Abstract
Smoking cessation is associated with increases in body weight, however, little is known about the relationship between participation in a weight loss intervention and smoking.
Objective
To determine whether a) weight losses at 1 year differ as a function of baseline smoking status (never smoker, current smoker, ex-smoker) and b) whether participation in a weight loss intervention affects smoking behavior.
Methods
This analysis addressed these questions using the publicly available database from Look AHEAD, a randomized trial comparing Intensive Lifestyle Intervention (ILI) and Diabetes Support and Education (DSE) (control condition) among individuals with overweight/obesity and Type 2 diabetes, and included 4387 participants who had self-reported smoking and objective weight measures available at baseline and at 1-year.
Results
Although participants in ILI lost a significantly greater percentage of weight than those in DSE at 1 year (ILI M = −8.8%, sd = 6.8; DSE M = −0.7%, sd = 4.7), there were no differences in weight loss outcomes between never smokers (N=2297), ex-smokers (N=2115), and current smokers (N=188) within either condition. Participation in ILI was not associated with compensatory smoking or likelihood of quitting smoking or relapsing.
Conclusions
Smokers in a weight loss intervention had reductions in weight that were comparable to individuals who did not smoke without any evidence of compensatory smoking to manage eating and appetite. Smokers with obesity should be encouraged to pursue weight loss without concerns regarding the impact on smoking behavior.
Keywords: smoking, obesity, behavioral weight loss, diabetes
Smokers are more likely to develop diabetes than non-smokers (Willi, Bodenmann, Ghali, Faris, & Cornuz, 2007). Weight trajectories over time vary as a function of smoking status with smokers gaining less weight than nonsmokers (Audrain-McGovern & Benowitz, 2011; Veldheer, Yingst, Zhu, & Foulds, 2015). Yet, approximately 9 million US smokers are obese (Healton, Vallone, McCausland, Xiao, & Green, 2006); smokers with overweight/obesity report more weight-control motivated smoking (i.e., smoking to control hunger, eating, and weight) than smokers of normal weight (Murphy & MacKillop, 2013). The current NIH guidelines on obesity treatment recommend that when treating smokers, the primary emphasis is on smoking cessation and advise against initiating in smoking cessation and weight loss simultaneously (National Heart Lung and Blood Institute, 1998). Thus, encouragement of weight loss in this population is typically postponed or deemphasized in an effort to promote cessation. Yet, smokers with obesity report more concern about gaining weight after quitting smoking and have less confidence in their ability to maintain their weight without smoking (Levine, Bush, Magnusson, Cheng, & Chen, 2012). Thus, they may be less motivated to quit smoking until they have lost weight and/or developed weight management skills. There are limited data examining whether smokers and nonsmokers differ in outcomes in a weight loss program and conversely whether participation in a weight loss program affects smoking behavior. Addressing these questions is of greater significance for several reasons. First, there is a synergistic effect of smoking and weight-related risks on mortality (Freedman et al., 2006) making this a vulnerable, high-risk, group in need of effective interventions. Second, understanding how smoking and weight loss interact in the context of a controlled clinical intervention is important for determining the extent to which smokers with obesity should be encouraged to enroll in weight loss programs. Third, this information is critical when making evidence-based recommendations regarding the most effective way to order interventions targeting both of these behaviors.
There are a few previous studies which have actually examined the associations between smoking status and weight loss outcomes; weight loss studies sometimes include smoking status as a covariate in models predicting outcomes or exclude smokers altogether on the basis of anticipated relationships between smoking and weight. Smoking status was not associated with weight loss success at 6 months among participants in a trial of dietary intervention strategies (Mediterranean, low-carbohydrate, low-fat diet) (Greenberg, Stampfer, Schwarzfuchs, & Shai, 2009), at 12 months among participants in a combined pharmacotherapy and behavior therapy trial (Fabricatore et al., 2009), or at 6 or 12 months among participants in a randomized trial of nonclinic-based weight-loss interventions (Jeffery et al., 2003). In contrast, differences in weight outcomes between smokers and nonsmokers were observed in a randomized trial of weight gain prevention (Jeffery, McGuire, & French, 2002), with smokers being twice as likely to report large weight losses (≥5% of body weight) despite the intervention’s emphasis on making small changes to prevent weight gain rather than to produce weight loss.
Despite these equivocal findings, research in other relevant domains provides several reasons to believe that weight losses could be poorer in smokers than in never or ex-smokers. A dose-response type of relationship has been shown with increasing smoking status category (never smoker, ex-smoker, current smoker) associated with an unhealthier diet (Osler, Tjonneland, Suntum, & Thomsen, 2002). Smokers make fewer healthful dietary choices (e.g., less fruit/vegetables, high fiber grains, low fat milk; more alcohol, soft drinks, French fries, bacon/luncheon meats) and have lower levels of physical activity including leisure activity relative to non-smokers and ex-smokers (Chiolero, Wietlisbach, Ruffieux, Paccaud, & Cornuz, 2006; French, Hennrikus, & Jeffery, 1996; Kaczynski, Manske, Mannell, & Grewal, 2008; Subar, Harlan, & Mattson, 1990). Given less healthful practices, weight loss may require making more extensive lifestyle changes and be more difficult. Additionally, frequent self-weighing, a strategy associated with successful weight loss (Sciamanna et al., 2011), is less common among those who smoke (Linde, Jeffery, French, Pronk, & Boyle, 2005). Synergistic effects of smoking and obesity (Freedman et al., 2006; Koster et al., 2008) may result in poorer health and cause greater difficulty adhering to behavioral recommendations for weight loss and smokers may have greater central fat accumulation and insulin resistance than non-smokers (Chiolero, Faeh, Paccaud, & Cornuz, 2008). Thus, differences in health and health habits may make successfully achieving and maintaining weight loss more challenging for smokers than it would be among individuals who do not smoke.
Alternatively, smokers could be hypothesized to achieve greater weight loss in formal programs than non-smokers since smoking is thought to have anorexic or metabolic effects (Audrain-McGovern & Benowitz, 2011; Chiolero et al., 2008; Filozof, Fernandez Pinilla, & Fernandez-Cruz, 2004; Li, Kane, & Konu, 2003; Perkins et al., 1991; Perkins, Epstein, Stiller, Marks, & Jacob, 1989). Theoretically, smokers may use cigarettes as a “tool” when attempting to lose weight which could confer a weight loss advantage relative to non-smokers and ex-smokers. This could be especially true if smoking effectively curbs hunger when following a calorie-restricted diet to produce weight loss, albeit with deleterious consequences on smoking behavior. Many smokers endorse a belief that smoking helps control their weight and weight concerns may motivate some individuals to smoke (Boles & Johnson, 2001; Cavallo, Duhig, McKee, & Krishnan-Sarin, 2006; Copeland & Carney, 2003); one-third of smokers reported having used smoking as a dieting strategy to lose weight in the past 6 months (Klesges & Klesges, 1988).
This also raises the other important question—do smokers with overweight/obesity in behavioral weight loss treatment increase their smoking over time? Smokers may increase their smoking while following reduced-calorie weight loss regimens to manage their appetite and also to replace the loss of one reinforcing activity (i.e., eating particular food) with another (smoking cigarettes). Animal research suggests that rats under chronic food restriction show considerable increases in drug-seeking behavior (D’Cunha, Sedki, Macri, Casola, & Shalev, 2013). Research findings from several small studies on smokers following acute food deprivation, fasting weight loss protocols, or reduced calorie diets have resulted in mixed findings with some studies finding little evidence of increased cigarette consumption (Kendzor, Baillie, Adams, Stewart, & Copeland, 2008; Lawson, Bulik, Rodefer, Scanlon, & Borger, 1997; Zacny & de Wit, 1990, 1992), another finding evidence of increased cigarette smoking following one low-calorie diet but not another (Cheskin, Hess, Henningfield, & Gorelick, 2005), and others showing possible increases in aspects of smoking behavior not directly measured such as number of puffs/cigarette or puff duration (Kendzor et al., 2008; Niaura, Clark, Raciti, Pera, & Abrams, 1992; Zacny & de Wit, 1990).
Instead of causing increases in smoking, successful weight loss may promote a reduction or cessation of smoking. Given substantial overlap between obesity and addictive behavior (Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Volkow, Wang, Fowler, & Telang, 2008; Volkow, Wang, Tomasi, & Baler, 2013), once an individual develops skills for health behavior change (e.g., recognizing triggers and cues, managing urges, problem-solving), these skills may generalize and facilitate making other changes. Data from the present study could support this in two ways: first, if greater success with weight loss is associated with greater decreases in smoking (i.e., changes in diet/exercise facilitating changes in smoking), and, second, if a history of successfully quitting smoking is associated with success when attempting to lose weight (i.e., changes in smoking facilitating changes in diet/exercise). Indeed, individuals who had previously participated in substance abuse treatment or mental health treatment had improved weight loss following bariatric surgery compared to individuals who had not previously been in treatment (Clark et al., 2003).
The current study is a secondary analysis of the Look AHEAD (Action for Health in Diabetes) clinical trial of adults with overweight/obesity and type 2 diabetes who were randomly assigned to Intensive Lifestyle Intervention (ILI) that included a calorie-restricted diet and increased physical activity (PA) to produce weight loss or to a Diabetes Support and Education (DSE) control condition. Outcomes of this study have previously been reported including significantly greater weight loss at 1-year among participants in ILI compared to DSE (Look AHEAD Research Group et al., 2007; Ryan et al., 2003). In the current analysis, we used the publicly available database to examine: (1) whether smoking status (never smoker, current smoker, ex-smoker) at baseline was associated with weight loss at 1 year in ILI or DSE and (2) whether participation in the ILI weight loss intervention versus the DSE control condition affected smoking behavior or the chances of quitting or relapsing at 1-year follow-up. We hypothesized that baseline smokers would be less successful in their weight loss attempts due to greater difficulties associated with clustering of health-injurious lifestyle behaviors in this population. We hypothesized that participation in the ILI would not be associated with increased risk for smoking or relapse relative to DSE.
Method
Participants
Eligibility criteria for Look AHEAD included type 2 diabetes, 45–76 years of age, being overweight or obese (BMI ≥25 kg/m2; >27 kg/m2 if taking insulin), HbA1C <11%, blood pressure <160 (systolic) <100 (diastolic) mmHg, and triglycerides <600 mg/dL. In addition, participants were excluded based on underlying diseases/comorbid conditions that were anticipated to limit adherence to or affect the safety of the interventions (Ryan et al., 2003).
Procedures
Consistent with the requirements of institutional review boards of the 16 clinical centers of the trial (Ryan et al., 2003) and the Helsinki Declaration, informed consent was obtained before screening and at enrollment. Prior to randomization, all participants attended a 1-hour diabetes education class which provided basic education about diabetes such as self-monitoring of blood glucose, management of hypoglycemia, and symptoms of cardiovascular disease. Smokers were encouraged to quit but did not receive smoking cessation counseling or strategies as part of the study. Eligible participants were randomized 1:1 to either ILI or the DSE comparison condition.
Intervention Conditions
The ILI and DSE conditions have been described at length previously (Look AHEAD Research Group, 2006b; Ryan et al., 2003; Wadden et al., 2009). The present description includes only a description of activities occurring during the first year.
ILI
Participants in this condition participated in a weight loss intervention that included a reduced calorie diet and increased physical activity designed to produce a weight loss of ≥7% of initial body weight. During the first 6 months, participants were seen weekly, with three group sessions and one individual session each month. During months 7–12, participants were provided with two group sessions and one individual session per month. Sessions were led by interventionists trained in nutrition and exercise counseling. Participants were given a calorie goal of 1200-1500 kcal/day (≤250 lbs) or 1500-1800 kcal/day (>250 lbs) and provided with meal replacement products to promote adherence to the calorie goals. Physical activity recommendations increased gradually from 50 to 175 minutes/week using activities similar in intensity to a brisk walk.
DSE
During the first year, participants in DSE were invited to attend three educational/social support group sessions, focused on diet/nutrition, physical activity/exercise, and social support. Sessions were informational and provided opportunities for discussing topics but specific behavioral strategies related to diet and physical activity were not provided.
Measures
Weight
Weight was measured using a balance beam scale by assessors masked to intervention condition at baseline and 1-year follow-up. Height was assessed using a standard stadiometer. BMI was calculated using the standard formula (weight in pounds * 703)/height in inches2 and percent reduction in weight at 1 year from baseline was calculated.
Tobacco use
Smoking was assessed using a self-reported questionnaire at baseline and 1-year follow-up. At both time points, participants were asked “Have you smoked at least 100 cigarettes during your entire life? (yes/no)”. Those answering “no” were coded as “never smokers” and received no additional questions. Individuals who endorsed “yes” were asked “Do you smoke cigarettes now? (yes/no)”. Individuals who answered “yes” were coded as “current smokers” and were subsequently asked “On the days that you smoke, about how many cigarettes do you usually smoke per day?” Individuals who answered “no” to smoking cigarettes currently were coded as “ex-smokers” and were subsequently asked “About how old were you when you quit smoking cigarettes (fairly regularly)?”.
Data Analysis Approach
Preliminary analyses
The subset selected for analyses were those with smoking and weight data available at baseline and who gave consent for data to be used in secondary analyses (N = 4837; 94.0% of 5145 randomized). Analyses of study hypotheses were conducted excluding individuals with missing year 1 data (n = 237) on a final sample N = 4600 (89.4% of randomized). The percentage of participants that were missing year 1 data differed by intervention condition (91 in ILI (3.8%); 146 in DSE (6.0%); χ2(1, N = 4837) = 13.57, p < .01), consistent with previous reports (Wadden et al., 2009), and by baseline smoking status (104 never smokers (4.3%), 117 ex-smokers (5.2%), and 16 current smokers (7.8%); χ2(2, N = 4837) = 7.18, p = .03). Analyses were repeated using the baseline carried forward imputation method (zero weight loss, no change in smoking status) for individuals with missing year 1 data; there were no substantive differences from the pattern of findings reported below (data not shown).
One-way analysis of variance (ANOVA) and chi square tests were used to examine baseline characteristics of never, ex-, and current smokers. Any demographic characteristic that differed significantly by smoking status at baseline and also predicted percent weight loss in a multiple linear regression (accounting for differences attributable to intervention condition) was included as a model covariate in primary analyses of weight loss outcomes.
Primary statistical analyses
The first set of analyses examined differences in percent weight loss at year 1 as a function of baseline smoking status (never, current, ex-smoker). A univariate general linear model (GLM) was used to compare changes in weight from baseline to follow-up in ILI and DSE by smoking status, controlling for statistically significant baseline differences. Among baseline ex-smokers, regression analyses were conducted separately in ILI and DSE to determine whether years since quitting was a significant predictor of percent weight loss with relevant demographic covariates included.
The second set of analyses examined changes in smoking habits in ILI vs DSE from baseline to 1 year. Logistic regression was used to determine whether participants who were current smokers at baseline differed in the likelihood of quitting smoking at year 1 as a function of treatment condition. The number of cigarettes smoked at baseline and follow-up was also compared among individuals who reported smoking at both time points (quitters excluded) using a 2 × 2 mixed (condition × time) analysis of variance (ANOVA) to test for differential changes in smoking (e.g., compensatory smoking) related to randomized intervention condition. Finally, logistic regression was used to determine whether the likelihood of relapsing to smoking (i.e., identifying as an ex-smoker at baseline and a current smoker at year 1) differed between ILI and DSE. Effect sizes were calculated as η2 for continuous dependent variables, odds ratios (OR) for dichotomous ones.
Results
Baseline participant characteristics by smoking status are presented in Table 1. The two treatment conditions did not differ significantly on any baseline characteristics confirming success of randomization consistent with previously reported findings (data not shown) (Look AHEAD Research Group, 2006a; Look AHEAD Research Group et al., 2007). The number of smokers, ex-smokers, and never smokers, randomized to each study condition did not significantly differ χ2 (2, N = 4600) = 1.24, ns.
Table 1.
Baseline Participant Characteristics by Smoking Status
| Never Smokers | Ex-Smokers | Current Smokers | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ILI (N=1145) |
DSE (N=1152) |
ILI (N=1081) |
DSE (N=1034) |
ILI (N=100) |
DSE (N=88) |
p | |
| Weight (kg) | 98.9 (19.6) | 99.4 (18.5) | 103.1 (19.7) | 103.4 (18.9) | 99.6 (17.7) | 100.2 (17.9) | <.01b |
| Body Mass Index (BMI) | 36.0 (6.1) | 36.0 (5.8) | 35.8 (5.9) | 36.0 (5.7) | 35.0 (5.0) | 35.7 (5.1) | 0.30b |
| Duration of Diabetes (years) | 6.6 (6.5) | 6.7 (6.3) | 6.7 (6.5) | 6.9 (6.3) | 7.4 (7.1) | 5.1 (5.6) | 0.51b |
| Cigarettes/day | – | – | – | – | 11.0 (8.8) | 13.3 (11.9) | – |
| Years since quitting smoking | – | – | 21.4 (11.4) | 21.3 (11.3) | – | – | – |
| Female | 788 (69%) | 784 (68%) | 522 (48%) | 495 (48%) | 54 (54%) | 52 (59%) | <.01a |
| Age | 58.8 (6.7) | 59.0 (6.9) | 60.4 (6.7) | 60.7 (6.5) | 57.6 (6.5) | 57.7 (6.8) | <.01b |
| Race/Ethnicity | <.01a | ||||||
| Black/African American | 189 (17%) | 185 (16%) | 164 (15%) | 156 (15%) | 32 (32%) | 27 (31%) | |
| Hispanic | 187 (16%) | 187 (16%) | 107 (10%) | 105 (10%) | 17 (17%) | 17 (19%) | |
| Other/Mixed | 53 (5%) | 40 (3%) | 35 (3%) | 37 (4%) | 0 (0%) | 2 (2%) | |
| White/Caucasian | 716 (63%) | 740 (64%) | 775 (72%) | 736 (71%) | 51 (51%) | 42 (48%) | |
| Education* | <.01a | ||||||
| <13 years | 239 (21%) | 232 (21%) | 186 (17%) | 162 (16%) | 22 (22%) | 17 (20%) | |
| 13-16 years | 370 (33%) | 412 (37%) | 409 (38%) | 404 (40%) | 51 (51%) | 37 (44%) | |
| >16 years | 510 (46%) | 479 (43%) | 473 (44%) | 442 (44%) | 27 (27%) | 30 (36%) | |
Note. ILI = Intensive Lifestyle Intervention; DSE = Diabetes Support and Education. Values shown are means (sd) or frequency counts (with percentages).
χ2-test.
Analyses of variance (one-way).
N=4502 due to missing data. Column percentages differ from 100% in instances due to rounding.
There were significant baseline differences between smokers, ex-smokers, and never smokers on measures of weight, education, race, sex, and age. A multiple linear regression based on these variables (df = 4502) showed that education (coded as 0 = < 13 years, 1 = 13-16 years, 2 = >16 years) and sex (coded as 0 = male, 1 = female) did not significantly predict percent weight loss, t = .35, ns, and t = .79, ns, respectively. However, baseline weight (kilograms), age (years), and race (coded as 0 = Hispanic, African-American, and “other” participants (“other” category consisted of individuals identifying as other/mixed race (~57% of “other”), Asian/Pacific Islander (~35% of “other”), and non-excluded American Indian/Alaskan Natives (~8% of “other”)), 1 = non-Hispanic white participants) were significant predictors of percent weight loss at year 1 (t = 3.74, p < .01, t = 3.25, p < .01, and t = 6.75, p < .01, respectively). Therefore, these three demographic variables were controlled in analyses of weight loss outcomes in order to avoid confounding effects.
Effects of Baseline Smoking Status on Weight Loss Outcomes by Treatment Condition
The first set of analyses focused on differences in weight loss as a function of baseline smoking status. Figure 1 depicts percent weight loss as a function of baseline smoking status in the ILI and DSE conditions. As expected, there was a main effect of the intervention condition, confirming that individuals in ILI had greater percent weight loss (M = −8.8%, sd = 6.8) compared to individuals in DSE (M = −0.7%, sd = 4.7) at 1 year (F(1,4591) = 675.16, p < .01, η2 = .13). There was not a significant effect of smoking status on percent weight loss (F(2, 4591) = .07, p = .93, η2 = .00), nor a significant interaction between smoking status and intervention condition (F(1, 4591) = .31, p = .74, η2 = .00). Among baseline ex-smokers, time since quitting smoking did not significantly predict percent weight loss in either ILI, β = −.04, t(1073) = −1.10, p = .27, or DSE, β = −.06, t(1024) = −1.89, p = .06.
Figure 1.
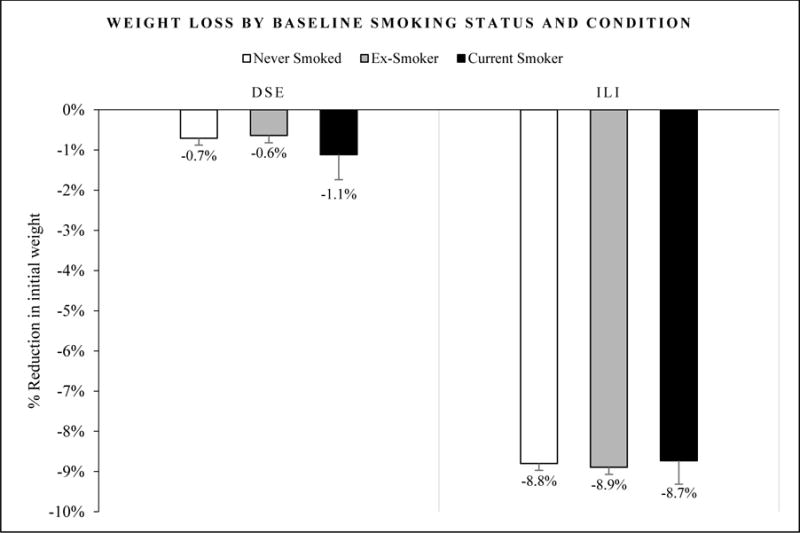
Percent reduction in weight (kg) as a function of baseline smoking status by intervention condition from baseline to 1-year follow-up. Standard error bars are shown. Differences between smoking groups were not significant. ILI = Intensive Lifestyle Intervention; DSE = Diabetes Support and Education.
Effects of Intervention Type on Smoking Behavior in Current or Ex-Smokers
The second set of analyses looked at the relationship between treatment condition and changes in smoking habits. Frequency counts and percentages of baseline current and ex-smokers who reported smoking at follow-up are shown in Table 2. Among individuals endorsing current smoking at baseline, intervention condition did not predict likelihood of smoking at follow-up (OR = 0.75, CI = 0.37 – 1.52, p = .42). Among those identified as current smokers at both baseline and 1-year follow-up (i.e., non-quitters), there was no effect of the intervention condition (F(1, 148) = 2.79, p = .10, η2 = .02), of time (F(1, 148) = .01, p = .91, η2 = .00), nor a time by intervention condition interaction (F(1, 148) = .06, p = .81, η2 = .00) on number of cigarettes smoked per day (shown in Table 2). Finally, among baseline ex-smokers, intervention condition assignment did not predict likelihood of smoking at follow-up (OR = 0.83, CI = .43 – 1.62, p = .59).
Table 2.
Smoking Habits Reported at 1-year Follow-up
| Baseline Current Smokers
| ||
|---|---|---|
| Do you smoke cigarettes now? | ILI (N=100)
|
DSE (N=88)
|
| No | 18 (18%) | 20 (23%) |
| Yes | 82 (82%) | 68 (77%) |
| *On the days that you smoke, about how many cigarettes do you usually smoke per day? | ||
| Baseline | 11.7 (9.0) | 14.3 (11.6) |
| Follow-up | 11.5 (9.4) | 14.4 (11.7) |
| Baseline Ex-Smokers
| ||
|---|---|---|
| Do you smoke cigarettes now? | ILI (N=1081)
|
DSE (N=1034)
|
| No | 1061 (98%) | 1018 (98%) |
| Yes | 20 (2%) | 16 (2%) |
Note. ILI = Intensive Lifestyle Intervention; DSE = Diabetes Support and Education.
Values shown are means (sd) for those identified as current smokers at both baseline and 1-year follow-up only (i.e., non-quitters).
Discussion
The results of the present study suggest that among individuals with overweight/obesity and type 2 diabetes mellitus, smoking status did not affect weight loss outcomes at 1 year. Moreover, there was no evidence that participation in a weight loss program affected the odds of quitting smoking, relapsing to smoking, or the number of cigarettes smoked per day relative to the control group. Both of these findings suggest that weight loss interventions have great promise in improving the health of smokers with excess weight.
Participants in ILI lost considerably more weight over time relative to the control condition without any differences attributable to smoking status. Given the increased risk of mortality from combined smoking and obesity (Freedman et al., 2006; Peeters et al., 2003), the fact that smokers in the current study achieved weight loss outcomes that were equivalent to non-smokers is promising in this population. Despite many smokers reporting using cigarettes to help control hunger, weight, and prevent overeating (Piper et al., 2004), any putative effects of smoking on appetite, satiety, or metabolism conferred no advantage (or disadvantage) for weight loss outcomes in the present study. In addition, having successfully quit smoking did not affect success at weight loss despite the fact that there are many similarities between the skills needed to make and maintain behavior change and prevent relapse for weight loss and smoking cessation (Brownell et al., 1986). Notably, baseline ex-smokers in the present study reported having quit smoking approximately two decades earlier, on average, based on differences between reported quit age and current age. Therefore, the knowledge and strategies used when quitting may no longer have been salient to them and, thus, may not have facilitated greater weight loss.
The effects of the intervention on smoking outcomes were also encouraging. Despite caloric restriction being the predominant method used to achieve weight loss in the ILI condition, smokers assigned to ILI did not exhibit compensatory smoking from this restriction; instead, nearly 20% of ILI smokers quit, fewer than 2% of former smokers reported they were current smokers again at the 1-year follow-up, and number of cigarettes smoked per day did not change over time. In all of these domains, those in ILI did not differ significantly from those in DSE. Notably, even after long-term abstinence, relapse to smoking may still occur (Krall, Garvey, & Garcia, 2002).
The lack of an increase in cigarettes smoked daily is consistent with findings from short-term food deprivation studies (Kendzor et al., 2008; Lawson et al., 1997; Zacny & de Wit, 1990, 1992) but inconsistent with another study that reported participants assigned to one of two low-calorie (700 calories/day deficit for 6 days) conditions tended to smoke more cigarettes than when consuming a normal calorie diet (2,000-2,800 calories/day) in an inpatient research ward (Cheskin et al., 2005). However, the study setting, small sample size (N=17), and the exclusion of participants with overweight or obesity all limit the generalizability of this finding. Another small study of female smokers with obesity reported increases in salivary cotinine concentrations during a very low calorie diet weight-loss treatment program but no information concerning smoking patterns was gathered, so the cause for the increase could not be established (Niaura et al., 1992). The current study expands on earlier findings using a large sample of individuals with overweight/obesity randomly assigned to 12-months of ILI or a comparison condition and supports the finding of no evidence of increased smoking secondary to calorie restriction.
The proportion of smokers reporting quitting at year 1 across conditions (>20%) is notable given that no smoking cessation resources were provided as part of the study - just brief advice that quitting smoking would benefit their health. Smokers who are trying to lose weight are more likely to want to quit smoking and to have made an attempt to quit smoking in the preceding year than those who are not trying to lose weight (Wee, Rigotti, Davis, & Phillips, 2001). For that reason, smoking cessation should be encouraged during weight loss, particularly for individuals with type 2 diabetes. Leveraging momentum when an individual is already in the process of making health behavior changes may be key. Providing brief advice about quitting smoking has been shown to increase the likelihood of quitting among smokers (Stead, Bergson, & Lancaster, 2008). As participants in both groups were encouraged to quit smoking, it may be that this advice impacted subsequent behavior or that self-reports did not accurately reflect actual smoking behavior.
Despite numerous beneficial outcomes for smokers in the current study, preliminary analyses indicated that smokers had the highest percent of missing data at 1 year suggesting this group may be particularly susceptible to being lost to follow-up in clinical weight loss trials. Some evidence suggests that smoking status may be associated with differences in attending and completing treatment. Smokers were less likely to initially engage in the Veterans Affairs MOVE!® weight control program following a referral (Funderburk, Arigo, & Kenneson, 2016). Similarly, participants with incomplete data in a weight gain prevention trial were more likely to be smokers (Jeffery & French, 1999). In a review of predictors of dropout in weight loss interventions, Moroshko, Brennan, and O’brien (2011) reported three studies in which smoking was associated with greater attrition (Bradshaw, Horwath, Katzer, & Gray, 2010; Clark, Niaura, King, & Pera, 1996; Greenberg et al., 2009), however, no association was observed in two other studies (Fabricatore et al., 2009; Inelmen et al., 2005). Thus, extra steps may be necessary to reduce attrition when working with this population.
The strengths of the current study include using large sample with prospective data on weight and smoking over 1 year. Participants were randomly assigned to participate in an ILI or a DSE control condition and weight was measured by assessors blind to intervention condition. Nevertheless, this secondary analysis of data is limited by the reliance on only self-report measures of smoking behavior. Although the use of self-report may have inflated quit rates in the current study, typically the magnitude of such inflation is small and does not vary across intervention conditions (SRNT Subcommittee on Biochemical Verification, 2002). In addition, Look AHEAD did not collect self-reported reasons for smoking, quitting, or relapse. Thus, the extent to which weight control motivated smoking behavior in the present study cannot be determined. Finally, data from 545 of the 5145 participants randomized to treatment (10.6%) were not included in the current analyses due to consent limitations (n = 255 participants from Native American sites did not provide consent for their data to be part of the public database) and missing data (n = 301). While imputation for missing data suggested comparable patterns of findings to those reported herein, study attrition limits generalizability, particularly, given the different rates of attrition noted.
In conclusion, smokers participating in ILI lost considerably more weight than those in DSE without evidence of compensatory smoking and without treatment differences in rates of quitting smoking. Moreover, the weight losses of smokers were comparable to those in non-smokers or ex-smokers. These findings suggest that smoking status should not be a deterrent to enrolling in weight loss interventions. Smokers with overweight or obesity who are interested in losing weight should be encouraged to do so in order to improve health; weight loss and smoking cessation both may be viable initial targets when encouraging multiple health behavior changes.
Acknowledgments
Financial support: Dr. Murphy’s effort on data analysis and preparation of this manuscript was provided by grant number T32 DA016184 from the National Institute on Drug Abuse at the National Institutes of Health.
Footnotes
Author Contributions: CMM performed the analyses and wrote the manuscript. RRW and DJR participated in the development and writing and provided critical feedback. KCJ reviewed the work and provided critical feedback. KCJ and RRW also contributed to data collection in Look AHEAD. CMM takes responsibility for the accuracy of the data analysis.
Trial Registration: ClinicalTrials.gov-NCT00017953.
Disclosures: The authors declared no conflict of interest.
Contributor Information
Cara M. Murphy, Center for Alcohol and Addiction Studies, Brown University School of Public Health
Damaris J. Rohsenow, Center for Alcohol and Addiction Studies, Brown University School of Public Health
Karen C. Johnson, Dept. of Preventive Medicine, University of Tennessee Health Science Center
Rena R. Wing, Weight Control & Diabetes Research Center, The Miriam Hospital
References
- Audrain-McGovern J, Benowitz N. Cigarette smoking, nicotine, and body weight. Clinical Pharmacology & Therapeutics. 2011;90(1):164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles SM, Johnson PB. Gender, weight concerns, and adolescent smoking. Journal of Addictive Diseases. 2001;20(2):5–14. doi: 10.1300/J069v20n02_02. [DOI] [PubMed] [Google Scholar]
- Bradshaw AJ, Horwath CC, Katzer L, Gray A. Non-dieting group interventions for overweight and obese women: what predicts non-completion and does completion improve outcomes? Public Health Nutr. 2010;13(10):1622–1628. doi: 10.1017/S1368980009992977. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41(7):765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Cavallo DA, Duhig AM, McKee S, Krishnan-Sarin S. Gender and weight concerns in adolescent smokers. Addictive Behaviors. 2006;31(11):2140–2146. doi: 10.1016/j.addbeh.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 2005;179(2):430–436. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: a population-based survey. Prev Med. 2006;42(5):348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Clark MM, Balsiger BM, Sletten CD, Dahlman KL, Ames G, Williams DE, Sarr MG. Psychosocial factors and 2-year outcome following bariatric surgery for weight loss. Obesity Surgery. 2003;13(5):739–745. doi: 10.1381/096089203322509318. [DOI] [PubMed] [Google Scholar]
- Clark MM, Niaura R, King TK, Pera V. Depression, smoking, activity level, and health status: pretreatment predictors of attrition in obesity treatment. Addictive Behaviors. 1996;21(4):509–513. doi: 10.1016/0306-4603(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Carney CE. Smoking expectancies as mediators between dietary restraint and disinhibition and smoking in college women. Exp Clin Psychopharmacol. 2003;11(3):247. doi: 10.1037/1064-1297.11.3.247. [DOI] [PubMed] [Google Scholar]
- D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacology. 2013;225(1):241–250. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Heymsfield SB, Nguyen AM. Predictors of attrition and weight loss success: Results from a randomized controlled trial. Behaviour Research and Therapy. 2009;47(8):685–691. doi: 10.1016/j.brat.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5(2):95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Sigurdson AJ, Rajaraman P, Doody MM, Linet MS, Ron E. The mortality risk of smoking and obesity combined. Am J Prev Med. 2006;31(5):355–362. doi: 10.1016/j.amepre.2006.07.022. [DOI] [PubMed] [Google Scholar]
- French SA, Hennrikus DJ, Jeffery RW. Smoking status, dietary intake, and physical activity in a sample of working adults. Health Psychology. 1996;15(6):448. doi: 10.1037//0278-6133.15.6.448. [DOI] [PubMed] [Google Scholar]
- Funderburk J, Arigo D, Kenneson A. Initial engagement and attrition in a national weight management program: demographic and health predictors. Translational behavioral medicine. 2016;6(3):358–368. doi: 10.1007/s13142-015-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) Journal of the American College of Nutrition. 2009;28(2):159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. Bmj. 2006;333(7557):25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inelmen EM, Toffanello ED, Enzi G, Gasparini G, Miotto F, Sergi G, Busetto L. Predictors of drop-out in overweight and obese outpatients. International Journal of Obesity. 2005;29(1):122–128. doi: 10.1038/sj.ijo.0802846. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, French SA. Preventing weight gain in adults: the pound of prevention study. Am J Public Health. 1999;89(5):747–751. doi: 10.2105/ajph.89.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, McGuire MT, French SA. Prevalence and correlates of large weight gains and losses. Int J Obes Relat Metab Disord. 2002;26(7):969–972. doi: 10.1038/sj.ijo.0802015. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Sherwood NE, Brelje K, Pronk NP, Boyle R, Boucher JL, Hase K. Mail and phone interventions for weight loss in a managed-care setting: Weigh-To-Be one-year outcomes. International Journal of Obesity. 2003;27(12):1584–1592. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: a systematic review. Am J Health Behav. 2008;32(1):93–110. doi: 10.5555/ajhb.2008.32.1.93. [DOI] [PubMed] [Google Scholar]
- Kendzor DE, Baillie LE, Adams CE, Stewart DW, Copeland AL. The effect of food deprivation on cigarette smoking in females. Addictive Behaviors. 2008;33(10):1353–1359. doi: 10.1016/j.addbeh.2008.06.008. http://dx.doi.org/10.1016/j.addbeh.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges RC, Klesges LM. Cigarette smoking as a dieting strategy in a university population. International Journal Of Eating Disorders. 1988;7(3):413–419. [Google Scholar]
- Koster A, Leitzmann MF, Schatzkin A, Adams KF, van Eijk JT, Hollenbeck AR, Harris TB. The combined relations of adiposity and smoking on mortality. The American journal of clinical nutrition. 2008;88(5):1206–1212. doi: 10.3945/ajcn.2008.26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine & Tobacco Research. 2002;4(1):95–100. doi: 10.1080/14622200110098428. [DOI] [PubMed] [Google Scholar]
- Lawson RH, Bulik CM, Rodefer JS, Scanlon W, Borger MD. The effect of a reduced energy diet and meal patterns on smoking and coffee drinking in women. International Journal Of Eating Disorders. 1997;21(2):137–145. doi: 10.1002/(sici)1098-108x(199703)21:2<137::aid-eat4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Levine MD, Bush T, Magnusson B, Cheng Y, Chen X. Smoking-related weight concerns and obesity: differences among normal weight, overweight, and obese smokers using a telephone tobacco quitline. Nicotine & Tobacco Research. 2012;15(6):1136–1140. doi: 10.1093/ntr/nts226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Kane JK, Konu O. Nicotine, body weight and potential implications in the treatment of obesity. Current topics in medicinal chemistry. 2003;3(8):899–919. doi: 10.2174/1568026033452203. [DOI] [PubMed] [Google Scholar]
- Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Annals of Behavioral Medicine. 2005;30(3):210–216. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) Research Study. Diabetes & vascular disease research: official journal of the International Society of Diabetes and Vascular Disease. 2006a;3(3):202. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md) 2006b;14(5):737. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroshko I, Brennan L, O’brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obesity Reviews. 2011;12(11):912–934. doi: 10.1111/j.1467-789X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- Murphy CM, MacKillop J. Smoking, Obesity, and Preventable Disease, Death, and Disability. Paper presented at the APA Divisions 28 & 50 Collaborative Perspectives on Addiction Conference; Atlanta, GA. 2013. [Google Scholar]
- National Heart Lung and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Bethesda, MD: National Institutes of Health; 1998. (NIH pub no 98-4083) [PubMed] [Google Scholar]
- Niaura R, Clark MM, Raciti MA, Pera V, Abrams DB. Increased saliva cotinine concentrations in smokers during rapid weight loss. J Consult Clin Psychol. 1992;60(6):985–987. doi: 10.1037//0022-006x.60.6.985. [DOI] [PubMed] [Google Scholar]
- Osler M, Tjonneland A, Suntum M, Thomsen BL. Does the association between smoking status and selected healthy foods depend on gender? A population-based study of 54 417 middle-aged Danes. European journal of clinical nutrition. 2002;56(1):57. doi: 10.1038/sj.ejcn.1601280. [DOI] [PubMed] [Google Scholar]
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, Demography Compression of Morbidity Research, G. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Fernstrom MH, Sexton JE, Jacob RG, Solberg R. Acute effects of nicotine on hunger and caloric intake in smokers and nonsmokers. Psychopharmacology (Berl) 1991;103(1):103–109. doi: 10.1007/BF02244083. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr. 1989;50(3):545–550. doi: 10.1093/ajcn/50.3.545. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, Loken E. Practices associated with weight loss versus weight-loss maintenance: results of a national survey. American Journal of Preventive Medicine. 2011;41(2):159–166. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. The Cochrane Library. 2008 doi: 10.1002/14651858.CD000165.pub3. [DOI] [PubMed] [Google Scholar]
- Subar AF, Harlan LC, Mattson ME. Food and nutrient intake differences between smokers and non-smokers in the US. Am J Public Health. 1990;80(11):1323–1329. doi: 10.2105/ajph.80.11.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldheer S, Yingst J, Zhu J, Foulds J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003-2012. Int J Obes (Lond) 2015;39(12):1727–1732. doi: 10.1038/ijo.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Reviews. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Vitolins MZ. One-year weight losses in the Look AHEAD Study: factors associated with success. Obesity. 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee CC, Rigotti NA, Davis RB, Phillips RS. Relationship between smoking and weight control efforts among adults in the united states. Arch Intern Med. 2001;161(4):546–550. doi: 10.1001/archinte.161.4.546. [DOI] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. Effects of a 24-hour fast on cigarette smoking in humans. Addiction. 1990;85(4):555–560. doi: 10.1111/j.1360-0443.1990.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. The effects of a restricted feeding regimen on cigarette smoking in humans. Addictive Behaviors. 1992;17(2):149–157. doi: 10.1016/0306-4603(92)90019-r. [DOI] [PubMed] [Google Scholar]


