Abstract
Background
Hematic cyst is a rare orbital condition that has a wide range of clinical presentation and is characterized pathologically by lack of endothelial lining.
Purpose
To correlate clinical and radiological features of hematic cysts, to tissue diagnosis, and investigate the possible etiology behind this condition, its relation to trauma and other interesting histopathological findings.
Methods
Retrospective case series at King Khaled Eye Specialist Hospital (KKESH) and King Abdulaziz University Hospital (KAUH) of all orbital lesions with tissue findings supporting the clinical and/or radiological diagnosis of hematic cyst.
Results
A series of 13 cases was studied, 8 males and 5 females. Age ranged from 2 to 84 years with a median of 54. Most cases presented with proptosis (76.9%) and limitation of eye movements (69.2%). History of trauma was confirmed in only 2/13. The clinical diagnosis of hematic cyst was made prior to surgery in 38.4%. Magnetic Resonance Imaging (MRI) confirmed the presence of blood in the orbit in 7/7. Surgical intervention was the mainstay of treatment. Histopathologically, these lesions demonstrated variable constituents including blood break-down products (hemosiderin), macrophages, mononuclear inflammatory cells, hemorrhage, absent endothelial lining, reactive fibrosis and capsule-like formation. Cholesterol clefts with typical granulomas and multinucleated giant cells were present in 2 cases. A clue to an underlying vascular lesion was found histopathologically in 30.8%. None of the patients developed recurrence or long-term complications with an average follow up period of 1 year.
Conclusion
Hematic cyst is a challenging clinical diagnosis that can be aided by radiological examination and histopathological confirmation. Trauma does not seem to play a major role while presence of a pre-existing vascular lesion with spontaneous hemorrhage may be an etiologic factor. Associated cholesterol granuloma is an interesting controversial finding. Surgical intervention is curative with possible persisting motility disturbance and/or the eye deviation and worse prognosis in post-traumatic cases.
Keywords: Orbital, Hematic cyst, Hemorrhage, Vascular, Granuloma, Hemosiderin
Introduction
Hematic cyst is a term used to describe a blood-derived lesion in the orbit.1 The characteristic features to define hematic cysts compared to other blood-derived lesions is actually the lack of endothelial lining and the fact that they are encapsulated by a layer of fibro-collagenous material.2 Hematic cyst is considered a rare condition and usually presents with symptoms due to its anatomical effect in the orbital cavity that includes proptosis, diplopia, visual disturbance and strabismus.3
Orbital imaging has played a major rule in the diagnosis of hematic cyst. Computerized tomography (CT) imaging typically shows a well-demarcated homogeneous mass but can show heterogeneity with layering of blood products in some cases. MRI is the imaging modality for the evaluation of the cyst nature because it is useful in confirming the presence of blood in the lesion as well as in assessing the stage of hemorrhage according to the signal intensity variation depending on the age of the hemorrhage.4
The aim of this retrospective study is to establish a correlation between the clinical, radiological, and pathological findings that can aid in a better understanding of these lesion, investigate the possible etiology and provide better approach to the management of such cases.
Methods
This is a retrospective case series including all patients diagnosed with hematic cyst by histopathological examination after undergoing surgical removal at 2 Eye centers in Saudi Arabia: King Khaled Eye Specialist Hospital (KKESH) and King Abdulaziz University Hospital (KAUH). Thirteen cases where included from the histopathology data base of the 2 institutions over the period 2000–2017. The demographic data (age, sex and nationality) and basic clinical information was collected by chart review. The pre-operative radiological studies were reviewed by an experienced neuro-radiologist. Data was completed by histopathological review of the histologic sections and the reports to confirm the clinical and/or the radiological diagnosis of hematic cyst, identify the cases with the specific findings of a cholesterol granuloma and to observe any clue suggestive of an underlying vascular lesion or anomaly.
Results
We had a total of 13 cases with histopathological findings that are consistent with the clinical and/or radiological impression of an orbital hematic cyst. 8 cases were males and 5 were females. The age ranged from 2 to 84 years with a mean of 44 years and median of 54 years. The right orbit was involved in 9/13.
History of trauma was confirmed in 2 cases only (15.3%). One of these was a 9-year old boy with history of undefined trauma few weeks prior to his presentation with proptosis and blurred vision on the left side (Case 4). He had an extensive hematic cyst extending to the left orbital apex with complete loss of vision in the affected eye, relative afferent pupillary defect (RAPD), and frozen globe. The other patient (Case 10) was a 70-year-old healthy lady presenting with stable left eye protrusion 4 months following a history of fall and facial trauma. She had counting finger vision in that eye, RAPD and limitation of supraduction and abduction. History of surgery (without trauma) was documented in one 25-year old patient who was 6 months post-secondary orbital implant (Case 7). One case had worsening of symptoms following therapeutic Botox injection for squint management (Case 9). No clinical history of a pre-existing orbital vascular lesion was found in any of the cases. Summary of the cases is provided in Table 1.
Table 1.
Summary of the 13 hematic cysts cases with their main clinical and radiological features in addition to the important additional pathological findings.
| Case number | Age | Gender | Side | Main Symptom(s) | Sign(s) | Radiological findings | Other histopathological findings |
|---|---|---|---|---|---|---|---|
| 1 | 69 | Female | Right | Proptosis: sudden onset | Proptosis EOM: limitation of supra/infraduction and adduction |
MRI: well-defined inferior extraconal right orbital mass exhibiting intra-lesional hemorrhage and fat content | Intravascular papillary endothelial hyperplasia Venous malformation |
| 2 | 14 | Male | Right | Proptosis: 1 week Blurred vision Diplopia in upward gaze |
Proptosis EOM: Painful and restricted Conjunctival injection |
MRI: well-encapsulated intraconal mass, more heterogeneous than expected for hemangioma | Old hemorrhage. No underlying vascular anomaly |
| 3 | 84 | Female | Right | Proptosis: 3 days Pain Restricted eye movements |
Proptosis: 16mm EOM: limitation Conjunctival injection Dermatochalasis and fatty herniation |
MRI: intraconal lesion infero-nasally extending from behind the globe to the apex, seems avascular except for a posterior hemorrhagic component |
Hemosiderin-lined vascular spaces. No underlying vascular anomaly |
| 4 | 9 | Male | Left | Proptosis Decreased vision Trauma: few weeks |
Proptosis EOM: frozen globe Fixed dilated pupil APD NLP Choroidal folds |
MRI: intraconal vascular lesion at the left orbital apex with some intracranial extension |
Extensive reactive fibrosis surrounding the thrombosed hemorrhage. No underlying vascular anomaly |
| 5 | 71 | Male | Left | Mass LUL: 3 months | Cystic swelling: LUL | Not done | Lymphatic venous vascular malformation |
| 6 | 2 | Male | Right | Proptosis Upper lid swelling: medially |
Proptosis with dystopia Globe displacement: laterally and downward |
CT scan: extraconal mass consisting of an anterior cystic component and solid portion posteriorly | Lymphangioma Chronic inflammation |
| 7 | 25 | Male | Right | Bleeding: socket behind the prosthesis | Reddish lesion removed in the minor treatment room | Not done | Chronic inflammation Hemosiderin-laden macrophages. |
| 8 | 61 | Female | Right | Mass: RUL: superonasally Narrowing of the palpebral fissure |
Ptosis: RUL EOM: limitation of up-gaze Palpable mass medially |
Not done | Lymphatic venous vascular malformation |
| 9 | 8 | Female | Left | Proptosis: since infancy Squint: treated with Botox Decreased vision |
Axial proptosis: 7mm Esotropia VA: 20/400 EOM: limitation of supraduction and abduction |
CT scan: well-defined intraconal homogenous mass MRI: well-encapsulated intraconal mass |
No underlying vascular anomaly |
| 10 | 70 | Female | Left | Proptosis Trauma: to face, 4 months earlier |
Proptosis EOM: limitation of supraduction and abduction RAPD VA: CF |
MRI: well-encapsulated intraconal mass, iso tense on T1 and hyper intense on T2 images | No underlying vascular anomaly |
| 11 | 55 | Male | Right | Proptosis: of 1-month Headache | Proptosis Upper lid mild edema Subconjunctival hemorrhage EOM: full |
MRI: well-encapsulated extraconal mass | Cholesterol granuloma Chronic inflammation Hemosiderin-laden macrophages. |
| 12 | 54 | Male | Right | Proptosis slowly progressing over 15 years Blurred vision for 3 months |
Proptosis: 4 mm Hypoglobus: 4 mm EOM: mild restriction of superior gaze A palpable mass above the globe |
CT scan: large extraconal supero-temporal orbital cyst | Cholesterol granuloma Calcification Chronic inflammation Hemosiderin-laden macrophages |
| 13 | 50 | Male | Right | Proptosis | RUL Ptosis Proptosis EOM: restricted |
MRI: well-encapsulated intraconal mass | No underlying vascular anomaly |
RAPD: Relative afferent pupillary defect. EOM: Extra-ocular motility. VA: Visual acuity. CF: Counting fingers. RUL: Right upper lid. LUL: Left upper lid.
Proptosis was the commonest presenting symptom in 10/13 (76.9%) followed by limitation of eye movements in 9 cases (69.2%).Other less common unusual presentations included decreased vision in the affected eye (4/13), upper lid swelling (3/13), socket-related bleeding after enucleation and squint in one patient each (case 7 and case 9 respectively). Interestingly, the clinical diagnosis of hematic cyst was made prior to the surgery in 5 cases (38.4%), in whom the impression was even questionable.
Radiological imaging was performed in a total of 10 patients as summarized in Table 1 and revealed an intraconal lesion in 6/10. The 7 cases who underwent MRI had confirmation of the presence of blood in the orbit, thus the impression of hematic cyst was raised. CT scan was performed in 3 cases, 2 of which were extraconal lesions. The clinico-radiological findings of 3 cases are demonstrated in Fig. 1, Fig. 2, Fig. 3 in addition to Fig. 4A through D.
Fig. 1.
Case (9) A: The clinical appearance of an 8-year-old girl (Case 9) at presentation with left eye proptosis and squint since infancy. She received 3 botox injections, left eye for esotropia. B: CT scan done showing a well-defined intraconal homogenous left orbital mass. (C) FIESTA high resolution MRI T2-weighted axial image showing an intraconal isotense lesion. (D) Post-operative resolved proptosis with residual eye deviation and full extraocular motility following surgical intervention.
Fig. 2.
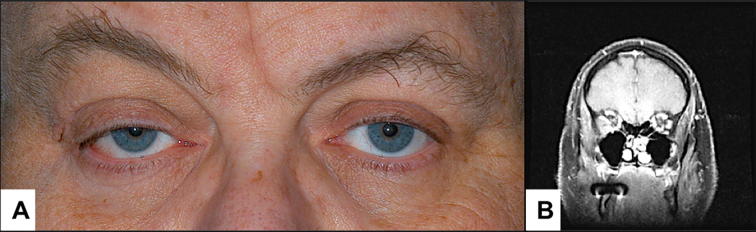
(Case 13) A: The clinical appearance of the proptosis and right upper lid ptosis ij a 50-year old gentleman with hematic cyst. B: The coronal magnetic resonance imaging showing the corresponding enlarging intraconal mass.
Fig. 3.
(Case 2) and (Case 4) A and B: T1-weighted coronal and axial MRI of the orbits, showing a high signal intensity lesion in the left orbital apex and non-traumatic sub-periosteal orbital hemorrhage (red arrows). C and D: T1 and T2-weighted coronal MRI of the orbits showing the typical appearance of an early subacute bleeding as rounded and well-circumscribed lesion (white arrow) in relation to the inferior and medial rectus muscles, where the belly of the inferior rectus muscle is seen running along the superior medial aspect of the lesion (white arrow head).
Fig. 4.
(Case 10) A: The clinical presentation of a 70-year-old lady with post-traumatic left eye protrusion and restricted motility with no diplopia, headache or numbness. B and C: Axial left proptosis on T1 and T2-weighted MRI images highlighting the intraconal mass as a homogenous isointense and hyper-intense lesion respectively. D: 6 weeks postoperative picture with resolved left proptosis. E: The corresponding histopathological appearance of the excised mass showing typical hematic cyst fibrous wall with organized hemorrhage (Original magnification X100 Hematoxylin and Eosin). F: Higher power of the fibrous wall with hemosiderin deposits (black arrows) (Original magnification X200 Hematoxylin and Eosin).
Surgical intervention was the mainstay of treatment with a variable surgical orbitotomy approach depending on several factors most importantly the location of the lesion. Histopathologically all lesions demonstrated collection of blood (often thrombosed) with absent endothelial lining, variable proliferating reactive fibroblasts, and surrounding formation of a fibrous capsule (Fig. 4E). Other constituents included chronic inflammatory cells in 4/13 cases and blood break-down products (hemosiderin) in 4/13(Fig. 4F). We had 2 unique cases of cholesterol granulomas showing typical cholesterol clefts, hemosiderin-laden macrophages, and multinucleated giant cells (Cases 11 and 12, one of which is shown in Fig. 5A through D). A histopathological clue to an underlying vascular lesion (lymphatic/venous malformation in 3 and lymphangioma in 1) was found histopathologically in 30.8% of the cases. None had confirmed history or confirmed clinical diagnosis of this pre-existing vascular lesion prior to the recent presentation. None of the patients developed recurrence or long-term complications with a follow up period up to 1 year except for motility-related sequelae.
Fig. 5.
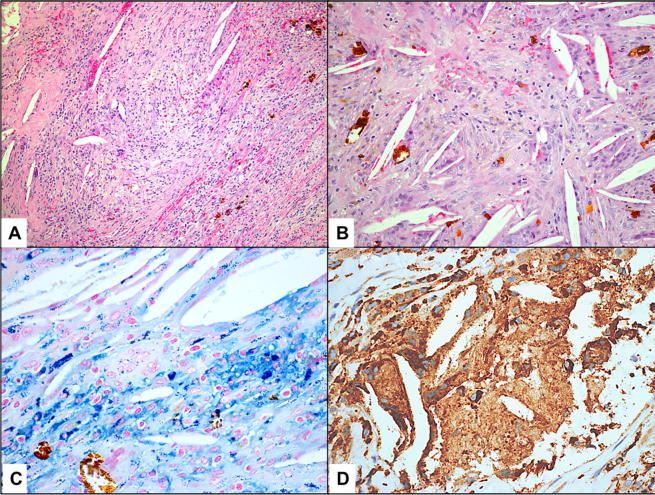
(Case 11) A: A case with hematic cyst showing the fibrous component and area of cholesterol granuloma (Original magnification X100 Hematoxylin and Eosin). B: Higher power of the adjacent area of cholesterol granuloma and hemosiderin-laden macrophages (Original magnification X200 Hematoxylin and Eosin). C: The area of cholesterol granuloma with hemosiderin deposits (Original magnification X400 Iron stain). D: The same area of cholesterol granuloma with epithelioid cells and few giant cells (Original magnification X400 CD68).
Discussion
We have aimed to study hematic cysts in the orbit since they are poorly understood with vague etiology. Shiparo and colleagues drew a connection of similarity between subdural hematoma and orbital hematic cysts.5 Bleeding in the orbit can be generally traumatic or non-traumatic. Spontaneous or non-traumatic orbital hemorrhage can be associated with other predisposing factors such as orbital vascular malformations, bleeding disorder, or sudden increase in venous pressure. This underlying presumed vascular malformation was found in approximately one third of our cases (30.8%) while history of trauma was confirmed in 2 patients only. Many theories have speculated other causes of bleeding in the absence of vascular malformations. Non-traumatic orbital hemorrhage was classified by McNabb into diffuse intraorbital, localized intraorbital (hematic cyst), sub-periosteal, extraocular muscle (EOM)-related and orbital floor implants-related.6 According to the previously mentioned classification, we had 11/13 non-traumatic orbital hemorrhage cases, out of which 10 were considered localized intraorbital hematic cyst and one was sub-periosteal. Also, activity of tissue plasminogen activator was noted in the outer areas of hematic cyst lesions, which has been proposed as an etiologic factor that allows more blood to accumulate.6, 7
Although the demographics of this lesion cannot be assessed with certainty based on a small case series, we can provide our observation that hematic cysts may tend to present in extreme age groups (in children with the youngest being 2 years-old and in patients in their fifties or older). The only young adult case of hematic cyst occurred in a 25-year old male in relation to previous surgical intervention and prosthesis. There was a slight male predominance with a male to female ratio of 8:5 and the right orbit was more commonly involved. In a local study of vascular lesions in our population, there has been predominance of vascular lesions in females.8 If we hypothesize that the presumed presence of a pre-existing vascular lesion may predispose to hemorrhage and eventual formation of hematic cyst, then we should have observed a parallel female predominance in the occurrence of hematic cyst, but this has not been observed in this series.8 Pathophysiology behind hematic cysts, however is still yet to be discovered. In one of our cases (Case 9) clinical worsening of the proptosis was noted after Botox injection, which has not been reported before in the literature, but a more likely explanation, would be the manipulation of the globe needed to access the medial rectus muscle by forced abduction and the use of a needle for injection that might have resulted in further bleeding. In relation to the etiology, trauma did not seem to be a major contributing factor in our series but seemed to be associated with worse prognosis since both our patients with documented history of trauma had significant loss of vision (NLP in one case), RAPD and marked limitation of motility. On the other hand, there is a well-known relationship of hematic cyst with orbital floor fracture where many studies documented hematic cysts development after orbital fractures repair. Formation of these cyst around orbital implants have been noted and some were symptomatic 5 years after surgical repair.9, 10, 11
Clinical features of hematic cysts in our cases were consistent with what has been mentioned in the literature. As any other space occupying lesion of the orbit hematic cysts has the potential to present with proptosis, limitation of eye movements, eyelid swelling and possibly vision changes.
Imaging has a major role in the diagnosis of hematic cysts. Hematic cyst may be associated with other vascular lesion or can mimic orbital varices particularly if the lesion is near the orbital apex, which is in agreement with Amrith and his co-authors who reported 3 cases of “spontaneous hematic cysts.” Their first case matched the definition of an unexpected hematic cyst, but the second occurred possibly in association with a vascular malformation. This can be differentiated by the presence or absenceofan endothelial lining in the wall.12 In our series, we had 4 cases with an underlying associated vascular malformation, however endothelial lining was absent around the hemorrhage even in the case where a pre-existing lymphangioma was detected. All our lesions histopathologically have shown free hemorrhage with surrounding reactive fibrosis similar to the so called: “spontaneous hematic cyst.”
MRI can indicate the sequence or timing of the evolution of the hemorrhage, which can be divided into 5 stages reflecting the steady breakdown of Blood.4, 13 McNabb described in detail these 5 stages in a systematic review where for example hyperacute bleeding in the lesion would appear isointense both in T1-weighted and T2-weighted images while, would appear isointense in the first and hyper intense in the later if chronic.6 This has been similarly observed in some of our cases with an early hemorrhage demonstrated in Fig. 3 as compared to Fig. 4 representing the radiological indication of a chronic post-traumatic hemorrhage. A computerized tomography (CT) scan may show homogenous or heterogeneous mass based on the stage of blood but can be misdiagnosed as an undefined orbital mass, thus limiting the diagnostic value of CT scan for cyst characterization. In the 3 cases where CT was performed, one has shown an anterior cystic component and a posteriorly located solid part and the radiological diagnosis raised the possibility of a vascular lesion-related hematic cyst (Case 6). However, the pre-operative radiological impression in our case was still suggestive of the proper diagnosis of hematic cyst and was proven afterwards by tissue diagnosis. The evolution characteristic signal intensity of the hemorrhage on T1 and T2 MRI helps in identify blood filled lesion this character depends on the stage.5, 6
Surgical removal of the cyst is successful in treating the signs and symptoms of this lesion. All of our patients underwent surgical excision and the surgical approach depends mainly on the location whether intraconal or extraconal. Surgery was described by other studies to be the mainstay of management for hematic cysts and is beyond the scope of this paper.14 Examination under the microscope reveals homogenous debris with loss of endothelial lining. Hemosiderin-laden macrophages with fine capillaries where also evident. These findings are noted in other studies that looked at the pathologic characteristics of hematic cysts with the importance of differentiation from other orbital pathologies.15 The hemorrhage in the case with underlying lymphangioma was not enclosed within an endothelial-lined space as evidenced also by negative CD34 and was surrounded by typical reactive fibrosis. Therefore, the lesion was not considered as a lymphangioma-related chocolate cyst. On the other hand, orbital cholesterol granuloma pathogenesis has been controversial and is less frequently associated with trauma compared to other sites in the head and neck.16 Interestingly, we have encountered this diagnosis in association with the presence of hematic cysts in 2 cases, none of which had any history of preceding trauma. However, this might support the hypothesis that these granulomas develop in relation to a non-absorbed hematoma.17
In conclusion, spontaneous hematic cysts in the orbit might be a challenging and often serious diagnosis especially in the absence of an underlying alarming vascular lesion. Trauma seems to negatively affect the outcome in some cases with permanent visual loss even after successful surgical excision of the hematic cyst. In other cases, pre-operative motility disturbance and/or the eye deviation secondary to the mass effect is likely to persist. Imaging plays an important role in the diagnosis, which then can be confirmed histopathologically. Development of cholesterol granuloma in association with orbital hematic cyst is an interesting finding that may be studied further.
Compliance with ethical standards
This case series was performed in accordance with the ethical standards of the institutional and national research Human Ethical Committee (HEC) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. HEC/IRB at KKESH has granted an expedited approval of this study. General Informed Consent was obtained from all patients included in this case series.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgment
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Pearson P.A., Rakes S.M., Bullock J.D. Clinicopathologic study of hematic cysts of the orbit. LetterAm J Ophthalmol. 1986;102:804–805. doi: 10.1016/0002-9394(86)90423-x. [DOI] [PubMed] [Google Scholar]
- 2.Henderson J.W., Farrow G.M. 2nd ed. B.C. Decker; New York: 1980. Orbital Tumors; pp. 107–111. [Google Scholar]
- 3.Loeffler M.L., Hornblass A. Hematic cyst of the orbit. Arch Ophthalmol. 1990;108:886–887. doi: 10.1001/archopht.1990.01070080130050. [DOI] [PubMed] [Google Scholar]
- 4.ersten R.C., Kersten J.L., Bloom H.R. Chronic hematic cyst of the orbit: role of magnetic resonance imaging in diagnosis. Ophthalmology. 1988;95:1549–1553. doi: 10.1016/s0161-6420(88)32974-x. [DOI] [PubMed] [Google Scholar]
- 5.Ito H., Komai T., Yamamoto S. Fibrino-lytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978;48:197. doi: 10.3171/jns.1978.48.2.0197. [DOI] [PubMed] [Google Scholar]
- 6.McNabb A.A. Nontraumatic orbital hemorrhage. SurvOphthalmol. 2014;59(2):166–184. doi: 10.1016/j.survophthal.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ito H., Yamamoto S., Komai T., Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neuro-surg. 1976;45:26. doi: 10.3171/jns.1976.45.1.0026. [DOI] [PubMed] [Google Scholar]
- 8.Alkatan HM, Al-Falah MA, Elkhamary S, Al Shaikh O. Prevalence of orbital vascular lesions in Saudi adults: a tertiary eye hospital experience. Acta Bioethica June 2016; 22 (2): 1096–1105.
- 9.Chao Daniel L., Ko Marcus J., Johnson Thomas E. Hematic cyst around orbital floor implant masquerading as choroidal mass. JAMA Ophthalmol. 2015;133(3):e143534. doi: 10.1001/jamaophthalmol.2014.3534. [DOI] [PubMed] [Google Scholar]
- 10.Glavas I., Lissauer B., Hornblass A. Chronic subperiosteal hematic cyst formation twelve years after orbital fracture repair with alloplastic orbital floor implant. Orbit. 2005 Mar;24(1):47–49. doi: 10.1080/01676830590892907. [DOI] [PubMed] [Google Scholar]
- 11.Kang S.J., Kwak I.H., 2nd Hematic cyst formation after repair of blow-out fracture. Korean J Ophthalmol. 1996 Jun;10(1):60–62. doi: 10.3341/kjo.1996.10.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Amrith S., Baratham G., Khoo C.Y. Spontaneous hematiccysts of the orbit presenting with acute proptosis. A report of three cases. OphthalPlastReconstr Surg. 1990;6:273e. doi: 10.1097/00002341-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Bradley W.G. MR appearance of hemorrhage in the brain. Radiology. 1993;189:15e26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 14.Iwata A., Matsumoto T., Mase M. Chronic, traumatic intraconal hematic cyst of the orbit removed through the fronto-orbital approach. Neurol Med Chir (Tokyo) 2000;40(2):106–109. doi: 10.2176/nmc.40.106. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa K., Fujisawa H., Kajiwara K. Cause of hematic cysts of the orbit: increased fibrinolysis and immunohistologic expression of tissue plasminogen activator. Ophthalmology. 2009;116(1):130–134. doi: 10.1016/j.ophtha.2008.08.041. Epub 2008 Nov 18. [DOI] [PubMed] [Google Scholar]
- 16.Roman-Romero L., Gonzalez-Garcia R. Cholesterol granuloma of the orbit. Report of cases and analysis of controversial treatment. J Maxillofac Oral Surg. 2011;10(2):166–169. doi: 10.1007/s12663-010-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiai H., Yamakawa Y., Fukushima T., Nakano S., Wakisaka S. Large cholesterol granuloma arising from the frontal sinus. Neurol Med Chir (Tokio) 2001;41(5):283–287. doi: 10.2176/nmc.41.283. [DOI] [PubMed] [Google Scholar]