Abstract
The mechanistic/mammalian target of rapamycin (mTOR) is a conserved protein kinase that controls several anabolic processes required for cell growth and proliferation. As such, mTOR has been implicated in an increasing number of pathological conditions, including cancer, obesity, type 2 diabetes and neurodegeneration. As part of the mTOR complex 1 (mTORC1), mTOR regulates cell growth by promoting the biosynthesis of proteins, lipids and nucleic acids. Several mTORC1 substrates have been shown to regulate protein synthesis, including the eukaryotic initiation factor 4E (eIF4E)-binding proteins (4E-BPs) and the ribosomal S6 kinases (S6Ks) 1 and 2. In this work, we focus on the signalling pathways that lie both upstream and downstream of mTORC1, as well as their relevance to human pathologies. We further discuss pharmacological approaches that target mTOR and their applications for the treatment of cancer.
Introduction
In order to divide, cells must first reach a critical size by promoting various anabolic processes required for growth. The evolutionarily conserved mechanistic/mammalian target of rapamycin (mTOR) has emerged as a critical node through which cells coordinate growth signals and nutrient availability to the macromolecular synthesis of proteins, lipids and nucleic acids (1). Deregulation of mTOR signalling is implicated in the development of several human diseases, including cancer, type 2 diabetes and obesity, highlighting the crucial role that mTOR plays in the maintenance of cellular homeostasis (2).
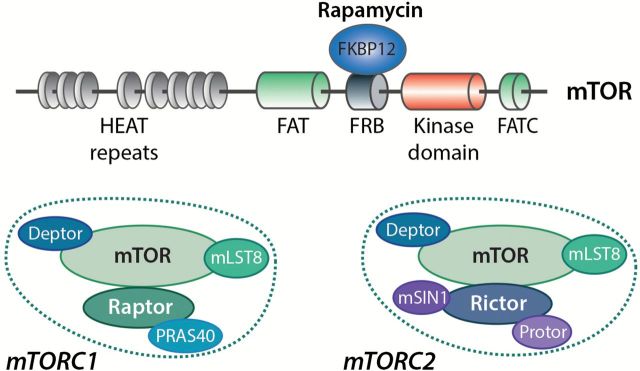
mTOR is a multidomain Ser/Thr kinase that belongs to the phosphoinositide 3-kinase (PI3K)-related kinase (PIKK) family (3). Structurally, mTOR harbours several distinct regions including N-terminal tandem HEAT repeats (found in huntingtin, elongation factor 3, a subunit of protein phosphatase 2A and TOR proteins) that mediate protein–protein interactions, followed by a FAT (FRAP, ATM and TRRAP) domain . The catalytic domain of mTOR, which shares high sequence homology with that of PI3K isoforms, is located in the C-terminal region between the FKBP12-rapamycin binding domain (FRB) and the FATC (FAT C-terminus) domain (Figure 1). While the FRB domain provides a docking site for the FKBP12/rapamycin complex, the FAT and FATC domains are thought to modulate mTOR kinase activity (4,5).
Figure 1.
Structural organisation of mTOR and composition of mTORC1 and mTORC2. The N-terminal region of mTOR is composed of tandem HEAT repeats that mediate protein–protein interactions. The kinase domain is located between the FRB (recognised by the FKBP12/rapamycin complex) and the FATC domains. mTOR is found in two protein complexes, mTORC1 and mTORC2, that are defined by their association with Raptor and Rictor, respectively. Deptor and mLST8 are found in both complexes, while PRAS40 is strictly associated with mTORC1, and mSIN1 and Protor interact only with mTORC2.
mTOR is the catalytic subunit of two functionally and structurally distinct multiprotein complexes known as mTOR complex 1 (mTORC1) and mTORC2 that are defined by their necessary components Raptor (regulatory-associated protein of mTOR) and rapamycin-insensitive companion of mTOR (Rictor), respectively (6). Both Raptor and Rictor serve as scaffolding proteins that facilitate the recruitment of regulators and substrates. Additional components are also exclusively present in each complex, including PRAS40 (proline-rich AKT substrate 40kDa) for mTORC1, and mSIN1 (mammalian stress-activated protein kinase-interacting protein 1) and Protor (Protein observed with Rictor) for mTORC2. Other regulatory components, such as mLST8 (mammalian lethal with Sec13 protein 8) and Deptor (DEP domain-containing mTOR-interacting protein) are present within both mTOR complexes (7).
mTOR was identified as the cellular target of the antifungal macrolide rapamycin by genetic screens in yeast (8), and was later characterised in mammals (9,10). After entering cells, rapamycin forms a complex with a small protein called FKBP12 (FK506-binding protein 12kDa), which specifically associates with the FRB domain of mTOR and potently interferes with mTORC1 function (11). While mTORC1 was initially described as being rapamycin-sensitive, recent studies revealed that some of its functions appear to be resistant to rapamycin treatment (12). It is noteworthy that although mTORC2 was initially shown to be rapamycin-insensitive, prolonged exposure to rapamycin in specific cell types was found to inhibit some its functions (13).
In this article, we will focus on the regulation and function of mTORC1. We will describe many of the upstream events that regulate mTORC1 activity as well as downstream effectors that promote anabolic processes, particularly protein synthesis. Finally, we will discuss the use of mTOR inhibitors in the treatment of different types of neoplasms.
Growth factors and nutrients regulate mTORC1 activity
Eukaryotic cells rely on mTORC1 to coordinate the availability of environmental signals, such as growth factors, nutrients, glucose and oxygen, with the activation of intracellular signalling pathways that regulate cellular homeostasis (14). These factors regulate mTORC1 through multiple mechanisms, including the direct modification of mTORC1 components and the regulation of upstream factors such as the small GTPases Rheb (Ras-homolog enriched in brain) and Rag (Ras-related GTP-binding protein) (15).
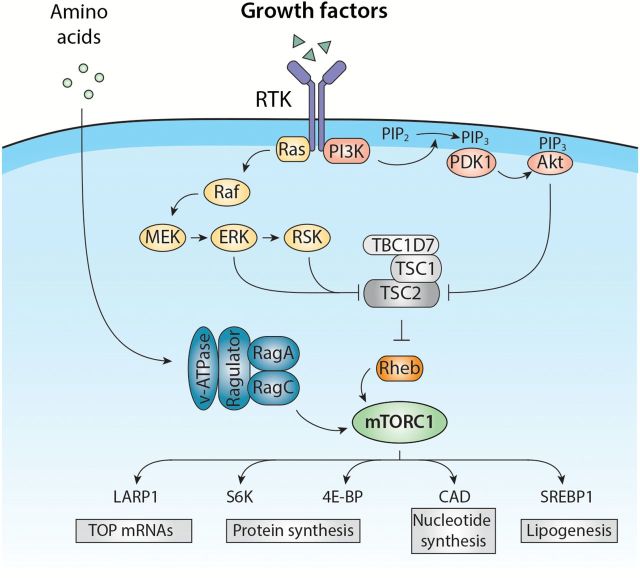
Growth factors and hormones, such as epidermal growth factor (EGF) and insulin, stimulate mTORC1 activity via two well-characterised signalling cascades, the PI3K/Akt and Ras/mitogen-activated protein kinase (MAPK) pathways, which are activated downstream of receptor tyrosine kinases (RTKs). Given that several components within these pathways are oncogenes or tumour suppressors, it is not too surprising that deregulation of mTORC1 signalling occurs in up to 80% of human cancers (16). The PI3K/Akt and Ras/MAPK pathways activate mTORC1 primarily by phosphorylating and inhibiting tuberous sclerosis complex 2 (TSC2) (17–21). TSC2 is part of a complex that also comprises TSC1 and TBC1D7 [Tre2-Bub2-Cdc16 (TBC) 1 domain family member 7], which are required for the stability and activity of the complex (22,23). The TSC complex is a negative regulator of mTORC1 that functions as a GTPase-activating protein (GAP) for Rheb and which maintains Rheb in a GDP-bound inactive state (24,25). Upon growth factor stimulation, the GTP-bound form of Rheb accumulates and directly activates mTORC1 by a mechanism that is not well defined (Figure 2). In addition to the inhibition of TSC2, the PI3K/Akt and Ras/MAPK pathways stimulate mTORC1 activity by directly targeting regulatory elements of mTORC1. For example, Akt-mediated phosphorylation of the repressor protein PRAS40 leads to its dissociation from mTORC1, while MAPK- and RSK (p90 ribosomal S6 kinase)-mediated phosphorylation of Raptor correlates with increased mTORC1 activity (26–29).
Figure 2.
mTORC1 activation by amino acids and growth factors. Growth factors stimulate mTORC1 through the activation of the Ras/MAPK and PI3K/Akt signalling pathways, which are triggered by RTK dimerisation and autophosphorylation. The Akt, ERK and RSK kinases directly phosphorylate and inhibit the TSC2 subunit of the TSC complex that functions as a GAP towards Rheb and prevents its ability to stimulate mTORC1. Once activated, mTORC1 promotes cell growth by regulating several anabolic processes including protein, lipid and nucleotide synthesis, which contribute to the increase in cell biomass.
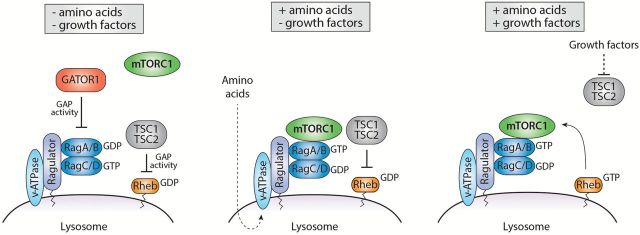
In addition to growth factors, mTORC1 activity is also sensitive to nutrient levels, such as amino acids, particularly leucine, arginine and glutamine (30–32). The mechanisms by which mTORC1 senses amino acids is not fully understood, but recent studies showed that mTORC1 localisation at the lysosomal surface is required for this process (33). mTORC1 regulation by amino acids involves a large number of proteins that orchestrate a series of events that activate the Rag GTPases (34). In mammals, there are four Rag GTPases (RagA, B, C and D) that function as heterodimers where RagA or B associate with RagC or D (35). The nucleotide-bound status of the heterodimers determines its ability to interact with Raptor and activate mTORC1 (34). More specifically, in the presence of amino acids, Rag proteins adopt their active configuration (RagA or B bound to GTP, and RagC or D bound to GDP) and interact with Raptor to promote mTORC1 recruitment at the lysosome where its upstream activator Rheb resides (34,36). In its GTP-loaded form (i.e. when growth factors are present), Rheb efficiently activates mTORC1. This spatial regulation of mTORC1 ensures that its activation occurs only when amino acids and growth factors are available (i.e. when growth conditions are optimal) (Figure 3).
Figure 3.
Integration of amino acids and growth factors at the lysosome. In the absence of amino acids and growth factors, Rag and Rheb GTPases are repressed by the GATOR1 and TSC complexes, which both localise at the lysosome. In the presence of amino acids, the Ragulator complex is activated in a v-ATPase-dependent manner and stimulates the active conformation of Rag proteins, leading to their direct interaction with mTORC1. Growth factors promote TSC complex release from the lysosome, thereby allowing the full activation of mTORC1 by Rheb.
Several mechanisms have been proposed to explain how amino acids regulate the activity of Rag proteins. Amongst these, two complexes known as Ragulator and GATOR1 (GAP activity toward Rags) control the Rag GTPases by acting as guanine nucleotide exchange factor (GEF) and as GAP towards RagA/B, respectively (37,38). When local concentrations of amino acids exceed a certain threshold, the Ragulator complex, which is anchored at the lysosomal membrane, interacts with Rag proteins and promotes their recruitment and activation at the lysosome by stimulating GTP-loading onto RagA and RagB (Figure 3). How amino acids are sensed by the Ragulator complex remains elusive, but this seems to involve the vacuolar (H+)-ATPase (33). Conversely, upon amino acid deprivation, the GATOR1 complex, which is negatively regulated by the GATOR2 complex, interacts with Rag proteins and stimulates the intrinsic GTPase activity of RagA and RagB, leading to mTORC1 inhibition (38,39). In addition to the Ragulator, the scaffold protein p62 interacts with both Rags and Raptor, and participates in the translocation of mTORC1 to the lysosomal membrane (40). Another important candidate is the folliculin protein (FLCN), which has been identified as a positive regulator of mTORC1 signalling that acts as a GAP towards RagC and RagD and thereby promotes the active conformation of Rags required for mTORC1 activation (41,42). Finally, mTORC1 activity is also controlled by the recruitment of the TSC complex at the lysosome. In absence of amino acids and growth factor stimulation, the TSC complex interacts with Rag proteins and translocates to the lysosome where it suppresses Rheb activity, thus leading to mTORC1 inhibition (Figure 3) (43,44).
mTORC1 is a central regulator of protein synthesis
Protein synthesis is the most energy-consuming process in the cell (45). It is thus not surprising that mRNA translation represents a tightly regulated process. mTORC1 senses mitogenic signals and nutrients availability in cells, and stimulates protein synthesis, particularly at the initiation step of mRNA translation (46,47). While mTORC1 stimulates global protein synthesis, it also promotes the selective translation of specific subsets of mRNAs. mTORC1 coordinates mRNA translation by phosphorylating components of the translational machinery, including its two best characterised substrates: the eukaryotic initiation factor 4E (eIF4E)-binding proteins (4E-BPs) and the ribosomal S6 kinases (S6Ks) 1 and 2 (48).
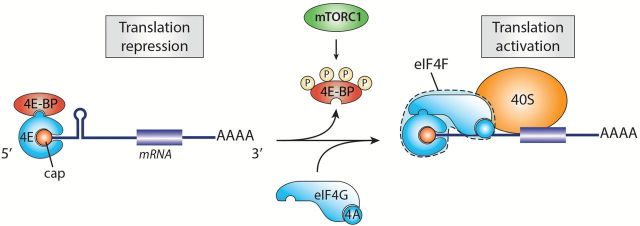
Translation initiation occurs when growth-promoting conditions stimulate assembly of the eIF4F (eukaryotic initiation factor 4F) complex, which assembles at the 5′ mRNA cap structure (49). eIF4F is a hetero-tripartite protein complex composed of the RNA helicase eIF4A that unwinds secondary structures in the mRNA 5′ untranslated region (5′UTR), required for proper scanning of the ribosome to the initiation codon, the cap-binding protein eIF4E and the large scaffolding protein eIF4G, which is responsible for the recruitment of additional translation initiation factors (50). Once assembled, the eIF4F complex promotes the recruitment of the ribosome and subsequent translation initiation of the transcript (51). This process represents an important limiting step in translation and is therefore tightly controlled. The 4E-BPs are small proteins that repress translation at the initiation step by interfering with assembly of the eIF4F complex at the 5′ cap of mRNAs (52,53). In quiescent cells, the 4E-BPs are kept in a hypophosphorylated state and strongly associate to eIF4E, thereby preventing eIF4G binding and eIF4F assembly. Once activated, mTORC1 phosphorylates Thr37 and Thr46 in human 4E-BP1, which are priming sites for subsequent phosphorylation at Ser65 and Thr70 (54,55). These phosphorylation events lead to 4E-BP1 release from eIF4E and subsequent initiation of translation (Figure 4) (53). By regulating eIF4E-dependent translational activity, the 4E-BPs specifically control the translation of mRNAs with a structured 5′UTR, many of which code for proteins involved in cell proliferation and survival (56,57). As mTORC1 directly controls assembly of the eIF4F complex, this finding implies that mTORC1 activity is particularly important for translation of these transcripts. It has been proposed that, while the 4E-BPs mediate cell proliferation downstream of mTORC1, the S6Ks regulate cell growth through complementary but distinct mechanisms (57).
Figure 4.
Stimulation of protein synthesis by mTORC1. In quiescent cells, 4E-BP is hypophosphorylated and tightly associated with eIF4E, thus preventing translation initiation. When activated, mTORC1 phosphorylates 4E-BP leading to its dissociation from eIF4E and assembly of the eIF4F complex. 4E-BP repression by mTORC1 stimulates global protein synthesis.
As mentioned, mTORC1 also promotes cell growth by phosphorylating and activating S6K1/2, which belong to the AGC (protein kinases A, G and C) protein kinase family (58). The S6Ks phosphorylate and regulate several protein substrates that are involved in mRNA metabolism and translation (58). Amongst these are the ribosomal protein S6 (59,60), the initiation factor eIF4B (61,62) and the tumour suppressor PDCD4 (programmed cell death 4) (63). An important role of the S6Ks is to activate the eIF4A helicase, which is done by promoting the degradation of PDCD4 (63), an inhibitory eIF4A-binding protein, and by stimulating its cofactor, eIF4B (61,62). These events promote 5′UTR unwinding and facilitate the translation initiation step (64). In addition to the initiation step, the S6Ks have also been reported to regulate translation elongation by phosphorylating and inhibiting the eukaryotic elongation factor 2 (eEF2) kinase, an important negative regulator of eEF2 (65). Although the S6Ks phosphorylate rpS6 on several residues (66), the impact of these phosphorylation events is not well understood (67–69). Finally, recent studies have shown that the S6Ks also regulate protein synthesis by controlling the transcription of genes required for ribosome biogenesis, termed the ‘Ribi’ transcriptional program (70), as well as other events that increase the protein synthetic capacity of the cell (2).
mTORC1 selectively controls the translation of specific subsets of mRNAs
While mTORC1 controls global protein synthesis, it also preferentially stimulates the translation of a select group of mRNAs, in particular those that contain relatively long and structured 5′UTRs also referred to as ‘eIF4E-sensitive’ mRNAs.(71) A second group of mRNAs that harbour a terminal oligopyrimidine (TOP) tract at their 5′ end was shown to be particularly sensitive to mTOR inhibitors (72), and these mRNAs encode for components of the translation apparatus, such as ribosomal proteins and elongation factors (73,74). In other words, 5′ TOP mRNAs encode proteins that represent the building blocks of the apparatus that enables their own translation and that of every cellular transcript. Consequently, the translation of 5′ TOP mRNAs is highly sensitive to stress and growth conditions, and behave as an ‘all-or-none’ phenomenon. As such, in stress conditions or during mitosis, their translation is halted while mitogenic stimuli promote their maximum translational efficiency. Importantly, recent studies using high-resolution transcriptome-scale ribosome profiling provided convincing evidence that the translation of 5′ TOP mRNAs is highly sensitive to mTORC1 activity (75,76). These studies demonstrated that the selective regulation of 5′ TOP mRNAs by mTORC1 depends on the regulation of eIF4E by the 4E-BPs, as their deletion rendered 5′ TOP mRNA translation resistant to mTORC1 inhibition. While these data suggest that the 4E-BPs are the main downstream regulators of 5′ TOP mRNA translation, a recent study that utilised various 4E-BP loss- and gain-of-function reagents challenged this view and suggested the involvement of yet unidentified factors (77). Moreover, though it was initially proposed that the S6Ks could be involved in mTORC1-mediated translation of 5′ TOP mRNAs, later studies showed that this was not the case (78). Extensive search over the years for transacting factors that positively regulate 5′ TOP mRNA translation has recently led to the identification of the La-related protein 1 (LARP1) as a positive regulator of 5′TOP mRNA translation (79). This study demonstrated that LARP1 associates with 5′TOP mRNAs and is recruited to the translation machinery in an mTORC1-dependent manner to promote efficient 5′TOP mRNA translation (Figure 4). While additional transacting factors that regulate 5′TOP mRNAs likely exist, these results underscore LARP1 as an important mTORC1 effector of cell growth.
Other anabolic processes controlled by mTORC1
In addition to being the driving force behind protein synthesis, emerging evidence demonstrate that mTORC1 is also involved in the biosynthesis of additional cellular building blocks, such as membrane lipids and nucleic acids (14). De novo lipid biogenesis is required for efficient cell growth as it provides essential elements that are necessary for the production of new membranes. Although the exact molecular mechanism is poorly known, lipid synthesis is regulated at the transcriptional level by the sterol-regulatory-element-binding proteins (SREBPs), which are key regulators of genes involved in the synthesis of both fatty acids and sterols (80). Interestingly, rapamycin was shown to block the expression of genes involved in lipogenesis and prevented nuclear translocation of SREBPs (81). Subsequent work using genomic approaches confirmed the role of SREBPs in mTORC1-regulated lipid synthesis and found that S6Ks mediate the regulation of SREBPs activity (82). Another study showed that mTORC1 also regulates lipid synthesis by directly phosphorylating and inhibiting Lipin-1, a phosphatidic acid phosphatase that represses SREBP activity (83).
In addition to lipid biogenesis, mTORC1 stimulates nucleotide synthesis, especially pyrimidine, providing precursors required not only for DNA and RNA but also for multiple growth processes such as glycogen synthesis, membrane phospholipids and protein glycosylation (84). Furthermore, mTORC1 promotes the expression of genes participating in the pentose phosphate pathway (PPP) (82) that provides essential intermediates for the production of ribose 5-phosphate (R5P), as well as for the synthesis of purine and pyrimidine and the formation of NADPH, which is an essential factor for biosynthetic reactions. In addition, mTORC1 induces the expression of rate limiting enzymes in PPP, an effect that is mediated by SREBP activation (82). Notably, two recent studies proposed a new mechanism by which mTORC1 stimulates pyrimidine biosynthesis (85,86). They found that the enzyme carbamoyl-phosphate synthetase 2, aspartate transcarbamylase and dihydro-orotase (CAD), which catalyses the first steps in pyrimidine synthesis, is directly phosphorylated and activated by S6K1. Treatment of cells with mTOR- or S6K1-specific inhibitors resulted in reduced pyrimidine synthesis, confirming the important role that the mTORC1/S6K1 pathway plays in this anabolic process.
Deregulation of mTORC1 in cancer
The mTOR signalling pathway plays an essential role in cell growth and proliferation by coordinating anabolic processes with oxygen, energy and nutrient availability, as well as extracellular cues. One fundamental characteristic of cancer cells resides in their ability to sustain chronic proliferation in the absence of growth-promoting signals. This proliferative advantage is achieved, at least in part, by genetic events that cause aberrant activation of mTORC1 signalling (87). Indeed, mTORC1 lies downstream of the Ras/MAPK and PI3K/Akt signalling pathways, where gain-of-function mutations in Ras, Raf, PI3K and Akt oncogenes, and loss-of-function mutations in the tumour suppressors neurofibromatosis-related protein-1 (NF-1), phosphatase and tensin homolog (PTEN) and TSC are found in up to 80% of human cancers (16). These mutational events promote constitutive activation of mTORC1 that, in turn, stimulate anabolic processes driving tumour cell growth and proliferation. Activating mutations in the gene coding for mTOR have also been described in different cancer types, including renal carcinoma (RCC) and colon carcinoma (88,89). The importance of mTORC1 in the pathogenesis of cancer is now well recognised and a substantial amount of effort is being invested in the development of novel approaches for the inhibition of mTOR-dependent signalling events.
Given that mTORC1 promotes cell growth and is hyperactivated in a large number of cancers, small molecules that specifically target mTORC1, such as rapamycin, a natural allosteric inhibitor of the complex, have the potential to suppress tumour progression and represent a promising anticancer therapy. Since the bioavailability and efficacy of rapamycin are poor, several rapamycin derivatives, better known as rapalogs, have been developed. Amongst these, temsirolimus and everolimus have been approved in 2007 and 2009 by the FDA for the treatment of advanced stage RCC (90,91). Some of these compounds are currently undergoing clinical trials for the treatment of specific types of cancer, such as osteosarcoma, hepatocellular carcinoma, gastric cancer and non-small cell lung cancer (92). Globally, rapalogs have only showed modest effects in limiting the growth of major solid tumours in patients, likely due to the cytostatic rather than cytotoxic effect of these drugs (93). These limited effects can be attributed to several mechanisms. First, rapalogs do not fully inhibit mTORC1 activity, especially in regards to its ability to promote protein synthesis by phosphorylating and inhibiting the 4E-BPs, a process that is involved in tumorigenesis (57,94). Second, mTORC1 controls several negative feedback loops that repress RTKs and downstream signalling. This is the case for insulin receptor substrate 1 (IRS1) and Rictor phosphorylation by S6K1, which lead to the inhibition of PI3K signalling and mTORC2, respectively, both of which being important for cell survival and proliferation (95–98). In addition to S6K1, mTORC1 directly phosphorylates and stabilises Grb10, causing a feedback inhibition of PI3K signalling (99,100). Thus, blocking mTORC1 activity with rapalogs derepresses mTORC1-mediated feedback inhibition and may contribute to the variable effects of these compounds (101). While using rapalogs as single therapeutic agents only showed modest efficacy in cancer treatment, their combination with other chemotherapeutic agents are expected to yield more promising results (92).
To overcome some of the limitations encountered with rapalogs, innovative strategies have led to the development of novel small molecules that directly target the kinase domain of mTOR, thereby inhibiting simultaneously both mTORC1 and mTORC2 in cells. Surprisingly, these compounds were found to inhibit cell growth and proliferation independently of mTORC2, suggesting that this new generation of ATP-competitive inhibitors primarily target rapamycin-resistant functions of mTORC1 (94). Another important characteristic of these inhibitors is their ability to inhibit PI3K, which shares a closely related kinase domain (102). Preclinical studies indicated that catalytic mTOR inhibitors prevent cancer cell growth and proliferation in vitro, as well as the development of tumours in vivo, and show stronger overall efficacy than rapalogs (103–105). The majority of catalytic mTOR inhibitors is currently in phase I clinical trial, and these compounds are being tested as single agents or in combination with other chemotherapeutic agents. Currently, these compounds are being tested against several types of cancer, including breast cancer, endometrial cancer, non-Hodgkin lymphoma and advanced stages of solid tumours (102). The efficacy of these second-generation inhibitors as anti-cancer agents is still under investigation, but high expectations are present among clinicians.
Conclusion
Since its discovery in the early 1990s as the cellular target of rapamycin, mTOR has attracted unprecedented attention among clinicians and scientists. This strong interest prompted an exponential understanding about the regulation and function of mTOR. These advances have led to the discovery and development of several new mTOR inhibitors with appealing anti-cancer properties. While the fundamental mechanisms by which mTOR signalling promotes tumorigenesis are being elucidated, the precise mechanisms by which mTOR stimulates the translation of select groups of mRNAs that fuel uncontrolled cell growth and proliferation require further investigation. The recent discovery of many downstream effectors of mTOR may open new therapeutic windows for inhibiting some of its function without completely blocking its activity. Targeting downstream components could also bypass issues linked to feedback mechanisms, avoid limited efficiency, limit the toxicity caused by completely blocking mTOR, and avoid targeting other kinases such as PI3K. Overall, whether or not these new agents are effective against cancer cells, they will most certainly provide researchers with valuable tools to further investigate the normal and pathological roles of mTOR.
Funding
The Roux laboratory is supported by grants from the Canadian Institutes of Health Research, the Cancer Research Society, the Natural Sciences and Engineering Research Council. M.C. was supported by a scholarship from the Fonds de Recherche en Santé - Québec (FRQS).
Acknowledgements
We thank all laboratory members for their insightful comments and suggestions on the manuscript. We apologise to those authors whose work was not cited due to space constraints.
Conflict of interest statement: None declared.
References
- 1. Dibble C. C., Manning B. D. (2013)Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol., 15, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laplante M., Sabatini D. M. (2012)mTOR signaling in growth control and disease. Cell, 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lempiäinen H., Halazonetis T. D. (2009)Emerging common themes in regulation of PIKKs and PI3Ks. EMBO J., 28, 3067–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang H., Rudge D. G., Koos J. D., Vaidialingam B., Yang H. J., Pavletich N. P. (2013)mTOR kinase structure, mechanism and regulation. Nature, 497, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang Q., Guan K. L. (2007)Expanding mTOR signaling. Cell Res., 17, 666–681. [DOI] [PubMed] [Google Scholar]
- 6. Caron E., Ghosh S., Matsuoka Y., Ashton-Beaucage D., Therrien M., Lemieux S., Perreault C., Roux P. P., Kitano H. (2010)A comprehensive map of the mTOR signaling network. Mol. Syst. Biol., 6, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zoncu R., Efeyan A., Sabatini D. M. (2011)mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol., 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heitman J., Movva N. R., Hall M. N. (1991)Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science, 253, 905–909. [DOI] [PubMed] [Google Scholar]
- 9. Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. (1994)RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell, 78, 35–43. [DOI] [PubMed] [Google Scholar]
- 10. Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. (1994)A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature, 369, 756–758. [DOI] [PubMed] [Google Scholar]
- 11. Chen J., Zheng X. F., Brown E. J., Schreiber S. L. (1995)Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc. Natl. Acad. Sci. U.S.A., 92, 4947–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choo A. Y., Yoon S. O., Kim S. G., Roux P. P., Blenis J. (2008)Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U.S.A., 105, 17414–17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006)Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell, 22, 159–168. [DOI] [PubMed] [Google Scholar]
- 14. Howell J. J., Ricoult S. J., Ben-Sahra I., Manning B. D. (2013)A growing role for mTOR in promoting anabolic metabolism. Biochem. Soc. Trans., 41, 906–912. [DOI] [PubMed] [Google Scholar]
- 15. Sengupta S., Peterson T. R., Sabatini D. M. (2010)Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell, 40, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menon S., Manning B. D. (2008)Common corruption of the mTOR signaling network in human tumors. Oncogene, 27 (Suppl 2), S43–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002)TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol., 4, 648–657. [DOI] [PubMed] [Google Scholar]
- 18. Manning B. D., Tee A. R., Logsdon M. N., Blenis J., Cantley L. C. (2002)Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell, 10, 151–162. [DOI] [PubMed] [Google Scholar]
- 19. Potter C. J., Pedraza L. G., Xu T. (2002)Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol., 4, 658–665. [DOI] [PubMed] [Google Scholar]
- 20. Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004)Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U. S. A., 101, 13489–13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005)Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell, 121, 179–193. [DOI] [PubMed] [Google Scholar]
- 22. Kwiatkowski D. J., Manning B. D. (2005)Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet., 14, R251–R258. [DOI] [PubMed] [Google Scholar]
- 23. Dibble C. C., Elis W., Menon S, et al. (2012)TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell, 47, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoki K., Li Y., Xu T., Guan K. L. (2003)Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev., 17, 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tee A. R., Manning B. D., Roux P. P., Cantley L. C., Blenis J. (2003)Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol., 13, 1259–1268. [DOI] [PubMed] [Google Scholar]
- 26. Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007)PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell, 25, 903–915. [DOI] [PubMed] [Google Scholar]
- 27. Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007)Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol., 9, 316–323. [DOI] [PubMed] [Google Scholar]
- 28. Carrière A., Cargnello M., Julien L. A., Gao H., Bonneil E., Thibault P., Roux P. P. (2008)Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr. Biol., 18, 1269–1277. [DOI] [PubMed] [Google Scholar]
- 29. Carriere A., Romeo Y., Acosta-Jaquez H. A., Moreau J., Bonneil E., Thibault P., Fingar D. C., Roux P. P. (2011)ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J. Biol. Chem., 286, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hara K., Yonezawa K., Weng Q. P., Kozlowski M. T., Belham C., Avruch J. (1998)Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem., 273, 14484–14494. [DOI] [PubMed] [Google Scholar]
- 31. Wang X., Campbell L. E., Miller C. M., Proud C. G. (1998)Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem. J., 334 (Pt 1), 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicklin P., Bergman P., Zhang B, et al. (2009)Bidirectional transport of amino acids regulates mTOR and autophagy. Cell, 136, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D. M. (2011)mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science, 334, 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008)The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science, 320, 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sekiguchi T., Hirose E., Nakashima N., Ii M., Nishimoto T. (2001)Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J. Biol. Chem., 276, 7246–7257. [DOI] [PubMed] [Google Scholar]
- 36. Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008)Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol., 10, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bar-Peled L., Schweitzer L. D., Zoncu R., Sabatini D. M. (2012)Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell, 150, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bar-Peled L., Chantranupong L., Cherniack A. D, et al. (2013)A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science, 340, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panchaud N., Péli-Gulli M. P., De Virgilio C. (2013)Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci. Signal., 6, ra42. [DOI] [PubMed] [Google Scholar]
- 40. Duran A., Amanchy R., Linares J. F., Joshi J., Abu-Baker S., Porollo A., Hansen M., Moscat J., Diaz-Meco M. T. (2011)p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell, 44, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsun Z. Y., Bar-Peled L., Chantranupong L., Zoncu R., Wang T., Kim C., Spooner E., Sabatini D. M. (2013)The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell, 52, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petit C. S., Roczniak-Ferguson A., Ferguson S. M. (2013)Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J. Cell Biol., 202, 1107–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Demetriades C., Doumpas N., Teleman A. A. (2014)Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell, 156, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menon S., Dibble C. C., Talbott G., Hoxhaj G., Valvezan A. J., Takahashi H., Cantley L. C., Manning B. D. (2014)Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell, 156, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buttgereit F., Brand M. D. (1995)A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J., 312 (Pt 1), 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma X. M., Blenis J. (2009)Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol., 10, 307–318. [DOI] [PubMed] [Google Scholar]
- 47. Sonenberg N., Hinnebusch A. G. (2009)Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell, 136, 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roux P. P., Topisirovic I. (2012)Regulation of mRNA translation by signaling pathways. Cold Spring Harb. Perspect. Biol., 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Topisirovic I., Svitkin Y. V., Sonenberg N., Shatkin A. J. (2011)Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip. Rev. RNA, 2, 277–298. [DOI] [PubMed] [Google Scholar]
- 50. Gingras A. C., Raught B., Sonenberg N. (1999)eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem., 68, 913–963. [DOI] [PubMed] [Google Scholar]
- 51. Jackson R. J., Hellen C. U., Pestova T. V. (2010)The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol., 11, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin T. A., Kong X., Haystead T. A., Pause A., Belsham G., Sonenberg N., Lawrence J. C.Jr. (1994)PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science, 266, 653–656. [DOI] [PubMed] [Google Scholar]
- 53. Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. (1994)Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature, 371, 762–767. [DOI] [PubMed] [Google Scholar]
- 54. Gingras A. C., Gygi S. P., Raught B., Polakiewicz R. D., Abraham R. T., Hoekstra M. F., Aebersold R., Sonenberg N. (1999)Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev., 13, 1422–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gingras A. C., Raught B., Gygi S. P, et al. (2001)Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev., 15, 2852–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petroulakis E., Parsyan A., Dowling R. J, et al. (2009)p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell, 16, 439–446. [DOI] [PubMed] [Google Scholar]
- 57. Dowling R. J., Topisirovic I., Alain T, et al. (2010)mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science, 328, 1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Magnuson B., Ekim B., Fingar D. C. (2012)Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J., 441, 1–21. [DOI] [PubMed] [Google Scholar]
- 59. Banerjee P., Ahmad M. F., Grove J. R., Kozlosky C., Price D. J., Avruch J. (1990)Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc. Natl. Acad. Sci. U. S. A., 87, 8550–8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kozma S. C., Ferrari S., Bassand P., Siegmann M., Totty N., Thomas G. (1990)Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc. Natl. Acad. Sci. U. S. A., 87, 7365–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raught B., Peiretti F., Gingras A. C., Livingstone M., Shahbazian D., Mayeur G. L., Polakiewicz R. D., Sonenberg N., Hershey J. W. (2004)Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J., 23, 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shahbazian D., Roux P. P., Mieulet V, et al. (2006)The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J., 25, 2781–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dorrello N. V., Peschiaroli A., Guardavaccaro D., Colburn N. H., Sherman N. E., Pagano M. (2006)S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science, 314, 467–471. [DOI] [PubMed] [Google Scholar]
- 64. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005)mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell, 123, 569–580. [DOI] [PubMed] [Google Scholar]
- 65. Wang X., Li W., Williams M., Terada N., Alessi D. R., Proud C. G. (2001)Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J., 20, 4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bandi H. R., Ferrari S., Krieg J., Meyer H. E., Thomas G. (1993)Identification of 40 S ribosomal protein S6 phosphorylation sites in Swiss mouse 3T3 fibroblasts stimulated with serum. J. Biol. Chem., 268, 4530–4533. [PubMed] [Google Scholar]
- 67. Meyuhas O. (2008)Physiological roles of ribosomal protein S6: one of its kind. Int. Rev. Cell Mol. Biol., 268, 1–37. [DOI] [PubMed] [Google Scholar]
- 68. Meyuhas O., Dreazen A. (2009)Ribosomal protein S6 kinase from TOP mRNAs to cell size. Prog. Mol. Biol. Transl. Sci., 90, 109–153. [DOI] [PubMed] [Google Scholar]
- 69. Ruvinsky I., Katz M., Dreazen A, et al. (2009)Mice deficient in ribosomal protein S6 phosphorylation suffer from muscle weakness that reflects a growth defect and energy deficit. PLoS One, 4, e5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chauvin C., Koka V., Nouschi A, et al. (2014)Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene, 33, 474–483. [DOI] [PubMed] [Google Scholar]
- 71. Koromilas A. E., Lazaris-Karatzas A., Sonenberg N. (1992)mRNAs containing extensive secondary structure in their 5’ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J., 11, 4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jefferies H. B., Reinhard C., Kozma S. C., Thomas G. (1994)Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc. Natl. Acad. Sci. U. S. A., 91, 4441–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Avni D., Biberman Y., Meyuhas O. (1997)The 5’ terminal oligopyrimidine tract confers translational control on TOP mRNAs in a cell type- and sequence context-dependent manner. Nucleic Acids Res., 25, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meyuhas O. (2000)Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem., 267, 6321–6330. [DOI] [PubMed] [Google Scholar]
- 75. Hsieh A. C., Liu Y., Edlind M. P, et al. (2012)The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature, 485, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thoreen C. C., Chantranupong L., Keys H. R., Wang T., Gray N. S., Sabatini D. M. (2012)A unifying model for mTORC1-mediated regulation of mRNA translation. Nature, 485, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miloslavski R., Cohen E., Avraham A, et al. (2014)Oxygen sufficiency controls TOP mRNA translation via the TSC-Rheb-mTOR pathway in a 4E-BP-independent manner. J. Mol. Cell Biol., 6, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tang H., Hornstein E., Stolovich M., Levy G., Livingstone M., Templeton D., Avruch J., Meyuhas O. (2001)Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell. Biol., 21, 8671–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tcherkezian J., Cargnello M., Romeo Y., Huttlin E. L., Lavoie G., Gygi S. P., Roux P. P. (2014)Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5’TOP mRNA translation. Genes Dev., 28, 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Espenshade P. J., Hughes A. L. (2007)Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet., 41, 401–427. [DOI] [PubMed] [Google Scholar]
- 81. Porstmann T., Santos C. R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J. R., Chung Y. L., Schulze A. (2008)SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab., 8, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Düvel K., Yecies J. L., Menon S, et al. (2010)Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell, 39, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Peterson T. R., Sengupta S. S., Harris T. E, et al. (2011)mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell, 146, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang M., Graves L. M. (2003)De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell. Mol. Life Sci., 60, 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Robitaille A. M., Christen S., Shimobayashi M, et al. (2013)Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science, 339, 1320–1323. [DOI] [PubMed] [Google Scholar]
- 86. Ben-Sahra I., Howell J. J., Asara J. M., Manning B. D. (2013)Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science, 339, 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guertin D. A., Sabatini D. M. (2007)Defining the role of mTOR in cancer. Cancer Cell, 12, 9–22. [DOI] [PubMed] [Google Scholar]
- 88. Sato T., Nakashima A., Guo L., Coffman K., Tamanoi F. (2010)Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene, 29, 2746–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grabiner B. C., Nardi V., Birsoy K., Possemato R., Shen K., Sinha S., Jordan A., Beck A. H., Sabatini D. M. (2014)A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov., 4, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hudes G., Carducci M., Tomczak P, et al. ; Global ARCC Trial. (2007)Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med., 356, 2271–2281. [DOI] [PubMed] [Google Scholar]
- 91. Motzer R. J., Escudier B., Oudard S, et al. ; RECORD-1 Study Group. (2008)Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet, 372, 449–456. [DOI] [PubMed] [Google Scholar]
- 92. Li J., Kim S.G., Blenis J. (2014)Rapamycin: one drug, many effects. Cell Metab., 19, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bissler J. J., McCormack F. X., Young L. R, et al. (2008)Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med., 358, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thoreen C. C., Kang S. A., Chang J. W, et al. (2009)An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem., 284, 8023–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harrington L. S., Findlay G. M., Gray A, et al. (2004)The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol., 166, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shah O. J., Wang Z., Hunter T. (2004)Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol., 14, 1650–1656. [DOI] [PubMed] [Google Scholar]
- 97. Julien L. A., Carriere A., Moreau J., Roux P. P. (2010)mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol., 30, 908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dibble C. C., Asara J. M., Manning B. D. (2009)Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol., 29, 5657–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hsu P. P., Kang S. A., Rameseder J, et al. (2011)The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science, 332, 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu Y., Yoon S. O., Poulogiannis G, et al. (2011)Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science, 332, 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. O’Reilly K. E., Rojo F., She Q. B, et al. (2006)mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res., 66, 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benjamin D., Colombi M., Moroni C., Hall M. N. (2011)Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov., 10, 868–880. [DOI] [PubMed] [Google Scholar]
- 103. García-Martínez J. M., Moran J., Clarke R. G., Gray A., Cosulich S. C., Chresta C. M., Alessi D. R. (2009)Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem. J., 421, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yu K., Shi C., Toral-Barza L, et al. (2010)Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res., 70, 621–631. [DOI] [PubMed] [Google Scholar]
- 105. Falcon B. L., Barr S., Gokhale P. C., Chou J., Fogarty J., Depeille P., Miglarese M., Epstein D. M., McDonald D. M. (2011)Reduced VEGF production, angiogenesis, and vascular regrowth contribute to the antitumor properties of dual mTORC1/mTORC2 inhibitors. Cancer Res., 71, 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]