.
Pseudomonas aeruginosa causes increases in vascular permeability accompanied by mtDNA DAMP release into the perfusionmedium. As shown in A, administration of intratracheal saline (i.e., positive control, n = 4) 30minutes before instillation of PA103 caused increases in the vascular filtration coefficient (Kf), which was prevented when DNase (i.e., experimental group, n = 4) was administered. As shown in B, intratracheal DNase administered 15 minutes after PA103 causes reversal of mtDNA DAMP-dependent lung injury compared to the positive control. *Different from control at p < 0.05.
Serum mtDNA DAMPs in Patients With Suspected VAP
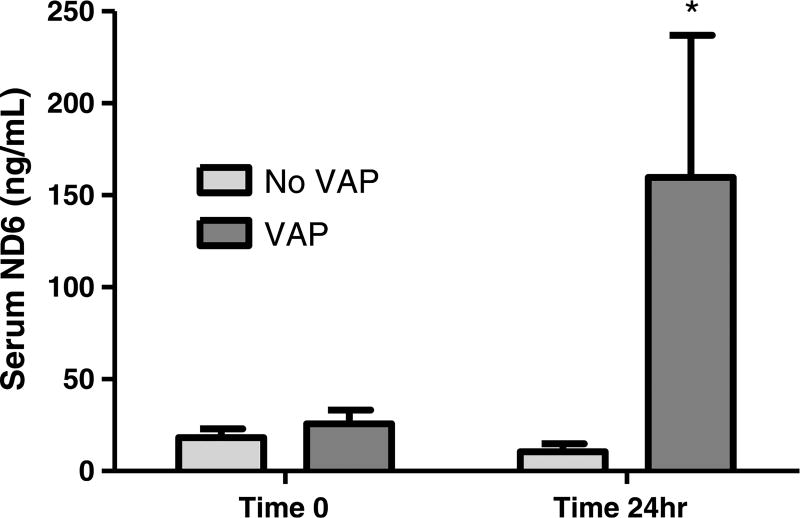
Thirty-one patients with suspected VAP were enrolled in the study. Fourteen displayed quantitative BAL consistent with VAP, where 17 patients did not. Bacterial species associated with the diagnosis of VAP are represented in Supplemental Digital Content 3 (http://links.lww.com/TA/A823). The groups with and without VAP were similar in terms of demographics and severity of injuries (Table 1). Serum levels of mtDNA DAMPs (ND6) measured at the time of suspected VAP did not differ between patients with and without VAP at the time of quantitative BAL for diagnosis (25.63 ± 7.51 vs. 18.30 ± 4.80, respectively). However, patients in whom the diagnosis of VAP was confirmed had significantly higher serum levels of mtDNA DAMPs at 24 hours when compared with patients that did not have VAP (159.60 ± 77.37 vs. 10.43 ± 4.36, p < 0.05; respectively) (Fig. 3).
TABLE 1.
Characteristics of Patient Population
| Demographics | VAP, n (%) | No VAP, n (%) | p |
|---|---|---|---|
| N | 14 | 17 | |
| Male | 13 (93) | 12 (71) | |
| Mechanism of injury | |||
| Blunt | 9 (64) | 11 (65) | |
| Penetrating | 3 (21) | 1 (6) | |
| Burn | 2 (14) | 1 (6) | |
| Other | 4 (24) | ||
| Injury Severity Score | 23 ± 8 | 27 ± 8 | NS |
| Pulmonary contusion | 4 (29) | 8 (47) | 0.15 |
| No. rib fractures | 2.6 (19) | 5.3 (47) | 0.08 |
| Hospital day of BAL, mean ± SD | 21 ± 32 | 8 ± 6 | 0.05 |
| Tracheostomy | 10 (71) | 12 (71) | 0.48 |
| Length of stay, mean ± SD, d | 66 ± 91 | 45 ± 40 | 0.20 |
| Ventilator-free days, mean ± SD | 16 ± 10 | 24 ± 29 | 0.17 |
| Hospital mortality | 0 | 0 | — |
| Renal failure (creatinine > 1.5) | 1 (7) | 4 (24) | 0.10 |
| Discharge to rehabilitation | 2 (14) | 3 (18) | 0.40 |
| Denver score > 3 | 0 (0) | 4 (24) | 0.03 |
| Initial PaO2/FiO2, mean ± SD | 143 ± 63 | 172 ± 70 | 0.49 |
| PaO2/FiO2 at 24 hours, mean ± SD | 178 ± 79 | 175 ± 84 | 0.28 |
Figure 3.
VAP is associated with a time-dependent increase in serum mtDNA DAMPs in a cohort of severely injured patients. *Different from patients without VAP at 24 hours, p < 0.05.
MtDNA DAMPs and Nuclear DNA in the BAL of Patients With Suspected VAP
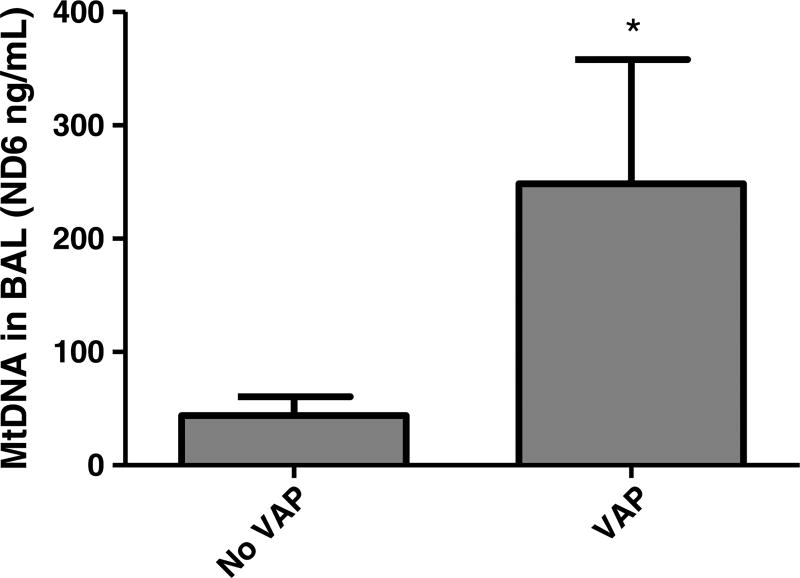
All patients with suspected VAP were subjected to BAL with quantitative culture to confirm or reject the diagnosis. MtDNA DAMPs (ND6) and nuclear DNA (hB2M) were quantified from the BAL and grouped according to the diagnosis of VAP. Patients with VAP had significantly higher levels of mtDNA DAMPs in the BAL when compared with patients that did not have VAP (248.70 ± 109.7 vs. 43.91 ± 16.61; p < 0.05) (Fig. 4). There was no difference in nuclear DNA in the BAL of patients with VAP when compared with patients without VAP (5,092 ± 1,570 vs. 7,556 ± 1,605).
Figure 4.
VAP is accompanied by mtDNA DAMPs in the bronchoalveolar lavage. *Different from No VAP at p < 0.05.
Bacterial DNA (16S) Quantity in BAL Specimens of Patients With Suspected VAP
To determine if bacterial DNA (bDNA) present in BAL specimens was associated with the occurrence of VAP, we quantified a bDNA sequence encoding the 16S rRNA transcript. Patients with VAP had significantly more bDNA when compared with patients that did not have VAP (0.28 ± 0.17 vs. 0.02 ± 0.02; p < 0.05).
DISCUSSION
Evidence that endogenously formed mtDNA DAMPs contribute to local and distant organ failure is compelling.2,3,12 The fact that patients with similar bacterial infections lead to very different inflammatory responses has vexed scientists and physicians for decades. A mechanism where overwhelming levels of oxidant stress from bacterial infection leading to the release of mtDNA DAMPs, thereby causing a feed-forward mechanism to propagate injury, both locally and distally, is an appealing explanation of this variability.4 If not tightly regulated, activation of such a pathway points to multiple avenues where a localized infection could engender local and/or distant organ failure despite the rapid eradication of bacteria with antibiotics. VAP is the ideal type of bacterial infection to evaluate such implications of mtDNA DAMPs because its nosocomial nature provides a known onset of disease. Herein, we tested the translationally relevant hypotheses that VAP is accompanied by intra-alveolar accumulation of proinflammatory mtDNA DAMPs and that enhancing the degradation of these extracellular mtDNA DAMPs prevents and reverses acute lung injury.
This research is significant because the pharmacological agent, recombinant human DNase I (Dornase), is approved by the Food and Drug Administration for administration to patients with cystic fibrosis to reduce the incidence of pneumonia, though mechanisms of these protective effects likely differ from its actions reported herein.13 Recombinant human DNase I has an excellent safety profile and has previously been shown to reduce the risk of respiratory tract infections requiring parenteral antibiotics in patients with CF.14,15 We submit that one of the most significant contributions of the current study, especially in light of the available data on safety and efficacy of recombinant human DNase I in other disorders, is that it provides equipoise for a randomized placebo controlled trial using nebulized DNase I to treat patients with VAP.
DNase I, a “waste-management” nuclease responsible for degrading extracellular DNA and chromatin, has the highest abundance in plasma.16 Importantly, there are significant biological implications for the presence of DNase in plasma, which may serve as a determinant of circulating mtDNA levels. DNases also may have pharmacologic significance. In this latter context, our group recently discovered that endothelial barrier disruption after intratracheal administration of Pseudomonas aeruginosa to isolated perfused rat lungs appears to be mediated by mtDNA DAMPs. One of the key lines of evidence supporting this concept is that degradation of mtDNA DAMPs in the perfusate with DNase I or the addition of a TLR-9 blocker (i.e., ODN) prevented lung injury in this same model of pseudomonas pneumonia. Most provocatively, we discovered that mtDNA DAMPs themselves caused increased mitochondrial oxidant stress leading to the production of more extracellular mtDNA DAMPs, suggesting a feed-forward cycle.4
To test the hypothesis that IT DNase prevents or reverses mtDNA DAMP-dependent lung injury in the setting of VAP, we conducted multiple experiments in an isolated rat lung model and an observational trial in a cohort of severely injured patients with suspected VAP. Based on our earlier finding that intravascular administration of DNase in isolated rat lungs challenged with intratracheal PA103 prevented accumulation of mtDNA DAMPs and lung injury,4 we first determined if IT DNase would be similarly protective. Additionally, we administered IT DNase or IT saline using prevention and reversal protocols to increase the relevance to treating patients with VAP. In the prevention model, IT DNase (administered 30 minutes before IT PA103) prevented lung injury and the accumulation of mtDNA DAMPs in the perfusate when compared with the IT saline. Likewise, in the reversal model consisting of IT DNase administered 15 minutes after IT PA103, mtDNA DAMP degradation also reversed the lung injury which was accompanied by decreased levels of mtDNA DAMPs. Although these timepoints generally do not correspond to the time course of VAP in human patients, they were selected based on our knowledge of this well-established rat model of bacterial-induced lung injury.
Next, we sought to determine if the concept that mtDNA DAMPS contribute to lung injury after pulmonary infection is translatable to patients with VAP, specifically testing the idea that accumulation of mtDNA DAMPs in either BAL or plasma would be associated with evolution of VAP. We prospectively analyzed the BAL and serum of all patients suspected of having VAP in a surgical trauma intensive care unit. All enrolled patients had a clinical pulmonary infection score greater than three at the time of suspected VAP.17 As expected, there was significantly more mtDNA DAMPs in the BAL of patients with VAP when compared with patients without VAP. Next, we measured mtDNA DAMPs in the serum to determine the relationship between VAP and serum mtDNA DAMPs. Collectively, we believe these observations in severely injured patients provide critical support for the hypothesis that mtDNA DAMPs are important mediators of VAP.
The most accurate way to diagnose VAP continues to be intensely debated in the literature and no “gold standard” measurement has emerged despite considerable effort. PCR analyses available for specific DNA sequences for certain bacteria are under development, but this technology is costly, time-consuming, and not available at most hospitals. The close homology between bacterial and mtDNA is well described, but there are some sequences contained in bDNA that are not found in mammalian mitochondrial genome.18 One of these is the gene encoding the 16S rRNA subunit which is not specific for the type of bacteria.19 Accordingly, we used qPCR to determine if the quantity of 16S in the BAL was associated with a quantitative culture containing greater than 10,000 CFU of any bacteria. Patients with VAP had significantly higher quantity of 16S DNA in comparison to patients without VAP. Although additional studies will be required to validate the concept, this finding points to the notion that VAP could be diagnosed within a few hours of performing a BAL using qPCR analysis of the DNA sequence encoding bacterial 16S rRNA. This rapid diagnostic test may enable clinicians to decrease the administration of inappropriate antibiotics in many clinical settings.
In conclusion, these findings in isolated perfused rat lungs and a cohort of severely injured patients reveal an association between bacterial pneumonia and accumulation of mtDNA DAMPs in the lung and serum. Furthermore, administration of intratracheal DNase I prevented and reversed the pulmonary dysfunction observed after a bolus of intratracheal pseudomonas in rats. Because of the excellent safety profile of this Food and Drug Administration–approved medication, we believe this report provides equipoise for conducting a clinical trial using nebulized DNase1 (dornase α) to prevent or treat VAP.
Supplementary Material
Acknowledgments
The research of J.D.S. and M.N.G. is supported by funding from the American Heart Association (J.D.S., 14CRP19010032), the American College of Surgeons (J.D.S., Clowes Award 2014–2019), the American Association for the Surgery of Trauma (J.D.S., Research Scholarship Award), and the National Institutes of Health (M.N.G., R01 HL58234, R01 HL113614 and J.D.S. K08 GM109113).
Footnotes
This study was presented as a Research and Education Scholarship Award presentation at the 74th annual meeting of the American Association for the Surgery of Trauma, September 9–12, 2016, in Las Vegas, Nevada.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
AUTHORSHIP
J.D.S. is the principle investigator, and contributed to the study design, data interpretation, writing of the article. D.R.F. contributed to the data interpretation and writing of the article. C.A.M. contributed to the collection of samples, PCR, data analysis, and writing of the article. B.O. contributed to the study design, isolated lung models, statistical analysis, data interpretation, and writing of the article. Y.L.L. contributed to the statistical analysis, data interpretation, and writing of the article. V.M.P. participated in the PCR, data analysis. S.B.B. contributed to the data analysis and writing of the article. M.N.G. contributed to the study design, data analysis, and writing of the article.
DISCLOSURE
The authors deny all other conflicts of interest regarding the research presented within this manuscript. Meeting: 2015 AAST, Las Vegas, NV.
References
- 1.Mangram AJ, Sohn J, Zhou N, Hollingworth AK, Ali-Osman FR, Sucher JF, Moyer M, Dzandu JK. Trauma-associated pneumonia: time to redefine ventilator-associated pneumonia in trauma patients. Am J Surg. 2015;210(6):1056–1061. doi: 10.1016/j.amjsurg.2015.06.029. discussion 1061–2. [DOI] [PubMed] [Google Scholar]
- 2.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258:591–596. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuck JL, Obiako BO, Gorodnya OM, Pastukh VM, Kua J, Simmons JD, Gillespie MN. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1078–L1085. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol. 2002;283:L205–L210. doi: 10.1152/ajplung.00443.2001. [DOI] [PubMed] [Google Scholar]
- 6.Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic Biol Med. 2011;50:1107–1113. doi: 10.1016/j.freeradbiomed.2010.10.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L892–L898. doi: 10.1152/ajplung.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebb SA, Decoux A, Waggoner A, Wilson GL, Gillespie MN. Mitochondrial DNA damage mediates hyperoxic dysmorphogenesis in rat fetal lung explants. Neonatology. 2013;103:91–97. doi: 10.1159/000342632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol. 2013;304:L287–L297. doi: 10.1152/ajplung.00071.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 11.Audia JP, Lindsey AS, Housley NA, Ochoa CR, Zhou C, Toba M, Oka M, Annamdevula NS, Fitzgerald MS, et al. In the absence of effector proteins, the Pseudomonas aeruginosa type three secretion system needle tip complex contributes to lung injury and systemic inflammatory responses. PLoS One. 2013;8:e81792. doi: 10.1371/journal.pone.0081792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23:1329–1350. doi: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990;87:9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 15.Shah PI, Bush A, Canny GJ, Colin AA, Fuchs HJ, Geddes DM, Johnson CA, Light MC, Scott SF, Tullis DE, et al. Recombinant human DNase I in cystic fibrosis patients with severe pulmonary disease: a short-term, double-blind study followed by six months open-label treatment. Eur Respir J. 1995;8:954–958. [PubMed] [Google Scholar]
- 16.Samejima K, Earnshaw WC. Trashing the genome: the role of nucleases during apoptosis. Nat RevMol Cell Biol. 2005;6:677–688. doi: 10.1038/nrm1715. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 18.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39:55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.