Abstract
Background:
Metastasis to the bone is a deleterious aspect of breast cancer and is a preferred site that results in bone loss. Hormones such as prolactin (PRL) have not yet been studied for their role in modulating the secondary tumor bone microenvironment.
Methods:
We used quantitative immunohistochemistry with 134 samples of human primary breast cancer and 17 matched primary breast cancers and bone metastases. A Cox proportional hazards regression model was fitted to evaluate the associations between high prolactin receptor (PRLR) expression and time to bone metastasis, adjusting for estrogen receptor status, lymph node status, and chemotherapy status. We assessed osteoclast differentiation, osteoclast size, and measured pit formation in dentine slices. Statistical tests were two-sided.
Results:
High PRLR expression in the primary breast tumor was associated with a shorter time to metastasis that includes bone (PRLR AQUA Max-per 100 unit hazard ratio = 1.04, 95% confidence interval = 1.00 to 1.07, P = .03). We observed the PRLR in rare samples of bone metastases and matched primary breast cancer. PRL treatment of breast cancer cells induced osteoclast differentiation and bone lysis via secreted factors and was abrogated by a PRLR antagonist (delta1-9-G129R-hPRL). We demonstrated that sonic hedgehog is a PRL-regulated cytokine in breast cancer cells and part of the mechanism that induces osteoclast differentiation.
Conclusions:
Our evidence indicates that PRL-PRLR can escalate the impact of breast cancer on bone metastasis and that the presence of the PRLR in the tumor microenvironment of breast cancer bone metastasis has the potential to modulate the microenvironment to induce lytic osteoclast formation.
Metastasis to the bone is a deleterious and debilitating aspect of breast cancer that occurs in up to 75% to 85% of women diagnosed with metastatic breast cancer ( 1 ). Breast cancer lesions in the bone are primarily osteolytic, resulting in bone loss rather than osteoblastic bone buildup. Breast cancer cells secrete factors that act on pre-osteoclasts, osteoblasts, or bone stromal cells to stimulate the production of mature osteoclasts, which degrade the bone, releasing growth factors that stimulate breast cancer cell proliferation and perpetuate a vicious osteolytic cycle ( 2 ). These factors are important targets for therapeutic intervention; however, the signaling pathways that feed into the vicious cycle are still unknown. Here we identified a new mechanism by which prolactin (PRL)-treated breast cancer cells directly promote the differentiation of functional PRL receptor (PRLR)–negative ( 3–5 ) osteoclasts capable of bone resorption, rather than through an intermediary osteoblast cell.
Large prospective studies determined that high-normal serum levels of PRL are associated with breast cancer risk ( 6 , 7 ). There is an overall worse survival in breast cancer patients ( 8 , 9 ) with an increase in occurrence of breast cancer metastasis ( 8 , 10–12 ). Both invasive ( 13 ) and invasive-suppressive properties ( 14 , 15 ) of PRL signaling have been reported. Advanced breast cancer patients often have elevated levels of PRL associated with poor response to treatment and poor prognosis ( 10 , 11 , 16 , 17 ). Constitutively active variants of the PRLR have been described in breast cancer patients ( 18 ). Expression of the PRLR is associated with poor prognosis ( 19 ). Therefore, there is a relationship of increased PRL and the PRLR in humans with increased breast cancer progression, metastases, and treatment resistance.
The effect of hormones known to impact breast cancer, such as PRL, on bone metastases is unknown. We sought to examine the relationship of PRLR levels in the primary breast tumor with receptor status and patient outcome. We investigated PRLR levels on circulating breast tumor cells (CTCs) and in matched bone metastases of primary breast tumors. We hypothesized that PRL-treated breast cancer cells induce the differentiation of osteolytic osteoclasts via secreted factors. We sought to understand the role of PRL and the PRLR in breast cancer to bone metastasis and identify a PRL-based mechanism that impacts bone metastasis.
Methods
Metastases Tissue Microarray (TMA) Series
Patients were selected based on three pre-assigned groups and criteria: 1) bone metastasis (radiographic evidence of bone involvement by tumor on bone scan; metastasis to other sites was allowed), 2) metastasis to other sites (radiographic disease evidence of distant disease on CT scan or MRI), and 3) no evidence of metastasis after five years (minimum five-year follow-up interval with no documented recurrence). Patients could have not evidence of distant metastasis at initial diagnosis, no previous cancer or synchronous lesion, documented diagnosis of invasive ductal or lobular breast cancer, and no prior experimental therapy.
Matched Primary Breast Tumor and Bone Metastasis Samples
Twenty primary breast tumor samples, seventeen with matched bone metastases, were studied. All metastases and two breast tumor samples were available as tissue sections. The remaining breast tumor samples were available as an array of 0.6mm cores. Institutional ethics review board approval was obtained to identify breast cancer patients with informed consent, both here and TMA.
Quantitative Immunohistochemistry
The Metastases TMA Series consisted of cores from paraffin blocks of 134 patients. Fluorescence immunohistochemistry was performed ( 20 ) using the anti-PRLR antibody 1A2B1 (extracellular domain) (Life Technologies, Burlington, Canada) 1:100, with secondary tyramide-Cy5 (Perkin–Elmer, Waltham, MA) ( 21 ). The epithelial (tumor) compartment was identified by staining with rabbit anti-pan-cytokeratin antibody (DAKO, Burlington, Canada) and an Alexa555-conjugated anti-rabbit secondary antibody (Invitrogen, Burlington, ON, Canada). Slides were scanned using the HistoRx PM-2000 system and analyzed by automated quantitative analysis (AQUA) software.
Preparation and Differentiation of Mouse Bone Marrow–Derived Osteoclasts
Bone marrow cultures were prepared from eight-week-old female Balb/c mice (University of Calgary) ( 22 ) with modifications. Animal care procedures followed the recommendations of the Life and Environmental Sciences Animal Care Committee. Cells were cultured with 100ng/mL M-CSF for 40 to 48 hours before seeded (9.0 x 10 5 cells/mL) in αMEM supplemented with 75ng/mL M-CSF and 50ng/mL RANKL with or without 20% breast cancer cell conditioned medium (CM) for six days.
Preparation of Breast Cancer Cell Conditioned Medium
Cell lines were plated (9.1 x 10 3 cells/cm 2 ) in appropriate medium and serum for 24 hours before treatment with ovine PRL (oPRL; Sigma Aldrich Oakville, Canada), recombinant human PRL (hPRL) ( 23 ), PRLR antagonist, Δ1-9-G129R-hPRL or vehicle for 48 hours. Recombinant hPRL and the PRLR antagonist were produced and purified ( 23 ).
Conditioned Media-Induced Differentiation of RAW264.7 Pre-osteoclasts and Tartrate-Resistant Acid-Phosphatase (TRAP) Assay
RAW264.7 were plated (7.0 x 10 3 cells/cm 2 ) in DMEM (ATCC) supplemented with 20% CM and serum to a total of 10% for six days (adapted from [ 24 ]). Cells were fixed, stained for TRAP ( 25 ) using a leukocyte acid phosphatase kit (Sigma-Aldrich Canada Co.) and counterstained with 10% hematoxylin. Stained cells were viewed under light microscopy using the Zeiss Axiovert 100 inverted microscope (Carl Zeiss Canada Ltd., Toronto, ON, Canada) and TRAP-positive multinucleate cells (containing three or more nuclei) counted.
Dentine Assay
Osteosite dentine discs (Immunodiagnostic Systems Inc., Scottsdale, AZ) ( 26 ) were prepared according to instructions. RAW264.7 cells were plated and stimulated as above on dentine discs. Cells were removed with a toothbrush and discs stained with 0.1% w/v toluidine blue (Sigma Aldrich). The area of dentine resorption was quantified using National Institute of Health ImageJ software. Intact cells on control discs were stained with DAPI.
Statistical Analysis
A paired Student’s t test or analysis of variance (ANOVA) with Tukey post-tests was used in osteoclastogenesis assays and enzyme-linked immunosorbent assays (ELISAs). A P value of .05 or less was required for statistical significance. Standard deviation was displayed as error bars. Detailed analysis and statistical models are described in the Supplementary Methods (available online). All statistical tests were two-sided.
Results
Quantitative Immunohistochemistry of the PRLR in Primary Tumors, CTCs, and Bone Metastases
To explore the potential involvement of the PRL pathway in clinical outcome, we performed quantitative immunohistochemistry of TMAs of clinical breast cancer cases. We optimized the staining procedure for the PRLR antibody 1A2B1 ( 27 ) using AQUA analysis on normal and cancerous breast tissue and breast cancer cell lines with distinct PRLR levels ( Figure 1A ) and carried out analyses on a panel of primary breast tumors from a retrospective discovery cohort with known outcomes (demographics in Table 1 additional description in Supplementary Methods , available online). There was no correlation (Pearsons test for correlation, see the Supplementary Methods , available online) of the PRLR with ER, PR, HER2 status, or Ki67 staining ( Supplementary Table 1 , available online), indicating that PRLR is an independent variable. Univariate analyses showed association of Ki67 and grade with disease-specific survival (DSS) ( Table 2 ). ER_max was a statistically significant continuous variable in univariate analysis (hazard ratio [HR] = 0.88, 95% confidence interval [CI] = 0.78 to 0.99, P = .04 for DSS of 8 years). The cutoff point of 4800 for high PRLR expression was determined by receiver operating characteristic (ROC) analysis and Youden’s J index. The area under the curve is 0.837 (95% CI = 0.75 to 0.92, P < .001) ( Figure 1B ). When unstratified patients were analyzed with respect to time to metastasis, we determined that high PRLR expression was associated with a shorter time to bone metastasis that includes other sites ( Figure 1C ), although not with any other site in the absence of bone metastasis. Multivariable Cox regression analysis indicates that the PRLR (PRLR AQUA Max-per 100 unit HR = 1.04, 95% CI = 1.00 to 1.07, P = .03), ER status, node status, and chemotherapy treatment were statistically significant independent variables only in disease with bone metastasis ( Table 2 ). All of the proportionality tests were nonsignificant ( P = .27 for PRLR; P = .31 for ER status; P = .25 for node status; P = .50 for chemotherapy status).
Figure 1.
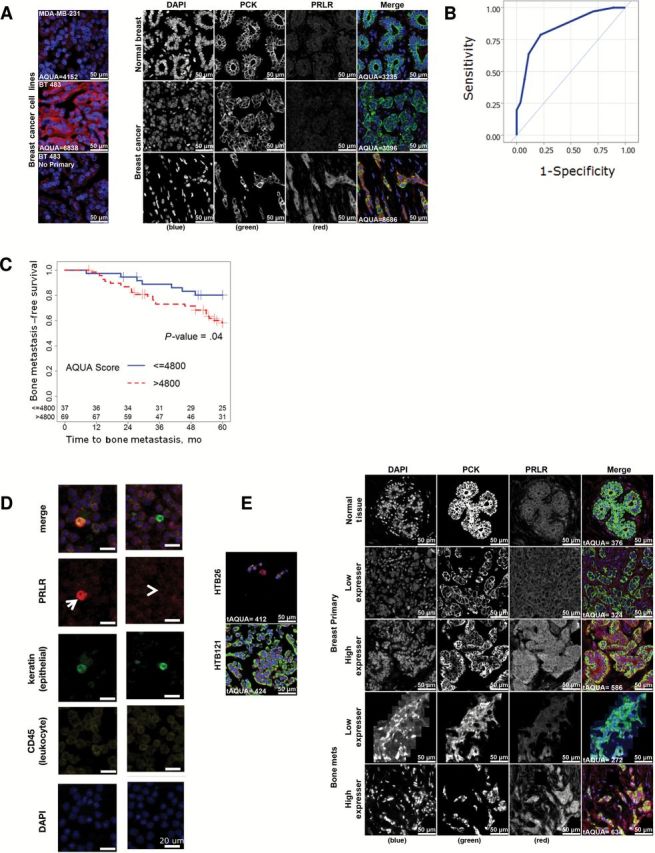
Prolactin receptor levels were tested in primary breast tumors and in bone metastases. A ) Prolactin receptor (PRLR) protein level quantification in breast cancer patient samples by AQUA scoring. The left panel indicates the specificity of the PRLR antibody used by demonstrating staining on the breast cancer cell line MDA-MB-231, which has low PRLR, and the higher PRLR breast cancer cell line BT 483. The right panel demonstrates the presence of PRLR in normal breast ducts ( top row ) as well as in breast cancer with low ( middle row ) or high ( bottom row ) expression. B ) The area under the curve for the PRLR cutpoint is presented. C ) Kaplan Meier plot of time to metastasis is stratified by site of metastasis for the metastasis TMA series. The PRLR AQUA score was used to dichotomize patients into low PRLR or high PRLR groups to analyze time to any bone metastasis. Patients whose maximal tumor AQUA score for PRLR was above 4800 were considered to be high expressors. Survival rate was calculated using the Kaplan-Meier method, and a log-rank test was used to compare statistical differences in survival among subgroups. D ) Isolation and biomarker characterization of circulating tumor cells (CTCs) from a breast cancer patient. Representative images of immunofluorescently stained CTCs mixed with peripheral blood mononucleated cells (PBMCs) show CTCs identified as CK-positive, CD45-negative, and nuclear intact cells. PBMCs are CK-negative and CD45-positive cells. The arrowhead points to a PRLR-negative CTC, and the arrow points to a PRLR - positive CTC. The scale bars represent 20 µM. E ) PRLR immunohistochemistry on matched primary breast cancer and bone metastases samples. The left panel panels shows staining of the PRLR on MDA-MB-231 cells (HTB26) and BT483 cells (HTB121). AQUA = automated quantitative analysis; PCK = pan cytokeratin PRLR = prolactin receptor.
Table 1.
Demographics table - categorical variables (n = 134), the FREQ procedure*
| Variable | Frequency | Percent | Cumulative frequency | Cumulative percent |
|---|---|---|---|---|
| Size | ||||
| Missing | 5 | - | - | - |
| <2 cm | 44 | 34.1 | 44 | 34.1 |
| ≥2 cm | 85 | 65.9 | 129 | 100.0 |
| Nodes | ||||
| missing | 5 | - | - | - |
| Nodes+ | 88 | 68.2 | 88 | 68.2 |
| Nodes- | 41 | 31.8 | 129 | 100.0 |
| ER | ||||
| Missing | 11 | - | - | - |
| ER+ | 91 | 74.0 | 91 | 74.0 |
| ER- | 32 | 26.0 | 123 | 100.0 |
| PR | ||||
| Missing | 7 | - | - | - |
| PR+ | 85 | 66.9 | 85 | 66.9 |
| PR- | 42 | 33.1 | 127 | 100.0 |
| HER2 | ||||
| Missing | 9 | - | - | - |
| HER2+ | 16 | 12.8 | 16 | 12.8 |
| HER2- | 109 | 87.2 | 125 | 100.0 |
| Radiotherapy | ||||
| Missing | 3 | - | - | - |
| No | 65 | 49.6 | 65 | 49.6 |
| Yes | 66 | 50.4 | 131 | 100.0 |
| Chemotherapy | ||||
| Missing | 3 | - | - | - |
| No | 43 | 32.8 | 43 | 32.8 |
| Yes | 88 | 67.2 | 131 | 100.0 |
| Tamoxifen | ||||
| Missing | 22 | - | - | - |
| No | 78 | 69.6 | 78 | 69.6 |
| Yes | 34 | 30.4 | 112 | 100.0 |
| Grade | ||||
| Missing | 30 | - | - | - |
| 1 | 18 | 17.3 | 18 | 17.3 |
| 2 | 40 | 38.5 | 58 | 55.8 |
| 3 | 46 | 44.2 | 104 | 100.0 |
| Grade | ||||
| Missing | 30 | - | - | - |
| 1+2 | 58 | 55.8 | 58 | 55.8 |
| 3 | 46 | 44.2 | 104 | 100.0 |
| 5-y bone met event status | ||||
| No | 89 | 66.4 | 89 | 66.4 |
| Yes | 45 | 33.6 | 134 | 100.0 |
| 5-y bone met–only event status | ||||
| No | 107 | 79.9 | 107 | 79.9 |
| Yes | 27 | 20.2 | 134 | 100.00 |
| 5-y other met–only event status | ||||
| No | 116 | 86.6 | 116 | 86.6 |
| Yes | 18 | 13.4 | 134 | 100.0 |
| Disease-specific survival event status | ||||
| Missing | 9 | - | - | - |
| No | 85 | 68.0 | 85 | 68.0 |
| Yes | 40 | 32.0 | 125 | 100.0 |
| 8-y disease-specific survival event status | ||||
| Missing | 9 | - | - | - |
| No | 89 | 71.2 | 89 | 71.2 |
| Yes | 36 | 28.8 | 125 | 100.0 |
* ER = estrogen receptor; HER2 = human epidermal growth factor receptor; PR = progesterone receptor.
Table 2.
Univariate and multivariable analyses for time to sites of metastasis other than bone (5 years), sites of metastasis including bone (5 years), and disease-specific survival (8 years)*
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Reference | Level | HR (95% CI) | P | HR (95% CI) | P |
| Sites of metastasis other than bone (OtherMetOnly) 5 y | ||||||
| Ki67_Avg | 1.06 (1.03 to 1.08) | <.001 | 1.06 (1.03 to 1.08) | <.001 | ||
| HER2 status | HER2- | HER2+ | 3.31 (1.17 to 9.42) | .03 | - | - |
| ER status | ER- | ER+ | 0.35 (0.13 to 0.90) | .03 | - | - |
| Sites of metastasis including bone (BoneMets) 5 y | ||||||
| ER status | ER- | ER+ | 1.91 (0.80 to 4.53) | .15 | 9.07 (1.17 to 70.59) | .04 |
| Nodes status | Nodes- | Nodes+ | 1.54 (0.76 to 3.15) | .23 | 12.29 (3.13 to 48.30) | <.001 |
| Chemotherapy | No | Yes | 0.60 (0.33 to 1.11) | .11 | 0.20 (0.08 to 0.50) | <.001 |
| PRLR_Max (per 100 unit) | 1.02 (0.99 to 1.04) | .17 | 1.04 (1.00 to 1.07) | .03 | ||
| Age | 1.02 (1.00 to 1.05) | .06 | - | - | ||
| Grade | 1+2 | 3 | 1.43 (0.75 to 2.76) | .28 | - | - |
| Disease-specific survival 8 y | ||||||
| Ki67_Avg | 1.04 (1.02 to 1.06) | <.001 | 1.04 (1.02 to 1.06) | <.001 | ||
| Grade | 1+2 | 3 | 2.06 (1.01 to 4.22) | .05 | - | - |
* P value was calculated using Wald Test. Variables with missing data were not statistically significant and were not included in the final statistical model; en dashes were placed within the cells above. We confirmed that there was no statistically significant difference in survival outcomes between 28 patients without automated quantitative analysis (AQUA) scores, and the other patients reported with full AQUA scores both by univariate Chi-square analysis and KM survival log-rank test. All reported P values were two-sided. CI = confidence interval; ER = estrogen receptor, HER2 = human epidermal growth factor receptor; HR = hazard ratio; PRLR = prolactin receptor.
To determine the PRLR presence on CTCs, we first enumerated CTCs from advanced breast cancer patients using Veridex CellSearch, then isolated CTCs separately using ficoll. The blood was processed and stained for the PRLR, CD45, and pan-cytokeratin (see the Supplementary Methods , available online). The staining for PRLR+ cells was heterogeneous between CTCs (cytokeratin + /CD45 - ) ( Figure 1D ).
We also analyzed 20 primary breast tumors and bone metastasis samples with quantitative immunohistochemistry ( Figure 1E ). Staining masks using pan-cytokeratin staining were used to identify the tumor areas vs bone marrow. All primary tumors scored positive for the PRLR, 10 with low and 10 with high PRLR based on their median staining signal. For the bone metastases, five scored negative and 15 scored positive (5 low and 10 high) for the PRLR. Seventeen of the primary and bone metastasis samples were directly matched within patients, and of those matched samples nine primary tumor samples had high PRLR staining and eight had low PRLR staining. Thirteen of the 17 matched bone metastases were positive, nine with high PRLR and eight with low PRLR levels (4 of these were negative). There was no pattern between high or low PRLR staining in the primary tumor with respect to high or low staining in the metastases ( Supplementary Table 2 , available online), which is possibly because of the heterogeneity of the colony-forming cells.
Together this indicates that high PRLR levels in the primary tumor are associated with a shorter time to metastasis that includes bone, which is supported observationally by the presence of the PRLR on metastatic circulating tumor cells and in tumor cells of bone metastases of PRLR-positive primary breast tumors.
The Effect of PRL on Breast Cancer Cell–Mediated Osteoclastogenesis
We hypothesized, based on the above results, that the PRLR was potentially contributing to the severity of the metastatic lesion. We evaluated the ability of prolactin-treated breast cancer cell lines to induce osteoclast differentiation.
Conditioned medium (CM) from breast cancer cells induced osteoclastogenesis in both PRL-dependent and PRL-independent manners. Using primary mouse bone marrow–derived osteoclasts (BMDOs), we observed the trend that breast cancer CM from untreated PRLR-positive SKBR3 cells enhanced the number of tartrate-resistant acid-phosphatase (TRAP)+/multinucleate cells ( P = .06) ( Figure 2 , A and C ) and increased their surface area ( Figure 2B ; Supplementary Methods , available online) and that PRL treatment of the breast cancer cells further enhanced both aspects of primary osteoclast cell differentiation. For ease of use and consistency, we continued experiments using the osteoclast cell line RAW264.7, a well-characterized murine macrophage lineage cell line routinely used in conjunction with human cancer cells for this purpose ( 24 , 28 ).
Figure 2.
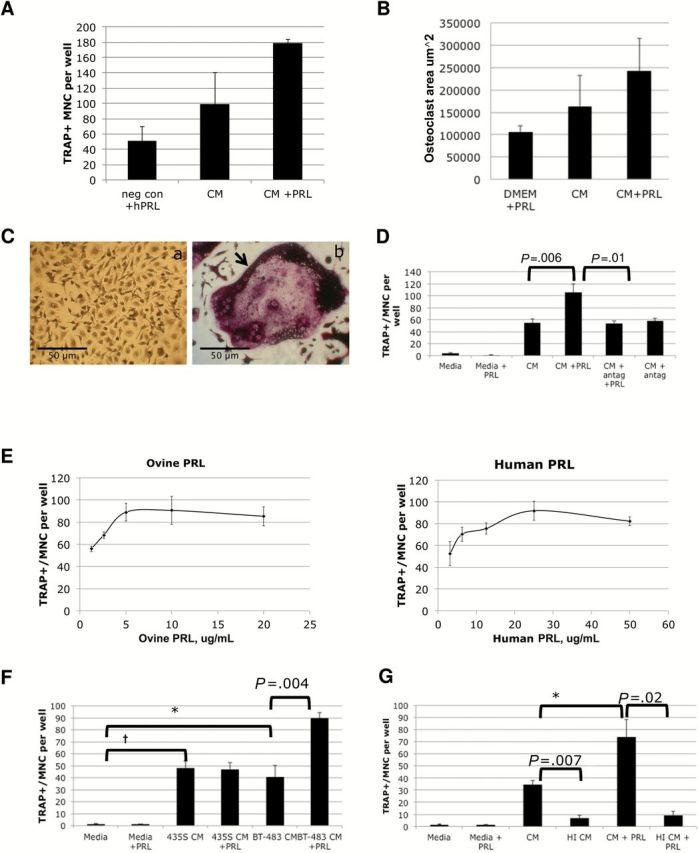
The effect of prolactin (PRL)–PRL receptor (PRLR) signaling on breast cancer–mediated osteoclastogenesis. A ) Primary bone marrow–derived osteoclasts (BMDOs) were plated in medium with RANKL (50ng/mL), M-CSF (75ng/mL), and hPRL (negative control) or stimulated with conditioned medium (CM) from SKBR3 cells treated with vehicle (CM) or 150ng/mL hPRL (CM+PRL). Tartrate-resistant acid-phosphatase (TRAP)+/multinucleate cells (MNC) were quantified from three replicates. B ) The surface area of each mature primary osteoclast within 10 random fields per well of panel 2A was calculated and averaged. C) (a ) Undifferentiated BMDOs, (b) a large TRAP+/multinucleate cell indicated by a black arrow . D ) Effect of a PRLR antagonist on TRAP+/multinucleate cell induction. RAW264.7 pre-osteoclasts were also cultured in growth media ± ovine PRL as negative controls or cultured in conditioned media from SKBR3 breast cancer cells treated with vehicle (CM - PRL), 5 µg/mL PRLR-antagonist ∆1-9-G129R-hPRL (CM + antag), 5 µg/mL ovine PRL (SKBR3 CM + PRL), or a combination. TRAP+/multinucleate cells were quantified for each condition. One of three experimental replicates with six internal replicates per experiment. E ) The effect of PRL-treated breast cancer CM on osteoclastogenesis. RAW264.7 pre-osteoclasts were cultured in conditioned media from SKBR3 breast cancer cells stimulated with increasing concentrations of ovine PRL or human recombinant PRL, and TRAP+/multinucleate cells were quantified. Sum of three experimental replicates. F ) Quantification of TRAP+/multinucleate cells treated with conditioned media from BT-483 breast cancer cells or MDA-MB-435S cells. Breast cancer cells were stimulated with vehicle or with 5 µg/mL oPRL for five days for preparation of conditioned media. RAW264.7 pre-osteoclasts were cultured in 20% conditioned media or growth media ± oPRL. TRAP-positive, multinucleate cells were quantified for each condition. One of three experimental replicates with six internal replicates per experiment. G ) Quantification of TRAP+/multinucleate cells after heat inactivation of SKBR3 breast cancer cell conditioned media. Ovine PRL-stimulated and -unstimulated SKBR3-conditioned media was heat-inactivated by incubation at 95°C for 10 minutes before addition to RAW264.7 cultures. One of three experimental replicates with four internal replicates per experiment. Statistical significance (* P < .05, † P < .01) was tested with the paired Student’s t test as indicated. Bars indicate standard deviation. CM = conditioned medium; MNC = multinucleate cell; PRL = prolactin; TRAP = tartrate-resistant acid-phosphatase.
To test the specificity of the PRL response for the PRLR, we used a PRLR antagonist, Δ1-9G129R-hPRL ( 23 ). When used to prepare the breast cancer CM, the receptor antagonist prevented PRL-enhanced increase in TRAP+/multinucleate RAW264.7 cells ( P = .01) without decreasing induction by untreated breast cancer cells ( Figure 2D ). These data indicated that the effect of PRL is PRLR-dependent ( P = .006) and suggested that there is no autocrine production of PRL in SKBR3 cells, respectively; the latter is supported by our published work on SKBR3 cells ( 29 ).
We observed that either ovine or recombinant human PRL produced similar dose-dependent responses, albeit at different concentrations to allow for the decreased sensitivity of ovine PRL for the human receptor ( 30 ) ( Figure 2E ). The dose range indicates that our results are potentially relevant for every patient, male or female. The results were also consistent with multiple cell lines. CM prepared with PRL from PRLR+ BT483 ( P = .004), but not melanoma MDA-MB-435S cells, increased TRAP+/multinucleate RAW264.7 cells to a greater extent than CM from untreated control cells ( Figure 2F ).
We heat-treated the CM, which abolished both PRL-dependent ( P = .02) and most of the breast cancer–induced (PRL-independent) ( P = .007) increase of TRAP+ multinucleate RAW264.7 cells ( Figure 2G ), consistent with a heat-sensitive factor(s) such as a protein, amino acid, or lipid-based vesicle, rather than an ion such as calcium. This is consistent with our hypothesis that PRL induces/enhances an osteoclastogenic secreted factor(s) from breast cancer cells.
The Effect of PRL on Breast Cancer Cell–Mediated Osteolysis
To study the effect of PRL on osteolysis, we plated pre-osteoclasts on dentine discs as a bone substitute ( 26 ) and induced differentiation with breast cancer cell CM ( Figure 3 ). Compared with the negative control ( P = .009) ( Figure 3 , A and E ) or non-PRL-treated SKBR3 cell CM ( P = .01) ( Figure 3 , B and E ), virtually only PRL-treated SKBR3 cell-CM ( Figure 3 , C and E ) was capable of inducing bone resorption in the equally loaded osteoclasts ( Figure 3D ), resulting in pit formation in the dentine. This supports the fact that there are more mature osteoclasts when treated with CM from PRL-treated breast cancer cells.
Figure 3.
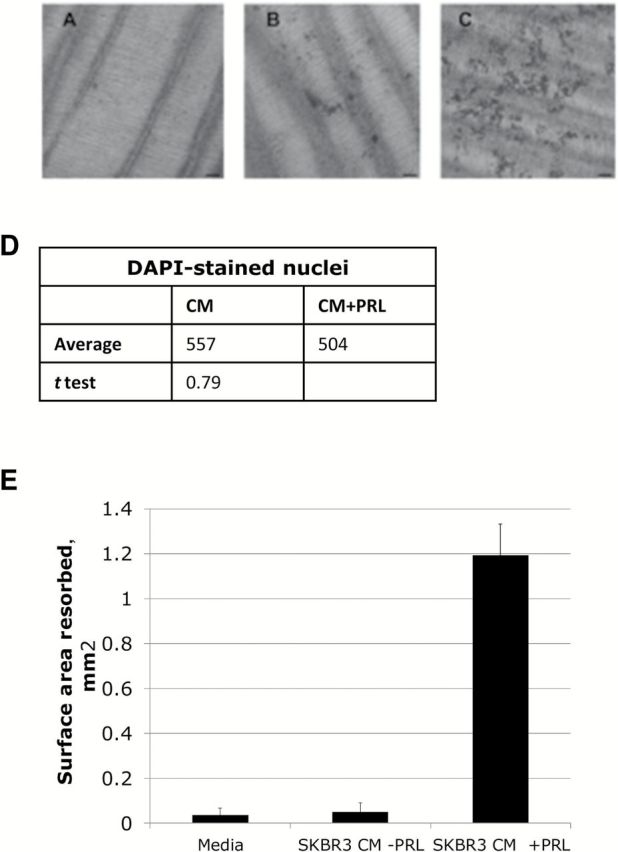
Assessment of dentine resorption as a measure of mature osteoclasts. RAW264.7 pre-osteoclasts were cultured on dentine discs in (A) growth medium or in (B) conditioned media from SKBR3 breast cancer cells treated with vehicle (CM-PRL) or (C) 5 µg/mL oPRL (CM +PRL). Scale bar = 100 µm. D ) Nuclei were alternately stained with DAPI and quantified to show equal loading. Five random fields of cells were counted in each sample. E ) Surface area of each pit was quantified using ImageJ software. Bars indicate standard deviation of three experimental replicates. Statistical significance ( P < .05) was tested with the paired Student’s t test. CM = conditioned media; PRL = prolactin.
The Role of PRL in the Induction of Breast Cancer Cell–Secreted SHH
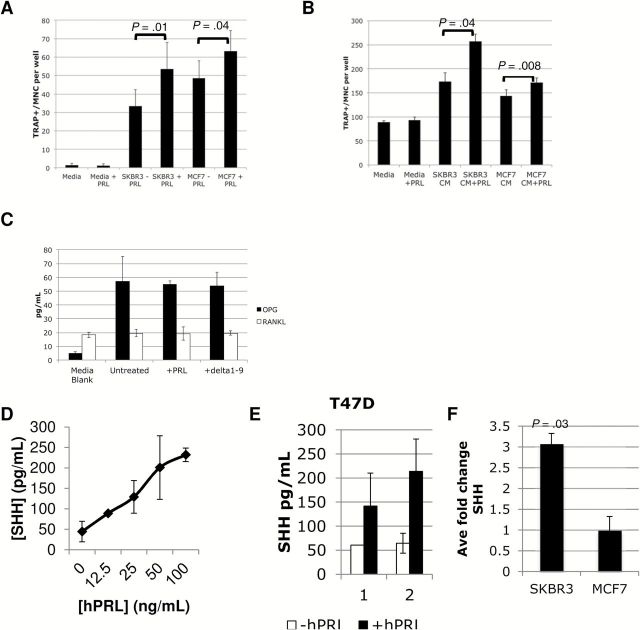
We used two different cytokine arrays and techniques, a quantitative Luminex array (cytokine/chemokine array of 65 proteins and bone panel array of 8 proteins) from SKBR3 +/- oPRL cells in triplicate and a large qualitative array (biotin-streptavidin based, high density L-507 glass, RayBiotech), to assess the cytokines secreted by both SKBR3 and MCF7 breast cancer cells +/- hPRL (See the Supplementary Methods , available online). SKBR3 and MCF7 cells induce osteoclastogenesis in direct coculture with RAW264.7 osteoclasts ( P = .01 and P = .04, respectively) ( Figure 4A ) and via secreted factors in the CM ( P = .04 and P = .008, respectively) ( Figure 4B ).
Figure 4.
Detection of sonic hedgehog (SHH), osteoprotegerin (OPG), and receptor activator of nuclear factor-Κappa B ligand (RANKL) secretion from breast cancer cells. A ) Direct coculture of MCF-7 and SKBR3 breast cancer cells with RAW264.7 pre-osteoclasts -/+ oPRL induces formation of tartrate-resistant acid-phosphatase (TRAP)–positive multinucleate cells. B ) Conditioned media (CM) from MCF-7 and SKBR3 breast cancer cells -/+ hPRL with RAW264.7 pre-osteoclasts induces formation of TRAP-positive multinucleate cells. C ) RANKL and OPG were detected using Luminex technology from unconditioned medium (media blank) or CM from three experimental samples of SKBR3 cells (2% FBS) that were untreated or treated with 5 ug/mL of oPRL or ∆1-9-G129R-hPRL. Three experiments pooled (A-C) , showing interexperimental variation. SHH is detected by enzyme-linked immunosorbent assay from (D) two samples of SKBR3 cells treated with a hPRL dose response, (E) two samples of T47D cells, or (F) three independent samples of SKBR3 or MCF7 cells treated with vehicle or 25ng/mL hPRL. Concentration of SHH was calculated using a linear regression standard curve. Error bars represent standard deviation. Statistical significance ( P < .05) with the paired Student’s t test as indicated. CM = conditioned medium; MNC = multinucleate cell; OPG = osteoprotegerin; PRL = prolactin; RANKL = receptor activator of nuclear factor-Κappa B ligand; SHH = sonic hedgehog; TRAP = tartrate-resistant acid-phosphatase.
We did not find any statistically significant PRL-regulated cytokines in the smaller Luminex arrays ( Supplementary Figure 1 , available online), although we identified known osteoclastogenic factors that may contribute to PRL-independent osteoclastogenesis ( Table 3 ). We validated the presence of interferon gamma-induced protein 10 (IP-10)/CXCL10 and monocyte chemoattractant protein-1 (MCP-1) by ELISA ( Supplementary Figure 2 and Supplementary Methods , available online). Interestingly, receptor activator of nuclear factor-Κappa B ligand (RANKL), a central ligand in osteoclastogenesis, was only detectable in the serum and was not increased in breast cancer cell CM nor induced by PRL ( Figure 4C ).
Table 3.
PRL-independent breast cancer secreted factors*
| Factors detected in SKBR3 breast cancer conditioned media | Osteoclastogenic | Breast cancer secreted and osteoclastogenic |
|---|---|---|
| Fractalkine | yes | ND |
| G-CSF | yes | ND |
| GM-CSF | yes | yes |
| GRO | ND* | ND |
| IL-15 | yes | ND |
| IL-1RA | yes | ND |
| IL-6 | yes | yes |
| INF-a2 | ND | ND |
| IP-10 | yes | ND |
| MCP-1 | yes | yes |
| MCP-3 | yes | ND |
| PDGF-BB | yes | yes |
| RANTES | yes | ND |
| SCF | yes | ND |
| TNF-a | yes | ND |
| VEGF | yes | yes |
* These factors were identified from SKBR3 cells using Luminex technology. G-CSF = granulocyte-colony stimulating factor; GM-CSF = granulocyte macrophage-colony stimulating factor; GRO = growth-regulated alpha protein; IL = interleukin; INF = interferon; IP = interferon-gamma inducible protein; MCP = monocyte chemoattractant protein; ND = not determined; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T cell expressed and secreted; SCF = stem cell factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
The results of both arrays overlapped in a qualitative fashion and were extended by the results of the larger array. By examining known osteoclastogenic candidates in the larger high-density array that were present above three times the background, we identified sonic hedgehog (SHH). As SHH shares some identity with Indian hedgehog (IHH) ( 31 ), IHH was assessed but not detected in SKBR3, MCF7, or T47D cells using ELISA. We determined that SHH was secreted into the SKBR3 breast cancer cell CM and that this was enhanced across a dose response PRL ( Figure 4D ), peaking at 3.1-fold in SKBR3 ( P = .03) and 2.8-fold in T47D ( Figure 4E ), but was not PRL-enhanced in MCF7 cells ( Figure 4F ). It is possible another PRL-regulated factor besides SHH is responsible or alternatively that SHH is not fully released by MCF7 cells. Altogether, we identified a number of osteoclastogenic factors in the breast cancer CM and identified SHH as a PRL-enhanced osteoclastogenic factor.
The Impact of Hedgehog Pathway Inhibition on PRL-Independent and PRL-Dependent Osteoclastogenesis
We tested the requirement of the hedgehog (HH) pathway in the osteoclasts using the HH pathway inhibitor cyclopamine. We observed that cyclopamine, albeit with some toxicity ( P = .04) ( Figure 5A ; Supplementary Methods , available online), prevented the PRL-dependent and -independent increase in the number of TRAP+/MNCs by CM ( Figure 5B ), consistent with the presence of SHH secreted by the breast cancer cells. CM from untreated, and more so from PRL-treated, breast cancer cells increased the size of mature osteoclasts over that of controls, while cyclopamine prevented the maturation of the osteoclasts resulting in small osteoclasts ( Figure 5C ). Recombinant SHH was also able to increase osteoclastogenesis ( Supplementary Figure 3 , available online). Treatment of pre-osteoclasts with cyclopamine prevented the induction of mature osteoclasts from both untreated and PRL-treated breast cancer cells consistent with the presence of breast cancer–secreted SHH and PRL-enhanced SHH in the CM.
Figure 5.
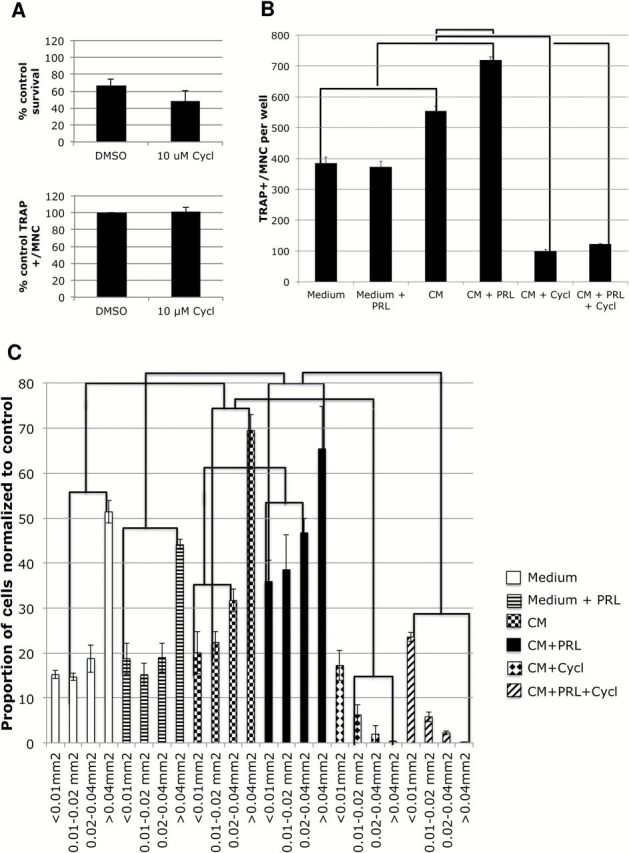
The role of the hedgehog (HH) pathway in osteoclastogenesis. RAW264.7 cells were cultured in the presence of 5ng/mL M-CSF and 7.5ng/mL RANKL in control media -/+ hPRL as negative controls or in conditioned media from SKBR3 cells treated with vehicle (-CM), 100 ng/mL hPRL (+CM), 10 µM cyclopamine, or a combination thereof. A ) Alamar Blue cell survival assay ( upper ) and quantification of tartrate-resistant acid-phosphatase (TRAP)+/multinucleate cells (MNC) in absence of CM ( lower ) (six replicates of negative controls). Student’s t test. B ) Quantification of TRAP+/MNC from three replicates. C ) Surface area of TRAP+ multinucleate cells. All osteoclasts in each well ( above ) were measured and divided into size ranges, and the percentage within that group was calculated and multiplied by the ratio of the group relative to control medium. Error bars represent standard deviation. A two-way analysis of variance was performed, followed by Tukey post-testing, and lines between pairs or groups of pairs indicate a P value of less than .05 for each paired bar between different treatments. CM = conditioned medium; MNC = multinucleate cell; PRL = prolactin; TRAP = tartrate-resistant acid-phosphatase.
Discussion
We discovered an intricate association of PRLR levels in the primary breast tumor with a shorter time to bone metastasis and identified part of the molecular mechanism.
The role of signaling pathways downstream of the PRLR ( 32 ) is not always consistent with the proposed roles of the PRLR in breast cancer progression. Evidence indicates that Stat5 activation is protective and indicative of good prognosis ( 14 , 15 ), with Stat5 phosphorylation decreasing with cancer progression ( 33 ). The Jak-Stat5 pathway, however, is not unique to PRLR activation, nor is it the only signaling pathway downstream of the PRLR. The specific PRL pathway that increases SHH secretion is unknown.
We identified that the majority of the paired primary breast cancers with matching bone metastases tested were PRLR-positive. The PRLR was previously detected in bone metastasis, with a statistically significant higher amount of the PRLR detected in bone metastases of carcinomas than in bone metastases of sarcomas ( 34 , and personal communication Drs V. Espina and A. Chiechi).
We identified 16 breast cancer–secreted factors in cytokine arrays including 14 known osteoclastogenic factors and five known to be breast cancer–secreted ( Table 3 ) ( 35 ) ( 1 and references therein). These factors likely contribute to the overall effect of PRL-independent, breast cancer–mediated induction of osteoclastogenesis.
At least part, if not all, of the PRL-dependent effect of breast cancer cells upon osteoclastogenesis was because of the subsequent activation of the HH pathway in the osteoclasts via the PRL-increased secretion of SHH. Breast cancer cell–secreted HH was previously shown to influence differentiation and activity of osteoclast precursors directly and indirectly through enhancement of osteoblast differentiation ( 36–39 ). SHH may not be the only PRL-regulated factor contributing to osteoclastogenesis, given we did not identify SHH in MCF7 cells.
The HH pathway is constitutively activated in many breast carcinomas ( 40–43 ). Unchecked HH signaling in breast cancer cells results in parathyroid hormone–related peptide expression and increased osteolysis ( 39 ). Despite its known expression in normal and malignant breast tissue, there is no previous evidence of PRL regulation of SHH.
PRL may be responsible for bone loss during lactation ( 44 ). Also, women with very high levels of PRL (hyperprolactinemia) experience osteopenia (bone thinning), thought to be either because of the indirect effect of PRL resulting in hypogonadism (thus low estrogen) or the direct action of PRL on osteoblasts, to increase osteoblast-induced differentiation of osteoclasts ( 45 ).
We view this discovery cohort, despite its size, as ideal for the identification of mechanisms driving bone metastasis. One other limitation to our study is the need for a validation cohort of breast cancer patients, such as the Breast Cancer to Bone (B2B) prospective cohort currently being assembled in Alberta, Canada ( 46 ).
In conclusion, our data indicate that high PRLR in the primary breast tumor is associated with a shorter time of breast cancer to metastasize to the bone, is also present in the tumor microenvironment of breast cancer bone metastasis, and has the potential to modulate the microenvironment to induce lytic osteoclast formation. PRL acts as an accelerator in the vicious cycle. This new PRL function highlights the need for targeted therapies for the treatment of breast cancer to bone metastases.
Funding
This work was supported by the Alberta Cancer Foundation (grant number 24133 and 25277) to CSS; The Breast Cancer Supportive Care Foundation to PT; the Institute of Cancer Research of the Canadian Institutes of Health Research to CSS (funding reference number 126447); the Canadian Breast Cancer Foundation to CSS; the Fondation ARC pour la Recherche sur le Cancer (grant number SFI 20101201635) to VG; and Inserm to VG.
Supplementary Material
The study sponsor had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; nor the decision to submit the manuscript for publication.
References
- 1. Chen YC, Sosnoski DM, Mastro AM . Breast cancer metastasis to the bone: mechanisms of bone loss . Breast Cancer Res . 2010. ; 12(6) : 215 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kingsley LA, Fournier PG, Chirgwin JM, et al. Molecular biology of bone metastasis . Mol Cancer Ther . 2007. ; 6(10) : 2609 – 2617 . [DOI] [PubMed] [Google Scholar]
- 3. Clement-Lacroix P, Ormandy C, Lepescheux L, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice . Endocrinology . 1999. ; 140(1) : 96 – 105 . [DOI] [PubMed] [Google Scholar]
- 4. Coss D, Yang L, Kuo CB, et al. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat . Am J Physiol Endocrinol Metab . 2000. ; 279(6) : E1216 - E1225 . [DOI] [PubMed] [Google Scholar]
- 5. Kelly PA, Binart N, Freemark M, et al. Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knockout mice . Biochem Soc Trans . 2001. ; 29 ( Pt 2 ): 48 – 52 . [DOI] [PubMed] [Google Scholar]
- 6. Tikk K, Sookthai D, Johnson T, et al. Circulating prolactin and breast cancer risk among pre- and postmenopausal women in the EPIC cohort . Ann Oncol . 2014. ; 25(7) : 1422 – 1428 . [DOI] [PubMed] [Google Scholar]
- 7. Tworoger SS, Eliassen AH, Zhang X, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development . Cancer Res . 2013. ; 73(15) : 4810 – 4819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel DD, Bhatavdekar JM, Chikhlikar PR, et al. Node negative breast carcinoma: hyperprolactinemia and/or overexpression of p53 as an independent predictor of poor prognosis compared to newer and established prognosticators . J Surg Oncol . 1996. ; 62(2) : 86 – 92 . [DOI] [PubMed] [Google Scholar]
- 9. Wang DY, Stepniewska KA, Allen DS, et al. Serum prolactin levels and their relationship to survival in women with operable breast cancer . J Clin Epidemiol . 1995. ; 48(7) : 959 – 968 . [DOI] [PubMed] [Google Scholar]
- 10. Holtkamp W, Nagel GA, Wander HE, et al. Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer . Int J Cancer . 1984. ; 34(3) : 323 – 328 . [DOI] [PubMed] [Google Scholar]
- 11. Bhatavdekar JM, Shah NG, Balar DB, et al. Plasma prolactin as an indicator of disease progression in advanced breast cancer . Cancer . 1990. ; 65(9) : 2028 – 2032 . [DOI] [PubMed] [Google Scholar]
- 12. Mujagic Z, Mujagic H . Importance of serum prolactin determination in metastatic breast cancer patients . Croat Med J . 2004. ; 45(2) : 176 – 180 . [PubMed] [Google Scholar]
- 13. Miller SL, Antico G, Raghunath PN, et al. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells . Oncogene . 2007. ; 26(32) : 4668 – 4678 . [DOI] [PubMed] [Google Scholar]
- 14. Nouhi Z, Chughtai N, Hartley S, et al. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells . Cancer Res . 2006. ; 66(3) : 1824 – 1832 . [DOI] [PubMed] [Google Scholar]
- 15. Sultan AS, Xie J, LeBaron MJ, et al. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells . Oncogene . 2005. ; 24(5) : 746 – 760 . [DOI] [PubMed] [Google Scholar]
- 16. Lissoni P, Barni S, Cazzaniga M, et al. Prediction of recurrence in operable breast cancer by postoperative changes in prolactin secretion . Oncology . 1995. ; 52(6) : 439 – 442 . [DOI] [PubMed] [Google Scholar]
- 17. Wang DY, Hampson S, Kwa HG, et al. Serum prolactin levels in women with breast cancer and their relationship to survival . Eur J Cancer Clin Oncol . 1986. ; 22(4) : 487 – 492 . [DOI] [PubMed] [Google Scholar]
- 18. Canbay E, Degerli N, Gulluoglu BM, et al. Could prolactin receptor gene polymorphism play a role in pathogenesis of breast carcinoma? Curr Med Res Opin . 2004. ; 20(4) : 533 – 40 . [DOI] [PubMed] [Google Scholar]
- 19. Bertucci F, Nasser V, Granjeaud S, et al. Gene expression profiles of poor-prognosis primary breast cancer correlate with survival . Hum Mol Genet . 2002. ; 11(8) : 863 – 872 . [DOI] [PubMed] [Google Scholar]
- 20. Bose P, Klimowicz AC, Kornaga E, et al. Bax expression measured by AQUAnalysis is an independent prognostic marker in oral squamous cell carcinoma . BMC Cancer . 2012. ; 12 : 332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hao D, Phan T, Jagdis A, et al. Evaluation of E-cadherin, beta-catenin and vimentin protein expression using quantitative immunohistochemistry in nasopharyngeal carcinoma patients . Clin Invest Med . 2014. ; 37(5) : E320 . [DOI] [PubMed] [Google Scholar]
- 22. Zhou Z, Immel D, Xi CX, et al. Regulation of osteoclast function and bone mass by RAGE . J Exp Med . 2006. ; 203(4) : 1067 – 1080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernichtein S, Kayser C, Dillner K, et al. Development of pure prolactin receptor antagonists . J Biol Chem . 2003. ; 278(38) : 35988 – 35999 . [DOI] [PubMed] [Google Scholar]
- 24. Nicolin V, Bortul R, Bareggi R, et al. Breast adenocarcinoma MCF-7 cell line induces spontaneous osteoclastogenesis via a RANK-ligand-dependent pathway . Acta Histochem . 2008. ; 110(5) : 388 – 396 . [DOI] [PubMed] [Google Scholar]
- 25. Verollet C, Gallois A, Dacquin R, et al. Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors . FASEB J . 2013. ; 27(9) : 3608 – 3618 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. You L, Temiyasathit S, Lee P, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading . Bone . 2008. ; 42(1) : 172 – 179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galsgaard ED, Rasmussen BB, Folkesson CG, et al. Re-evaluation of the prolactin receptor expression in human breast cancer . J Endocrinol . 2009. ; 201(1) : 115 – 128 . [DOI] [PubMed] [Google Scholar]
- 28. Guo Y, Tiedemann K, Khalil JA, et al. Osteoclast precursors acquire sensitivity to breast cancer derived factors early in differentiation . Bone . 2008. ; 43(2) : 386 – 393 . [DOI] [PubMed] [Google Scholar]
- 29. Perotti C, Liu R, Parusel C, et al. Heat shock protein 90alpha (Hsp90alpha), a prolactin-Jak2-Stat5 target gene identified in breast cancer cells, is involved in apoptosis regulation . Breast Cancer Res . 2008. ; 10(6) : R94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Utama FE, Tran TH, Ryder A, et al. Insensitivity of human prolactin receptors to nonhuman prolactins: relevance for experimental modeling of prolactin receptor-expressing human cells . Endocrinology . 2009. ; 150(4) : 1782 – 1790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pathi S, Pagan-Westphal S, Baker DP, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog . Mech Dev . 2001. ; 106 ( 1–2 ): 107 – 117 . [DOI] [PubMed] [Google Scholar]
- 32. Clevenger CV . Role of prolactin/prolactin receptor signaling in human breast cancer . Breast Dis . 2003. ; 18 : 75 – 86 . [DOI] [PubMed] [Google Scholar]
- 33. Nevalainen MT, Xie J, Torhorst J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis . J Clin Oncol . 2004. ; 22(11) : 2053 – 2060 . [DOI] [PubMed] [Google Scholar]
- 34. Chiechi A, Novello C, Magagnoli G, et al. Elevated TNFR1 and serotonin in bone metastasis are correlated with poor survival following bone metastasis diagnosis for both carcinoma and sarcoma primary tumors . Clin Cancer Res . 2013. ; 19(9) : 2473 – 2485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park BK, Zhang H, Zeng Q, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF . Nat Med . 2007. ; 13(1) : 62 – 69 . [DOI] [PubMed] [Google Scholar]
- 36. Das M, Harvey I, Chu LL, et al. Full-length cDNAs: more than just reaching the ends . Physiol Genomics . 2001. ; 6(2) : 57 – 80 . [DOI] [PubMed] [Google Scholar]
- 37. Das S, Samant RS, Shevde LA . Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis . J Biol Chem . 2011. ; 286(11) : 9612 – 9622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Das S, Tucker JA, Khullar S, et al. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases . PLoS One . 2012. ; 7(3) : e34374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sterling JA, Oyajobi BO, Grubbs B, et al. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells . Cancer Res . 2006. ; 66(15) : 7548 – 7553 . [DOI] [PubMed] [Google Scholar]
- 40. Hurchla MA, Weilbaecher KN . Hedgehog-targeted therapeutics uncouple the vicious cycle of bone metastasis . Oncoimmunology . 2012. ; 1(8) : 1411 – 1413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeng KS, Sheen IS, Jeng WJ, et al. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma . Onco Targets Ther . 2013. ; 7 : 79 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer . Cancer Res . 2004. ; 64(17) : 6071 – 6074 . [DOI] [PubMed] [Google Scholar]
- 43. O’Toole SA, Machalek DA, Shearer RF, et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer . Cancer Res . 2011. ; 71(11) : 4002 – 4014 . [DOI] [PubMed] [Google Scholar]
- 44. Suntornsaratoon P, Wongdee K, Goswami S, et al. Bone modeling in bromocriptine-treated pregnant and lactating rats: possible osteoregulatory role of prolactin in lactation . Am J Physiol Endocrinol Metab . 2010. ; 299(3) : E426 - E436 . [DOI] [PubMed] [Google Scholar]
- 45. Seriwatanachai D, Thongchote K, Charoenphandhu N, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio . Bone . 2008. ; 42(3) : 535 – 546 . [DOI] [PubMed] [Google Scholar]
- 46. Brockton NT, Gill SJ, Laborge SL, et al. The Breast Cancer to Bone (B2B) Metastases Research Program: a multi-disciplinary investigation of bone metastases from breast cancer . BMC Cancer . 2015. ; 15 : 512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.