Abstract
In analyses combining estrogen with or without progestin, some observational studies describe minimal breast cancer risk in obese and black women. Therefore, we examined these suggested interactions in the two Women’s Health Initiative (WHI) randomized hormone therapy trials. The estrogen plus progestin trial entered 16 608 postmenopausal women with a uterus, while the estrogen trial entered 10 736 postmenopausal women with prior hysterectomy. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values from log-rank x2 statistics were estimated from Cox proportional hazards models with subgroup analyses based on tests of interaction. All statistical tests were two-sided. Estrogen plus progestin statistically significantly increased breast cancer incidence (HR = 1.28, 95% CI = 1.11 to 1.48, P < .001), with hazard ratios greater than 1 in all body mass index (BMI) subgroups (Pinteraction = .58) and hazard ratios greater than 1 in black and white women (Pinteraction = .96). In contrast, estrogen alone statistically significantly decreased breast cancer incidence (HR = 0.79, 95% CI = 0.65 to 0.90, P = .02), with hazard ratios lower than 1 in all BMI subgroups (Pinteraction = .86) and hazard ratios lower than 1 in black and white women, where analyses with limited numbers suggest somewhat greater reduction in black women (Pinteraction = .09). In summary, estrogen plus progestin and estrogen alone have opposite effects on breast cancer incidence, with no statistically significant interactions by race/ethnicity or BMI. Therefore, observational studies should not combine these two regimens when examining breast cancer risk.
In the Women’s Health Initiative (WHI) trial evaluating estrogen plus progestin in postmenopausal women with a uterus (1,2), combined hormone therapy statistically significantly increased breast cancer incidence (HR = 1.28, 95% CI = 1.11 to 1.98, P < .001) (3) and statistically significantly increased deaths from breast cancer (4). In contrast, in the WHI trial in postmenopausal women with prior hysterectomy (1,5,6) with longer follow-up, estrogen alone statistically significantly decreased breast cancer incidence (HR = 0.79, 95% CI = 0.65 to 0.90, P = .02) and statistically significantly decreased deaths from breast cancer (7).
These randomized trial findings differ from the predominance of observational study reports where both hormone therapy regimens have been associated with increased breast cancers (8), with some observational studies continuing to report breast cancer results combining the two hormone therapy regimens (9–13). In addition, in some studies breast cancer risk with hormone therapy is substantially lower in obese women (14–17) and in black women (18,19), with a recent report suggesting that black and obese women, especially those with dense breasts, may experience “minimal excess breast cancer risk” with hormone therapy use (10).
Such findings, suggestive of minimal breast cancer risk for large subgroups of women, could influence clinical practice. Therefore, we examined estrogen plus progestin and estrogen alone influence on breast cancer incidence by body mass index (BMI) and race/ethnicity in the WHI randomized trials after 13 years of cumulative follow-up.
The studies conducted in the WHI hormone therapy trials have been published (1–3). In these trials, 16 608 postmenopausal women with a uterus (including 1122 black women) were assigned oral conjugated equine estrogen (estrogen) 0.625mg/d plus medroxyprogesterone acetate (progestin) 2.5mg/d or placebo, and 10 739 women with prior hysterectomy (including 1616 black women) were assigned estrogen 0.625mg/d or placebo. Median interventions were 5.6 and 7.2 years in the estrogen plus progestin and estrogen alone trials, respectively.
The studies are registered with ClinicalTrials.gov, number NCT 00000611. Eligible were postmenopausal women age 50 to 79 years with negative baseline mammogram, no prior breast cancer, and anticipated survival more than three years. The trials were approved by institutional review boards, and participants provided written, informed consent. Information on baseline characteristics was collected using standardized questionnaires. Measured body weight and height were used to calculate BMI. Race/ethnicity was by self-report. Breast cancers were confirmed by medical record review, with findings compared using hazard ratios (HRs), corresponding 95% confidence intervals (CIs), and P values from log-rank x2 statistics that were estimated from Cox proportional hazards models. Subgroup analyses were assessed similarly with statistical significance based on tests of interaction. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
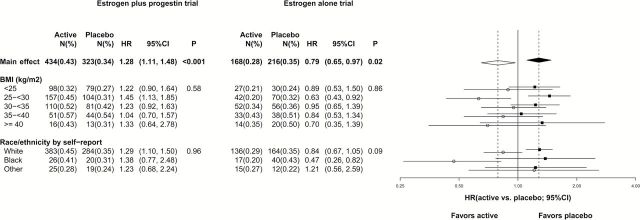
Baseline characteristics for randomization groups in each trial were well balanced for breast cancer risk factors for both white and black participants (2–4). Participant trial flow is described in Supplementary Figure 1 (available online). As previously reported, in the overall populations (20), estrogen plus progestin increased (HR = 1.28, 95% CI = 1.11 to 1.48,P < .001) and estrogen alone decreased (HR = 0.79, 95% CI = 0.65 to 0.90,P = .02) breast cancer incidence. In current subgroup analyses in the estrogen plus progestin trial, hazard ratios for breast cancer incidence were greater than 1 in all BMI groups (Pinteraction = .96). The hazard ratios in women with BMIs of less than 25 was similar to the hazard ratios in higher BMI groups (Figure 1), and no interaction with BMI was seen (Pinteraction = .58). In the estrogen alone trial, HRs for breast cancer incidence were less than 1 in all BMI groups and no interaction with BMI was seen (Pinteraction = .86) (Figure 1).
Figure 1.
Associations between hormone therapy and invasive breast cancer incidence in the overall study population and select subgroups in the Women’s Health Initiative estrogen plus progestin (n = 16 608) and estrogen alone (n = 10 739) randomized trials (intervention and postintervention periods). A solid (open) diamond represents the hazard ratio (HR; 95% confidence interval [CI]) for the main effect of the estrogen plus progestin (estrogen alone) trial. Solid (open) square (circle) and line represent HR (95% CI) for subgroups of the estrogen plus progestin (estrogen alone) trial. Dotted vertical reference line corresponds to estimates of the main effects. Two-sided P values were based on a log-rank (score) test and correspond to the test of main effects, or test of interactions for the subgroup analysis. A one-degree-of-freedom test for trend of the interaction was used for subgroups of body mass index, and a two-degree-of-freedom test was used for the subgroups of race/ethnicity. % = annualized percentage; CI = confidence interval; HR = hazard ratio; N = number of events; P = P value that corresponds to a test of the main effect or interactions.
For black women, the hazard ratio for breast cancer incidence with estrogen plus progestin use was 1.38 (95% CI = 0.77 to 2.48), comparable with that for white women (HR = 1.29, 95% CI = 1.10 to 1.50). For black women in the estrogen alone trial, a somewhat greater reduction in breast cancer incidence for estrogen use was suggested (17 vs 30 cases, respectively, HR = 0.47, 95% CI = 0.26 to 0.82) compared with white women (HR = 0.84, 95% CI = 0.67 to 1.05) (Pinteraction = .09) (Figure 1).
When the estrogen plus progestin results were initially reported in 2003 with 348 breast cancer case patients, the hazard in women with BMIs 30 or greater was 1.08 (95% CI = 0.78 to 1.49) (4), consistent with the common observational study null effect (21). Now with longer follow-up and 757 case patients, hazard ratios for estrogen plus progestin are substantially higher than 1 and comparable in lean (BMI < 25) and heavier women, suggesting adverse influence regardless of BMI (Figure 1). This issue may not be entirely settled, as the Million Women Study finds greater risk in lean than in obese women (21). Nonetheless, current evidence is insufficient to support use of estrogen plus progestin in obese women with “minimal breast cancer risk” (10).
Several factors may confound analyses of breast cancer risk in black and obese women in observational studies where estrogen alone and estrogen plus progestin findings are combined. Black and obese women are more likely to have a hysterectomy (13,22) and bilateral oophorectomy (23), the latter associated with lower breast cancer risk (24,25). In addition, women with hysterectomy are candidates for estrogen alone use. As a result, apparent lower breast cancer risk for hormone therapy use in obese and black women in observational studies can be confounded by disproportionate oophorectomy history and estrogen alone use. Study limitations in the current randomized trial include limited numbers in some subgroups and the potential for residual confounding despite random assignment.
Lower breast cancer incidence but higher breast cancer mortality is seen in black compared with white women in US populations (26–28), a finding not explained by consideration of socioeconomic factors (28,29), screening (30), cancer characteristics (31), or cancer therapy (32). Against that background, the finding that estrogen alone reduces breast cancer incidence in black women, based on analysis in a randomized trial involving 1616 black women, warrants additional study.
In conclusion, estrogen plus progestin and estrogen alone have opposite effects on breast cancer incidence, with no statistically significant interactions by race/ethnicity or BMI. Therefore, observational studies should not combine these two regimens in analyses examining breast cancer risk.
Funding
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 321115, 32118–32119, 32122, 42107–26, 42129–32, and 44221. Wyeth-Ayerst donated the study drugs.
Supplementary Material
Role of the Sponsors: The Women’s Health Initiative (WHI) project office at the National Heart, Lung, and Blood Institute (NHLBI), which was the sponsor, had a role in the design and conduct of the study; interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. Decisions concerning the above, as well as data collection, management, and analysis, resided with committees composed of WHI Investigators and included NHLBI representatives.
Conflict of interest disclosures: The authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Dr. Chlebowski reported receiving consulting fees or honoraria from Novartis, Amgen, AstraZeneca; fees for participation in review activities from Pfizer; payment for lectures from Novartis; and payment for educational activities from Educational Concepts Group. Chlebowski, Prentice, and Anderson reported receiving institutional grant support from the National Institutes of Health.
A Short List of Women’s Health Initiative Investigators: Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD). Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA). Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (State University of New York, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Rowan T. Chlebowski, (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles, CA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Women’s Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Additional Information: A full list of all the investigators who have contributed to Women’s Health Initiative science appears at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Additional Contributions: We thank the Women’s Health Initiative investigators, staff, and the trial participants for their outstanding dedication and commitment.
References
- 1. The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 2. Rossouw JE, Anderson GL, Prentice RL, et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Hendrix SL, Langer RD, et al. ; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. [DOI] [PubMed] [Google Scholar]
- 4. Chlebowski RT, Anderson GL, Gass M, et al. ; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson GL, Limacher M, Assaf AR, et al. ; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 6. Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized trial. JAMA. 2006;242:1048–1063. [DOI] [PubMed] [Google Scholar]
- 7. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104(7):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harlid S, Butt S, Ivarsson MI, et al. Interactive effect of genetic susceptibility with height, body mass index, and hormone replacement therapy on the risk of breast cancer. BMC Womens Health. 2012;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou N, Hong S, Wang W, Olopade OI, Dignam JJ, Huo D. Hormone replacement therapy and breast cancer: heterogeneous risks by race, weight, and breast density. J Natl Cancer Inst. 2013;105(18):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Méplan C, Dragsted LO, Ravn-Haren G, Tjønneland A, Vogel U, Hesketh J. Association between polymorphisms in glutathione peroxidase and selenoprotein P genes, glutathione peroxidase activity, HRT use and breast cancer risk. PLoS ONE. 2013;8(9):e73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obazee O, Justenhoven C, Winter S, et al. Confirmation of the reduction of hormone replacement therapy-related breast cancer risk for carriers of the HSD17B1_937_G variant. Breast Cancer Res Treat. 2013;138(2):543–548. [DOI] [PubMed] [Google Scholar]
- 13. Cui Y, Deming-Halverson SL, Beeghly-Fadiel A, et al. Interactions of hormone replacement therapy, body weight, and bilateral oophorectomy in breast cancer risk. Clin Cancer Res. 2014;20(5):1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 15. Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–491. [DOI] [PubMed] [Google Scholar]
- 16. Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92(4):328–332. [DOI] [PubMed] [Google Scholar]
- 17. Banks E, Canfell K, Reeves G. HRT and breast cancer: recent findings in the context of the evidence to date. Womens Health (Lond Engl). 2008;4(5):427–431. [DOI] [PubMed] [Google Scholar]
- 18. Ritte R, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012;14(3):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell Jenkins BW, Addison C, Wilson G, et al. Association of the joint effect of menopause and hormone replacement therapy and cancer in African American women: the Jackson Heart Study. Int J Environ Res Public Health. 2011;8(6):2491–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beral V, Reeves G, Bull D, Green J; Million Women Study Collaborators. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segars JH, Parrott EC, Nagel JD, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update. 2014;20(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larson CA. Prophylactic bilateral oophorectomy at time of hysterectomy for women at low risk: acog revises practice guidelines for ovarian cancer screening in low-risk women. Curr Oncol. 2014;21(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weitzel JN, Buys SS, Sherman WH, et al. Reduced mammographic density with use of a gonadotropin-releasing hormone agonist-based chemoprevention regimen in BRCA1 carriers. Clin Cancer Res. 2007;13(2 Pt 1):654–658. [DOI] [PubMed] [Google Scholar]
- 25. Benetti-Pinto CL, Brancalion MF, Assis LH, et al. Mammographic breast density in women with premature ovarian failure: a prospective analysis. Menopause. 2014;21(9):933–937. [DOI] [PubMed] [Google Scholar]
- 26. Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. [DOI] [PubMed] [Google Scholar]
- 27. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. [DOI] [PubMed] [Google Scholar]
- 28. Sineshaw HM, Gaudet M, Ward EM, et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010-2011). Breast Cancer Res Treat. 2014;145(3):753–763. [DOI] [PubMed] [Google Scholar]
- 29. Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt). 2009;18(6):883–893. [DOI] [PubMed] [Google Scholar]
- 30. Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553. [DOI] [PubMed] [Google Scholar]
- 31. Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. 2014;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livaudais JC, Lacroix A, Chlebowski RT, et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22(3):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.