Abstract
Background:
Little is known about the total patient burden associated with clinical development and where burdens fall most heavily during a drug development program. Our goal was to quantify the total patient burden/benefit in developing a new drug.
Methods:
We measured risk using drug-related adverse events that were grade 3 or higher, benefit by objective response rate, and trial outcomes by whether studies met their primary endpoint with acceptable safety. The differences in risk (death rate) and benefit (overall response rate) between industry and nonindustry trials were analyzed with an inverse-variance weighted fixed effects meta-analysis implemented as a weighted regression analysis. All statistical tests were two-sided.
Results:
We identified 103 primary publications of sunitinib monotherapy, representing 9092 patients and 3991 patient-years of involvement over 10 years and 32 different malignancies. In total, 1052 patients receiving sunitinib monotherapy experienced objective tumor response (15.7% of intent-to-treat population, 95% confidence interval [CI] = 15.3% to 16.0%), 98 died from drug-related toxicities (1.08%, 95% CI = 1.02% to 1.14%), and at least 1245 experienced grade 3–4 drug-related toxicities (13.7%, 95% CI = 13.3% to 14.1%). Risk/benefit worsened as the development program matured, with several instances of replicated negative studies and almost no positive trials after the first responding malignancies were discovered.
Conclusions:
Even for a successful drug, the risk/benefit balance of trials was similar to phase I cancer trials in general. Sunitinib monotherapy development showed worsening risk/benefit, and the testing of new indications responded slowly to evidence that sunitinib monotherapy would not extend to new malignancies. Research decision-making should draw on evidence from whole research programs rather than a narrow band of studies in the same indication.
Numerous analyses have chronicled high costs, attrition, and lengthy delays in drug development (1–3). In one recent study of US Food and Drug Administration (FDA)-approved drugs, the median time between start of clinical development and regulatory licensure was 6.5 years (4). In another, only one in 10 drugs entering clinical development is ultimately licensed for clinical application; the fraction of drugs receiving licensure in areas where clinical need is especially pressing—cancer and neurological drugs—is lower still (5,6). Such costs and attrition exact a heavy toll on health care systems, as expenses associated with drug development are ultimately absorbed into the cost of pharmaceuticals. They also impose burdens on patients participating in trials.
Little is known about the total patient burden associated with clinical development and where burdens fall most heavily during a drug development program. Clinical development is characterized by a range of different investigations: Drug developers conduct multiple trials in parallel with each other, continue testing long after a drug is licensed, and search for responding secondary clinical indications alongside lead indications. Thus, many of the aggregate figures about translation success and cost obscure patterns of success and trends in risk and benefit within a whole drug development program.
Many recent policy and research initiatives aim at accelerating the pace and improving success rates in clinical translation (7–10). Understanding patterns of research activity and burden in drug development can help identify opportunities for directing research resources more efficiently. It can also determine activities where burden is greatest, thus providing an evidence base for human protections.
To better understand the total risk/benefit associated with developing a drug and trends in risk/benefit spanning clinical development, we conducted a systematic review of monotherapy cancer trials for a successful drug, sunitinib, across 10 years of clinical development. We used objective response rate as a measure of benefit and grade 3 or above drug-related adverse events as a measure of harm.
Methods
The primary aim of this study was to capture a portfolio of published clinical trials for a single drug, sunitinib, and to quantify the total amount of patient burden and benefit encountered in clinical development. We defined a portfolio as all monotherapy trials testing sunitinib in oncology. Our secondary goals were to track the evolution of risk/benefit over the course of development and the relationship between risk/benefit with funding. Because sunitinib is licensed only as monotherapy and to limit the scope of this research effort, we focused our analysis on monotherapy trials.
Literature Search
We selected the drug sunitinib for analysis because it afforded 10 years of published trials in a variety of disease indications and a large but manageable number of trials. Trials were captured by searching Embase and Medline on March 11, 2015 for trials using the terms “sunitinib,” “Sutent,” and variations on “SU11248” and combined results with the exploded MeSH terms, including “randomized controlled trials,” “controlled clinical trials,” “random allocation,” “double-blind,” “single-blind,” “placebo,” “feasibility,” “pilot,” “proof of principle,” “open label,” “non-random,” “clinical trials” and “phase 1” to “phase 4” (Supplementary Methods, available online).
Trial publications were then screened by BC for the following inclusion criteria: 1) primary report, 2) final report (where a trial’s results were published more than once), 3) interventional trials, 4) human subjects, 5) phase 1 to phase 4, and 6) monotherapy trials. We excluded publications that were: 1) secondary reports, 2) interim results, 3) meta-analyses, 4) retrospective or observational studies, 5) laboratory analyses of ex vivo human tissues, 6) reviews, 7) preclinical studies, and 8) letters, editorials, guidelines, interviews, etc. During full-text analysis, abstract-only publications and poster presentations were excluded.
To explore a second dimension of drug development, we repeated the above for a second portfolio of sunitinib studies: combination studies involving the most intensively researched indication in our sample, renal cell carcinoma (RCC).
Extraction
We devised an extraction template that captured variables in the following domains: trial demographics, methods, hypothesis, trial design, measures of patient risk/burden and benefit (including adverse events, treatment duration, response, and investigator conclusions). Criteria for extraction were prespecified in a codebook, and coders underwent training before data collection.
We scored the number of grade 3 or 4 adverse events (defined by Common Terminology Criteria for Adverse Events [CTCAE] criteria [11]) in a trial conservatively, based on the highest number of events in a single category per trial. We based median duration of treatment on the length of time a patient must be enrolled in the trial to collect the primary endpoint. When not specified, duration of treatment was imputed by the product of the median number of cycles and the period of each cycle. Toxicities were only scored if they were defined as probably or definitely treatment related. Benefit was calculated in terms of the overall response rate (ORR; sum of complete and partial responses using RECIST criteria [12]) in individuals receiving sunitinib on an intent-to-treat basis. We used ORR because it is a widely used surrogate endpoint in cancer trials and allowed us to compare the magnitude of effect across diverse indications and trial phases. Indications were divided into meaningful categories identified in consultation with an oncologist (the exact indication for any node can be reviewed by reference to the numbered citation in our bibliography).
The success or failure of a study was determined based on whether the study reached its prespecified primary endpoint (usually ORR) and whether the regime was deemed tolerable by the authors. All studies were extracted by two independent coders using Numbat software—an open source meta-analysis management tool developed by BC (Numbat available from: http://bgcarlisle.github.io/Numbat/). Disagreements were reconciled by discussion between coders. In cases where opening and closure dates were unavailable, we contacted the corresponding authors for these details, with a reply rate of 35%. Our codebook is available upon request.
Analysis and Statistics
The differences in risk (death rate) and benefit (ORR) between industry and nonindustry trials were analyzed in R version 3.1.3 (13) using an inverse-variance weighted fixed-effect meta-analysis implemented as a weighted regression analysis. The outcome was regressed on a 0/1 indicator variable to identify industry trials. The individual trial results were weighted by sample size, as the variance of a rate estimate is directly proportional to sample size. We defined P values of less than .05 as statistically significant. All statistical tests were two-sided.
Results
Study Characteristics
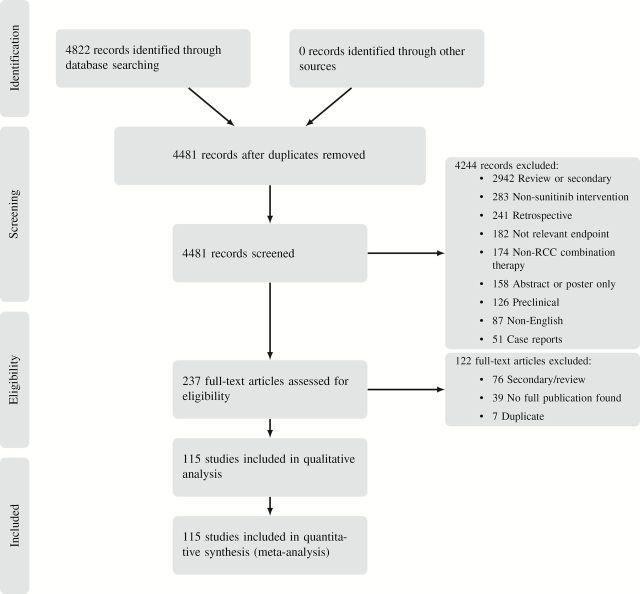
Our literature searches captured 115 primary publications of interventional trials of sunitinib (see Figure 1 for a PRISMA flow diagram and Supplementary Table 1, available online, for per-trial details). There were 103 monotherapy studies (14–116) and 12 combination therapy trials (117–128) spanning 11 different combination therapies. Properties of trials in our sample are described in Table 1 (see Supplementary Figure 1, available online).
Figure 1.
PRISMA flow diagram.
Table 1.
Properties of extracted trials in our sample
| Trial characteristics | Monotherapy (14-116) (n = 103) No. (%) |
Combination therapy (117-128) (n = 12) No. (%) |
|---|---|---|
| Phase | ||
| 1 | 15 (15) | 8 (67) |
| 1-2 | 1 (1) | 2 (17) |
| 2 | 80 (78) | 2 (17) |
| 3 | 7 (7) | |
| Sponsor | ||
| Industry only | 43 (42) | 8 (67) |
| Nonindustry support | 54 (52) | 3 (25) |
| Not stated | 6 (6) | 1 (8) |
| Number of centers | ||
| Single center | 36 (35) | 3 (25) |
| Multicenter | 63 (61) | 8 (67) |
| Not stated | 4 (4) | 1 (8) |
| Randomization | ||
| Randomized | 19 (18) | 1 (8) |
| Nonrandomized | 84 (82) | 11 (92) |
| Trials with a non-sunitinib comparator arm | 15 (15) | 2 (17) |
| Average duration, wk | 20.0 | 18.5 |
| Results | ||
| Positive | 48 (47) | 1 (8) |
| Inconclusive | 14 (14) | 2 (17) |
| Negative | 41 (40) | 9 (75) |
| Mean sample size | 88.3 | 20.1 |
| Location of corresponding author | ||
| N America | 58 (56) | 9 (75) |
| Europe | 32 (31) | 3 (25) |
| Asia | 11 (11) | |
| S America | 1 (1) | |
| Australia | 1 (1) | |
Total Patient Benefit and Burden
The 103 trials included in the sunitinib monotherapy analysis represent 9092 patients and 3991 patient-years of involvement over 10 years. Corresponding authors were based in 17 different countries. In the portfolio we identified, seven different doses, seven schedules, and 32 separate malignancies were tested. In total, 1052 patients in sunitinib monotherapy experienced objective tumor response (15.7% of intent-to-treat population, 95% confidence interval [CI] = 15.3% to 16.0%) and 98 died from drug-related toxicities (1.08%, 95% CI = 1.02% to 1.14%). There was a minimum of 1245 grade 3–4 drug-related toxicities (13.7%, 95% CI = 13.3% to 14.1%). In Figure 2A, we present the sequence of monotherapy and select combination therapy trials using an Accumulating Evidence and Research Organization (AERO) diagram (129). Based on start dates for these trials, an average of 10 new monotherapy trials were launched each year.
Figure 2.
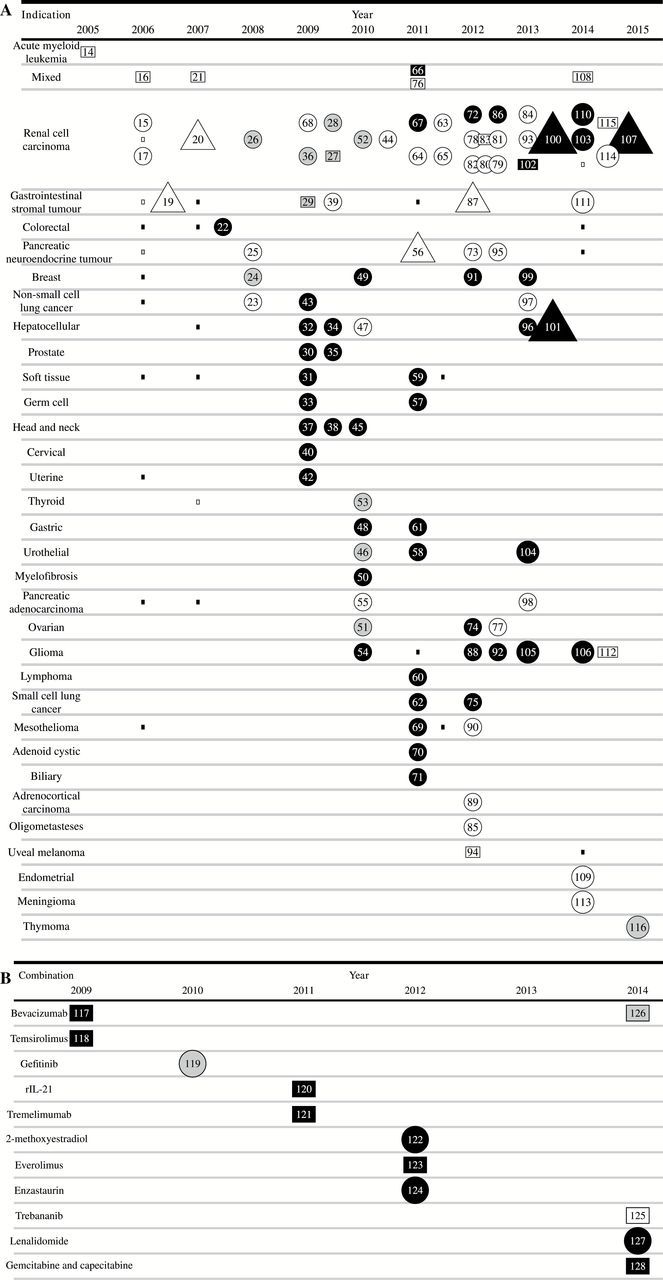
Accumulating Evidence and Research Organization (AERO) graphs for sunitinib therapy. A) monotherapy stratified by indication. Nodes represent all available sunitinib cancer trials testing anticancer activity, arranged according to first publication date horizontally and stratified by indication. Larger square nodes are phase 1 trials in the designated indication. Small squares represent presence of patients with a particular indication in a “mixed malignancy” phase 1 trial. Circular nodes are phase 2 trials. Triangles are phase 3 trials. White nodes indicate acceptable toxicity and positive effect, gray represents inconclusive results, and black nodes indicate negative results for their prespecified primary endpoint. Number of nodes may not sum to figures represented in Table 1 because of different classificatory schema used in AERO graph. B) AERO graph for sunitinib combination therapy. Nodes represent sunitinib cancer trials, stratified by combination therapy, arranged according to publication year. Number of nodes may not sum to data in Table 1 because of different classificatory schema used in AERO graph.
The phase 1 acute myeloid leukemia (AML) trial is white, despite not being followed up in phase 2 because it reports five (33%) partial responses; but these were short-lived and the authors of this report recommended against further development. There are three phase 2 trials in pancreatic adenocarcinoma, prostate cancer, and hepatocellular carcinoma (HCC) that met prespecified primary endpoints other than ORR (eg, disease control rate [DCR] or 12-month progression-free survival [PFS-12]). However, the authors of the pancreatic adenocarcinoma trial “argue against any usefulness of sunitinib in this patient population” (55). The prostate cancer trial authors qualified their use of PFS-12 as a “soft endpoint” (35), and the authors of the HCC trial admit that PFS-12 “should no longer be used as a primary endpoint for trials testing new compounds in HCC” (47). In each of these cases, the trials had very few objective responses (1 [1.4%], 2 [5.6%], and 1 [2.2%], respectively) and no licensure was obtained for these indications. Later trials in mesothelioma, adrenocortical carcinoma, oligometasteses, uveal melanoma, endometrial cancer, meningioma, and thymoma in Figure 2A appear to show evidence of success in identifying new responding indications. However, the positivity represents a switch in primary endpoint from response rate to progression-free survival. With the exception of endometrial cancer, all of these “positive” trials would have been considered negative had response rate been chosen for primary endpoint (ie, ORR was 5% or lower).
Figure 2B shows the exploration of 11 combination therapy regimes for RCC—an indication that responded and received licensure as monotherapy (117–128). In all, 241 patients and 111 patient-years of involvement in combination therapy studies over five years with corresponding authors based in four different countries, testing 11 different combination therapies, were sampled. In total, 64 patients experienced objective tumor response (32.2% of intent-to-treat population, 95% CI = 29.5% to 34.8%) and four patients died from treatment-related toxicity (1.7%, 95% CI = 1.4% to 1.9%). There was a minimum of 69 grade 3–4 drug related toxicities (28.6%, 95% CI = 25.2% to 32.1%).
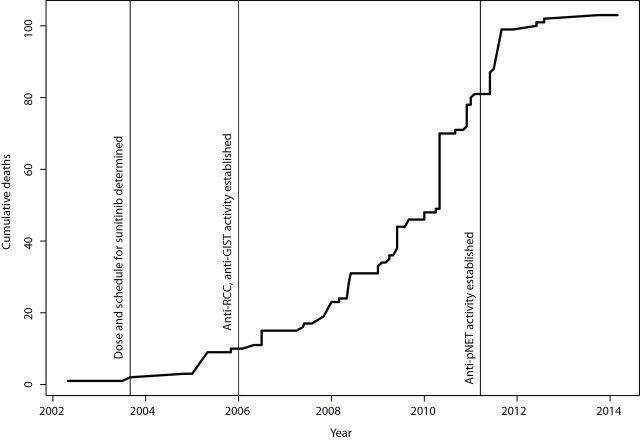
Sunitinib was first licensed by the FDA in 2006 for second-line gastrointestinal stromal tumor (GIST) and RCC and was licensed for pancreatic neuroendocrine tumors (pNET) in 2011. The relationship between achievement of milestones in sunitinib development and patient burden as measured by treatment-related deaths in the whole portfolio is depicted in Figure 3. Very few patient deaths occurred before appropriate dose, schedule, and initial responding indications were defined (3 deaths before FDA approval). However, burden accumulated before a third indication was established—81 treatment-related patient deaths in total.
Figure 3.
Cumulative treatment-related deaths in trials of sunitinib for key milestones. The total number of deaths in the whole portfolio is charted against time. Dates are based on trial closure.
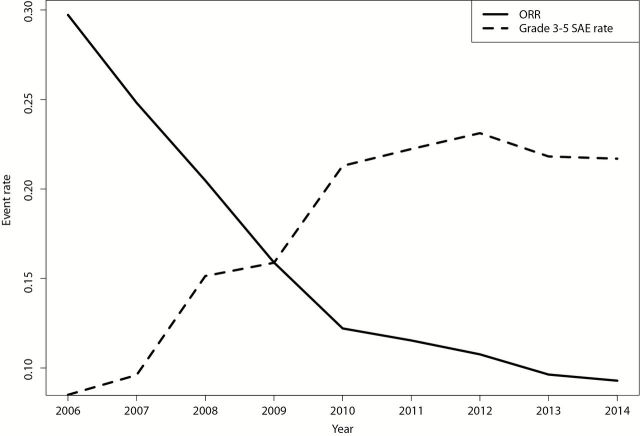
The above analysis suggests that most of the clinically useful applications of sunitinib were discovered very early on in clinical development, and our AERO diagram suggests a worsening risk/benefit balance with further testing of new malignancies. To characterize the evolution of risk and benefit, we plotted cumulative rates of objective response and drug-related life threatening morbidity and mortality for all monotherapy trials testing indications that were not yet FDA approved (Figure 4). Cumulative death rates increased, while cumulative ORR rates diminished with time (see Supplementary Figure 2, available online, for noncumulative risk and benefit proportions by year for studies in non–FDA approved indications).
Figure 4.
Cumulative risk and benefit proportions for studies in non–US Food and Drug Administration–approved indications. All events were classified by report authors as probably or definitely drug related. Dates are based on trial closure. ORR = overall response rate.
Patterns of Research, Coordination, and Outcomes
Our AERO diagram reveals 30 phase II trials testing indications that had been previously tested in phase I. In three instances, positive signal in phase I (defined as an objective response) led to positive phase II studies; in eight instances, absence of signal in phase I was followed by negative phase II studies of the same indication in a larger population. In 18 instances, indications were explored in phase II without prior reported testing of the same indication in phase I trials. We found many instances where single indications were tested in monotherapy studies before the results of earlier studies in the same indication were available. For RCC and GIST, phase II studies were initiated before closure to enrollment in phase I and phase III trials were initiated before closure to enrollment in phase II. In two instances, inconclusive initial phase II studies in a given indication were followed by negative trials in the same indication. We found 16 instances where negative phase II trials in an indication were followed by other, negative phase II trials in the same indication. We calculate that 22 patients died and 155 grade 3–4 events occurred because of drug toxicity in these potentially replicative studies. Based on inception and closure dates in trial reports, however, no replicative phase II trials were initiated after the first closed to recruitment. The mean time between closure to recruitment and publication was 26 months. Mean time to publication for negative studies in our cohort of trials was similar to that for positive studies (26 vs 27 months).
Industry would be expected to vigorously pursue malignancies that show the greatest prospect of approval. Since industry is the single largest funder, it would also be better positioned to synthesize the totality of evidence and coordinate investigations accordingly. We therefore tested whether industry-funded studies showed a more favorable risk/benefit profile for trials of indications not yet FDA approved. Industry-funded studies showed no statistically significant difference in ORR (10.0% vs 8.5%, P = .62) or in drug-related death rate (1.54% vs 1.53%, P = .98) when compared with nonindustry.
Discussion
We found striking discontinuities as sunitinib advanced toward postlicensure trials, with success rates and patient benefit diminishing as the research program matured. Our findings suggest potential deficiencies in the way information was used for planning studies and have implications for the planning and review of trials for new interventions.
Our analysis reveals patterns of risk and outcomes that would not be apparent from aggregate figures regarding success rates in translation. Though sunitinib clearly counts as a success story for drug development, the number of negative trials for indications not yet FDA approved far exceeded the number of positive trials, and risk/benefit worsened with time. Indeed, the total risk/benefit for the whole monotherapy portfolio, which included phase II and II studies leading to licensure, was similar to that reported for phase I monotherapy cancer studies in general (130).
Key elements for unlocking the clinical utility of sunitinib (responding indications, schedule, and dose) were established early on—and at a very modest burden for patients. The almost uniform inability to detect a clinically promising signal after the first three indications suggests that after initial discovery of responding indications researchers were unable to marshal preclinical evidence, knowledge of pathophysiology, or biomarkers to select new indications for further testing.
Policy discussions of clinical translation often tacitly portray the process of clinical translation as an orderly and methodical process, whereby early-phase studies beget safer late-phase studies that match test conditions (131). Our analysis of the whole portfolio of sunitinib monotherapy trials—and a slice of combination therapy studies—reveals a pattern that runs contrary to this portrayal. Phase III and additional phase II trials are launched before initial phase II trials in the same indication are completed, and trials exploring new indications are run concurrently and in rapid succession; the latter makes it difficult for researchers to use outcomes to plan subsequent studies. Exploration of new indications continued despite mounting evidence that responding indications had been saturated or that criteria for launching tests of new indications were not bearing fruit. The patient burden associated with this approach to indication exploration—at least for sunitinib—was considerable.
Our identification of potentially replicative studies, and a rapid fire approach to exploring indications, suggest that some burdens of translation, in particular, adverse events for participants and costs associated with unsuccessful primary endpoint attainment, arise not merely from inherent uncertainties, but also from poor coordination and information flow across the full research portfolio.
The patterns we observed—if they hold for other drugs—have implications for ethical review and data monitoring of trials and what is disclosed to prospective subjects during informed consent. For instance, patients might be told whether they are participating in a study that is preceded by studies with positive signal or not and what this might entail in terms of risk. Data monitoring committees should remain abreast of and respond to the outcomes of other new indication studies in a translation trajectory. When a study in an indication fails, institutional review boards and data safety monitoring boards should require explicit justification for initiating new or continuing underway studies in the same indication.
Our findings also have implications for initiatives addressing inefficiencies in drug development. Studies in our sample were funded and pursued by various actors and sponsors, and no single entity is synthesizing findings across different indications and coordinating further testing. The highly favorable risk/benefit profile of studies early in development represents the effect of incentives for drug developers to sprint to market with an agent that can begin to recoup the costs of development. Industry quickly capitalized on low-hanging fruit. After clear demonstration of activities in several different malignancies, we see a process of exploration in which the search for additional indications appears to have been poorly coordinated and may have involved a relaxation of evidentiary criteria.
These results should be interpreted in light of the following limitations. First, trends for sunitinib monotherapy may not generalize to other drugs. Sunitinib is a multikinase inhibitor; the prospect of many potential applications for a relatively novel drug—and strong signal of activity early on—may have driven an unusual level of indication exploration; many off-target effects likely drove the heavy burden. Second, to our knowledge, all of the studies enrolled patients who had exhausted established effective therapy. Given the absence of effective therapy for these life-threatening diseases, there are good reasons for vigorous research programs testing possible therapies. Nevertheless, patients with advanced disease are entitled to having their interests protected by proper planning, coordination, and risk minimization. Moreover, studies of second-line therapy and beyond still entail opportunity costs and draw on scarce research resources. Third, any single malignancy may have seemed—and may continue to appear—a plausible candidate for sunitinib monotherapy. Nevertheless, knowledge of how sunitinib monotherapy had fared against a host of other malignancies might have tempered the rationale for further exploration of indications and combination therapies. Fourth, some apparent redundancy reflected in our AERO diagram reflects important differences in trial hypotheses. For example, variation in trial outcomes in the RCC stratum in part reflects that some trials tested RCC subtypes, such as non–clear cell RCC, that are less responsive to sunitinib. Fifth, had investigators chosen different endpoints (eg, progression-free survival), the pattern of success and failure and evolution of risk/benefit might appear different. Last, our study relied on fully published research reports. According to one analysis, 61% of phase II studies are never published (132), and we found that 36% of registered sunitinib trials were not published within four years of the trials’ registry closure dates. However, we suspect that unpublished studies, if added to our analysis, would not improve the risk/benefit balance of the entire portfolio.
Our findings reinforce the suggestion, offered by others (133), that systematic review and research decision-making should draw on evidence from whole research programs rather than particular tranches within them. Any one protocol may appear to have a sound basis, but when the sunitinib monotherapy development portfolio is viewed as a whole there was a trend of worsening risk/benefit, and the testing of new indications responded slowly to accumulating evidence that sunitinib monotherapy would not extend to new malignancies. Most major codes of ethics prescribe the minimization of risk; a view of the entire portfolio and an assessment of cumulative risk/benefit offer a resource for achieving these ends.
Funding
This work was funded by the Canadian Institutes of Health Research (EOG111391).
Supplementary Material
Without intending to suggest their endorsement of our analysis, we thank Dean Fergusson, Abe Fuks, and Charles Weijer for various discussions; we also thank Janet Dancey for consultations. Faults remain our own.
The authors declare no competing interests related to the content of this paper.
References
- 1. Ioannidis JPA, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chalmers I, Bracken MB, Djulbegovic B, et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383(9912):156–165. [DOI] [PubMed] [Google Scholar]
- 3. Contopoulos-Ioannidis DG, Alexiou GA, Gouvias TC, Ioannidis JP. Life cycle of translational research for medical interventions. Science. 2008;321(5894):1298–1299. [DOI] [PubMed] [Google Scholar]
- 4. Moore TJ, Furberg CD. Development times, clinical testing, postmarket follow-up, and safety risks for the new drugs approved by the US Food and Drug Administration: the class of 2008. JAMA Int Med. 2014;174(1):90–95. [DOI] [PubMed] [Google Scholar]
- 5. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–716. [DOI] [PubMed] [Google Scholar]
- 6. Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32(1):40–51. [DOI] [PubMed] [Google Scholar]
- 7. President’s Council of Advisors on Science and Technology (U.S.). Report to the President On Propelling Innovation in Drug Discovery, Development, and Evaluation. Executive Office of the President, President’s Council of Advisors on Science and Technology; Washington, D.C; 2012. [Google Scholar]
- 8. Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA. 2011;306(23):2608–2609. [DOI] [PubMed] [Google Scholar]
- 9. U.S. Department of Health and Human Services, Food and Drug Administration. Critical Path Opportunities Report, 2006.
- 10. Collins FS. Reengineering translational science: the time is right. Sci Trans Med. 2011;3(90):90cm17–90cm17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 12. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 13. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria; 2015. [Google Scholar]
- 14. Fiedler W, Serve H, Döhner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–993. [DOI] [PubMed] [Google Scholar]
- 15. Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. [DOI] [PubMed] [Google Scholar]
- 16. Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. [DOI] [PubMed] [Google Scholar]
- 17. Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. [DOI] [PubMed] [Google Scholar]
- 18. Bello Carlo L, Sherman Laurie, Zhou Jihao, et al. Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs. 2006;17(3):353–358. [DOI] [PubMed] [Google Scholar]
- 19. Demetri GD, Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. [DOI] [PubMed] [Google Scholar]
- 20. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. [DOI] [PubMed] [Google Scholar]
- 21. Britten CD, Kabbinavar F, Hecht JR, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2008;61(3):515–524. [DOI] [PubMed] [Google Scholar]
- 22. Saltz LB, Rosen LS, Marshall JL, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25(30):4793–4799. [DOI] [PubMed] [Google Scholar]
- 23. Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(4):650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–1816. [DOI] [PubMed] [Google Scholar]
- 25. Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26(20):3403–3410. [DOI] [PubMed] [Google Scholar]
- 26. Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26(22):3743–3748. [DOI] [PubMed] [Google Scholar]
- 27. Thomas AA, Rini BI, Lane BR, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181(2):518–523. [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann K, Schmittel A, Steiner U, et al. Sunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenib. Oncology. 2008;76(5):350–354. [DOI] [PubMed] [Google Scholar]
- 29. Janeway KA, Albritton KH, Van Den Abbeele AD, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Ped Blood Cancer. 2009;52(7):767–771. [DOI] [PubMed] [Google Scholar]
- 30. Michaelson MD, Regan MM, Oh WK, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20(5):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feldman DR, Turkula S, Ginsberg MS, et al. Phase II trial of sunitinib in patients with relapsed or refractory germ cell tumors. Invest New Drugs. 2010;28(4):523–528. [DOI] [PubMed] [Google Scholar]
- 34. Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicenter, phase II study. Lancet Oncol. 2009;10(8):794–800. [DOI] [PubMed] [Google Scholar]
- 35. Sonpavde G, Periman PO, Bernold D, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2009:mdp323. [DOI] [PubMed] [Google Scholar]
- 36. Escudier B, Roigas J, Gillessen S, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(25):4068–4075. [DOI] [PubMed] [Google Scholar]
- 37. Choong NW, Kozloff M, Taber D, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs. 2010;28(5):677–683. [DOI] [PubMed] [Google Scholar]
- 38. Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, et al. A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol. 2010;65(4):649–660. [DOI] [PubMed] [Google Scholar]
- 39. Shirao K, Nishida T, Doi T, et al. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs. 2010;28(6):866–875. [DOI] [PubMed] [Google Scholar]
- 40. Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND. 184. Gynecol Oncol. 2010;116(2):163–167. [DOI] [PubMed] [Google Scholar]
- 41. Khosravan R, Toh M, Garrett M, et al. Pharmacokinetics and safety of sunitinib malate in subjects with impaired renal function. J Clin Pharmacol. 2010;50(4):472–481. [DOI] [PubMed] [Google Scholar]
- 42. Hensley ML, Sill MW, Scribner DR, et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2009;115(3):460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Novello S, Scagliotti GV, Rosell R, et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101(9):1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol. 2010;40(3):194–202. [DOI] [PubMed] [Google Scholar]
- 45. Machiels JPH, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28(1):21–28. [DOI] [PubMed] [Google Scholar]
- 46. Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol. 2010;28(8):1373–1379. [DOI] [PubMed] [Google Scholar]
- 47. Koeberle D, Montemurro M, Samaras P, et al. Continuous sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist. 2010;15(3):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29(6):1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wildiers H, Fontaine C, Vuylsteke P, et al. Multicenter phase II randomized trial evaluating antiangiogenic therapy with sunitinib as consolidation after objective response to taxane chemotherapy in women with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2010;123(2):463–469. [DOI] [PubMed] [Google Scholar]
- 50. Apostolidou E, Kantarjian H, Thomas D, Burger I, Borthakur G, Verstovsek S. Phase II Study of Sunitinib in Patients With Primary or Post-Polycythemia Vera/Essential Thrombocythemia Myelofibrosis. Clin Lymphoma Myeloma Leukemia. 2010;10(4):281–284. [DOI] [PubMed] [Google Scholar]
- 51. Biagi JJ, Oza AM, Chalchal HI, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group Study. Ann Oncol. 2011;22(2):335–340. [DOI] [PubMed] [Google Scholar]
- 52. Hellenthal NJ, Underwood W, Penetrante R, et al. Prospective clinical trial of preoperative sunitinib in patients with renal cell carcinoma. J Urol. 2010;184(3):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in fdg-pet-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16(21):5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neyns B, Sadones J, Chaskis C, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. [DOI] [PubMed] [Google Scholar]
- 55. O’Reilly EM, Niedzwiecki D, Hall M, et al. A Cancer and Leukemia Group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603). Oncologist. 2010;15(12):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 57. Oechsle K, Honecker F, Cheng T, et al. Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: a Canadian Urol Oncol Group/German Testicular Cancer Study Group cooperative study. Ann Oncol. 2011;22(12):2654–2660. [DOI] [PubMed] [Google Scholar]
- 58. Bellmunt J, Gonzalez-Larriba JL, Prior C, et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22(12):2646–2653. [DOI] [PubMed] [Google Scholar]
- 59. Mahmood ST, Agresta S, Vigil CE, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer. 2011;129(8):1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buckstein R, Kuruvilla J, Chua N, et al. Sunitinib in relapsed or refractory diffuse large B-cell lymphoma: a clinical and pharmacodynamic phase II multicenter study of the NCIC Clinical Trials Group. Leukemia Lymphoma. 2011;52(5):833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moehler M, Mueller A, Hartmann JT, et al. An open-label, multicenter biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer Eur J Cancer. 2011;47(10):1511–1520. [DOI] [PubMed] [Google Scholar]
- 62. Schneider BJ, Gadgeel SM, Ramnath N, et al. Phase II trial of sunitinib maintenance therapy after platinum-based chemotherapy in patients with extensive-stage small cell lung cancer. J Thorac Oncol. 2011;6(6):1117–1120. [DOI] [PubMed] [Google Scholar]
- 63. Négrier S, Gravis G, Pérol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12(7):673–680. [DOI] [PubMed] [Google Scholar]
- 64. Yildiz I, Sen F, Basaran M, et al. Response rates and adverse effects of continuous once-daily sunitinib in patients with advanced renal cell carcinoma: a single-center study in Turkey. Jpn J Clin Oncol. 2011:41(12):1380–1387. [DOI] [PubMed] [Google Scholar]
- 65. Jonasch E, McCutcheon IE, Waguespack SG, et al. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol. 2011;22(12):2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DuBois SG, Shusterman S, Ingle AM, et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a children’s oncology group study. Clin Cancer Res. 2011;17(15):5113–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bex A, Blank C, Meinhardt W, Tinteren H, Horenblas S, Haanen J. A phase II study of presurgical sunitinib in patients with metastatic clear-cell renal carcinoma and the primary tumor in situ. Urology. 2011;78(4):832–837. [DOI] [PubMed] [Google Scholar]
- 68. Barrios CH, Hernandez-Barajas D, Brown MP, et al. Phase ii trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma (mrcc): preliminary results. Eur J Cancer Suppl. 2009;7(2):429–430. [DOI] [PubMed] [Google Scholar]
- 69. Laurie SA, Gupta A, Chu Q, et al. Brief report: a phase II study of sunitinib in malignant pleural mesothelioma. the NCIC Clinical Trials Group. J Thorac Oncol. 2011;6(11):1950–1954. [DOI] [PubMed] [Google Scholar]
- 70. Chau NG, Hotte SJ, Chen EX, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23(6):1562–1570. [DOI] [PubMed] [Google Scholar]
- 71. Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicenter, multinational study. Eur J Cancer. 2012;48(2):196–201. [DOI] [PubMed] [Google Scholar]
- 72. Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30(1):335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strosberg JR, Weber JM, Choi J, et al. A phase II clinical trial of sunitinib following hepatic transarterial embolization for metastatic neuroendocrine tumors. Ann Oncol. 2012;23(9):2335–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Campos SM, Penson RT, Matulonis U, et al. A phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol Oncol. 2013;128(2):215–220. [DOI] [PubMed] [Google Scholar]
- 75. Han JY, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer. 2013;79(2):137–142. [DOI] [PubMed] [Google Scholar]
- 76. DuBois SG, Shusterman S, Reid JM, et al. Tolerability and pharmacokinetic profile of a sunitinib powder formulation in pediatric patients with refractory solid tumors: a Children’s Oncology Group study. Cancer Chemother Pharmacol. 2012;69(4):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baumann KH, Du Bois A, Meier W, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann Oncol. 2012;23(9):2265–2271. [DOI] [PubMed] [Google Scholar]
- 78. Baldazzi V, Tassi R, Lapini A, Santomaggio C, Carini M, Mazzanti R. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urol Oncol. 2012;30(5):704–710. [DOI] [PubMed] [Google Scholar]
- 79. Staehler M, Haseke N, Stadler T, et al. Feasibility and effects of high-dose hypofractionated radiation therapy and simultaneous multi-kinase inhibition with sunitinib in progressive metastatic renal cell cancer. 2012;30(3):290–293. [DOI] [PubMed] [Google Scholar]
- 80. Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012;23(8):2108–2114. [DOI] [PubMed] [Google Scholar]
- 81. Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30(12):1371–1377. [DOI] [PubMed] [Google Scholar]
- 82. Rini BI, Garcia J, Elson P, et al. The effect of sunitinib on primary renal cell carcinoma and facilitation of subsequent surgery. J Urol. 2012;187(5):1548–1554. [DOI] [PubMed] [Google Scholar]
- 83. Flörcken A, Takvorian A, Van Lessen A, et al. Sorafenib, but not sunitinib, induces regulatory T cells in the peripheral blood of patients with metastatic renal cell carcinoma. Anticancer Drugs. 2012;23(3):298–302. [DOI] [PubMed] [Google Scholar]
- 84. Zhao J, Zhu Y, Zhang C, et al. Sorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrence. Urol Oncol. 2013;31(8):1800–1805. [DOI] [PubMed] [Google Scholar]
- 85. Tong CCL, Ko EC, Sung MW, et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PloS One. 2012;7(6):e36979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62(6):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Demetri GD, Garrett CR, Schöffski P, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pan E, Yu D, Yue B, et al. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol. 2012;110(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metabol. 2012;97(10):3495–3503. [DOI] [PubMed] [Google Scholar]
- 90. Nowak AK, Millward MJ, Creaney J, et al. A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol. 2012;7(9):1449–1456. [DOI] [PubMed] [Google Scholar]
- 91. Yardley DA, Dees EC, Myers SD, et al. Phase II open-label study of sunitinib in patients with advanced breast cancer. Breast Cancer Res Treat. 2012;136(3):759–767. [DOI] [PubMed] [Google Scholar]
- 92. Kreisl TN, Smith P, Sul J, et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111(1):41–48. [DOI] [PubMed] [Google Scholar]
- 93. Neri B, Vannini A, Brugia M, et al. Biweekly sunitinib regimen reduces toxicity and retains efficacy in metastatic renal cell carcinoma: A single-center experience with 31 patients. Int J Urol. 2013;20(5):478–483. [DOI] [PubMed] [Google Scholar]
- 94. Mahipal A, Tijani L, Chan K, Laudadio M, Mastrangelo MJ, Sato T. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res. 2012;22(6):440–446. [DOI] [PubMed] [Google Scholar]
- 95. Ito T, Okusaka T, Nishida T, et al. Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drugs. 2013;31(5):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Barone C, Basso M, Biolato M, et al. A phase II study of sunitinib in advanced hepatocellular carcinoma. Dig Liver Dis. 2013;45(8):692–698. [DOI] [PubMed] [Google Scholar]
- 97. Reynolds C, Spira AI, Gluck L, et al. Sunitinib malate in previously untreated, nonsquamous, non-small cell lung cancer patients over the age of 70 years: results of a Phase II trial. Invest New Drugs. 2013;31(5):1330–1338. [DOI] [PubMed] [Google Scholar]
- 98. Reni M, Cereda S, Milella M, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer. 2013;49(17):3609–3615. [DOI] [PubMed] [Google Scholar]
- 99. Curigliano G, Pivot X, Cortés J, et al. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast. 2013;22(5):650–656. [DOI] [PubMed] [Google Scholar]
- 100. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. [DOI] [PubMed] [Google Scholar]
- 101. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013:JCO-2012. [DOI] [PubMed] [Google Scholar]
- 102. Kim HR, Park HS, Kwon WS, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol. 2013;72(4):825–835. [DOI] [PubMed] [Google Scholar]
- 103. Chevreau C, Ravaud A, Escudier B, et al. A Phase II Trial of Sunitinib in Patients With Renal Cell Cancer and Untreated Brain Metastases. Clin Genitourin Cancer. 2014;12(1):50–54. [DOI] [PubMed] [Google Scholar]
- 104. Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120(5):692–701. [DOI] [PubMed] [Google Scholar]
- 105. Hutterer M, Nowosielski M, Haybaeck J, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neuro-oncol. 2014;16(1):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Balaña C, Gil MJ, Perez P, et al. Sunitinib administered prior to radiotherapy in patients with non-resectable glioblastoma: results of a Phase II study. Target Oncol. 2014;9(4):321–329. [DOI] [PubMed] [Google Scholar]
- 107. Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol. 2014:JCO-2013. [DOI] [PubMed] [Google Scholar]
- 108. Lankheet NAG, Kloth JSL, Hooijdonk CGM, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumors. Br J Cancer. 2014;110(10):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Castonguay V, Lheureux S, Welch S, et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: A study of the Princess Margaret, Chicago and California Consortia. Gynecol Oncol. 2014;134(2):274–280. [DOI] [PubMed] [Google Scholar]
- 110. Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014:JCO-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Adenis A, Blay JY, Bui-Nguyen B, et al. Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: A randomized controlled open-label trial. Ann Oncol. 2014;25(9):1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wuthrick EJ, Curran WJ, Camphausen K, et al. A Pilot Study of Hypofractionated Stereotactic Radiation Therapy and Sunitinib in Previously Irradiated Patients With Recurrent High-Grade Glioma. Int J Radiat Oncol Biol Phys. 2014;90(2):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2014;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Motzer RJ, Hutson TE, Hudes GR, et al. Investigation of novel circulating proteins, germ line single-nucleotide polymorphisms, and molecular tumor markers as potential efficacy biomarkers of first-line sunitinib therapy for advanced renal cell carcinoma. Cancer Chemother Pharmacol. 2014;74(4):739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Matsushita H, Enomoto Y, Kume H, et al. A pilot study of autologous tumor lysate-loaded dendritic cell vaccination combined with sunitinib for metastatic renal cell carcinoma. J Immunother Cancer. 2014;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16(2):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(9):1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7(1):24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Motzer RJ, Hudes GR, Ginsberg MS, et al. Phase I/II trial of sunitinib plus gefitinib in patients with metastatic renal cell carcinoma. Am J Clin Oncol. 2010;33(6):614–618. [DOI] [PubMed] [Google Scholar]
- 120. Grünwald V, Desar IME, Haanen J, et al. A phase I study of recombinant human interleukin-21 (rIL-21) in combination with sunitinib in patients with metastatic renal cell carcinoma (RCC). Acta Oncol. 2011;50(1):121–126. [DOI] [PubMed] [Google Scholar]
- 121. Rini BI, Stein M, Shannon P, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117(4):758–767. [DOI] [PubMed] [Google Scholar]
- 122. Bruce JY, Eickhoff J, Pili R, et al. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest New Drugs. 2012;30(2):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118(7):1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schmidinger M, Szczylik C, Sternberg CN, et al. Dose escalation and pharmacokinetics study of enzastaurin and sunitinib versus placebo and sunitinib in patients with metastatic renal cell carcinoma. Am J Clin Oncol. 2012;35(5):493–497. [DOI] [PubMed] [Google Scholar]
- 125. Hong DS, Gordon MS, Samlowski WE, et al. A phase I, open-label study of trebananib combined with sorafenib or sunitinib in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2014;12(3):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bruce JY, Kolesar JM, Hammers H, et al. A phase I pharmacodynamic trial of sequential sunitinib with bevacizumab in patients with renal cell carcinoma and other advanced solid malignancies. Cancer Chemother Pharmacol. 2014;73(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rini B, Redman B, Garcia JA, et al. A phase I/II study of lenalidomide in combination with sunitinib in patients with advanced or metastatic renal cell carcinoma. Ann Oncol. 2014;25(9):1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bellmunt J, Suarez C, Gallardo E, et al. Phase I Study of Sunitinib in Combination With Gemcitabine and Capecitabine for First-Line Treatment of Metastatic or Unresectable Renal Cell Carcinoma. Oncologist. 2014;19(9):917–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hey SP, Heilig CM, Weijer C. Accumulating Evidence and Research Organization (AERO) model: a new tool for representing, analyzing, and planning a translational research program. Trials. 2013;14(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Roberts TG, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292(17):2130–2140. [DOI] [PubMed] [Google Scholar]
- 131. Kimmelman J, London AJ. The Structure of Clinical Translation: Efficiency, Information, and Ethics. Hastings Cent Rep. 2015;45(2):27–39. [DOI] [PubMed] [Google Scholar]
- 132. Hoeg RT, Lee JA, Mathiason MA, et al. Publication outcomes of phase II oncology clinical trials. Am J Clin Oncol. 2009;32(3):253–257. [DOI] [PubMed] [Google Scholar]
- 133. Ioannidis JPA, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ. 2010;341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.