Abstract
Loss-of-function mutations in progranulin (GRN) are a major autosomal dominant cause of frontotemporal dementia (FTD), a neurodegenerative disorder in which social behavior is disrupted. Progranulin-insufficient mice, both Grn+/− and Grn−/−, are used as models of FTD due to GRN mutations, with Grn+/− mice mimicking the progranulin haploinsufficiency of FTD patients with GRN mutations. Grn+/− mice have increased social dominance in the tube test at 6 months of age, though this phenotype has not been reported in Grn−/− mice. In this study, we investigated how the tube test phenotype of progranulin-insufficient mice changes with age, determined its robustness under several testing conditions, and explored associated cellular mechanisms. We observed biphasic social dominance abnormalities in Grn+/− mice: at 6–8 months, Grn+/− mice were more dominant than wild-type littermates, while after 9 months of age, Grn+/− mice were less dominant. In contrast, Grn−/− mice did not exhibit abnormal social dominance, suggesting that progranulin haploinsufficiency has distinct effects from complete progranulin deficiency. The biphasic tube test phenotype of Grn+/− mice was associated with abnormal cellular signaling and neuronal morphology in the amygdala and prefrontal cortex. At 6–9 months, Grn+/− mice exhibited increased mTORC2/Akt signaling in the amygdala and enhanced dendritic arbors in the basomedial amygdala, and at 9–16 months Grn+/− mice exhibited diminished basal dendritic arbors in the prelimbic cortex. These data demonstrate a progressive change in tube test dominance in Grn+/− mice and show that partial, but not complete progranulin deficiency disrupts this behavior in mice.
Keywords: Progranulin, Frontotemporal Dementia, Dominance, Social, Tube Test, Preclinical, Behavior, Neurodegeneration, Haploinsufficiency, Aging
Introduction
Progranulin is a secreted glycoprotein that is expressed throughout the body, where it acts as a growth factor and modulates inflammation (Bateman & Bennett, 2009, Nguyen et al., 2013). Progranulin performs both of these actions in the brain, where it has neurotrophic effects and regulates the inflammatory response of microglia (Gass et al., 2012, Martens et al., 2012, Ryan et al., 2009, Van Damme et al., 2008, Yin et al., 2010a). These actions of progranulin are important for maintaining the function of neural networks in the brain, as loss-of-function mutations causing haploinsufficiency of progranulin (GRN) are a major genetic cause of frontotemporal dementia (FTD), causing around 5–10% of FTD cases (Baker et al., 2006, Cruts et al., 2006, Gass et al., 2006).
FTD includes multiple clinical and pathological subtypes (Rascovsky et al., 2011). The most common subtype, behavioral variant FTD (bvFTD), causes personality and behavior changes resulting in apathy, disinhibition, and impaired social behavior (Rascovsky et al., 2011). This impaired social behavior is characterized by socially inappropriate actions, reduced interest in others, lack of empathy, and an impaired ability to interpret emotions and facial expressions (Adenzato et al., 2010, Barsuglia et al., 2014, Rascovsky et al., 2011). The personality and behavioral changes in bvFTD are associated with degeneration of the salience network, a neural network that responds to emotional stimuli (Seeley et al., 2009, Zhou et al., 2010).
Progranulin-insufficient mice (both Grn+/− and Grn−/−) have been studied as models of FTD with GRN mutations, though Grn+/− mice are the closer genetic model since the complete progranulin deficiency modeled by Grn−/− mice causes a different disease, neuronal ceroid lipofuscinosis (Smith et al., 2012). By around 6 months of age, both Grn+/− and Grn−/− mice develop abnormal social behavior that may model social deficits in FTD (Filiano et al., 2013, Ghoshal et al., 2012, Kayasuga et al., 2007, Yin et al., 2010b). These social deficits include reduced social interaction in the three-chamber sociability test (Filiano et al., 2013, Yin et al., 2010b) and the resident-intruder test, in which some studies reported increased aggression (Ghoshal et al., 2012, Kayasuga et al., 2007). In addition, we found that 4–6-month-old Grn+/− mice have increased social dominance versus wild-type littermates in the tube test for social dominance (Filiano et al., 2013).
The tube test for social dominance was first described in mice over fifty years ago, and has since become a common test for assessing social behavior in mice (Lindzey et al., 1961). The test is a simple, quick assay in which two mice are released into opposite ends of a tube and meet in the middle. One mouse will typically push the other out of the tube, and is considered the “winner” or more dominant mouse. When used to assess dominance hierarchies within a cage of mice, the tube test reveals linear hierarchies in which mice consistently win or lose when paired with specific cagemates (Wang et al., 2011). However, the degree to which tube test dominance is an index of general social dominance has been debated, as tube test dominance correlates with some other measures of dominance, but has a less clear relationship with others. Tube dominance correlates with barbering and urine marking in male mice, but there are mixed results on whether tube test dominance correlates with aggressive behavior or competition for access to food, water, or female mice (Benton, 1980, Greenberg et al., 2014, Lindzey et al., 1961, Rodriguiz et al., 2004, Van De Weerd et al., 1992, Wang et al., 2011). Tube test dominance also appears to correlate with other non-hierarchical social behaviors, as abnormal tube test behavior has been observed in many mouse models that display deficits in social interaction (Filiano et al., 2013, Jiang-Xie et al., 2014, Koh et al., 2008, Lijam et al., 1997, Long et al., 2004, Moretti et al., 2005, Nishijima et al., 2006, Shahbazian et al., 2002, Spencer et al., 2005, Veenstra-Vanderweele et al., 2012).
In this study, we further investigated social dominance in progranulin-insufficient mice by testing the effect of age on tube test behavior in Grn+/− mice and by testing for the presence of a social dominance phenotype in Grn−/− mice. To evaluate the potential of this test for preclinical studies, we tested the effects of methodological variables such as repeated testing, habituation to the tube, design of the test apparatus, mouse sex, and experimenter sex. We also used the tube test to study the effects of progranulin insufficiency on within-cage dominance hierarchies. Finally, to understand the mechanism underlying these changes, we investigated cell signaling pathways and neuronal morphology in the amygdala, a brain region in which Grn+/− mice show signs of dysfunction, and the prefrontal cortex, a brain region that is critical in mediating tube dominance (Filiano et al., 2013, Wang et al., 2011).
Materials and Methods
Animals
Grn+/− and Grn−/− mice were generated and crossed onto a C57BL/6J background as previously described (Filiano et al., 2013, Martens et al., 2012). The mouse line used for this study had been backcrossed onto the C57Bl/6J background over at least 11–12 generations. Only mice on a congenic C57Bl/6J background were used for this study, but we previously observed similar behavioral deficits between Grn+/− and Grn−/− mice on a mixed 129Sv, FVB/N, C57Bl/6J background (Filiano et al., 2013). All mice used in this study were bred and housed in our barrier housing facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Mice used for tube test studies were generated by breeding wild-type and Grn+/− mice to generate wild-type and Grn+/− offspring, or by breeding Grn+/− mice with Grn+/− mice to generate wild-type, Grn+/−, and Grn−/− offspring. Offspring from multiple litters were pooled to generate the cohorts of mice used for tube testing, and all testing was conducted between mice from the same cohort. Male and female mice were used for all experiments except for Golgi staining, in which only male mice were used. Mice were kept on a 12:12 hour light/dark cycle with lights on at 6 am, and all testing was conducted during the light phase. The mice were given ad libitum access to food (Harlan NIH31 diet #7917) and water throughout all experiments. All experiments were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Tube Test
Mice of the same sex, but opposite genotype (Grn+/+ vs. Grn+/− or Grn−/−) from separate cages were paired for testing. The mice received no prior training or habituation to the tube, but some groups of mice were tested multiple times across their lifespan. Testing was carried out in the mouse housing room in a cage changing hood with the hood lights on, but the blower motor off. The cages containing the desired mice were placed in the hood, and each pair of mice was removed from their home cage immediately before testing. Mice of each genotype were assigned evenly to the left and right sides of the tube to avoid any potential side bias, and the experimenter was blind to mouse genotype. The mice were gently placed into either end of the tube and released when both mice were completely inside the tube. The first mouse with two feet out of the tube was considered the “loser” or less dominant mouse. Tests lasting longer than two minutes were aborted and run again at the end of the session. Testing between non-cagemates was conducted over three rounds, with each mouse facing a different mouse of the opposite genotype in each round. Each round was conducted sequentially, with no waiting period between rounds. Clear vinyl tubing purchased at a local home improvement store was used for all tests. All tubes used for testing were 30.5 cm long. Tubes with several internal diameters (ID) were used based on mouse age and sex to prevent mice from crawling over each other, while still providing space to allow the mice to enter and move through the tube. A 2.5 cm ID tube was used for all mice at 2–3 months of age, and females less than 9 months old, a 3.2 cm ID tube was used for females greater than 9 months or males aged 6–9 months, and a 3.8 cm ID tube was used for males greater than 9 months.
Tube Test with Antechambers
The tube test with antechambers used a very similar protocol as our standard design, but was performed with an apparatus that had 12 cm × 12 cm antechambers at either end of a 3.2 cm ID, 30.5 cm long tube. The tube and antechambers were made from clear acrylic. The center of the tube was encased in a dark compartment made of black acrylic to encourage entry into the tube, and was blocked with a door to allow the experimenter to control when the mice initiated contact. Mice were trained to run through the tube for two days prior to testing. Each mouse was allowed to freely explore the apparatus for two sessions of two minutes each day. During training, mice that did not pass through the tube at least once during each session were placed into the tube and gently prodded with a 25 mL pipette to encourage passage through the tube. All mice quickly and voluntarily entered and traversed the tube by the last training session. On the test day, mice were put into the appropriate antechamber to begin the test. After both mice entered the tube, the door was lifted to initiate contact between the mice, and the winner was determined as in the standard protocol.
Within-Cage Tube Testing
For preliminary within-cage tube testing, cages containing 4–5 male or female wild-type mice were tested using the standard tube test protocol in a round-robin fashion with all mice matched against each of their cagemates once a day for 5 days. The number of wins by each mouse was used to determine its dominance score, with top mouse winning all of its matches (dominance score = 5), and the bottom mouse losing all of its matches (dominance score = 1). Thus, mice from 5-mouse cages were assigned dominance scores from 1–5, and mice from 4-mouse cages were assigned scores of 1, 2, 4, or 5. When two mice had the same number of wins, their head-to-head pairing was used to determine which mouse received the higher rank, and the within-cage hierarchy was still considered to be linear. If three mice achieved the same number of wins, all three mice were assigned the middle rank of the three ranks in question, and this was considered a non-linear hierarchy. At the end of the testing period, an “aggregate dominance score” was calculated for each mouse based on the overall winner of each mouse pairing over the five days of testing. In addition to calculating daily and aggregate dominance scores for each mouse, the number of rounds that gave linear hierarchies with no mice tied for the same dominance score was recorded for each sex. The percent of matches that were consistent with the aggregate dominance score was also calculated as a measure of the stability of the within-cage hierarchies. Four cages of mice were run for each sex.
For assessment of within-cage genotype effects, wild-type, Grn+/−, and Grn−/− mice were subjected to one round of round-robin within-cage testing. Each cage contained five mice. Genotype differences in dominance were assessed with the dominance score as described above, and by comparing the number of wins between each genotype pairing as in the between cage tests. Only female mice were used for this experiment due to the lack of male cages containing 5 mice. Under our housing conditions, we do not combine male mice from different cages after weaning to prevent fighting and injuries. As a result, most male cages no longer contain 5 mice by 9–16 months of age due to mouse deaths, use in experiments and breeding, etc.
Three-chamber Sociability
Three-chamber sociability testing was conducted under red lighting as previously described (Filiano et al., 2013). Wild-type and Grn+/− mice were transported to the testing room and allowed at least one hour to acclimate to the room. The mice were then placed in the center chamber of the three-chambered testing compartment and allowed to explore for 10 minutes. The mice were then confined to the center chamber while a novel mouse (a 6–12 month-old male C57Bl/6J mouse that was habituated to the wire cage each day) or a novel object (a plastic block) were placed into the wire cages in each side chamber. The doors to the side chambers were then removed and the mice were allowed to freely explore the testing compartment for 10 minutes. Sessions were recorded and scored with video tracking software (Cleversys). The social ratio for each mouse was determined by dividing the time spent investigating the novel mouse by the time spent investigating the novel object. Data from multiple cohorts were compiled, and data from each cohort was normalized to the average social ratio of wild-type mice.
Nesting
Wild-type and Grn+/− mice were single-housed for 24 hours in standard cages with a cotton nestlet (IsoBLOX, Harlan) and ad libitum access to food and water. At the end of the testing period, nests were scored using a scale adapted from that of Deacon, et al (Deacon, 2012). The nestlets were weighed before and after testing to aid in scoring. A modified scale was generated to account for nests that seemed to fall between scores on the 5-point scale generated by Deacon, et al (Deacon, 2012). The modified scale was as follows: 0 – nestlet untouched, 1 – nestlet slightly chewed (<25%), 1.5 – <25% of nestlet chewed, rudimentary nest formed, 2 – 25–49% of nestlet chewed, but no clear nest, 2.5 25–49% of nestlet chewed, rudimentary nest formed, 3 – at least 50% of nestlet chewed, no clear nest, 3.5 50–74% of nestlet chewed, nest formed, 4 – at least 75% of nestlet chewed, nest, 5 – essentially 100% of nestlet chewed, nest. Data from multiple cohorts were compiled, and data from each cohort was normalized to the average nest score of wild-type mice.
Tissue preparation
Tissue samples for western blot and Golgi staining were generally obtained from separate groups of mice and thus cannot be directly correlated. However, the same procedure was used to collect tissue from all groups. Mice were anesthetized with pentobarbital (100 mg/kg, Fatal Plus, Vortech Pharmaceuticals), followed by transcardial perfusion with 0.9% saline. The brains were then removed and the left hemibrain was immediately processed for Golgi staining as described below, while the right hemibrain was frozen on dry ice and stored at −80°C. The frozen hemibrains were later thawed and cut into 1 mm sections using a brain matrix (Zivic Instruments). The amygdala was dissected and stored at −80 °C.
Antibodies
The following antibodies from Cell Signaling Technologies were used for western blot: p-rpS6 (Ser235/236, #4858), p-rpS6 (Ser240/244, #5364), rpS6 (#2317), p-Akt (Ser473, #4060), Akt (pan, #4691), p-mTOR (Ser2448, #2971), mTOR (#2972), p-ERK1/2 (Thr202/Tyr204, #4370), ERK1/2 (#9102), p-S6K (Thr421/Ser424, #9204), S6K (#2708).
Western Blot
Frozen amygdala samples were thawed and homogenized in RIPA buffer (50 mM Tris, 150 mM NaCl, 5mM EDTA, 0.1% SDS, 0.1% Triton X-100, 0.5 % Sodium Deoxycholate), then centrifuged for 10 minutes at 5000 × g. Protein concentration of the lysates was determined by Bradford assay (Coomassie Plus, Thermo Scientific). Samples were then diluted in LDS buffer (Life Technologies) with Bolt Reducing Agent (Life Technologies) and loaded onto 4–12% Bis-Tris gels (Life Technologies). Electrophoresis was conducted with MOPS-SDS running buffer. The proteins were then transferred to PVDF membranes (Immobilon-FL, EMD Millipore), which were blocked with either 50% Odyssey blocking buffer in TBS (Li-COR Biosciences) or Casein blocking buffer (Sigma) prior to overnight incubation at 4°C with the antibodies described above. The following day, the membranes were incubated with IR-dye-conjugated secondary antibodies (Li-COR Biosciences), and scanned with an Odyssey scanner (Li-COR Biosciences).
Golgi Staining
Golgi staining was conducted on brains from male wild-type and Grn+/− mice as previously described using the FD Rapid GolgiStain kit (FD NeuroTechnologies) and the manufacturer’s protocol (Filiano et al., 2013). Hemibrains collected as described above were incubated in impregnation solution for 14 days prior to incubation in cryoprotectant for at least 48 hours. The hemibrains were then sliced into 200 μm sections on a cryostat (Leica) and mounted onto gelatin-subbed slides. The slides were then incubated in developing solution prior to dehydration with graded ethanol and clearing in xylene. The slides were coverslipped with Permount (Fisher Scientific). 20X z-stacks (1 μm per slice) were taken of 3–5 neurons per mouse using a light microscope (Nikon). Neuronal morphology was then analyzed by manual tracing and Sholl analysis using Neurolucida (MBF Bioscience).
Statistics
The number of wins by each genotype is not an independent measure, so it was analyzed by the binomial test for observed versus expected distributions, with the expected distribution set at 50% wins for each genotype. The distribution of wins per mouse was analyzed by Chi-square test. In cases where the criteria for chi-square testing were not met (no empty cells, at least 20% of cells containing values greater than 5), the number of wins was combined into larger bins of 0–1 or 2–3 wins per mouse. Within-cage dominance scores were analyzed by Kruskal-Wallis test followed by Dunn’s post-hoc test. Comparisons of win distribution between mouse experience, tube design, mouse sex, and experimenter sex were performed with Fisher’s Exact Test. Analysis of win distribution over three rounds of testing was performed by Chi-square test. Correlation analysis of within-cage dominance scores from pilot studies was performed using Spearman correlation due to the nonparametric nature of dominance scores. The number of linear within-cage hierarchies for male and female mice was compared by Mann-Whitney test, and the percent of trials consistent with aggregate dominance scores was compared by t-test. Data from the three-chamber sociability test were analyzed by t-test, and nest scores were analyzed by Mann-Whitney test. Western blot data for p-rpS6 (Ser235/236) and p-Akt (Ser473) across multiple age groups were analyzed by ANOVA with age and genotype as factors, and significant main effects or interactions were followed by Sidak’s post-hoc test to compare wild-type and Grn+/− mice at each age. The correlation of p-rpS6 (Ser235/236) and p-Akt (Ser473) was analyzed using Pearson correlation. All other western blot data only included 6–9 month-old mice and were analyzed by t-test. Sholl analysis of Golgi-stained neurons was analyzed by repeated measures ANOVA with genotype and distance from the soma as factors. Total dendritic length and the number of nodes from Golgi-stained neurons were analyzed by two-way ANOVA with genotype and dendritic type as factors. Signficant main effects or interactions were followed with Sidak’s post-hoc test to compare wild-type and Grn+/− mice at each distance. All of the above analyses were performed with Graphpad Prism 6.0 (Graphpad, La Jolla, CA) with α set at 0.05. Determination of kappa values to quantify the agreement between different experimenters or with different testing designs was performed with the freely-available online kappa calculator from Graphpad Quick Calcs (Graphpad, La Jolla, CA)
Results
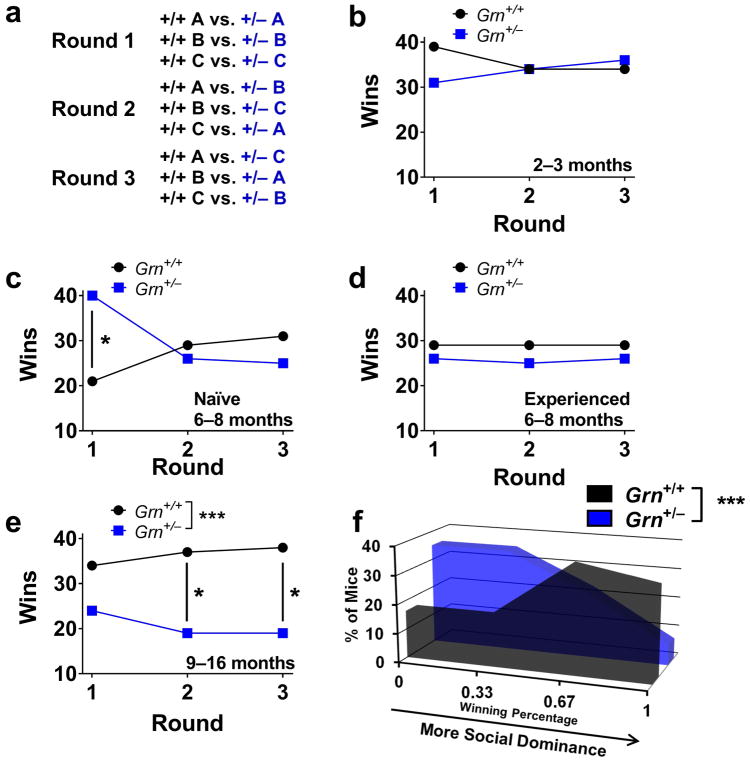
We previously reported increased social dominance in the tube test in Grn+/− mice at around 6 months of age, using a protocol in which each mouse was tested only once (Filiano et al., 2013). In follow-up experiments, we conducted a cross-sectional study of Grn+/− mice of different ages to determine the time course of this dominance phenotype. We conducted these experiments using a round-robin design in which each mouse was paired with three non-cagemate mice of the same sex but different genotype (Fig. 1a). In addition to the number of wins for each genotype, this protocol provides the individual measure of wins per mouse (out of three total). This measure provides more resolution of the severity of phenotype in an individual mouse than the binary win-lose outcome of a single match.
Figure 1.
We did not detect any social dominance abnormalities in 2–3 month-old Grn+/− mice (Fig. 1b), which is consistent with the general lack of behavioral changes in Grn+/− mice prior to 6 months of age (Filiano et al., 2013). Interestingly, the increased dominance of 6–8 month-old Grn+/− mice was sensitive to repeated testing and prior test experience (Fig. 1c, d). 6–8 month-old Grn+/− mice that were naïve to the tube test exhibited a dominant phenotype over wild-type littermates in the first round of testing as previously reported (Fig. 1c) (Filiano et al., 2013). However, this phenotype was not maintained in rounds 2 and 3 (Fig. 1c), and 6–8 month-old Grn+/− mice with prior test experience (at 2–3 months of age) failed to exhibit abnormalities (Fig. 1d). The sensitivity of this winning phenotype to repeated testing indicates a requirement for tube novelty in the dominant behavior of Grn+/− mice at around 6 months of age.
We then tested 9–16 month-old Grn+/− mice to determine how the social dominance phenotype of Grn+/− mice changes with age. In contrast to younger mice, these mice exhibited reduced social dominance, a phenotype that was maintained with repeated testing (Fig. 1e, f). This losing phenotype was significant when data were collapsed across rounds, and reached individual significance in the second and third rounds of testing (Fig. 1e). Grn+/− mice also had a significant losing phenotype as measured by wins per mouse, with Grn+/− mice shifted toward a lower number of wins for each mouse than wild-type littermates (Fig. 1f). There was not a significant difference in the performance of tube-naïve and tube-experienced 9–16 month-old mice (Fig. S1, p = 0.53, Fisher’s exact test). The maintenance of reduced social dominance across multiple rounds of testing in older Grn+/− mice indicates stable changes in dominance behavior in the tube test.
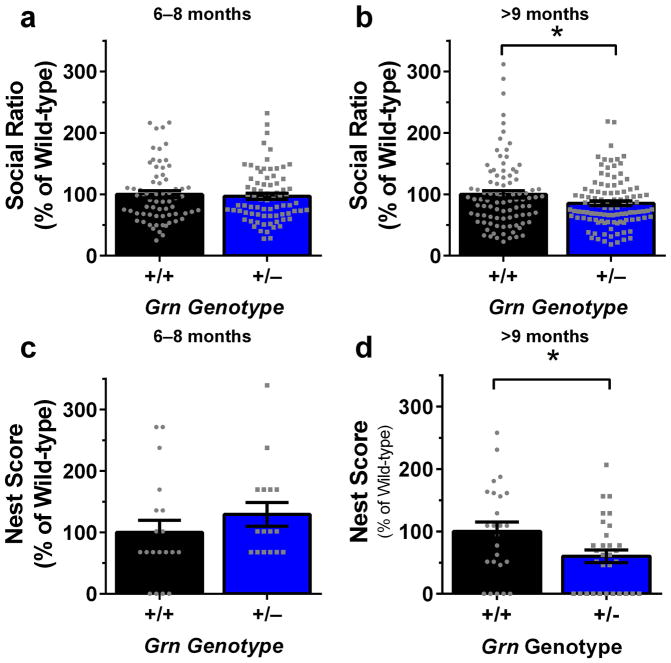
We next sought to determine if the biphasic behavioral phenotype observed with the tube test in Grn+/− mice extends to other tests by testing Grn+/− mice in the three-chamber sociability and testing tests. As previously reported, we detected deficits in social behavior in Grn+/− mice aged 9 months and older in the three-chamber sociability test (Fig. 2b) (Filiano et al., 2013). However, we did not observe reduced sociability in Grn+/− mice aged 6–8 months (Fig. 2a). Similarly, older Grn+/− mice exhibited deficits in nesting behavior (Fig. 2d), while 6–8 month-old Grn+/− mice did not exhibit this abnormality (Fig. 2c). These data do not support the presence of a more general biphasic behavioral phenotype in Grn+/− mice.
Figure 2.
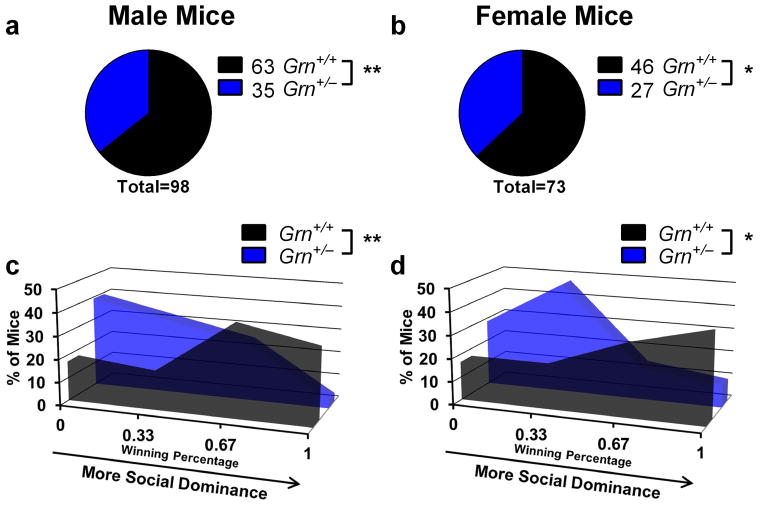
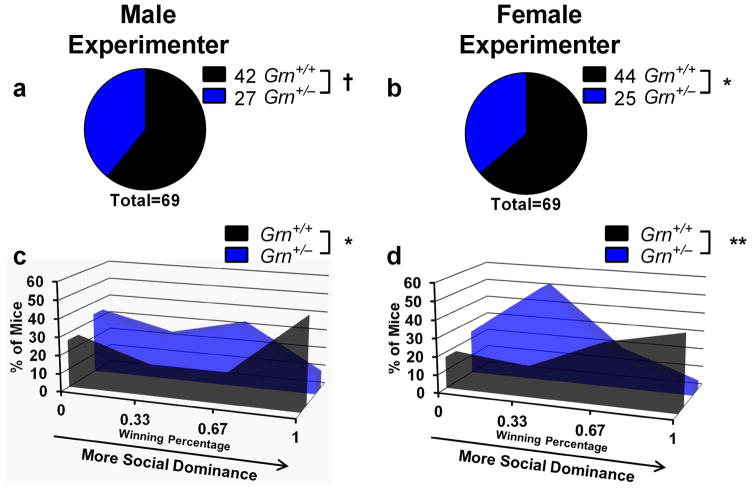
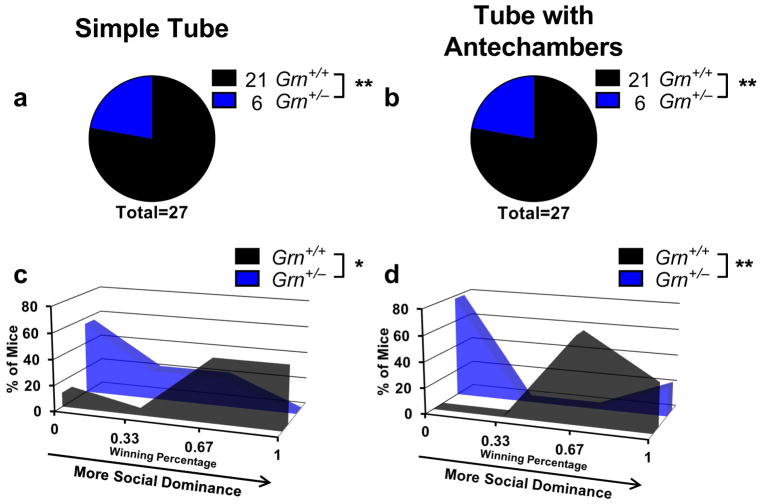
Insofar as Grn+/− mice model FTD, their social dominance phenotype could provide a useful assay for preclinical evaluation of potential therapeutics, including agents that would increase progranulin expression from the remaining allele, particularly since the tube test is rapid and inexpensive to perform. However, to be useful as a preclinical assay, it would have to provide consistent results under different conditions. Therefore, we conducted several experiments to determine the robustness of the tube test assay. First, we examined test-retest effects, matching the same mice in tube test trials on successive days. Under these conditions, 40 of 47 matches achieved the same result, producing a kappa value indicating “good” agreement (κ = 0.693). Second, we tested the effects of mouse sex on the tube test phenotype of Grn+/− mice. Comparison of male and female 9–16-month-old mice from the experiment shown in Fig. 1 revealed no significant difference between male and female Grn+/− mice (Fig. 3). Both sexes had significant phenotypes at each age, and there was not a significant between-sex difference using Fisher’s exact test (p = 0.87). Third, we tested the effects of experimenter sex, which has recently been demonstrated to affect some behavioral tests (Sorge et al., 2014). We observed similar results when the same group of mice was tested by both a male and a female examiner (Fig. 4, Fisher’s exact test, p = 0.86). Fourth, we tested whether prior habituation to the testing apparatus and voluntary entry into the tube affects the low dominance phenotype of older Grn+/− mice. We tested a group of 12-month-old Grn+/− mice in both the standard tube test in which the experimenter places the mice into the tube, and a related apparatus in which mice voluntarily enter the tube from antechambers at either end of the tube. In the latter case, mice performed two training sessions for habituation to the apparatus and to ensure voluntary entry into the tube. Despite the differences in test design, we found identical winning percentages for Grn+/− mice across both designs (Fig. 5). These data indicate that the losing phenotype of older Grn+/− mice is not dependent upon the stress of forced tube entry.
Figure 3.
Figure 4.
Figure 5.
We next asked whether the social dominance phenotypes observed in the tube test reflect changes that are manifest in “real life”. Group-housed mice form social dominance hierarchies that may be measured with the tube test, in which male mice form stable, linear hierarchies (Wang et al., 2011). We therefore decided to test within-cage dominance hierarchies among wild-type, Grn+/−, and Grn−/− mice using the tube test.
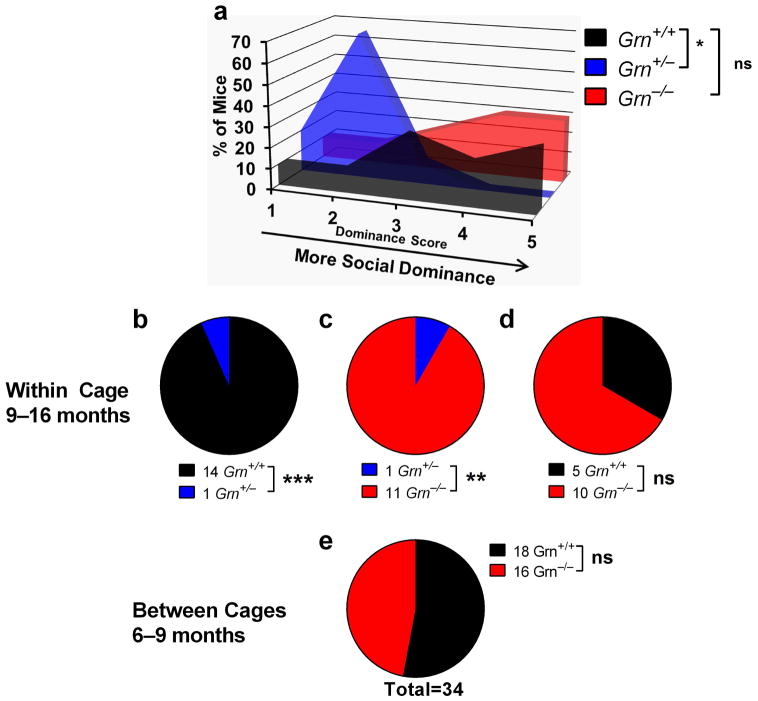
In preliminary experiments with wild-type mice, we replicated findings of stable, generally linear hierarchies in male mice, and found that female mice also establish stable dominance hierarchies (Fig. S2) (Wang et al., 2011). After testing mice daily for 5 days, we found that within-cage hierarchies were relatively stable across the testing period, with the dominance score of mice from the first day strongly correlated with both the score from the last day of testing and with the aggregate dominance score for the entire 5-day period (Fig. S2). We therefore compared the within-cage dominance scores of wild-type, Grn+/−, and Grn−/− mice from a single bout of within-cage testing.
For this experiment, we bred Grn+/− mice together to produce all three genotypes, and selected cages containing a full complement of 5 mice with a mixture of Grn+/+, Grn+/−, and/or Grn−/− littermates. The mice were tested at ages 9–16 months. We tested all mice in each cage against their cagemates in a round-robin design to determine the social dominance hierarchy. The number of wins by each mouse was used to rank the dominance of the mice, with the most dominant mouse winning all matches (dominance score = 5) and the least dominant mouse losing all matches (dominance score = 1). There was an overall genotype effect on within-cage dominance (Fig. 6a, Kruskal-Wallis, p = 0.0063), with Grn+/− mice, but not Grn−/− mice, exhibiting lower scores than wild-type mice. Analysis of the win totals from between-genotype pairings confirmed that Grn+/− mice lost a significant proportion of their matches versus both wild-type (Fig. 6b) and Grn−/− mice (Fig. 6c). The losing phenotype of Grn+/− mice is consistent with the results obtained when testing against non-cagemates (Figs. 1, 3–5), and indicates that progranulin insufficiency affects the social behavior of mice living together in their home cage.
Figure 6.
Interestingly, Grn−/− mice did not exhibit a difference in social dominance (Fig. 6a, d). To confirm the lack of tube test phenotype in Grn−/− mice, we also tested a group of 6–9-month-old male and female Grn−/− mice against wild-type mice from separate cages (Fig. 6e), just as we had done with Grn+/− mice. We observed no social dominance changes in these mice, in contrast to the dominant phenotype that Grn+/− mice have under similar testing conditions (Fig. 1c). These data suggest that there are distinct effects of partial and complete progranulin deficiency on social dominance in the tube test.
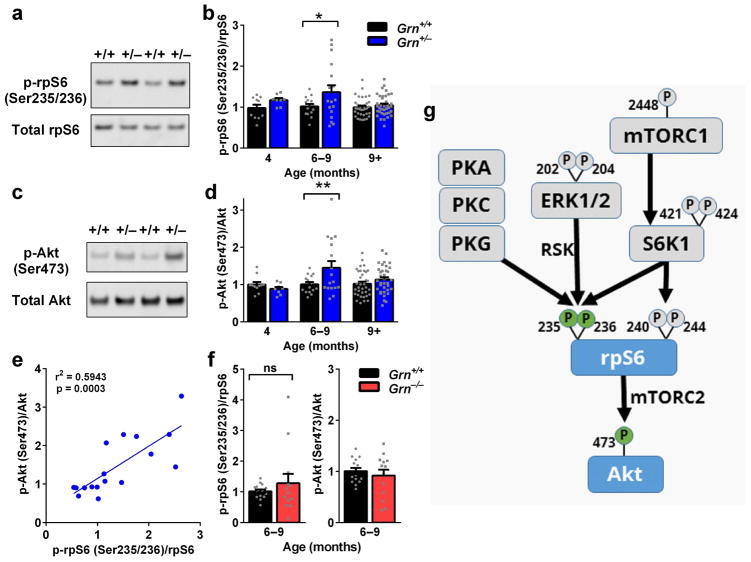
Finally, we investigated the potential mechanisms underlying the biphasic tube test phenotype of Grn+/− mice. Our initial investigation focused on cellular signaling pathways in the amygdala, a brain region in which we have previously observed signs of dysfunction in Grn+/− mice (Filiano et al., 2013). Available data on cellular signaling in progranulin-deficient models suggested changes in the ribosomal protein S6 (rpS6) phosphorylation pathway (Almeida et al., 2012, Tanaka et al., 2013). We therefore hypothesized that Grn+/− mice might have abnormal rpS6 phosphorylation. At 6–9 months, when Grn+/− mice showed increased dominance on the tube test, rpS6 phosphorylation at Ser 235/236 was increased (Fig. 7a, b). However, at 4–5 months, when Grn+/− mice have no social dominance abnormalities, and at >9 months, when they have lower social dominance, rpS6 phosphorylation was normal (Fig. 7b). Thus, there is a monophasic increase in rpS6 phosphorylation in the amygdala that corresponds to the stage in which Grn+/− mice have increased social dominance.
Figure 7.
We next investigated the cellular signaling pathways upstream of increased p-rpS6 (Ser235/236) in 6–9 month-old Grn+/− mice (Fig. 7g). S6 kinase 1 and 2 (S6K1 and S6K2) are major kinases that phosphorylate rpS6 at Ser235/236 and an additional site at Ser240/244, and previous work has indicated abnormal expression or phosphorylation of these kinases in progranulin-deficient model systems (Almeida et al., 2012, Magnuson et al., 2012, Tanaka et al., 2013). mTOR signaling through mTORC1 is a major driver of S6K1 activity, so we hypothesized that elevated mTORC1/S6K signaling could lie upstream of the elevated p-rpS6 (Ser235/236) in 6–9 month-old Grn+/− mice (Magnuson et al., 2012, Meyuhas, 2008). To test this hypothesis, we immunoblotted for phospho-proteins at multiple levels of the mTORC1 signaling pathway in the amygdala of 6–9 month-old Grn+/− mice. We also immunoblotted for total levels of S6K2. Our analysis of mTORC1 signaling in the amygdala of 6–9 month-old Grn+/− mice revealed no detectable genotype differences in levels of p-mTOR (Ser2448) or p-S6K1 (Ser421/Thr424) (Fig. S3). We also observed no genotype differences in total S6K2 levels (Fig. S3). In addition, we examined two phosphorylation sites on rpS6, Ser240/244, that are uniquely phosphorylated by S6K1/S6K2 (Pende et al., 2004). Unlike Ser235/236, there was no significant increase in rpS6 phosphorylation at Ser240/244 (Fig. S3). Together, these data are inconsistent with elevated mTORC1 signaling as the source of elevated p-rpS6 (Ser235/236) in the amygdala of 6–9 month-old Grn+/− mice. We next examined other signaling pathways that are associated with rpS6 phosphorylation at Ser235/236 but not at Ser240/244. ERK kinase activation of p90 ribosomal S6 kinase (p90 RSK) is a major driver of rpS6 phosphorylation at Ser235/236 (Pende et al., 2004, Roux et al., 2007). To investigate whether this pathway was more active in Grn+/− mice, we immunoblotted for levels of phospho-ERK1/2, but again observed no genotype differences at 6–9 months of age (Fig. S3), suggesting that ERK-p90RSK signaling is unlikely to be the main driver of rpS6 phosphorylation in grn1 mice. rpS6 can be phosphorylated by several additional kinases at Ser235/236, including protein kinase A, protein kinase C, and protein kinase G (Fig. 7g, Biever et al., 2015, Yano et al., 2014) and exploring these pathways will be an important goal in future studies of Grn+/− mice (Fig. 7g).
To better understand the relevance of elevated rpS6 phosphorylation at Ser235/236 to tube test behavior in Grn+/− mice, we investigated potential downstream pathways that could result in behavioral changes. rpS6 has been reported to activate mTORC2 signaling, which phosphorylates Akt at Ser473 (Fig. 7g) (Sarbassov et al., 2005, Yano et al., 2014). This mTORC2/Akt signaling regulates the actin cytoskeleton and can modulate neuronal morphology, as mTORC2 inactivation impairs dendritic arbors (Thomanetz et al., 2013). Thus, we hypothesized that the elevated p-rpS6 (Ser235/236) in 6–9 month-old Grn+/− mice could increase mTORC2 signaling, activate Akt, and enhance dendritic arbors of amygdala neurons. We tested for increased mTORC2 signaling by immunoblotting for p-Akt (Ser473) in the amygdala of Grn+/− mice. mTORC2 activity is required for phosphorylation of Akt at Ser473, making p-Akt (Ser473) a useful readout of mTORC2 activity (Sarbassov et al., 2005, Thomanetz et al., 2013). Similarly to p-rpS6 (Ser235/236), we found that p-Akt (Ser473) was elevated specifically in Grn+/− mice at 6–9 months of age, during the phase in which the mice have increased dominance on the tube test, and not at younger or older ages (Fig. 7c,d). Levels of p-Akt (Ser473) were strongly correlated with levels of p-rpS6 (Ser235/236) (Fig. 7e), consistent with the idea that they are linked in a common signaling pathway, likely involving mTORC2 (Fig. 7g). To further test the association between increased rpS6/Akt signaling and tube test behavior, we examined this pathway in Grn+/− mice, which do not have tube test abnormalities (Fig. 6). There were no significant differences in either p-rpS6 (Ser235/236) or p-Akt (Ser473) in Grn+/− mice at this age (Fig. 7f), consistent with the idea that these changes are linked to social dominance abnormalities.
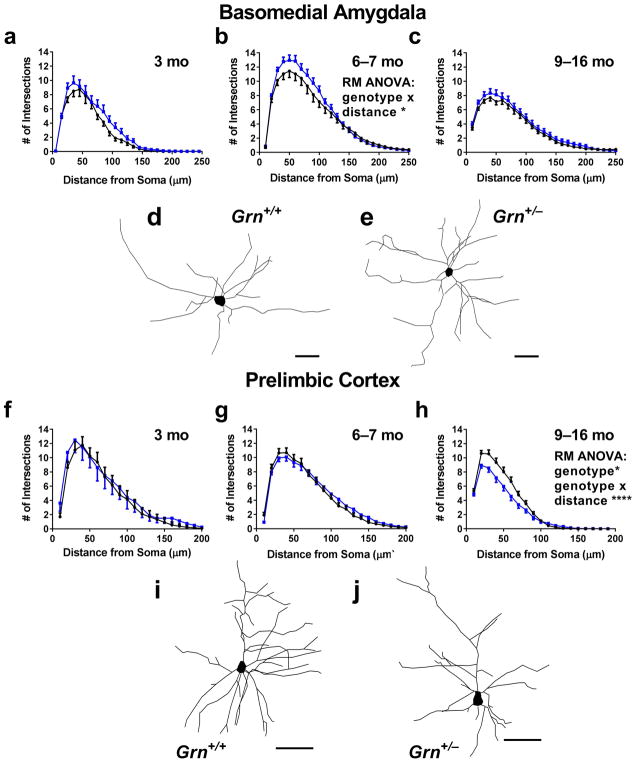
To further test our hypothesis that increased mTORC2/Akt signaling in 6–9 month-old Grn+/− mice could enhance dendritic arbors, we analyzed Golgi-stained neurons in the basomedial amygdala of Grn+/− mice at 6–7 months of age. Consistent with our hypothesis, we observed enhanced dendritic arbors in the amygdala of 6–7 month-old Grn+/− mice (Fig. 8b, RM ANOVA genotype x distance interaction, p = 0.0286). Similar to the signaling data, we did not observe enhanced dendritic arbors in Grn+/− mice at ages before the onset of a tube test phenotype (Fig. 8a, 3 months) or after the onset of a losing phenotype (Fig. 8c, 9–16 months). Taken together, these data lead us to propose that abnormal cell signaling, potentially through mTORC2/Akt, in the amygdala of 6–9 month-old Grn+/− mice enhances the dendritic arbor of amygdala neurons and contributes to their transient dominant phenotype.
Figure 8.
To investigate the mechanism behind the low dominance phenotype of Grn+/− mice after 9 months of age, we again investigated neuronal morphology. The prelimbic cortex is an important driver of tube test behavior, with greater activity in this brain region associated with greater dominance (Wang et al., 2011). We therefore analyzed Golgi-stained pyramidal neurons in layer II/III of the prelimbic cortex of wild-type and Grn+/− mice, and found an impairment in the basal dendritic arbor of 9–16 month-old Grn+/− mice (Fig. 8h) that was not present at earlier ages (3 months, Fig. 8f; 6–7 months, Fig. 8g). This impairment was specific for the basal dendrites, as the apical arbor was not significantly changed (Fig. S4). Given the established role of the prelimbic cortex in tube test behavior, these basal dendritic impairments represent a potential mechanism contributing to the low dominance phenotype of Grn+/− mice at ages older than 9 months (Wang et al., 2011).
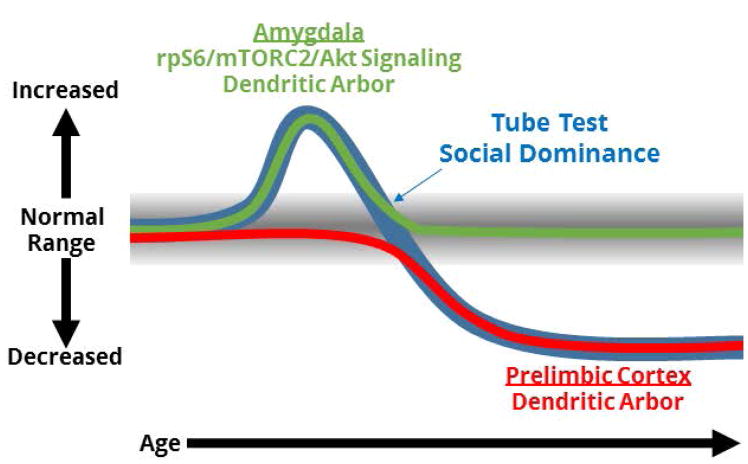
In summary, our data on amygdala signaling pathways and neuronal morphology in the amygdala and prelimbic cortical dendritic arbors suggest a model in which separate mechanisms across a distributed network give rise to the biphasic tube test phenotype of Grn+/− mice. In this model (Fig. 9), elevated rpS6/mTORC2/Akt signaling in the amygdala at 6–8 months enhances dendritic arbors and contributes to a dominant phenotype, and ongoing degeneration of basal dendritic arbors in the prelimbic cortex contributes to a low dominance phenotype as Grn+/− mice age.
Figure 9.
Discussion
In this study, we found that the tube test phenotype of Grn+/− mice is age-dependent, with a dominant phenotype that develops around 6 months of age and progresses to a submissive phenotype after 9 months of age. The submissive phenotype in older mice is more robust and seen in both male and female mice across a range of experimental conditions, including in assessments of dominance hierarchies established during home-cage behavior, while the dominant phenotype in younger mice is not seen with repeated testing. We further found that this biphasic tube test phenotype in Grn+/− mice is associated with biochemical and morphological changes in the amygdala and prelimbic cortex, with the transient dominant phenotype associated with elevated p-rpS6 (Ser235/236) and p-Akt (Ser473) levels and enhanced dendritic arbors in the amygdala, and the later submissive phenotype associated with reduced basal dendritic arbors in the prelimbic cortex. The elevated p-rpS6 and p-Akt levels in 6–9 month-old Grn+/− mice may reflect an increase in mTORC2 signaling, as mTORC2 is required for Akt phosphorylation at Ser473, and p-rpS6 can activate mTORC2 signaling (Sarbassov et al., 2005, Thomanetz et al., 2013, Yano et al., 2014). There are interesting differences between Grn+/− and Grn−/− mice, as Grn−/− mice do not have abnormalities in either tube test social dominance or the mTORC2/Akt signaling pathway that develop in Grn+/− mice.
Our data lead us to propose that the biphasic tube test phenotype of Grn+/− mice is at least partially driven by changes in the dendritic arbor of neurons in the amygdala and prelimbic cortex (Fig. 9). This hypothesis is consistent with data on the roles of these brain regions in social behavior in humans and animal models. Both the prefrontal cortex and amygdala are part of networks that respond to social hierarchies in humans and non-human primates (Noonan et al., 2014, Zink et al., 2008). Additional data from rodents indicates that both brain regions affect dominance behavior. While the results vary with experimental paradigm, lesions of the amygdala generally reduce dominance and aggression in rodents (Bunnell, 1966, Lukaszewska et al., 1984, Miczek et al., 1974, Vochteloo & Koolhaas, 1987). However, it is difficult to conclusively tie increased basomedial amygdala dendritic arbors to increased tube test dominance, as the relationship between amygdala activity and dominance behavior is complex given the anatomic and cellular heterogeneity of the amygdala. Additionally, the role of the amygdala in tube test behavior remains poorly understood. The association of reduced prelimbic dendritic arbors with low dominance behavior in older Grn+/− mice provides a clearer potential mechanism, as greater activity in the prelimbic cortex drives greater tube test dominance in mice (Wang et al., 2011).
The abnormalities in the prefrontal cortex and amygdala of Grn+/− mice are also consistent with dysfunction of these brain regions in FTD patients and in animal models of FTD. The prefrontal cortex and amygdala are part of the salience network, which degenerates in behavioral variant FTD, and degeneration of these brain regions is associated with abnormal behavior in FTD patients (Le Ber et al., 2006, Rosen et al., 2005, Seeley et al., 2009, Zamboni et al., 2008). Abnormal AMPA receptor expression in the medial prefrontal cortex has been tied to abnormal social behavior in a mouse model of FTD due to mutations in CHMP2B (Gascon et al., 2014). Multiple animal models of FTD have reported amygdala dysfunction, as Grn+/− mice display hypoactivation of the amygdala after exposure to a novel, social environment, and rTg4510 mice expressing tau with the FTD-associated P301L mutation display behavioral abnormalities and pathology consistent with amygdala dysfunction (Cook et al., 2014, Filiano et al., 2013). The age-dependent changes in neuronal morphology and tube test behavior observed in Grn+/− mice may therefore be modeling dysfunction in nodes of the salience network similar to that observed in behavioral variant FTD.
The simplification of prelimbic dendritic arbor with age in Grn+/− mice appears to be a relatively straightforward age-dependent degenerative phenotype. Understanding the mechanisms upstream of the impaired dendritic arbors will be an important direction for future study and may provide insight into potential FTD therapies. The transient increase in p-rpS6 (Ser235/236) and p-Akt (Ser473) with accompanying increase in amygdala dendritic arbors is an interesting phenotype that is presumably due to a transient alteration in an upstream signaling pathway. We were unable to elucidate upstream signaling cascades in this study, but our data argue against increases in mTORC1 or ERK signaling. rpS6 can also be phosphorylated at Ser235/236 by several other kinases (Fig. 7g), so it is possible that a signaling pathway involving G-protein coupled receptors is involved (Biever et al., 2015, Yano et al., 2014). As with the impaired prelimbic arbors, elucidating this upstream pathway is an important target for future investigation that could highlight potential FTD therapies. It will also be important to investigate the relationship between both phenotypes. In addition to being part of the salience network in humans, and a network that responds to social hierarchies in both humans and mice, the prelimbic cortex and amygdala are directly connected, as the prelimbic cortex innervates the central amygdala (Noonan et al., 2014, Seeley et al., 2009, Vertes, 2004, Zink et al., 2008). This raises the possibility that the age-dependent phenotypes reported in the prelimbic cortex and amygdala may not be independent. The relationship between the enhanced amygdala rpS6/Akt signaling and dendritic branching and the impaired prelimbic cortical basal arbors will be an important area for future investigation that could yield further insight into the mechanisms by which progranulin deficiency disrupts behavior.
Unlike Grn+/− mice, Grn−/− mice had neither tube test abnormalities (Fig. 6) nor the signaling abnormalities seen in Grn+/− mice (Fig. 7f). The lack of a tube test phenotype in Grn−/− mice fits with a growing body of data regarding the divergent effects of partial and complete progranulin deficiency. Grn−/− mice develop widespread lipofuscinosis and gliosis in the brain, while Grn+/− mice do not (Ahmed et al., 2010, Filiano et al., 2013, Gotzl et al., 2014, Wils et al., 2012, Yin et al., 2010b). This difference in pathology is similar to that seen in humans, as individuals heterozygous for loss-of-function GRN mutations develop FTD, while those homozygous for loss-of-function GRN mutations develop a lysosomal storage disorder, neuronal ceroid lipofuscinosis (Baker et al., 2006, Canafoglia et al., 2014, Cruts et al., 2006, Gass et al., 2006, Smith et al., 2012). Despite the fact that they may model different diseases, we did not previously observe differences between Grn+/− and Grn−/− mice in the three-chamber sociability test or fear conditioning (Filiano et al., 2013). Other studies have also reported abnormal behavior in Grn−/− mice, but did not compare them to Grn+/− mice (Ghoshal et al., 2012, Yin et al., 2010b). Another study that tested both Grn+/− and Grn−/− mice reported increased aggression in Grn−/−, but not Grn+/−, mice (Kayasuga et al., 2007). The elevated p-rpS6 and p-Akt levels in Grn+/−, but not Grn−/− mice may explain the presence of a dominant phenotype only in Grn+/− mice at around 6 months of age. Additionally, there are likely as yet uncharacterized differences in neuronal dysfunction that could explain the emergence of a low dominance phenotype in older Grn+/−, but not Grn−/− mice.
The sensitivity of the dominant phenotype of young Grn+/− mice to repeated testing highlights the importance of the testing protocol to reproducing tube test data. While no standard tube test protocol has been widely adopted, the 3-round design used in this study is comparable to other studies that have used repeated testing with 3–7 matches per mouse (Chachua et al., 2014, Long et al., 2004, Nishijima et al., 2006, Rodriguiz et al., 2004, Shahbazian et al., 2002, Spencer et al., 2005). Some of these studies have also observed a change in phenotype after testing for several rounds. Fmr1−/− mice lost a significant proportion of their matches versus wild-type mice in their first test, but no longer had a significant phenotype by their third test (Spencer et al., 2005). Conversely, male Brd2+/− mice did not exhibit a significant phenotype in their first test, but developed a dominant phenotype over tests 2 and 3 (Chachua et al., 2014). Neither of these studies reported habituating mice to the tube, so it is possible that the novelty of the tube affected performance in the first round as it appears to have done with 6–8 month-old Grn+/− mice.
We did not observe a significant sex difference in tube test behavior in Grn+/− mice, which is consistent with our previous observations of social behavior and fear conditioning in these mice (Filiano et al., 2013). However, others have noted greater impairment of male than female Grn−/− mice in other behavioral paradigms such as the open field, elevated plus maze, and novel object recognition tests (Chiba et al., 2009, Kayasuga et al., 2007, Petkau et al., 2012). The lack of a sex difference in tube test behavior of Grn+/− mice agrees with other tube test studies showing that this test may be used with female mice, though sex differences in behavior may occur in some mouse models (Chachua et al., 2014, Lijam et al., 1997, Lindzey et al., 1961).
In addition to more fully characterizing the social dominance phenotype of Grn+/− mice, another goal of this study was to evaluate the potential utility of the tube test for preclinical testing of potential FTD therapies in Grn+/− mice, given the rapid and simple administration of the test. Using the three-round testing design adopted for this study, the low social dominance phenotype of Grn+/− mice at age 9 months and older was robust, detected with repeated testing and a variety of experimental conditions including different apparatus designs, and independent of both mouse sex and experimenter sex. The three-round design we used in this study allows for efficient use of mice, as it provides the individual measure of winning percentage, which can be tracked to provide a before-after measure for each mouse in response to an experimental intervention. The robustness of the low social dominance phenotype to repeated testing makes such before-after testing possible, which is a useful feature for potential preclinical studies. Future studies should further evaluate how the tube test performs as a preclinical assay and should focus on the more robust, low dominance phenotype of older Grn+/− mice.
An important caveat to the discussion of the tube test dominance phenotypes of Grn+/− mice is that they may not reflect overall changes in dominance. Some studies fail to find correlation of tube test dominance with more ethologically relevant measures such as competition for food or access to female mice, though other studies have found that tube test dominance does predict these other behaviors (Benton, 1980, Greenberg et al., 2014, Lindzey et al., 1961, Rodriguiz et al., 2004, Van De Weerd et al., 1992, Wang et al., 2011). Instead, the low dominance phenotype of older Grn+/− mice could reflect a more general social withdrawal, given their reduced social interaction in the three-chamber sociability test. Similarly, many other mouse models with reduced social interaction also exhibit low dominance phenotypes in the tube test (Filiano et al., 2013, Jiang-Xie et al., 2014, Koh et al., 2008, Lijam et al., 1997, Long et al., 2004, Moretti et al., 2005, Nishijima et al., 2006, Shahbazian et al., 2002, Spencer et al., 2005, Veenstra-Vanderweele et al., 2012). Future studies with other tests for dominance behavior could help distinguish between these possibilities.
In summary, this study characterized a biphasic tube test phenotype in Grn+/−, but not Grn−/− mice that is associated with abnormal cell signaling and neuronal morphology in brain regions that are affected in FTD. The losing phenotype of older Grn+/− mice is robust and maintained across repeated testing and multiple testing conditions. These characteristics may make the tube test a useful preclinical measure for studies of potential therapeutics. Additionally, further study of the mechanisms upstream of the abnormal cell signaling and neuronal morphology described in this study could reveal aspects of the basic biology underlying FTD.
Supplementary Material
Acknowledgments
We thank James Black and Miriam Roberson for help with mouse colony maintenance and Robert Farese, Jr. for supplying progranulin knockout mice. This work was supported by a grant from the Consortium for Frontotemporal Dementia Research to E.D.R, an NICHD training grant (T32HD071866) and a Glenn/AFAR postdoctoral fellowship to A.E.A., and NRSA fellowships to A.E.A. (F32NS090678) and B.A.W. (F30AG046088). The authors declare no conflict of interest.
Abbreviations
- GRN
Progranulin
- FTD
Frontotemporal dementia
References
- Adenzato M, Cavallo M, Enrici I. Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48:2–12. doi: 10.1016/j.neuropsychologia.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S, Zhang Z, Coppola G, Mao W, Futai K, Karydas A, Geschwind MD, Tartaglia MC, Gao F, Gianni D, Sena-Esteves M, Geschwind DH, Miller BL, Farese RV, Jr, Gao FB. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell reports. 2012;2:789–798. doi: 10.1016/j.celrep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Barsuglia JP, Kaiser NC, Wilkins SS, Joshi A, Barrows RJ, Paholpak P, Panchal HV, Jimenez EE, Mather MJ, Mendez MF. A scale of socioemotional dysfunction in frontotemporal dementia. Arch Clin Neuropsychol. 2014;29:793–805. doi: 10.1093/arclin/acu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Benton D, Dalrymple-Alford John C, Brain Paul F. Comparisons of Measures of Dominance in the Laboratory Mouse. Animal Behaviour. 1980;28:1274–1279. [Google Scholar]
- Biever A, Puighermanal E, Nishi A, David A, Panciatici C, Longueville S, Xirodimas D, Gangarossa G, Meyuhas O, Herve D, Girault JA, Valjent E. PKA-dependent phosphorylation of ribosomal protein S6 does not correlate with translation efficiency in striatonigral and striatopallidal medium-sized spiny neurons. J Neurosci. 2015;35:4113–4130. doi: 10.1523/JNEUROSCI.3288-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell BN. Amygdaloid lesions and social dominance in the hooded rat. Psychon Sci. 1966;6:93–94. [Google Scholar]
- Canafoglia L, Morbin M, Scaioli V, Pareyson D, D’Incerti L, Fugnanesi V, Tagliavini F, Berkovic SF, Franceschetti S. Recurrent generalized seizures, visual loss, and palinopsia as phenotypic features of neuronal ceroid lipofuscinosis due to progranulin gene mutation. Epilepsia. 2014;55:e56–59. doi: 10.1111/epi.12632. [DOI] [PubMed] [Google Scholar]
- Chachua T, Goletiani C, Maglakelidze G, Sidyelyeva G, Daniel M, Morris E, Miller J, Shang E, Wolgemuth DJ, Greenberg DA, Veliskova J, Velisek L. Sex-specific behavioral traits in the Brd2 mouse model of juvenile myoclonic epilepsy. Genes Brain Behav. 2014;13:702–712. doi: 10.1111/gbb.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Matsuwaki T, Yamanouchi K, Nishihara M. Alteration in anxiety with relation to the volume of the locus ceruleus in progranulin-deficient mice. J Reprod Dev. 2009;55:518–522. doi: 10.1262/jrd.20239. [DOI] [PubMed] [Google Scholar]
- Cook C, Dunmore JH, Murray ME, Scheffel K, Shukoor N, Tong J, Castanedes-Casey M, Phillips V, Rousseau L, Penuliar MS, Kurti A, Dickson DW, Petrucelli L, Fryer JD. Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia. Neurobiol Aging. 2014;35:1769–1777. doi: 10.1016/j.neurobiolaging.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. Journal of visualized experiments : JoVE. 2012:e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Martens LH, Young AH, Warmus BA, Zhou P, Diaz-Ramirez G, Jiao J, Zhang Z, Huang EJ, Gao FB, Farese RV, Jr, Roberson ED. Dissociation of frontotemporal dementia–related deficits and neuroinflammation in progranulin haploinsufficient mice. J Neurosci. 2013;33:5352–5361. doi: 10.1523/JNEUROSCI.6103-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Lynch K, Ruan H, Almeida S, Verheyden JM, Seeley WW, Dickson DW, Petrucelli L, Sun D, Jiao J, Zhou H, Jakovcevski M, Akbarian S, Yao WD, Gao FB. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat Med. 2014;20:1444–1451. doi: 10.1038/nm.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL, 3rd, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Gass J, Lee WC, Cook C, Finch N, Stetler C, Jansen-West K, Lewis J, Link CD, Rademakers R, Nykjær A, Petrucelli L. Progranulin regulates neuronal outgrowth independent of sortilin. Mol Neurodegener. 2012;7:33. doi: 10.1186/1750-1326-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal N, Dearborn JT, Wozniak DF, Cairns NJ. Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol Dis. 2012;45:395–408. doi: 10.1016/j.nbd.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, Janssens J, van der Zee J, Lang CM, Kremmer E, Martin JJ, Engelborghs S, Kretzschmar HA, Arzberger T, Van Broeckhoven C, Haass C, Capell A. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–860. doi: 10.1007/s00401-014-1262-6. [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Howerton CL, Trainor BC. Fighting in the home cage: Agonistic encounters and effects on neurobiological markers within the social decision-making network of house mice (Mus musculus) Neurosci Lett. 2014;566:151–155. doi: 10.1016/j.neulet.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang-Xie LF, Liao HM, Chen CH, Chen YT, Ho SY, Lu DH, Lee LJ, Liou HH, Fu WM, Gau SS. Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol Autism. 2014;5:32. doi: 10.1186/2040-2392-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Koh HY, Kim D, Lee J, Lee S, Shin HS. Deficits in social behavior and sensorimotor gating in mice lacking phospholipase Cbeta1. Genes Brain Behav. 2008;7:120–128. doi: 10.1111/j.1601-183X.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, Decousus M, Hannequin D, Vera P, Lacomblez L, Camuzat A, Didic M, Puel M, Lotterie JA, Golfier V, Bernard AM, Vercelletto M, Magne C, Sellal F, Namer I, Michel BF, Pasquier J, Salachas F, Bochet J, Brice A, Habert MO, Dubois B French research network on FFM. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–3065. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Lukaszewska I, Korczynski R, Kostarczyk E, Fonberg E. Food-motivated behavior in rats with cortico-basomedial amygdala damage. Behav Neurosci. 1984;98:441–451. doi: 10.1037//0735-7044.98.3.441. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L, Finkbeiner S, Huang EJ, Robert V, Farese J. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122:3955–3959. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O. Physiological roles of ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol. 2008;268:1–37. doi: 10.1016/S1937-6448(08)00801-0. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Brykczynski T, Grossman SP. Differential effects of lesions in the amygdala, periamygdaloid cortex, and stria terminalis on aggressive behaviors in rats. J Comp Physiol Psychol. 1974;87:760–771. doi: 10.1037/h0036971. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Nguyen TA, Martens LH, Mitic LL, Farese RV., Jr Progranulin: at the interface of neurodegenerative and metabolic diseases. Trends in endocrinology and metabolism: TEM. 2013;24:597–606. doi: 10.1016/j.tem.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima I, Yamagata T, Spencer CM, Weeber EJ, Alekseyenko O, Sweatt JD, Momoi MY, Ito M, Armstrong DL, Nelson DL, Paylor R, Bradley A. Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior. Hum Mol Genet. 2006;15:3241–3250. doi: 10.1093/hmg/ddl402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Mars RB, Neubert FX, O’Reilly JX, Andersson JL, Mitchell AS, Bell AH, Miller KL, Rushworth MF. A neural circuit covarying with social hierarchy in macaques. PLoS Biol. 2014;12:e1001940. doi: 10.1371/journal.pbio.1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G, Feldman HH, Mackenzie IR, Raymond LA, Leavitt BR. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis. 2012;45:711–722. doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CL, Baranowski DC, Chitramuthu BP, Malik S, Li Z, Cao M, Minotti S, Durham HD, Kay DG, Shaw CA, Bennett HP, Bateman A. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci. 2009;10:130. doi: 10.1186/1471-2202-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Matsuwaki T, Yamanouchi K, Nishihara M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience. 2013;250:8–19. doi: 10.1016/j.neuroscience.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Thomanetz V, Angliker N, Cloetta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Ruegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol. 2013;201:293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weerd HA, van den Broek FA, Beynen AC. Removal of vibrissae in male mice does not influence social dominance. Behav Processes. 1992;27:205–208. doi: 10.1016/0376-6357(92)90177-F. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vochteloo JD, Koolhaas JM. Medial amygdala lesions in male rats reduce aggressive behavior: interference with experience. Physiol Behav. 1987;41:99–102. doi: 10.1016/0031-9384(87)90137-5. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van Dam D, Cuijt I, Joris G, De Deyn PP, Haass C, Van Broeckhoven C, Kumar-Singh S. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol. 2012;228:67–76. doi: 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- Yano T, Ferlito M, Aponte A, Kuno A, Miura T, Murphy E, Steenbergen C. Pivotal role of mTORC2 and involvement of ribosomal protein S6 in cardioprotective signaling. Circ Res. 2014;114:1268–1280. doi: 10.1161/CIRCRESAHA.114.303562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010a;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Dumont M, Banerjee R, Ma Y, Li H, Lin MT, Beal MF, Nathan C, Thomas B, Ding A. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J. 2010b;24:4639–4647. doi: 10.1096/fj.10-161471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71:736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.