Abstract
A tracheary element (TE) is a typical example of a cell type that undergoes programmed cell death in the developmental processes of vascular plants. The loss of the selective permeability of the tonoplast, which corresponds to tonoplast disintegration, occurred after the cells commenced secondary wall thickening and played a pivotal role in the programmed cell death of TEs in a zinnia (Zinnia elegans L.) cell culture. A search for events specifically associated with the TE vacuole provided an important clue to the understanding of the cell death mechanism. The transport of fluorescein, a fluorescent organic anion, across the tonoplast declined drastically in differentiating TEs. The capacity of the vacuole to accumulate the probe was also impaired. Treatment with probenecid, an inhibitor of organic anion transport, caused rapid cell death of TEs and led to the ultimate disruption of the vacuole even in other types of cultured cells. These changes in vacuolar properties during TE development were suppressed by cycloheximide. Specific mRNA accumulation in cells cultured in a TE differentiation-inductive condition was abolished by probenecid. These results suggest that a change in vacuolar membrane permeability promotes programmed cell death in TEs.
In recent years, much attention has been directed to the characterization of programmed cell death (PCD) mechanisms in plant cells (Greenberg, 1996; Jones and Dangl, 1996). As in animals, PCD is indispensable for the integral development or maintenance of various tissues and organs in multicellular plant species (Pennel and Lamb, 1997). Plant PCD occurs in leaf cells undergoing the hypersensitive response to preclude pathogen spread (e.g. Greenberg, 1997), in senescent leaf cells for the translocation of their components to other younger, growing parts (e.g. Smart, 1994), in reproductive organ cells to support fertilization or to supply nutrients for gametophyte and zygote development (e.g. Greenberg, 1996), and in root cortex cells to form aerenchyma for the efficient internal diffusion of oxygen (e.g. Justin and Armstrong, 1987). It remains unclear whether common regulatory mechanisms mediate these varied examples of PCD in plants (Fukuda, 1997b; Pennel and Lamb, 1997).
In the vascular systems, PCD plays a role in the construction of conduits such as vessels and tracheids that supply water. These conductive tissues consist of dead tracheary element (TE) cells that are highly differentiated to form a rigid, waterproof structure for long-distance water transport. Differentiation into such cells requires various genetically controlled mechanisms, including those involved in PCD (e.g. Jones and Dangl, 1996; Fukuda, 1997a).
Transdifferentiation of isolated zinnia (Zinnia elegans) mesophyll cells has been used to analyze TE differentiation both at the physiological and the molecular level (Fukuda and Komamine, 1980). In suspension culture, nearly one-half of all cells can be induced to become TEs semisynchronously (Fukuda and Komamine, 1980). This system very precisely reflects most of the genetic events that occur during the progression of TE differentiation in plants. For example, genes expressed prior to the initiation of secondary wall thickening (SWT) (TED2-4, CCoAMT, CAOMT, and ZePel) in cultured cells are also detected around the site of TEs of zinnia hypocotyls or roots in a temporally and spatially regulated manner (Demura and Fukuda, 1993, 1994; Ye et al., 1994; Ye and Varner, 1995; Domingo et al., 1998). Furthermore, Sato et al. (1993, 1995) characterized several peroxidases of zinnia, one of which was specifically activated in the differentiation-inductive culture. They proposed that this protein was selectively involved in lignin deposition in the secondary wall of TEs. The p48h-17 gene that encodes a Cys protease (Ye and Varner, 1996) and the ZRNaseI gene (Ye and Droste, 1996) were isolated as genes specifically expressed in the inductive condition and were demonstrated to localize around the vessels in situ. Such hydrolytic enzymes are assumed to function in the autolytic processes that produce hollow TEs (e.g. Fukuda, 1997a).
The cascade of gene expression underlying the cytological phenotype of cultured TEs can represent TE differentiation in situ (Fukuda, 1996). One of the TE-associated events, vacuole disruption, is known to occur prior to heavy lignin deposition and drastic autolysis (Burgess and Linstead, 1984a). The mixing of the vacuolar contents with the cytoplasm disorganizes the whole intracellular structure. Burgess and Linstead (1984b) confirmed its consistency by comparing TEs in culture with those in situ, and it has since been widely recognized as the critical event that leads to cell death and subsequent autolysis (Fukuda, 1992; Groover et al., 1997).
Various hydrolytic enzymes are synthesized for the progression of autolysis (Thelen and Northcote, 1989; Minami and Fukuda, 1995; Ye and Droste, 1996; Ye and Varner, 1996; Beers and Freeman, 1997; Fukuda, 1997a; Aoyagi et al., 1998). The enzymes are thought to accumulate in the TE vacuole until disruption of the vacuole, so that they do not begin to destroy the working molecular apparatus in the cytoplasm. The maintenance of vacuolar compartmentation should be necessary for differentiating TE cells to produce a complete set of these enzymes (Fukuda et al., 1998). If this is the case, some unknown mechanisms that bring about the disintegration of the vacuolar membrane and the release of enzymes must have a key role in the cell death program. A major question, therefore, is whether the vacuolar membrane of TEs exhibits specific visible characteristics leading to the disintegration. To address this question, a simple and efficient method to detect and quantify vacuolar malfunction and disruption in TEs is needed.
In this report, the transport of a fluorescent organic anion probe across the tonoplast was assayed in cultured cells to monitor vacuolar function. A drastic change in the distribution and thus the transport kinetics of the organic anion could be observed in cells before and after the initiation of SWT. This effect was controlled by some genetic program expressed in TEs, and interestingly was very similar to that caused by probenecid, an inhibitor of organic anion transport (Cole et al., 1990; Oparka et al., 1991; Wright and Oparka, 1994). Treatment with this compound was followed by acceleration of TE cell death and by the disruption of the vacuole even in other cultured zinnia cells. Probenecid affected the accumulation of mRNA of a marker gene for SWT in culture. This intrinsic inhibitory effect on the organic anion transport was coupled with the cell death program of TE differentiation.
MATERIALS AND METHODS
Plant Material and Culture
The first leaves of 14-d-old seedlings of zinnia (Zinnia elegans L. cv Canary Bird [Takii Shubyo, Kyoto]) were used for the isolation of mesophyll cells in suspension culture according to the method of Fukuda and Komamine (1980). All experiments were performed with cells cultured in inductive D medium that contained 0.1 mg/L α-naphthylacetic acid and 0.2 mg/L benzyladenine as hormones. The percentages of total (T), living (L), or dead (D) TEs were defined as follows: T = TEs/(TEs + other living cells) × 100, L = living TEs/(TEs + other living cells) × 100, D = dead TEs/(TEs + other living cells) × 100, where living cells mean those stained with fluorescein diacetate (FDA) so that their cytoplasm and vacuole were distinguishable. More than 500 cells were examined as one sample for the determination of these values. All assays were performed in triplicate within each experiment.
Microscopy
Observations were carried out with an epifluorescence microscope (model BH2-RFL-T2 or BX-50-FLA, Olympus, Tokyo) and an inverted microscope (IX 70, Olympus). To visualize the fluorescence of fluorescein, an excitation filter of 490 nm and a dichroic mirror of 500 nm were used. The filter and mirror were changed to 545 and 570 nm, respectively, for the observation of FM 4-64 fluorescence. The autofluorescence of chloroplasts was eliminated with a 460-nm barrier filter if necessary. Photographs were taken using a model PM-CBSP or PM-30 camera (Olympus) with black and white film (PREST 400, Fuji Photo Film, Tokyo), color film (Super G 400, Fuji Photo Film), or reversal film (Ektachrome 400, Kodak, Rochester, NY), and were selected to best illustrate the phenomenon described.
Treatment with Reagents and Inhibitors
FDA (Aldrich, Milwaukee, WI) and FM 4-64 (Molecular Probes, Eugene, OR) were added to the medium of each sample to a final concentration of 0.1 and 1 μg/mL, respectively. Probenecid (Wako Pure Chemical Industry, Osaka) was used at 100 μm unless otherwise specified. Cycloheximide (CHX) (Wako Pure Chemical Industry) was used at a concentration of 50 μm based on previous results (Fukuda and Komamine, 1983). The effects of the chemicals were assessed by microscopic analyses. For the determination of the number of cells excluding fluorescein from their vacuole, cells were subjected to a further 1-h incubation with FDA at the same temperature of the culture condition before observation.
RNA Gel-Blot Analysis
Zinnia cells were lysed by snap-freezing and subsequent incubation with phenol in buffer (200 mm Tris-Cl, 100 mm NaCl, and 10 mm EDTA, pH 8.0) in immediate vigorous vortexing. Total RNA was isolated by mixing the lysate with an equal volume of extraction buffer (50 mm Tris-Cl, 300 mm NaCl, and 5 mm EDTA, pH 8.0) followed by the microcentrifugation and precipitation of the contents in aqueous phase with ethanol. Remnant DNA was removed by RNA precipitation with one-third volume of 10 m LiCl at 4°C. RNA gel-blot analyses were carried out according to the method of Sambrook et al. (1989). The TED3 and ZCP4 probes were generated by PCR and labeled with digoxigenin-11-dUTP (Boehringer Mannheim, Basel) using cloned cDNAs as templates (Demura and Fukuda, 1993; Yamamoto et al., 1997). For signal detection, blocking reagent, anti-digoxigenin Fab fragment, and CDP-Star were used according to the manufacturer's instructions (Boehringer Mannheim). Signals were recorded by the exposure of membranes to radiographic films (Hyperfilm ECL, Amersham International, Buckinghamshire, Little Chalfont, UK).
RESULTS
Selective Permeability of the Tonoplast in Differentiating TEs
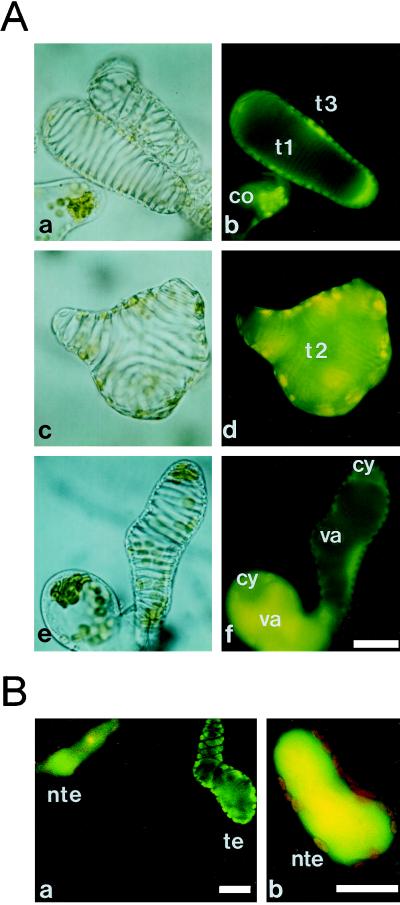
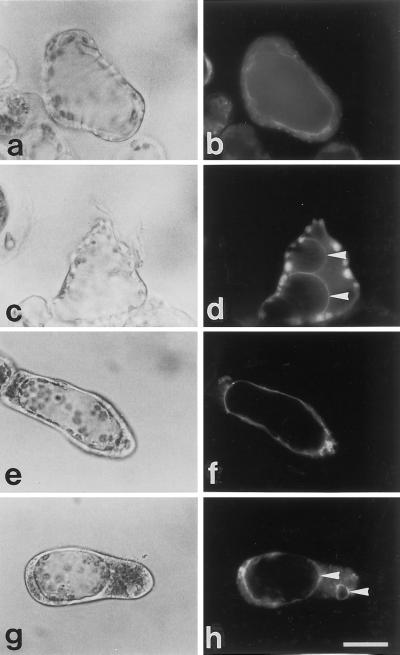
TEs are highly specialized, mortal cells that form the rigid, lignified secondary wall. Electron microscopic observations by Burgess and Linstead (1984a, 1984b) showed that there are many cells with both SWT and apparently intact subcellular components, indicating that TEs can survive for a certain period after the initiation of SWT. Since cells within the secondary wall always die, PCD has already been or is being expressed at this stage (Fukuda, 1997a). To analyze the kinetics of living TEs and to determine their longevity in zinnia cell culture, FDA, a vital dye, was applied. FDA is de-esterified in living cells to become fluorescein that can fluoresce. When FDA was added to culture at 55 h, three characteristic staining patterns of differentiating TE cells could be observed (Fig. 1A). In contrast with other intact cells, stained TEs could be categorized into those with green fluorescence in the cytoplasm (Fig. 1A, b, t1), those with yellow fluorescence in the whole cell (Fig. 1A, d, t2), and those with no fluorescence (Fig. 1A, b, t3), which correspond to living, dying, and dead TEs, respectively. These characteristics were designated as type 1 (Fig. 1A, b, t1), type 2 (Fig. 1A, d, t2), and type 3 (Fig. 1A, b, t3) for further analyses. FDA can diffuse across the plasma membrane, causing cytoplasmic fluorescence to appear immediately (Fig. 1A), whereas, in general, vacuole staining occurs very gradually (e.g. Yoshida, 1994) due to the physicochemical properties of the dye and membrane lipids (see “Discussion”). TEs immediately filled with yellow fluorescence (Fig. 1A, d, t2) indicate the lack of a functional vacuole in these cells.
Figure 1.
Light (a, c, and e) and fluorescence (b, d, and f) images of zinnia cells stained with FDA, some of which are differentiating into TEs. A, The staining patterns of TEs were categorized into living type 1 (t1, a and b), dying type 2 (t2, c and d), and dead type 3 (t3, a and b). After a 1-h incubation with FDA, fluorescein was transported to the vacuole of non-TE cells but remained in the cytoplasm of TEs (e and f). co, Cell that either has not yet differentiated or will never differentiate into a TE; cy, cytoplasm; va, vacuole. B, After a further 2-h incubation, non-TE cells (nte) sequestered fluorescein completely in their vacuole (a and b) but many TE cells (te) still excluded the dye (a). The red autofluorescence of the chloroplasts in the cytoplasm is visible in the non-TE cell. The bars indicate 20 μm.
After incubation with 0.1 μg/mL FDA for about 1 h, fluorescein in the cytoplasm usually began to enter the vacuole. Figure 1A, e and f, show the staining pattern of a TE and non-TE cell 1 h after FDA treatment. While non-TE cells allowed the dye to enter their vacuole, many TEs still had fluorescein only in their cytoplasm. More than one-half of all TEs exhibited this staining pattern. Because the cytoplasm and the vacuole were differently stained by this method, the vacuolar compartment was discernible even after the entrance of the dye into the vacuole (Fig. 1A, f). Further incubation resulted in the disappearance of cytoplasmic green fluorescence in all non-TE cells, because fluorescein was sequestered into vacuoles (Fig. 1B). However, many TEs still had fluorescein in their cytoplasm only (Fig. 1B). TEs therefore appeared to have a defect on fluorescein transport into the vacuole.
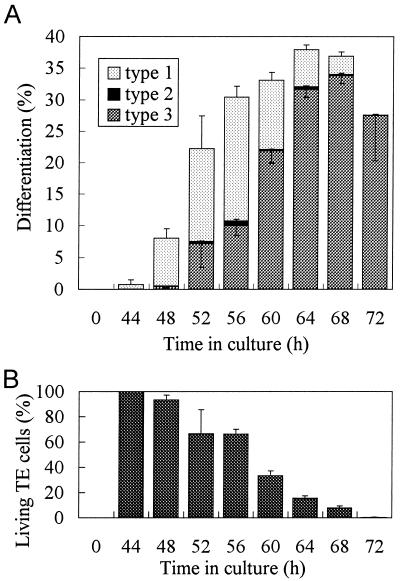
The time course of the appearance of each cell type was investigated through the differentiation process. Figure 2A shows how the percentages of TE cells with the characteristic staining changed. Until 4 h after the first appearance of TE cells, most of the TEs corresponded to type 1 cells. The percentage of type 1 cells then decreased, while the percentage of type 3 cells increased. At a maximum (at 56 h), about 20% of living TEs could be seen at a time. The number of type 2 cells was always small and constant throughout the entire culture process. All type 1 cells at each point were transformed to type 3 cells between 4 and 8 h, indicating that the longevity of living TEs in culture was about 6 h after the onset of SWT. Consequently, the percentage of living TEs in culture decreased gradually from 44 to 72 h (Fig. 2B).
Figure 2.
A, Time course of the appearance of TE types 1 to 3. Zinnia cells cultured for the indicated times were stained with FDA and examined immediately. The percentages of living (type 1), dying (type 2), and dead (type 3) TEs were calculated. B, The percentages of living TEs in the culture were recorded. Error bars represent sd.
Inhibition of Fluorescein Transport into the Vacuole of TEs
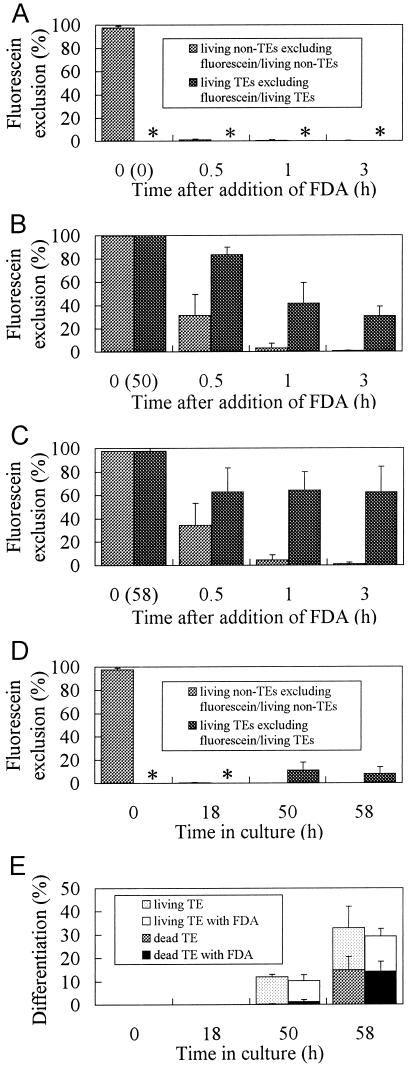
Since the above result (Fig. 1A, e and f) suggested that the tonoplast of TEs became less permeable to fluorescein, the number of TE and non-TE cells that excluded the dye from the vacuole was counted following FDA administration. Cells at 0, 50, or 58 h of culture were loaded with FDA and the percentages of TEs and non-TE cells excluding fluorescein from their vacuole were calculated separately (Fig. 3, A–C). As a control, cells incubated with FDA from the start of the culture were also examined (Fig. 3D). Figure 3A shows that almost all zinnia cells began to transport fluorescein into their vacuoles within 30 min after the addition of FDA to the medium. At 50 and 58 h (Fig. 3, B and C), when a substantial percentage of TEs had become apparent (Fig. 3E), fluorescein uptake also occurred but at a much slower rate. About 40% (B) or 60% (C) of living TEs excluded the dye from the vacuole even at 3 h. Thus, the accumulation of the dye into the TE vacuole was significantly inhibited. If FDA was loaded at the start of culture, the dye was completely sequestered in the vacuole of most mesophyll cells, but the majority of TEs exhibited both green and yellow fluorescence in the cytoplasm and vacuole, respectively. It was striking that 10% of TEs in the culture containing FDA from the beginning also excluded the dye at 50 and 58 h, albeit once they had sealed the dye in the vacuole before SWT (at 18 h, Fig. 3D). Because these TEs still contained fluorescein in their cytoplasm, the exclusion of fluorescein from the vacuole occurred concurrently with the inhibition of uptake. The difference in fluorescein exclusion of TEs at almost the same developmental stage (50 or 58 h) in Figure 3, B to D, strongly suggested that fluorescein can fluoresce even in the TE vacuole and that the lack of fluorescein in TEs (Fig. 3, B and C) was due to an inhibition of dye transport and not to degradation or the influence of vacuolar pH. Experiments designed to examine the direct uptake of fluorescein molecules by these cells through acid loading gave the same results (data not shown). The results shown in Figure 3E suggest that the amount of FDA used here had no effect on TE differentiation.
Figure 3.
Exclusion of fluorescein from the TE vacuole. Cells cultured for 0 (A), 50 (B), or 58 h (C) were stained with FDA for the indicated times at 26°C. The percentages of TEs and non-TE cells that do not contain fluorescein in their vacuole at all were calculated. Cells from the same culture but from another batch containing FDA from the start were sampled at indicated times and examined for the presence of fluorescein in vacuoles (D). The percentages of total TEs and the ratios of dead to living TEs in cultures in the presence or absence of FDA are shown in E. Asterisks (*) indicate that the parameter could not be defined at this time because TE formation had not yet occurred. Error bars represent sd.
Effects of Probenecid on the Viability of TE
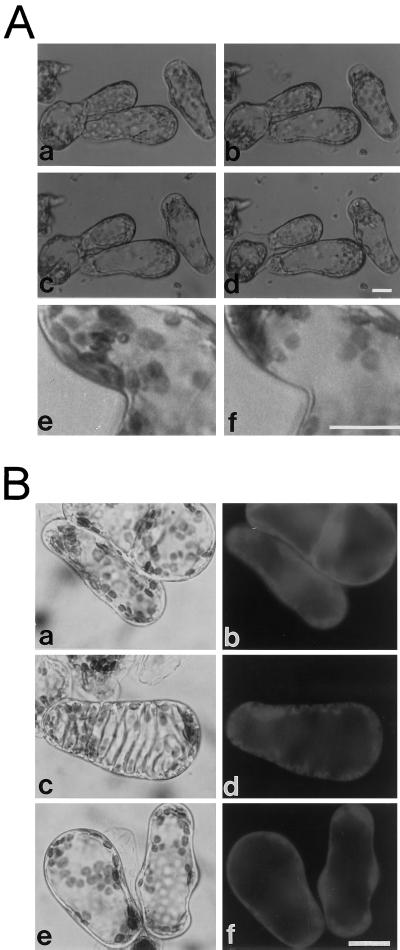
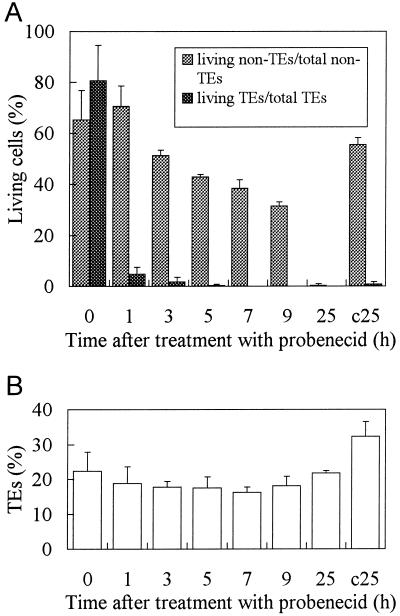
The effect of probenecid on TE development was tested because it is known to inhibit the transport of organic anions such as fluorescein derivatives across the tonoplast (Wright and Oparka, 1994). Probenecid altered the structure of cultured cells, and some laterally located cytoplasm disappeared (Fig. 4A). To compare non-TEs with TEs, cells were stained with FDA in the presence or absence of 100 μm probenecid at 55 h. Figure 4B, a and b, shows the fluorescent cytoplasm of non-TE cells. Like most plant mesophyll cells, those of zinnia leaves have a large central vacuole without any intervening cytoplasmic strands and basically maintain such an intracellular structure until about 72 h. The cytoplasm was distributed in a relaxed mode around the central vacuole of non-TE cells (Fig. 4B, a and b). But the cytoplasm of living TEs was compressed to form a very thin layer (Fig. 4B, c and d) as differentiation progressed. This layer ran along the inward protrusion of the thickened secondary walls. After probenecid treatment, the cytoplasm of non-TE cells became thinner, tense, and compressed around the vacuole due to vacuolar swelling, similar to those of TEs (Fig. 4B, e and f). Because all probenecid-treated non-TE cells continued to exclude fluorescein from their vacuole for more than 3 h, a concentration of 100 μm probenecid was also effective in blocking fluorescein transport across the tonoplast in zinnia cells. This concentration was significantly lower than those used in other cases (Oparka et al., 1991). Curiously, under these conditions, type 1 TEs were very rare even at 55 h, when they are usually abundant (Fig. 2). In fact, the percentage of living to total TEs was drastically decreased by probenecid treatment (Fig. 5A). When 100 μm probenecid was added 51 h after induction, almost all living TEs were dead within 1 h (Fig. 5A), in contrast to controls (Fig. 2B). Non-TE cells gradually died beginning at 3 h, and were completely dead by 25 h. Probenecid can therefore efficiently kill differentiating TE cells. The percentage of TEs to total cells at each time point was almost equal to that before addition (Fig. 5B), indicating that further TE formation was consequently aborted by probenecid-induced TE cell death.
Figure 4.
A, Alteration of cellular structure by probenecid. Zinnia cultured cells before (a) and after (b–d) treatment with probenecid were observed using an inverted microscope and a Petri dish. Several 1-μL drops of 0.1 mg/mL probenecid were carefully added to the cell suspension cultured for 50 h until visible changes occurred. Micrographs are arranged sequentially (a, before probenecid addition; b, 15 min after probenecid addition; c, 1 h after probenecid addition; d, after a prolonged incubation). Upon higher magnification of other cell images (e and f), the structural change is obvious and the cytoplasm before probenecid addition (e) became localized to the pole of cells by the treatment (f). B, Comparison of the morphological features of TEs with those of non-TE cells treated with probenecid. Light (a, c, and e) and fluorescence (b, d, and f) micrographs of zinnia cells cultured for 55 h and then stained with FDA for less than 10 min. Non-TE cells (a and b), TEs (c and d), and non-TE cells treated with probenecid (e and f) accumulated fluorescein in their cytoplasm, but the morphology of the cytoplasm and the vacuole of control non-TE cells was different from those of TEs and non-TE cells treated with probenecid. Swelling of the vacuole was evident in TEs and non-TE cells treated with probenecid. The bars indicate 20 μm.
Figure 5.
Cell death caused by probenecid. Cells were cultured for 51 h and then treated with 100 μm probenecid. A, The percentages of living TEs and non-TE cells were recorded separately. At 51 h, T and L values (%) were as follows: T(51) = 30.68 ± 6.08, L(51) = 24.18 ± 1.30. B, The percentage of TEs to total cells (including dead cells) in the culture was shown. In this case, the conventional T value does not make sense because non-TE cells were also killed by this treatment. c25, Sample of cells in a control batch in which cells have been cultured for 76 h in the absence of probenecid. Error bars represent sd.
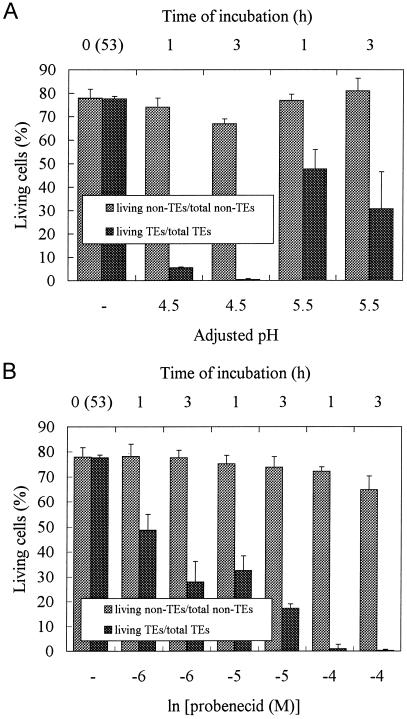
Probenecid is a weak acid and its permeation into cells is influenced by medium pH, so the effect of pH on TE viability was investigated. Cells cultured for 53 h were incubated in the presence of 100 μm probenecid for 1 to 3 h at either pH 4.5 or 5.5 (Fig. 6A). The medium pH at 53 h of culture declined to 4.8 (Roberts and Haigler, 1994) and was adjusted with 1 n KOH just after the addition of probenecid. More TEs were killed by 100 μm probenecid at pH 4.5 than at pH 5.5, due to the abundance of the undissociated form of probenecid at pH 4.5 (Fig. 6A). Therefore, probenecid may only be effective once it has entered TEs. Under the same pH conditions, an increase in probenecid concentration had a more severe effect on TEs within 1 h, while the viability of non-TE cells was almost unaffected up to 3 h (Fig. 6B). The culture medium was replaced with 20 mm 2-(N-morpholino)-ethanesulfonic acid (MES) medium (pH 4.7) just before the experiment to prevent the differential effect of probenecid by medium pH. The probenecid effect on living TEs is therefore dose dependent.
Figure 6.
Effect of pH and probenecid concentration on cell viability. A, Dependence of the probenecid effect on medium pH. Probenecid at 100 μm was added at 53 h to culture media, the pH of which was adjusted to 4.5 or 5.5 with the appropriate amounts of 1 n KOH just after probenecid addition. The percentages of TE and non-TE cells were examined separately at 1 and 3 h after the addition of probenecid. T(53) = 23.62 ± 4.69, L(53) = 18.34 ± 3.64. B, Dependence of the probenecid effect on the probenecid concentration under the same pH (4.7) medium. The medium of zinnia cell culture was buffered with 20 mm MES (pH 4.7) at 53 h after induction and supplied with 100 to 1 μm probenecid. The percentages of TE and non-TE cells were calculated separately 1 and 3 h after the treatment with probenecid. T(53) = 23.62 ± 4.69, L(53) = 18.34 ± 3.64. Error bars represent sd.
To determine whether probenecid also killed non-TE cells by disrupting the vacuole, vacuole morphology was examined using FM 4-64, which specifically stains the yeast vacuolar membrane (Vida and Emr, 1995). FM 4-64 selectively stained the tonoplast of zinnia cells (Fig. 7) when added to the culture from the start, although it significantly retarded and reduced TE differentiation (data not shown). The tonoplast of TEs formed a wave along the pattern of secondary walls (Fig. 7, a and b). When the vacuole disrupted, it shrank and fragmented (Fig. 7, c and d). The swollen vacuole of non-TE cells (Fig. 7, e and f) also ruptured and shrank after 6 h of incubation in 100 μm probenecid, similar to TEs (Fig. 7, g and h).
Figure 7.
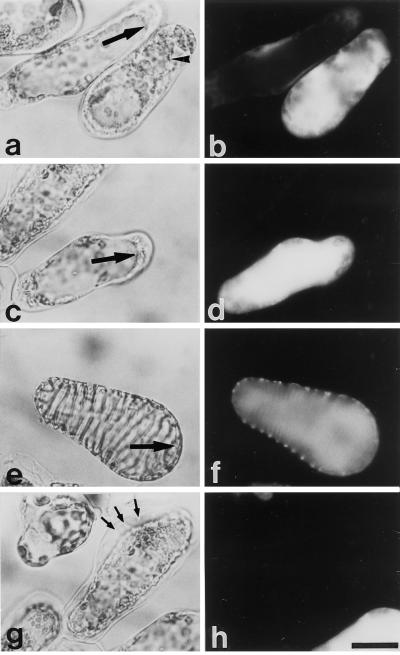
Vacuole disruption visualized by FM 4-64. FM 4-64 was added to the culture from the beginning for the selective staining of the vacuole. The light (a, c, e, and g) and fluorescence (b, d, f, and h) micrographs of zinnia cells are shown. TE (a and b) and non-TE cells treated with 100 μm probenecid for 1 h (e and f) have a large central vacuole. The vacuole of TEs ruptured to form small, fragmented vacuoles (arrowhead, c and d). Similarly, treatment with probenecid for 6 h caused the rupture, fragmentation, and shrinkage of the vacuole of non-TE cells (arrowhead, g and h). The bar indicates 20 μm.
The relationship between tonoplast selective permeability and vacuole shape was also investigated using probenecid and FDA. Although the dye was confined to the cytoplasm in all living cells treated with 100 μm probenecid at this time of culture (55 h), incubation for another 6 h killed non-TEs significantly (Fig. 5A). Many non-TE cells were first filled evenly with fluorescein, after which the fluorescence soon faded away. Among such cells, one cell filled with fluorescein, one cell precluded dye entry into the vacuole, and a third cell was dead as shown at the right, center, and left, respectively in Figure 8, a and b. The tonoplast of the cell at the right clearly lost selective permeability, like type 2 TE cells (Fig. 1A, c and d), whereas that of the middle cell retained selective permeability to fluorescein. In a bright-field image, the large compartment of the central vacuole was not seen in the cell (at the right), and a spherical, balloon-like structure was observed instead. This was composed of the fragmented tonoplast and was probably the same as those found in dying TE cells (Fig. 8, e and f; Groover et al., 1997). These morphological data indicate that probenecid can also induce cell death in non-TEs via the loss of tonoplast selective permeability, and that the resultant fragmented vacuole loses membrane integrity. Furthermore, another cell shown in Figure 8, c and d, had lost its tonoplast selective permeability during probenecid treatment, although the shape of its central vacuole apparently re-mained intact. Figure 8, e and f, shows dying TEs that still had the boundary between the cytoplasm and the central vacuole. The existence of these cells strongly suggested that the loss of tonoplast selective permeability occurred before the physical fragmentation of the vacuolar membrane both in TEs and in probenecid-treated non-TE cells.
Figure 8.
Tonoplast disintegration of cells treated with probenecid. Light (a, c, e, and g) and fluorescence (b, d, f, and h) micrographs of zinnia cells. Non-TE cells that were treated with 100 μm probenecid for 6 h after 55 h of culture (a–d) were stained with FDA for 1 h. Large arrows point to the tonoplast (a, c, and f). The arrowhead indicates the fragmented, spherical vacuole formed after the disruption of the central vacuole (a). TEs incubated with FDA for 1 h (e and f) lost their tonoplast selective permeability without any drastic changes in the boundary between the cytoplasm and the vacuole (large arrow). Dead non-TE cells and differentiating TE cells killed by 100 μm probenecid (g and h) did not autolyze their contents. Small arrows indicate the slightly thickened secondary wall of a very young TE cell killed by probenecid. The bar indicates 20 μm.
In contrast to the formation of TE (Fukuda 1997a, 1997b; Groover et al., 1997), non-TE cells killed by probenecid plasmolyzed and did not autolyze (Fig. 8, g and h). This probenecid treatment produced many dead cells with only slight SWT, the contents of which also remained undigested (Fig. 8, g and h). Therefore, probenecid could disrupt the vacuole but could not induce autolysis.
Cell Death Suppressed by Cycloheximide (CHX)
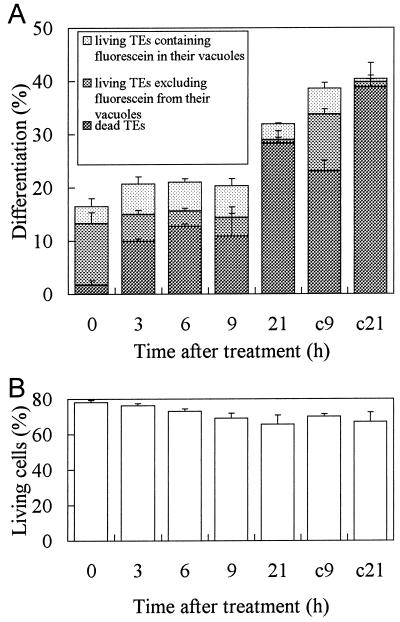
To examine whether the inhibition of organic anion transport into the TE vacuole was regulated by the cell death program, CHX, an inhibitor of cellular protein synthesis, was applied to the culture. The influence of CHX on TE formation differed depending on the timing of inhibitor added to the culture. When zinnia mesophyll cells were treated with CHX from the start of the culture (Fukuda and Komamine, 1983), SWT was completed prevented. At 50 h, when about 20% of cells had commenced wall synthesis, they responded to this antibiotic in a different manner. As shown in Figure 9A, CHX treatment up to 9 h prevented the differentiation of cells into TEs, as shown by the low percentage of TE cells (22%) relative to the untreated control (40%). Moreover, the increase in dead TEs was also blocked from 3 to 9 h. The proportion of TEs excluding fluorescein from their vacuole decreased dramatically among the total living TEs. Therefore, differentiation, cell death, and fluorescein exclusion from the vacuole of TEs were all suppressed by CHX treatment. The parallel changes in cell death and vacuolar exclusion of fluorescein suggest that biochemical changes had occurred, resulting in a loss of tonoplast integrity. Fluorescein exclusion by the vacuole of living TEs also clearly requires new or continuous expression of a certain protein(s) associated with TE formation.
Figure 9.
Effect of CHX on TE differentiation, cell death, and the inhibition of fluorescein transport across the tonoplast. A, CHX at 50 μm was added to culture at 50 h. The percentages of TEs, living TEs, and living TEs whose vacuole was not fluorescent were determined. The parameters presented were dead TEs, living TEs containing fluorescein in their vacuole, living TEs excluding fluorescein from the vacuole. B, The percentages of living non-TE cells were not affected by the 50 μm CHX treatment of this experiment. c9 and c21, Patterns of differentiating TEs in control batches in which cells have been cultured for 59 and 71 h in the absence of CHX, respectively. Error bars represent sd.
The effect of CHX was attenuated by 21 h possibly due to partial CHX detoxification as seen in yeast growth (Meyers et al., 1992), such that differentiation and cell death could progress to nearly the control level (Fig. 9A). The percentage of living non-TE cells in culture was almost constant irrespective of CHX treatment (Fig. 9B). CHX at 50 μm did not have any significant nonspecific toxic effect on zinnia cells. The ratio of non-TE cells excluding fluorescein from their vacuole to total living non-TE cells was much smaller than that of TEs throughout this experiment (data not shown). The treatment did not affect the fluorescein uptake into the vacuole of non-TE cells.
Accumulation of Marker Genes for TE Differentiation Could Be Altered by Probenecid
To investigate the effect of probenecid on mRNA accumulation in cultured cells, RNA gel-blot analyses were performed using probes for two marker genes, TED3 and ZCP4. Although in situ studies showed RNA expression of the TED3 and the Cys protease genes (p48-17 and ZCP4) associated with TE cells (Demura and Fukuda, 1994; Ye and Varner, 1996; Igarashi et al., 1998; A. Minami and H. Fukuda, unpublished results), the regulation of expression appears to differ markedly. TED3 mRNA is steadily expressed after 36 h, but ZCP4 mRNA only appears from 48 to 72 h in this culture system (Fukuda, 1997a). All known TE-associated genes exhibit one of these expression patterns in cultured cells (Fukuda, 1997a). The expression of these genes represents two distinct regulatory points in the TE differentiation program, although visible changes such as SWT or the production of other hydrolytic enzymes start temporally together with the latter ZCP4 expression.
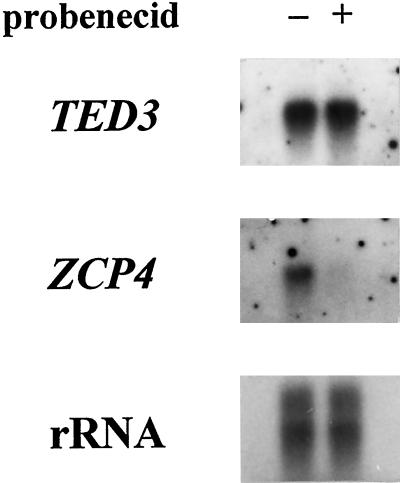
Total RNA samples from cells incubated for 1 h with or without 100 μm probenecid from 57 h after induction, when transcripts of these genes had accumulated as described previously (Fukuda, 1997a), were probed with TED3 and ZCP4. The results shown in Figure 10 indicate that ZCP4 mRNA accumulation was abolished by a 1-h treatment with probenecid, whereas TED3 mRNA was maintained at a constant level in 2 μg of total RNA. These data strongly suggest that ZCP4 mRNA accumulated mainly in cells that could be selectively killed by probenecid treatment, and strongly support the observation of selective TE cell death that resulted from this treatment (Fig. 5A). Meanwhile, TED3 was equally expressed in these cells irrespective of the difference in susceptibility to probenecid. Probenecid therefore affects the cells that have expressed the latter TE-differentiation program, suggesting the involvement of the latter regulatory point in the changes in the vacuolar properties of TEs. These results are summarized in Figure 11.
Figure 10.
RNA gel-blot analyses using digoxigenin-labeled cDNA probes for TED3 and ZCP4 genes. The total RNA of cells cultured for 57 h and subsequently treated with (+) or without (–) 100 μm probenecid for 1 h was extracted. In each lane 2 μg of total RNA was loaded. rRNA was displayed by staining with methylene blue (Sambrook et al., 1989).
Figure 11.
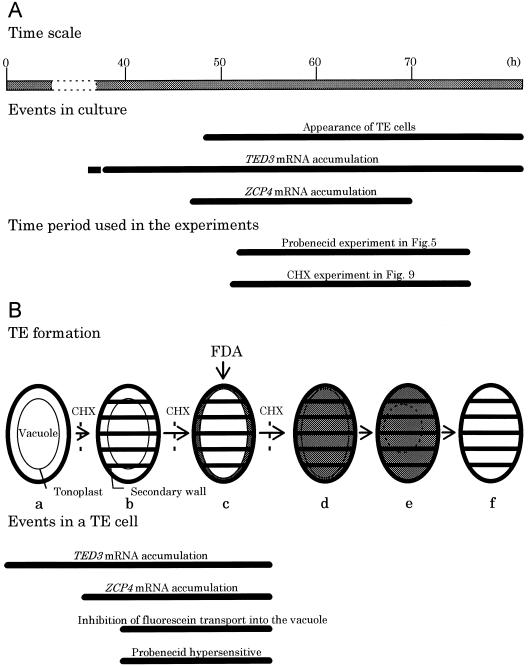
A simple model of events occurring during TE PCD. A, Related events occurring in cultured cells (Fukuda, 1997a) after hormonal induction (0 h) are displayed with time periods selected for two experiments studying the effect of probenecid and cycloheximide (Figs. 5 and 9). B, Events occurring in a single, differentiating TE are shown with an emphasis on the changes in the vacuole. A general model of TE PCD was made by Groover et al. (1997). Shaded areas indicate the distribution of fluorescein when FDA is added to the culture at stage c. CHX suppresses progression to stage b, c, and d (Fig. 9). Related events and vacuolar properties are also shown. a, Before TE differentiation; b, secondary wall formation; c, vacuole swelling, inhibition of fluorescein transport into the vacuole, and fluorescein exclusion from the vacuole; d, loss of the selective permeability of the tonoplast; e, tonoplast shrinkage and fragmentation; f, a dead TE.
DISCUSSION
Change in Tonoplast Permeability during TE Formation
FDA has been used as a probe to monitor vacuolar function and/or properties during TE differentiation. This dye is highly lipophilic and readily passes through the plasma membrane of a cell; its acetyl ester groups are then cleaved by intracellular esterase activity to generate fluorescein. Most of the fluorescein molecules in the cytoplasm are in the polar, dissociated form and are therefore retained within the cell (Oparka, 1991). The selective permeability of the membrane restricts free passage of electrolytes across the membrane and maintains cell integrity. This dye was used to identify living and dead TEs in the zinnia cell culture (Fig. 1A, a–d). Three characteristic staining patterns were recognized: cells with green fluorescence in their cytoplasm, cells with yellow fluorescence in the whole cell, and cells with no fluorescence. The difference in these fluorescence spectra reflects the variant molecular forms of fluorescein affected by the pH of the milieu where the dye is present (Martin and Lindqvist, 1975). The cytoplasmic and vacuolar pH are generally about 7.0 and 5.5, respectively. At physiological range, the fluorescence of the yellow wavelength area becomes conspicuous as fluorescein is exposed to the solutions of lower pH. The first and the last cell types obviously correspond to the living and dead states of TEs, respectively. The second type of TE has just lost the tonoplast selective permeability to both fluorescein and H+, and only accounted for a small number of dying cells undergoing tonoplast disintegration (Fig. 2A). Closer examination indicated that after the ester groups of FDA were cleaved, the dye was transported to the vacuole of non-TE cells, but remained in the cytoplasm of differentiating live TEs (Fig. 1A, f, and Fig. 1B). The time course of the appearance of cells with or without fluorescein in their vacuoles revealed that the inhibition of fluorescein transport across the tonoplast was correlated with the formation of TEs (Fig. 3, B and C). Moreover, since the number of TEs excluding fluorescein from their vacuoles was variable depending on the timing of FDA addition to culture (Fig. 3, B–D), one can conclude that both the inhibition of uptake across the tonoplast and the exclusion of fluorescein from the vacuole accompany TE formation.
Oparka et al. (1991) and Wright and Oparka (1994) determined that a probenecid-sensitive, ATP-dependent organic anion transporter is responsible for the transport of fluorescein derivatives into the vacuoles of plant cells. Organic anion transporters that can be affected by probenecid are also detected in vitro in the tonoplast of barley or rye leaf cells (Fig. 1; Blake-Kalff and Coleman, 1996; Klein et al., 1997). Probenecid addition appeared to promote and reinforce the inhibition of transport in differentiating, living TEs, whereas it caused the initiation of transport inhibition in non-TE cells. Thus, probenecid killed living TEs rapidly and selectively and then killed non-TE cells after a significant time lag (Fig. 5A). The possible target of probenecid in TE cells is not on the outer surface but in the cytoplasm (Fig. 6A), and the deleterious effect of probenecid is dose dependent (Fig. 6B). Thus, the probenecid effect could at least in part mimic developmental changes in the TE vacuole.
In addition, many features of differentiating, living TEs are similar to those of probenecid-treated cells. In contrast to fluorescein, Lucifer Yellow, a hydrophilic dye with a much lower pKa value, did not leak from the vacuole following probenecid treatment (Oparka et al., 1991). When the dye was added to culture at the beginning, substantial accumulation could be observed in the vacuole of all cells but not in the cytoplasm. TEs could be formed even in such a situation but, unlike fluorescein, Lucifer Yellow did not leak from the TE vacuole until the very moment of its disruption (data not shown). Also, the vacuoles of TEs became swollen as differentiation progressed (Fig. 4B, c and d). This vacuole swelling also occurred in non-TE cells treated with probenecid (Fig. 4B, e and f; Oparka et al., 1991). Enhanced vesiculation in the cytoplasm is reported to occur both in TEs (Groover et al., 1997) and in onion epidermal cells fed with probenecid (Oparka et al., 1991). Furthermore, Groover et al. (1997) showed that the vacuolar membrane shrinks inwardly when the TE vacuole collapses (Fig. 7, c and d). Non-TE cells treated with probenecid also died from the disintegration of their tonoplasts (Fig. 7, e–h; Fig. 8, a–d) in the same manner as TEs (Fig. 8, e and f). The small, spherical structure of the fragmented tonoplast that results from shrinkage was formed in dying non-TE cells (Fig. 7, g and h; Fig. 8, a and b) in the same manner as TEs (Fig. 7, c and d). These findings show that all of the known effects of probenecid can occur in differentiating, living cells, and suggest that these effects play an important role in vacuole disruption.
It is possible that the effects of probenecid on TE development are inevitable consequences of anion transport inhibition. For example, the unbalanced distribution of electrolytes across the membrane could perturb the membrane potential and the total transport system. Disruption of cellular osmoregulation could thus have deleterious effects on cellular homeostasis. Probenecid might alter the water potential gradient across the tonoplast by blocking organic anion transporters and possibly other transport systems, leading to tonoplast disintegration. Probenecid has been shown to perturb the osmoregulatory mechanisms of guard cells, forcing them to remain swollen (Schwartz et al., 1995). Furthermore, a certain class of ATP-dependent drug transporters prevent the swelling-induced membrane disintegration of animal cells (Roman et al., 1997). This class of transporters on plant cell vacuoles (Rea et al., 1998) may perform a similar function in zinnia cultured cells, so that probenecid inhibition of those transporters can lead to a loss of membrane integrity. Developing TEs have intrinsic inhibition of organic anion transport (Figs. 1 and 3). Probenecid may reinforce this effect and cause rapid cell death of TEs through tonoplast disintegration. These functions are distinct from those of proton pumps or aquaporins. Organic anion uptake by the vacuoles is independent from vacuolar pH and H+-ATPase inhibition (Blake-Kalff and Coleman, 1996; Klein et al., 1997), and the alkalization of the vacuole does not seem to cause vacuole disruption in cultured cells (Matsuoka et al., 1997). Aquaporins (Maurel, 1997) cannot continue to transport water against a water potential gradient until a membrane compartment disrupts.
One criterion to discriminate PCD processes from necrotic death is the dependence of cell death on specific gene expression. Figure 9A shows the response of living TEs to CHX, which suppressed SWT, death, and the exclusion of fluorescein molecules from the vacuole of TEs. These findings suggest that not only vacuole disruption but also the change in permeability of the tonoplast resulted from the function of a certain gene product(s) that appears at TE formation. The possibility that some necrotic effects such as spontaneous lipid deterioration are responsible for the tonoplast disintegration was completely ruled out. Even after the initiation of SWT, the vacuole disruption of TEs is still under the control of a genetic program.
Transport Inhibition and TE Cell Death Program
Possible regulatory mechanisms of plant PCD include Ca2+-mediated processes (Greenberg, 1997; O'Brien et al., 1998) and oligosaccharide-dependent processes (McCann, 1997). These molecules also function in TE differentiation (Roberts and Haigler, 1989, 1990; Roberts et al., 1997; Domingo et al., 1998). In particular, Ca2+ influx and extracellular proteolysis mediate the death of zinnia cells (Groover and Jones, 1999). However, events directly related to the vacuolar membrane of TEs had not been found. As indicated above, the organic anion transport system across the tonoplast was impeded in cultured zinnia cells that began to show SWT. Since cells that develop SWT eventually die from vacuole disruption, events that occur specifically in the TE vacuole must be important in the cell death of TEs. The inhibitory effect on organic anion transport could lead to vacuole disruption (Figs. 5A, 7, and 8) even in non-TE cells. Moreover, the effects of transport inhibition are distinct from those of the reagents tested by Groover and Jones (1999), which killed all zinnia cultured cells immediately. Probenecid may act on another, novel event in the process of vacuole disruption.
RNA gel-blot analyses revealed that probenecid abolished ZCP4 mRNA accumulation within 1 h (Fig. 10). Cells expressing ZCP4 were killed rapidly and selectively, suggesting that the intrinsic inhibitory effect of fluorescein transport, which could be promoted by probenecid treatment, occurred in parallel with ZCP4 expression. It is unlikely, however, that probenecid, a xenobiotic compound, has a key role in the actual differentiation process of TEs in vivo. Some genetic mechanism(s) may exist that can bring about the same effect. The result of the experiment with CHX (Fig. 9) strongly supports this idea.
In mammalian hepatocytes, probenecid treatment invokes the expression of a particular gene (Gant et al., 1995). However, probenecid drastically decreased ZCP4 mRNA in culture and did not induce ZCP4 expression in non-TE cells (Fig. 10). Probenecid probably does not induce specific genes to exert its disruptive effect, much less activate the entire genetic program of TE formation. Not all of the TE formation processes (Groover et al., 1997) were mimicked by probenecid-treated non-TE cells. Non-TE cells and immature TEs killed by probenecid did not autolyze their contents (Fig. 8, g and h) but, rather, morphologically resembled those reported on PCD in a diluted carrot suspension culture (McCabe et al., 1997; Pennell and Lamb 1997). The coordinated progress of the program of TE formation may require other regulatory mechanisms that would establish a background such as sufficient production of hydrolytic enzymes to function in autolysis. The inhibition of organic anion transport into the vacuole alone does not regulate total TE formation. However, given the critical role of vacuole collapse as the trigger of TE cell death, further analysis of intrinsic transport inhibition at the molecular level may reveal the fatal signal generated by the TE cell death program.
ACKNOWLEDGMENTS
Many thanks to Prof. H. Fukuda (University of Tokyo) for critical reading of the manuscript and helpful discussions and to his colleagues for their kind gifts of cDNA clones.
Footnotes
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and the Japan Society for the Promotion of Science.
LITERATURE CITED
- Aoyagi S, Sugiyama M, Fukuda H. BEN1 and ZEN1 cDNAs encoding S1-type DNases that are associated with programmed cell death in plants. FEBS Lett. 1998;429:134–138. doi: 10.1016/s0014-5793(98)00563-8. [DOI] [PubMed] [Google Scholar]
- Beers EP, Freeman TB. Proteinase activity during tracheary element differentiation in zinnia mesophyll cultures. Plant Physiol. 1997;113:873–880. doi: 10.1104/pp.113.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake-Kalff MMA, Coleman JOD. Detoxification of xenobiotics by plant cells: characterization of vacuolar amphiphilic organic anion transporters. Planta. 1996;200:426–431. [Google Scholar]
- Burgess J, Linstead P. In-vitro tracheary element formation: structural studies and the effect of tri-iodobenzonic acid. Planta. 1984a;160:481–489. doi: 10.1007/BF00411135. [DOI] [PubMed] [Google Scholar]
- Burgess J, Linstead P. Comparison of tracheary element differentiation in intact leaves and isolated mesophyll of Zinnia elegans. Micron Microscop Acta. 1984b;15:153–160. [Google Scholar]
- Cole L, Coleman J, Evans D, Hawes C. Internalization of fluorescein isothiocyanate and fluorescein isothiocyanate-dextran by suspension-cultured plant cells. J Cell Sci. 1990;96:721–730. [Google Scholar]
- Demura T, Fukuda H. Molecular cloning and characterization of cDNAs associated with tracheary element differentiation in cultured Zinnia cells. Plant Physiol. 1993;103:815–821. doi: 10.1104/pp.103.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Fukuda H. Novel vascular cell-specific genes whose expression is regulated temporally and specially during vascular system development. Plant Cell. 1994;6:967–981. doi: 10.1105/tpc.6.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Roberts K, Stacey N, Connerton I, Ruíz-Teran F, McCann M. A pectate lyase from Zinnia elegans is auxin inducible. Plant J. 1998;13:17–28. doi: 10.1046/j.1365-313x.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Tracheary element formation as a model system of cell differentiation. Int Rev Cytol. 1992;136:289–332. [Google Scholar]
- Fukuda H. Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:299–325. doi: 10.1146/annurev.arplant.47.1.299. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Tracheary element differentiation. Plant Cell. 1997a;9:1147–1156. doi: 10.1105/tpc.9.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H. Programmed cell death during vascular system formation. Cell Death Differ. 1997b;4:684–688. doi: 10.1038/sj.cdd.4400310. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Changes in the synthesis of RNA and protein during tracheary element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Plant Cell Physiol. 1983;24:603–614. [Google Scholar]
- Fukuda H, Watanabe Y, Kuriyama H, Aoyagi S, Sugiyama M, Yamamoto R, Demura T, Minami A. Programming of cell death during xylogenesis. J Plant Res. 1998;111:253–256. [Google Scholar]
- Gant TW, O'Connor CK, Corbitt R, Thorgeirsson U, Thorgeirsson SS. In vivo induction of liver P-glycoprotein expression by xenobiotics in monkeys. Toxicol Appl Pharmacol. 1995;133:269–276. doi: 10.1006/taap.1995.1151. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Groover A, DeWitt N, Heidel A, Jones A. Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma. 1997;196:197–211. [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Demura T, Fukuda H. Expression of the Zinnia TED3 promoter in developing tracheary elements of transgenic Arabidopsis. Plant Mol Biol. 1998;36:917–927. doi: 10.1023/a:1005977624631. [DOI] [PubMed] [Google Scholar]
- Jones AM, Dangl JL. Logjam at the Styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characterization of roots and plant response to soil flooding. New Phytol. 1987;106:465–495. [Google Scholar]
- Klein M, Martinoia E, Weissenböck G. Transport of lucifer yellow CH into plant vacuoles: evidence for direct energization of a sulphonated substance and implications for the design of new molecular probes. FEBS Lett. 1997;420:86–92. doi: 10.1016/s0014-5793(97)01492-0. [DOI] [PubMed] [Google Scholar]
- Martin MM, Lindqvist L. The pH dependence of fluorescein fluorescence. J Lumin. 1975;10:381–390. [Google Scholar]
- Matsuoka K, Higuchi T, Maeshima M, Nakamura K. A vacuolar-type H+-ATPase in a nonvacuolar organelle is required for the sorting of soluble vacuolar protein precursors in tobacco cells. Plant Cell. 1997;9:533–546. doi: 10.1105/tpc.9.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Levine A, Meijer P-J, Tapon NA, Pennell RI. A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 1997;12:267–280. [Google Scholar]
- McCann MC. Tracheary element formation: building up to a dead end. Trends Plant Sci. 1997;2:333–338. [Google Scholar]
- Meyers S, Schauer W, Balzi E, Wagner M, Goffeau A, Golin J. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr Genet. 1992;21:431–436. doi: 10.1007/BF00351651. [DOI] [PubMed] [Google Scholar]
- Minami A, Fukuda H. Transient and specific expression of a cysteine endopeptidase during autolysis in differentiating tracheary elements from Zinnia mesophyll cells. Plant Cell Physiol. 1995;36:1599–1606. [PubMed] [Google Scholar]
- O'Brien IEW, Baguley BC, Murray BG, Morris BAM, Ferguson IB. Early stages of the apoptotic pathway in plant cells are reversible. Plant J. 1998;13:803–814. [Google Scholar]
- Oparka KJ. Uptake and compartmentation of fluorescent probes by plant cells. J Exp Bot. 1991;42:565–579. [Google Scholar]
- Oparka KJ, Murant EA, Wright KM, Prior DAM, Harris N. The drug probenecid inhibits the vacuolar accumulation of fluorescent anions in onion epidermal cells. J Cell Sci. 1991;99:557–563. [Google Scholar]
- Pennel RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea PA, Li Z-S, Lu Y-P, Drozdowicz YM, Martinoia E. From vacuolar GS-X pumps to multispecific ABC transporters. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:727–760. doi: 10.1146/annurev.arplant.49.1.727. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Donovan SG, Haigler CH. A secreted factor induces cell expansion and formation of metaxylem-like tracheary elements in xylogenic suspension cultures of zinnia. Plant Physiol. 1997;115:683–692. doi: 10.1104/pp.115.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Haigler CH. Rise in chlorotetracycline fluorescence accompanies tracheary element differentiation in suspension cultures of Zinnia. Protoplasma. 1989;152:37–45. [Google Scholar]
- Roberts AW, Haigler CH. Tracheary-element differentiation in suspension-cultured cells of Zinnia requires uptake of extracellular Ca2+ Planta. 1990;180:502–509. doi: 10.1007/BF02411447. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Haigler CH. Cell expansion and tracheary element differentiation are regulated by extracellular pH in mesophyll cultures of Zinnia elegans L. Plant Physiol. 1994;105:699–706. doi: 10.1104/pp.105.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz G. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Labolatory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sato Y, Sugiyama M, Górecki RJ, Fukuda H, Komamine A. Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia. Planta. 1993;189:584–589. [Google Scholar]
- Sato Y, Sugiyama M, Komamine A, Fukuda H. Separation and characterization of the isoenzymes of wall-bound peroxidase from cultured Zinnia cells during tracheary element differentiation. Planta. 1995;196:141–147. [Google Scholar]
- Schwartz A, Ilan N, Schwartz M, Scheaffer J, Assmann SM, Schroeder JI. Anion-channel blockers inhibit S-type anion channels and abscisic acid responses in guard cells. Plant Physiol. 1995;109:651–658. doi: 10.1104/pp.109.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytol. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Thelen MP, Northcote DH. Identification and purification of a nuclease from Zinnia elegans L.: a potential marker for xylogenesis. Planta. 1989;179:181–195. doi: 10.1007/BF00393688. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Oparka KJ. Physicochemical properties alone do not predict the movement and compartmentation of fluorescent xenobiotics. J Exp Bot. 1994;45:35–44. [Google Scholar]
- Yamamoto R, Demura T, Fukuda H. Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol. 1997;38:980–983. doi: 10.1093/oxfordjournals.pcp.a029262. [DOI] [PubMed] [Google Scholar]
- Ye Z-H, Droste DL. Isolation and characterization of cDNAs encoding xylogenesis-associated and wounding-induced ribonucleases in Zinnia elegans. Plant Mol Biol. 1996;30:697–709. doi: 10.1007/BF00019005. [DOI] [PubMed] [Google Scholar]
- Ye Z-H, Kneusel RE, Matern U, Varner JE. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell. 1994;6:1427–1439. doi: 10.1105/tpc.6.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE. Differential expression of two o-methyltransferases in lignin biosynthesis in Zinnia elegans. Plant Physiol. 1995;108:459–467. doi: 10.1104/pp.108.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE. Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol Biol. 1996;30:1233–1246. doi: 10.1007/BF00019555. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Low temperature-induced cytoplasmic acidosis in cultured mung bean (Vigna radiata [L.] Wilczek) cells. Plant Physiol. 1994;104:1131–1138. doi: 10.1104/pp.104.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]