Abstract
Previous studies suggested that human immunodeficiency virus (HIV) infected patients at risk of poor adherence were not distinguishable only based on the baseline characteristics. This study is to identify patient characteristics that would be consistently associated with poor adherence across regimens and to understand the associations between initial and long-term adherence. HIV treatment-naïve patients initiated on protease inhibitors, nonnucleoside reverse transcriptase inhibitors, or integrase strand transfer inhibitors were identified from the Veteran Health Administration system. Initial adherence measured as initial coverage ratio (ICR) and long-term adherence measured as thereafter 1-year proportion days covered (PDC) of base agent and complete regimen were estimated for each patient. The patients most likely to exhibit poor adherence were African-American, with lower socioeconomic status, and healthier. The initial coverage ratio of base agent and complete regimen were highly correlated, but the correlations between ICR and thereafter 1-year PDC were low. However, including initial adherence as a predictor in predictive model would substantially increase predictive accuracy of future adherence.
Keywords: adherence, initial adherence, long-term adherence, predictive model, proportion days covered
1. Introduction
It is estimated that more than 1.1 million people in the United States are living with human immunodeficiency virus (HIV).[1] Owing to early treatment, the life expectancy of HIV patients has been prolonged to near normal.[2] The primary goals of antiretroviral therapies (ARTs) are to control HIV replication, restore and preserve the immune system, decrease HIV transmission and infections, reduce complications caused by HIV, and improve quality of life and survival.[3]
Studies have shown that adherence to ARTs is a critical factor that determines virologic/immunologic outcomes among patients infected with HIV.[4–8] Unfortunately, patients are prone to have a lower adherence level when they take medicine over a long period, with long-term adherence rates reported to be as low as 50% to 75%.[9] Among patients with HIV, common reasons for poor adherence may include health beliefs, side-effects, heavy pill burden, busy schedule, other comorbid conditions, substance abuse, and fear of disclosing HIV-positive status to others.[10]
US guidelines recommend that physicians delay initiating ARTs among patients who would potentially have poor adherence, as suboptimal adherence is associated with an increased risk of adverse outcomes.[10] According to published studies, it is difficult to distinguish patients who would have poor adherence from those who would not if only based on patients’ baseline demographic characteristics alone[11–13]; prediction accuracy was reportedly not higher than 0.70, which is the lowest acceptable discrimination level.
Patient health beliefs were also found to critically determine patient adherent behavior; however, this information is usually not captured in administrative databases.[14] Patients’ initial adherence might be primarily dominated by their health belief, as the initial adherence has not yet been influenced by other risk factors (i.e. treatment effect, side-effects, or drug resistance, and so on.) for poor adherence that occur when patients have taken medicines for a while. Two studies found that initial adherence is a good predictor for future adherence in the long term,[15,16] but no study has been done among HIV patients.
In the present study, we aimed to identify patient characteristics that would be consistently associated with poor adherence across regimens, understand the correlation between initial and long-term adherence, and create an adherence predictive model targeted at maximizing prediction accuracy.
2. Methods
2.1. Patient selection
Antiretroviral-naïve veterans with incident HIV infection initiating ARTs in the Veterans Health Administration (VHA) system between January 1, 1999 and December 31, 2015, were selected for study. Patients were included if satisfying the following inclusion/exclusion criterion: having at least 1 ICD-9 code of HIV-1 or acquired immune deficiency syndrome (AIDS) 042 or V08; being an adult (18 years or older) at ARTs initiation; receiving ARTs consisting of 3 or more antiretroviral medications including 1 first-line base agent of protease inhibitors (PIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), or integrase strand transfer inhibitors (INSTIs); having virologic and immunologic lab tests before the index date; and having viral load reading before the index date. Patients were excluded if they had any evidence showing they would be treatment experienced.[17] Evidence included: viral load was less than 500 cells/mL any time before ARTs initiation; initiated regimens included 5 or more agents or used a regimen containing 2 base agents (‘5 or more agents’ defined as there are 4 or more antiretroviral agents filled within ± 15 days of the first fill of a base agent; ‘2 base agents’ defined as there was another base agent filled within 15 days after the first fill of a base agent; initiated ARTs was a single antiretroviral agent, defined as no antiretroviral agent filled within ± 30 days of the first fill of a base agent; received any antiretroviral agent earlier than 30 days before the first fill of a base agent; or the first fill of a base agent was not 30 days of supply.
2.2. Patient characteristics
Patient baseline characteristics of this study included age, sex, race/ethnicity, socioeconomic status (SES), viral load, CD4 counts, initiated regimen, pill burden, Deyo-adapted Charlson comorbidity index, and individual comorbid conditions.
2.3. Adherence measures
As a complete regimen should include 1 base agent and at least 2 other nucleoside reverse transcriptase inhibitors, adherences were calculated for a base agent and a complete regimen, respectively. The base agent was classified as an unboosted PI, boosted PI, NNRTI, and INSTI, which was same as the classification for the complete regimen. The initial adherence was measured as a coverage ratio (called an initial coverage ratio [ICR]); the thereafter 1-year adherence was measured as proportion days covered (PDC). Formulas are listed in Table 1. The index date was defined as the first fill date of base agent. ARTs discontinuation was defined as that the patient did not have a second fill of base agent within 60 days after the index date, as the days of supply for the first fill of base agent was 30 days. If patients switched the initiated regimen class, then the observations at the switch date were censored.
Table 1.
Initial adherence and thereafter 1-year PDC formulas.

2.4. Statistical analysis
Patient characteristics were summarized using mean (±standard deviation) or number (%) for continuous and categorical variables, respectively. Characteristics between patients initiated on different regimens were compared. As there were 4 treatment groups to compare, one-way analysis of variance (ANOVA) tests were used for continuous variables regardless of data distribution, because one-way ANOVA is considered a robust test against the normality assumption. Chi-square tests or Fisher exact test were used for categorical variables depending on whether the expected number for each cell of the variable was >5 or not.
Pearson correlations were calculated between the ICRs and PDCs. Proportions of patients at initial coverage ratio of complete regimen (ICRCR) of ≥95%, 80% to≤95%, 65% to ≤80%, 50% to≤65%, and <50% moving to specific thereafter 1-year proportion of days covered of complete regimen (PDCCR) categories were displayed in the figures for each initiated regimen category. For each regimen category, a Kappa coefficient between the Initial coverage ratio of base agent (ICRBA) and 1-year PDCCR was also calculated.
Multiple logistic regressions were used to predict binary 1-year adherence to a complete regimen with and without including initial adherence as a predictor and to compare predictive performance. It is generally accepted that near-perfect adherence (≥95%) is important to help achieve optimal outcomes in patients with HIV. [10] Poor 1-year adherence was defined as <95%. In the sensitivity analyses, other thresholds were also used to define poor adherence: <90%, <85%, <80%, and <75%. Logistic regression models were conducted using different thresholds to define poor adherence.
The University of Utah Institutional Review Board and the Salt Lake City Veteran Affairs (VA) Health Care System Office of Research and Development approved this study.
3. Results
A total of 53,427 veterans with at least 1 diagnosis of HIV-1 or AIDS from 1999 to 2015 were identified from the VHA databases; of these, 34,598 patients were treated with ARTs. After executing inclusion/exclusion criteria, a total of 10,274 veterans remained in the final cohort.
3.1. Patient characteristics
The cohort was relatively young with a mean age of 47.3 years old (Appendix: A1). More than half were identified as African-American, and about 29% were white. A total of 36.6% veterans had reached a very high level of viral load ≥100,000 copies/mL when they initiated ARTs. About 9.3% of veterans initiated ARTs with a higher than normal (≥500 cells/mL) CD4 count level. About 17.7% and 25.3% patients had AIDS conditions and opportunistic infections at the baseline, respectively. There were 976 (9.5%), 2291 (22.3%), 6374 (62.0%), and 633 (6.2%) patients initiated on unboosted PIs, boosted PIs, NNRTIs, and INSTIs, respectively. One-third of patients were treated on single-pill ARTs. About 44.2% patients had no comorbid conditions.
3.2. Discontinuation and switching
There were 1678 (16.3%) patients who did not have a second fill in 60 days. Patients initiated on unboosted PIs had the highest discontinuation rate at 20.9%, compared with a boosted PIs discontinuation rate of 17.4%, NNRTIs of 15.9%, and INSTIs of 10.1%. A total of 1502 (14.6%) patients had switched their initiated regimen during the follow-up period. About 25.8% patients initiated on unboosted PIs switched to another regimen category, followed by 19.2% for boosted PIs, 12.0% for NNRTIs, and 7.1% for INSTIs.
3.3. Initial adherence
Patients initiated with unboosted PIs had the lowest ICR with ICRBA of 0.84 and ICRCR of 0.83. Patients initiated with INSTIs had the highest initial coverage with ICRBA of 0.90 and ICRCR of 0.89. Detailed results may be found in Table 2. More than 50% of patients had both ICRBA and ICRCR ≥95% across all the regimens. However, there was still a large proportion of patients with very low level ICRBA and ICRCR at <65%, with the proportion range of 14.7% to 28.5%.
Table 2.
ICR among HIV antiretroviral-naïve patients.

3.4. Patient characteristics comparison between different initial adherence levels
Patient characteristics at different initial adherence levels were compared for each initiated regimen category. Patients most likely to exhibit poor adherence were African-American, had a lower SES, a lower baseline viral load, and a higher CD4 counts; these characteristics were consistently associated with poorer adherence for all initiated regimens. For other characteristics, patients with poor adherence and treated with unboosted PIs were those who were younger, male, less comorbid, and had less severe HIV conditions; patients with poor adherence and treated with boosted PIs were those who were older, female, less comorbid, and had more severe HIV conditions; patients with poor adherence and treated with NNRTIs were those who were older, female, more comorbid, and had more severe HIV conditions; patients with poor adherence and treated with INSTIs were those who were younger, female, less comorbid, and had less severe HIV conditions.
3.5. Thereafter 1-year PDC
Patients were censored when they switched their initiated regimens. There were 171 patients who switched regimens on the second fill, so they were excluded for calculating thereafter 1-year PDC. Similar to the pattern for ICRs, patients on INSTIs had the highest proportion of days covered of base agent and PDCCR, followed by NNRTI, boosted PI, and unboosted PI. Results are shown in Table 3.
Table 3.
Risk of poor thereafter 1-year adherence to a complete regimen (<95%).

3.6. Correlation between initial and thereafter adherence
The correlation between ICRBA and ICRCR was very strong, with the correlation estimate in the range of 0.92 to 0.96 for various initiated regimens. However, the correlations between ICRs and thereafter 1-year PDCs were of medium strength with the estimate in the range of 0.54 to 0.63. The correlation estimates are included in Appendix A2 and A3.
The patterns of patients changing adherence levels are shown in Figure 1. Among patients who initially had a near-to-perfect adherence (ICRBA ≥95%), those initiated with INSTIs were most likely to stay at a high adherence level, and those initiated with unboosted PIs were most likely to move to a low adherence level over the long term. Similarly, among patients who initially had a low adherence rate (ICRBA 50–≤65%), those initiated with INSTIs were least likely to stay at a low adherence level, whereas those initiated with unboosted PIs were most likely to stay at a low adherence level for the long term.
Figure 1.
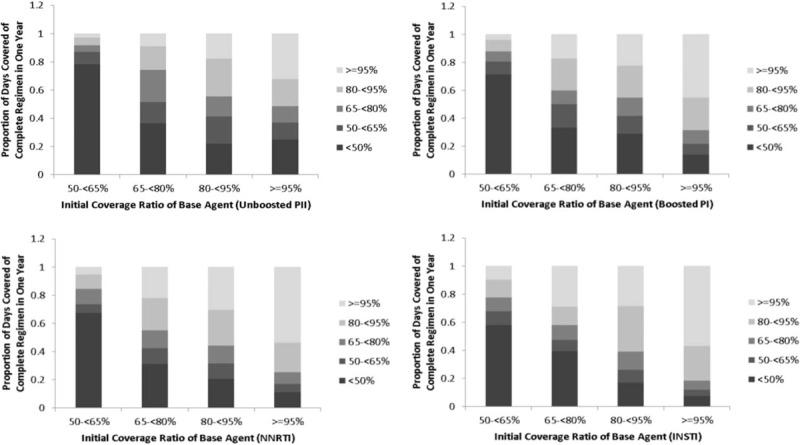
Adherence Change Pattern by Initiated Regimen.
The Kappa coefficients were also calculated to estimate interrater agreement between ICRBA and thereafter 1-year PDCCR based on the categories classified in Figure 1. The Kappa coefficients indicated fair strength of the agreements, as all coefficients were in the range of 0.21 to 0.40 (Appendix A4). In comparison, INSTIs and NNRTIs had the highest Kappa coefficient, followed by boosted PIs and unboosted PIs.
3.7. Predicting thereafter 1-year PDC
Compared with the model that excluded initial adherence, the model that included initial adherence as a predictor had an improved prediction accuracy for thereafter 1-year adherence to a complete regimen < 95%, as the c-statistic increased from 0.68 to 0.78 as shown in Table 3. Therefore, when ICRBA increased by 10%, then the risk of being at poor thereafter 1-year adherence would be reduced by 6%. Both models indicated that older age was independently associated with a decreased risk of poor adherence. African-Americans were almost twice as likely to have poor adherence as white Americans after adjusting for other covariates including SES. Decreased baseline viral load level was independently associated with an increased risk of poor adherence; however, baseline CD4 count was not an independent risk factor. Compared to patients who initiated NNRTIs, those who initiated INSTIs had similar risk of being at poor adherence, as the odds ratio (OR) was not statistically significant, whereas patients who initiated with PI-based regimens, regardless of unboosted or boosted PIs, had significantly increased risk of poor adherence. Increased pill burden was also found to be independently associated with an increased risk of poor adherence.
Results of the sensitivity analysis are shown in Table 4. ICRBA was a consistent predictor of poor thereafter 1-year adherence with the OR of 0.94, no matter how the threshold to define poor adherence was changed. If the initial adherence was not included in the models, then the c-statistic was around 0.68 to 0.69; but when the initial adherence was included in the models, the c-statistic only increased to 0.78 to 0.81, indicting good discrimination. As unboosted PI-based regimens are not recommended in the current guidelines, the predictive models were also created without unboosted PI-based regimens, and the results (not reported in the article) were very similar to those in Tables 3 and 4.
Table 4.
Sensitivity analysis with changing threshold to define poor thereafter 1-y adherence to a complete regimen.

4. Discussion
This study cohort was relatively young and healthy, and more than 50% were African-Americans. About one-third of patients had a high baseline viral load (viral load ≥100,000 copies/mL). One-tenth of patients initiated ARTs with a CD4 count ≥500 cells/mL. This reflects the US guideline's recommendations that patients should be initiated with ARTs once they are diagnosed with HIV regardless of immunosuppressive state.[10] Patients who were African-Americans and had a lower SES, lower baseline viral load, and higher baseline CD4 counts consistently had lower adherence than other patients across all regimens. These findings were consistent with the results of other published studies relative to the existence of racial disparities in HIV treatment adherence; African-Americans had the lower adherence than other races including whites and Latinos.[18] A recent meta-analysis reported that patients who did not perceive their disease as severe or as a threat were > 1.5 times more likely to be nonadherent.[14]
The cohort had a high ICR with a mean of 0.84 to 0.90 for base agent and 0.83 to 0.89 for the complete regimen. Among the cohort, patients initiated on INSTIs had the highest coverage. This might be because of the superiority of INSTIs in first-line HIV therapy in term of fewer adverse effects and better tolerance.[19] Patients initiated on PIs had the lowest coverage. This might be because of the high pill burden and treatment-associated adverse events.[10] Patients on unboosted PIs have even lower adherence than those on boosted PIs, perhaps because patients on unboosted PIs were younger and healthier when patient characteristics were compared across regimens. For thereafter 1-year adherence, the same pattern was also observed. About 45.1% patient on INSTIs showed adherence levels at ≥95%; however, the proportion was only 21.2% for patients on unboosted PIs. The mean of thereafter 1-year PDCCR was found to be 0.60 to 0.79 for various regimens. This finding is consistent with another adherence study among the US veterans treated with ARTs, which reported that the median of first year PDC to ARTs was 0.73 with the interquartile range of 0.41 to 0.97.[20] Owing to the low genetic barrier to drug resistance and the potential for cross resistance within the NNRTIs, patients on NNRTIs had lower thereafter 1-year PDC than those on INSTIs.[21]
The ICRBA and ICRCR were highly correlated. However, the correlations between the ICR and thereafter 1-year PDC were low. One previous study has shown that it is hard to distinguish patients who would be not adherent to ARTs based on patients’ baseline characteristics.[22] The present findings also show interaction between adherence and type of regimen, which means ARTs may have an influence on long-term adherence. For example, if patients’ initial adherence was low and they were also initiated on a PI-based regimen, then patients were more likely to be at low adherence; in comparison, if patients’ initial adherence was high and they were initiated on NNRTI-based regimen, then patients were more likely to be at high adherence. These suggest that a regimens’ side-effects and barriers to drug resistance may influence long-term adherence.
This finding confirms that although short-term adherence could not perfectly predict long-term adherence, the models improved predictive performance as the c-statistic increased from less than 0.7 to around 0.8 after adding short-term adherence as a predictor. Similarly, a recently published study confirmed that initial filing behavior could strongly predict adherence in the future.[15] This finding may help physicians identify targeted patients and provide interventions to enhance their adherence.
As this is a retrospective study based on an existing database, some limitations are unavoidable. The first limitation is related to the inclusion/exclusion criteria. Although rules were made to maximally exclude patients that would be treatment experienced, it is still possible to misclassify patients. Second, adherence were calculated according to refill records, which might not really reflect patient adherence behavior. Although unlikely, there is also a possibility that patients obtained medications outside the VHA system. Third, an assumption was made to calculate initial adherence. If there was no second fill of base agent within 60 days, patients were assumed to have discontinued the regimen, resulting in a calculation of patients’ ICRBA as 0.5. This is the conservative way to calculate adherence, as these patients are highly likely to have even lower adherence or did not take ARTs at all. This study would have limited generalizability, as it is based on the VA population, among whom the majority are men.
5. Conclusion
In summary, patients most likely to exhibit poor adherence were African-American, from a lower SES, and healthier (at lower baseline viral load and higher CD4 counts) for all initiated regimens. The ICRBA and ICRCR were highly correlated, but correlations between ICR and thereafter 1-year PDC were low. However, including initial adherence as a predictor in a predictive model would substantially increase predictive accuracy of future adherence.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, ARTs = antiretroviral therapies, CCI = Charlson comorbidity index, HIV = human immunodeficiency virus, ICR = initial coverage ratio, ICRBA = initial coverage ratio of base agent, ICRCR = initial coverage ratio of complete regimen, INSTI = integrase strand transfer inhibitors, NNRTI = nonnucleoside reverse transcriptase inhibitor, NRTIs = nucleoside reverse transcriptase inhibitors, PDC = proportion days covered, PDCBA = proportion of days covered of base agent, PDCCR = proportion of days covered of complete regimen, PI = protease inhibitor, SES = socioeconomic status, VHA = veterans health administration.
The authors have no conflicts of interest to disclose.
References
- [1].Singh R, Song R, Johnson AS, et al. HIV incidence, prevalence, and undiagnosed infections in men who have sex with men. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington; 2017. [Google Scholar]
- [2].Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pandhi D, Ailawadi P. Initiation of antiretroviral therapy. Indian J Sex Trans Dis 2014;35:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr 2002;30:105–10. [DOI] [PubMed] [Google Scholar]
- [5].Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001;15:1181–3. [DOI] [PubMed] [Google Scholar]
- [6].Carrieri MP, Raffi F, Lewden C, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antivir Ther 2003;8:585–94. [DOI] [PubMed] [Google Scholar]
- [7].Hong SY, Jerger L, Jonas A, et al. Medication possession ratio associated with short-term virologic response in individuals initiating antiretroviral therapy in Namibia. PloS One 2013;8:e56307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials 2007;8:282–92. [DOI] [PubMed] [Google Scholar]
- [9].Adherence to HIV Antiretroviral Therapy. HIC InSite, 2006. (Accessed June 15, 2017, at http://hivinsite.ucsf.edu/InSite?page=kb-03-02-09). [Google Scholar]
- [10].Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. U.S. Department of Health and Human Services, 2016. (Accessed June 15, 2017, at https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0/). [Google Scholar]
- [11].Gross IM, Hosek S, Richards MH, et al. Predictors and profiles of antiretroviral therapy adherence among African American adolescents and young adult males living with HIV. AIDS Patient Care STDS 2016;30:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krumme AA, Franklin JM, Isaman DL, et al. Predicting 1-year statin adherence among prevalent users: a retrospective cohort study. J Manag Care Spec Pharm 2017;23:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blumenthal DM, Singal G, Mangla SS, et al. Predicting non-adherence with outpatient colonoscopy using a novel electronic tool that measures prior non-adherence. J Gen Intern Med 2015;30:724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 2007;45:521–8. [DOI] [PubMed] [Google Scholar]
- [15].Franklin JM, Krumme AA, Shrank WH, et al. Predicting adherence trajectory using initial patterns of medication filling. Am J Manag Care 2015;21:e537–44. [PubMed] [Google Scholar]
- [16].Kozma CM, Phillips AL, Meletiche DM. Use of an early disease-modifying drug adherence measure to predict future adherence in patients with multiple sclerosis. J Manag Care Spec Pharm 2014;20:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS 2007;21:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simoni JM, Huh D, Wilson IB, et al. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. J Acquir Immune Defic Syndr 2012;60:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Messiaen P, Wensing AM, Fun A, et al. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PloS One 2013;8:e52562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohl ME, Perencevich E, McInnes DK, et al. Antiretroviral adherence among rural compared to urban veterans with HIV infection in the United States. AIDS Behav 2013;17:174–80. [DOI] [PubMed] [Google Scholar]
- [21].Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015. at http://aidsinfo.nih.gov/guidelines). Accessed February 13, 2016. [Google Scholar]
- [22].Cheng Y, Nebeker JR, Knippenberg KA, et al. PIN78 - Predicting Human Immunodeficiency Virus Veterans’ Adherence to Antiretroviral agents via optimal data analysis. Value Health 2015;18:A241.DOI: doi.org/10.1016/j.jval.2015.03.1402. [Google Scholar]


