Abstract
Studies on the association of maternal diabetes with autism spectrum disorders (ASDs) in offspring provide inconsistent findings; therefore an updated and comprehensive literature review and meta-analysis is necessary to perform in order to evaluate the available evidences.
After searching databases systematically, we established the inclusion criteria and selected the eligible studies. In both overall and stratified analyses, the estimated effects were synthesized dependent on the presence or absence of heterogeneity.
Twelve articles involving 16 studies were included and synthesized, demonstrating a significant association of maternal diabetes with ASDs among children (relative risk [RR] = 1.48). However, high heterogeneity was observed (I2 = 56.3%) and publication bias was identified. In terms of the analyses on reliable evidences from case-control studies, heterogeneity and publication bias disappeared, and the risk of ASDs was increased by 62% among diabetic mothers compared with non-diabetic mothers.
Maternal diabetes, especially gestational diabetes mellitus, is associated with ASDs in offspring based on a limited number of convincing case-control studies. More large-scale population-based prospective studies are still needed to draw firm conclusions.
Keywords: autism spectrum disorders, maternal diabetes, meta-analysis, risk factor
1. Introduction
Autism spectrum disorders (ASDs) are defined as neurodevelopmental disorders manifested with persistent impairments in social interaction and communications and restricted and repetitive patterns of behaviors, interests, or activities.[1] In addition to these common manifestations, a series of pilot studies has innovatively revealed significant deficits in executive functions,[2] sensory perception,[3] sleep habits,[4] and autonomic regulation[5] among children with ASDs. Autistic disorder, including Asperger syndrome and pervasive developmental disorders (PDDs), is the most severe form of ASDs. A recent meta-analysis reports an estimated 52 million cases of ASDs in 2010, indicating a prevalence of 7.6 per 1000.[6] The diagnosis of ASDs has increased substantially over time.[7] Although this increment may be caused by increased public awareness and changing diagnostic standard, it is also possible that a true rise is occurring.[8] The causes and contributing factors for autism are poorly understood. Conventional knowledge indicates autism is a neurobiological disorder of development with strong genetic basis.[9] Furthermore, accumulating evidences provide a novel insight that prenatal environmental factors are associated with ASD via affecting fetal brain development.[10,11]
During gestation, a hyperglycemic environment of intrauterine negatively impacts the development of fetal brain.[12] With the constantly growing prevalence of maternal diabetes,[13] it is plausible to observe a parallel rise in ASD diagnosis over years. The association of maternal diabetes with ASD in offspring has been evaluated by several case-control or cohort studies, with controversial conclusions.[1,14–24] For example, a prospective birth study in 2016 (N = 2743 mother–child pair) showed a weak association of gestational diabetes mellitus (GDM) with ASD (hazard ratio [HR] 1.86; 95% confidence interval [CI] 0.92–3.76) after adjusting crucial variables.[21] On the contrary, a retrospective longitudinal cohort study based on larger sample size (N = 322,323) revealed that GDM was not related to ASD (HR, 1.04; 95% CI, 0.91–1.19).[1] Therefore, it is necessary to obtain more data to evaluate the relationship between maternal diabetes and ASD in offspring. In this study, we comprehensively searched electronic databases until June 2017 to identify all the available investigations, and conducted a meta-analysis to reassess the possible risk of ASD in offspring conferred by maternal diabetes.
2. Methods
The study doesn’t involve any patients and animals. Ethical approval and patient consent is not applicable.
2.1. Literature search and identification of eligible studies
A comprehensive literature search in the electronic databases (PubMed and Web of Science) was performed to identify all the relevant studies. The key words were “autism,” “autism spectrum disorder,” “ASD,” “Asperger syndrome,” “pervasive developmental disorder,” “PDD,” and “maternal diabetes.” Initially, the irrelevant records were removed according to title and abstract screening. The remaining articles were manually checked based on the inclusion criteria. The inclusion criteria were: original article; case-control study or cohort study aimed to investigate the relationship between risk of ASD in offspring and maternal diabetes; effect size such as odds ratio (OR), relative risk (RR), or hazard ratio (HR), with 95% confidence interval (95% CI) was provided, otherwise the number of participants in exposure/non-exposure group for cohort study, or in case/control group for case-control study must be presented. When a cohort was reported in articles repeatedly, the newest publication was selected in order to avoid inclusion of overlapping data. Review, editorial, conference article, or comment were excluded.
2.2. Data extraction and quality assessment
The following items were extracted by 2 investigators independently: first author, publication year, study design, characteristics of the participants, diagnostic standards of ASD and maternal diabetes, effect size with 95% CI (preferentially adjusted effect size), and controlled covariates. Effect size was manually calculated from the original data when necessary. Any disagreement was resolved by further discussion.
The quality of eligible study was evaluated based on Newcastle-Ottawa Scale.[25] A “star system” was used to judge the quality of study on 3 broad perspectives: the selection of the study groups (4 stars); the comparability of the groups (2 stars); and the ascertainment of either the exposure for case-control study, or of the outcome of interest for cohort study (3 stars). Studies with 0 to 3, 4 to 6, or 7 to 9 stars were designated as low, moderate, and high quality, respectively.
2.3. Data synthesis
The heterogeneity within studies was assessed with Q-statistic, in which the significance level was defined as 0.1.[26] The extent of the inconsistency was measured by I2 value, which indicated the percent of the total variance across studies due to heterogeneity rather by chance. Heterogeneity was classified into high, medium, or low, represented as I2 ≥ 50%, 50% > I2 ≥ 25%, or 25% > I2, respectively.[27] If an I2 was smaller than 25%, Mantel–Hansel method in fixed effect was used to pool outcomes, otherwise data were accumulated by Dersimonian and Laird method in random effect model.[28] Publication bias was evaluated by the symmetry of funnel plot visually and Egger linear regression test statistically.[26] Sensitivity analysis was performed with omitting each study and observing whether the synthesized result altered significantly. All statistical analyses were conducted by Stata 9.0 (Stata Crop LP, College station, TX). All P values were 2-sided and identified as significant if <0.05, unless otherwise specified.
3. Results
3.1. Selection of eligible studies and characteristics of the included studies
As illustrated in Fig. 1, 188 records were initially found through database searching. After removal of duplicated records, 79 studies were screened according to titles and abstracts and 61 of them were excluded. Then the remaining 18 articles were reviewed. Two of them[29,30] were excluded due to insufficient data; 2 of them[15,31] reported the same Swedish population, therefore dropping the older one[31]; and another 2 studies[14,32] were excluded because the entire national populations were chosen as control groups. Finally, a total of 12 eligible articles[1,15–24,33] were selected in the meta-analysis.
Figure 1.
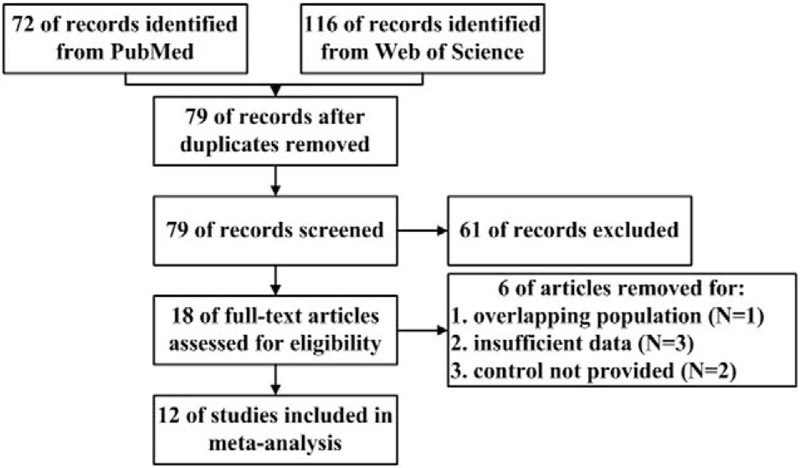
Flow diagram of the process of study selection.
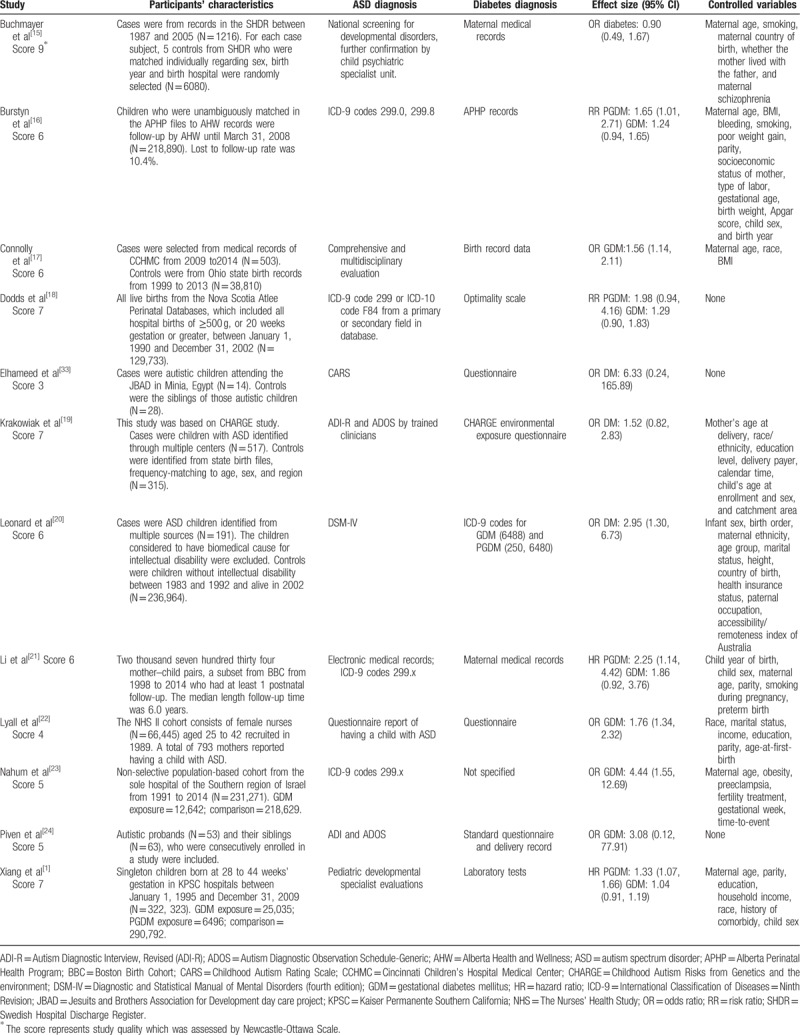
Among the 12 articles, 7 were case-control studies[15,17,19,20,22,24,33] and 5 were cohort study.[1,16,18,21,23] Most of the studies were conducted in USA,[1,17,19,21,22,24] 2 of them were from Canada,[16,18] and the rest were from Sweden,[15] Israel,[23] Australia,[20] and Egypt.[33] Therefore, findings in the present study might be restricted to specific populations. The majority of the included studies reported estimated effects after adjusting crucial variables, except studies of Dodds et al,[18] Elhameed et al,[33] and Piven et al.[24] The diagnostic criteria of ASD and maternal diabetes were clarified in each study (Table 1).
Table 1.
Characteristics of the included studies.
3.2. Meta-analyses on maternal diabetes and risk of ASD in offspring
The overall analysis demonstrated that gestational diabetes increased the risk of ASD by 48% (RR: 1.48; 95% CI: 1.26–1.75) (Table 2, Fig. 2). However, this result was unstable due to presence of inconsistency (I2 = 56.3%, P = .003) and publication bias (P < .001). In order to find more stable results, we subsequently performed several stratified analyses, as shown in Table 2. The data pooling of the 8 articles that reported OR as effect size[15,17,19,20,22–24,33] did not show heterogeneity (I2 = 31.8%, P = .174), nor publication bias (P = .351). In this strata, the risk was relatively higher, accounting for OR of 1.67 (95% CI: 1.40–1.99). However, the combinations of the studies reporting RR values[16,18] or HR values[1,21] presented either heterogeneity or publication bias. In addition, we also performed subgroup analyses based on type of diabetes, study design, country, and study quality. For more details, see Table 2.
Table 2.
Main results of the meta-analyses.
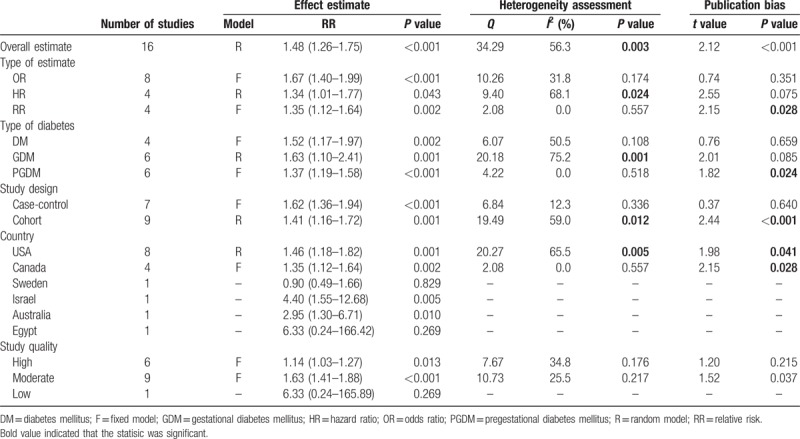
Figure 2.
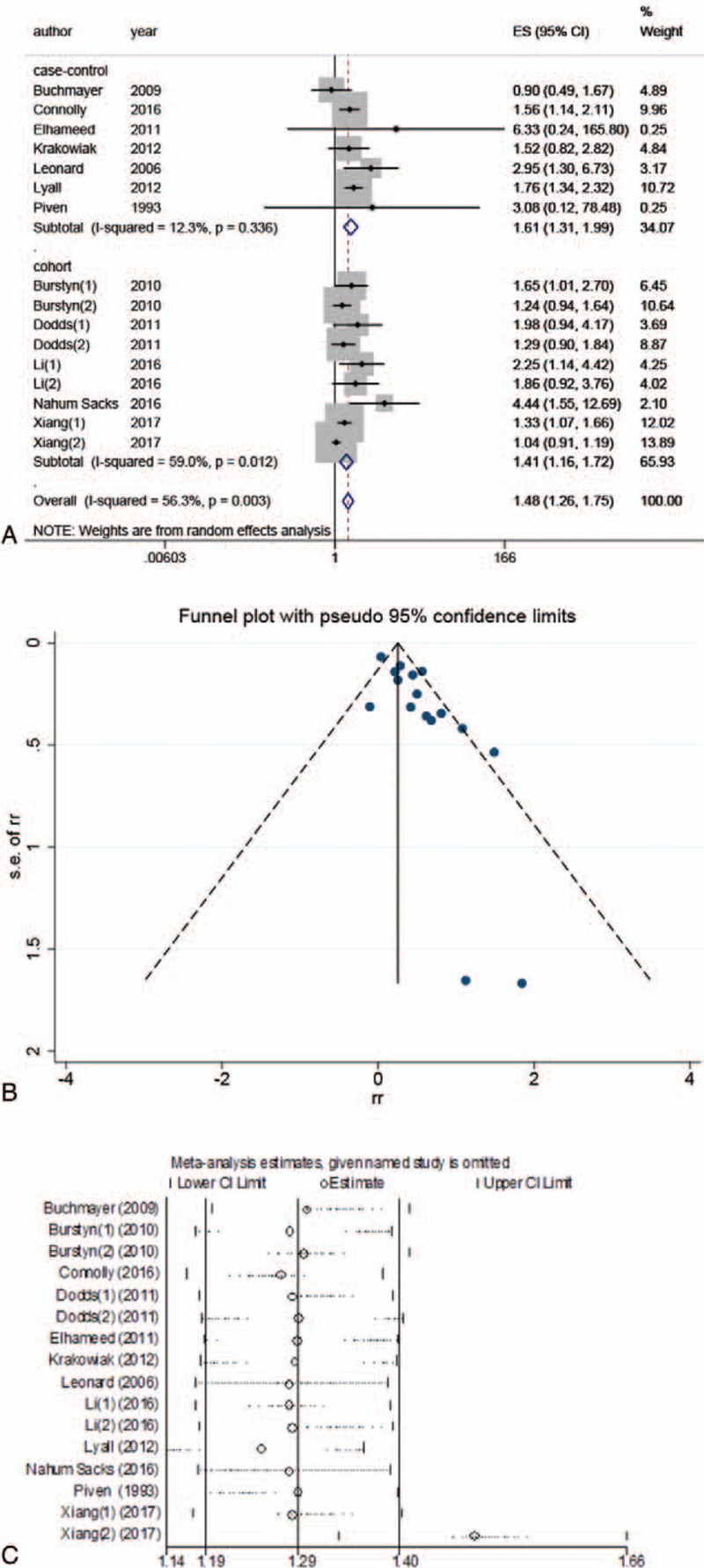
Meta-analysis of all included studies. (A) Forest plot of the meta-analysis; (B) funnel plot of the meta-analysis; (C) sensitivity analysis.
3.3. Meta-analyses on maternal diabetes and risk of ASD in offspring based on moderate and high-quality case-control studies
Of note, based on the stratified analyses, the pooled case-control studies did not present inconsistency and publication bias. But the strata combining cohort studies showed both of them. Therefore, the case-control studies seemed to be an ideal source of reliable and consistent evidences, from which we could draw a more robust conclusion.
Thus, another group of meta-analyses synthesized case-control studies with moderate[17,20,22,24] or high quality.[15,19] Generally, the combined data indicated that maternal diabetes could increase the risk of ASD by 62% (RR: 1.62; 95% CI: 1.35–1.94) (Table 3, Fig. 3), without detecting significant heterogeneity (I2 = 19.0%, P = .290) and publication bias (P = .847). In the strata based on pregestational diabetes, the inconsistency across studies was not detected (I2 = 0.0%, P = .859), while the estimated effect was higher (RR: 1.72; 95% CI: 1.34–2.21; Table 3).
Table 3.
Main results of the meta-analyses of moderate and high quality case-control studies.
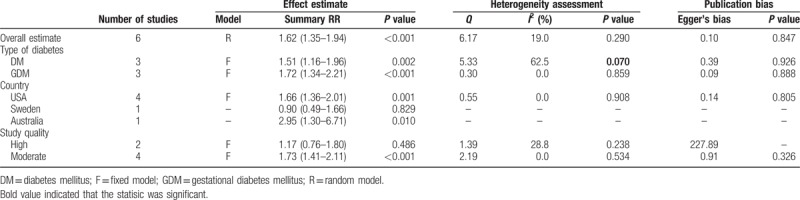
Figure 3.
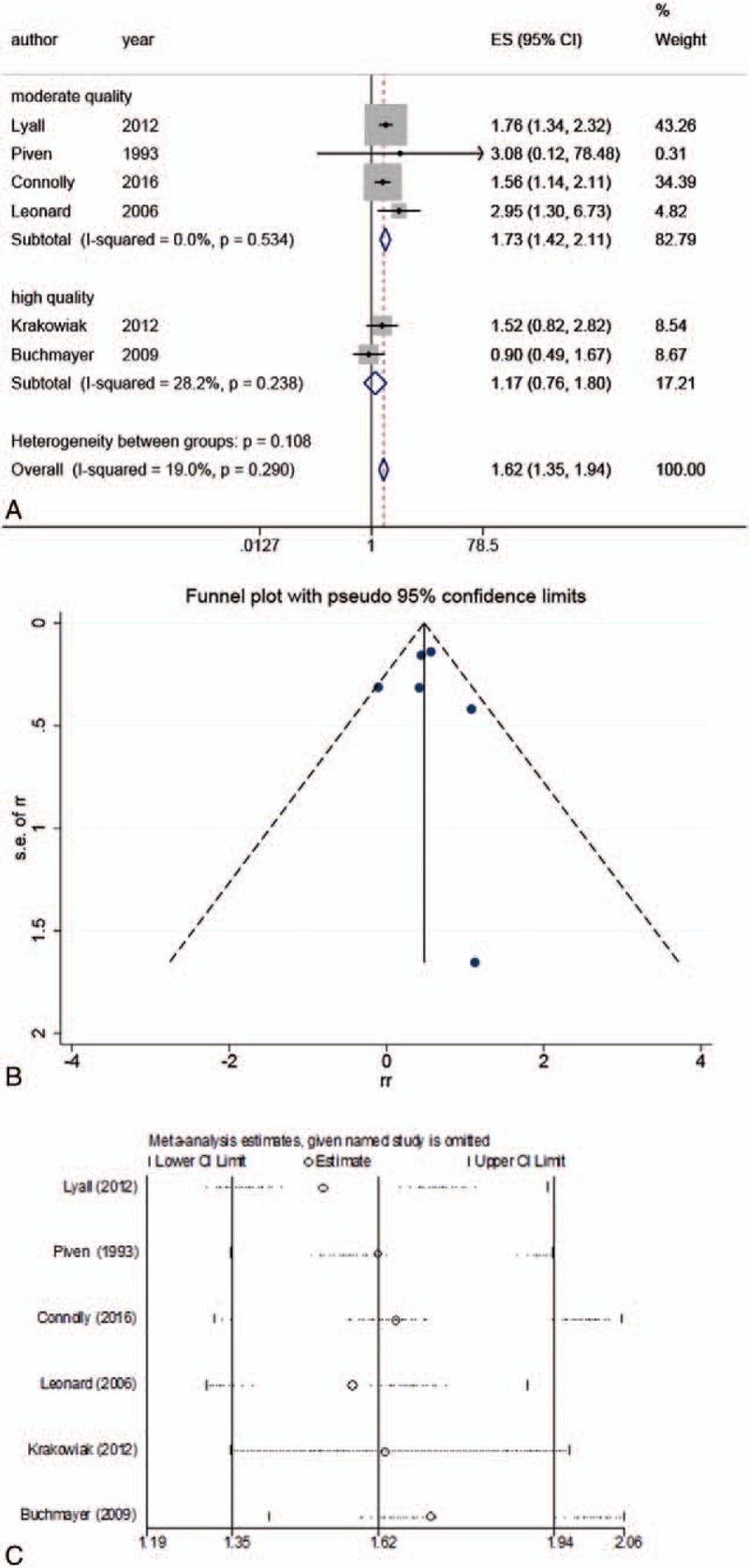
Meta-analysis of moderate and high quality case-control studies. (A) Forest plot of the meta-analysis; (B) funnel plot of the meta-analysis; (C) sensitivity analysis.
4. Discussion
In the present study, it was demonstrated that maternal diabetes was associated with an increased risk of ASD in offspring (RR: 1.48; 95% CI: 1.26–1.75). This was consistent with the results of 2 previous meta-analyses.[34,35] However, moderate heterogeneity (I2 = 56.3) and significant publication bias (P < .001) were also observed, therefore, we conducted several subgroup analyses to find more rigorous and reliable results. On the one hand, the combined data from retrospective studies revealed an unfavorable effect of gestational diabetes (RR: 1.62; 95% CI: 1.36–1.94), without detecting heterogeneity and publication bias. This result was in line with other retrospective studies based on large sample size. For example, a study involving 231,271 individuals demonstrated that GDM was associated with 3.44-fold higher risk of ASD in offspring and this impact was independent of crucial covariates such as maternal age, obesity, and gestational week.[23] Another retrospective study recruited >40,000 participants and consistently found an adverse effect, showing a 0.56-fold increased risk among mothers with GDM.[17] The studies reporting null results were often based on small sample size and flaw design.[24,33] On the other hand, in the subgroup combining prospective studies, both heterogeneity (I2 = 59.0) and publication bias (P < .001) were detected. Hence, a robust conclusion was unlikely to be drawn by synthesizing cohorts. Nevertheless, each high-quality prospective study is capable to provide us with reliable evidences that support the association and clarify the temporal relationship of it. A large cohort included 322,323 individuals and found significant risks of ASD among women with pre-existing DM (HR = 1.21) and GDM by 26 weeks’ gestation (HR = 1.39) after adjustment of crucial variables.[1]
The majority of the included studies clarified the time of DM diagnosis and separated pregestational DM from GDM. Thus, we further evaluated whether the temporal relationship between DM diagnosis and conception was a determinant for higher ASD risk. Surprisingly, we found that both pregestational (RR = 1.37; P-value for publication bias = 0.024) and gestational DM (RR = 1.63; I2 = 75.2%) were associated with ASD. Since heterogeneity and publication bias were detected, we did not believe the result was reliable, so we reassessed the evidences derived from trustworthy (moderate and high quality) case-control studies. In this scenario, GDM was identified as a risk factor (RR = 1.72), without detecting heterogeneity and publication bias. No quality case-control study aimed to investigate the role of pregestational DM.
Since the history of gestational complications was commonly obtained by questionnaires, recall bias might occur. Fortunately, the majority of the convincing case-control studies used medical record data,[15,17,20,24] thus recall bias was probably prevented. Regarding the studies using questionnaire,[19,22] a recent validation study demonstrated that, for etiological study, self-reported diabetes during periconception showed high validity among mothers compared with medical records.[36] In addition, diabetic mothers should be aware of the intensive management of this disease during pregnancy, the changes in diet and life style, and the medication for glycemic control. Therefore, we were confident that the quality case-control studies provided with rigorous diagnosis of pregnant complications.
Although robust epidemiological evidences are limited and findings are controversial, several biological mechanisms are proposed in order to elucidate how DM may cause brain malformation and aberrant neurodevelopment, which in turn supports our findings. In a diabetic mouse model that mimicking pregestational DM, abnormal morphogenesis, and histological structure of brain in mouse fetuses were identified.[37] Enhanced cell apoptosis and activated oxidative stress were detected in mouse fetal brains, where Nrf2 signaling played a crucial role.[37]The role of oxidative stress on pathogenesis of autism was further supported by idiopathic autism mouse model.[38,39] It was demonstrated that gestational exposure to chemical trigger of oxidative stress strengthened some of the autistic-like traits, including delayed motor maturation and increased vocalization rate.[38,39] Apart from oxidative stress, dysregulated immune responses during fetal neurodevelopment are also believed to participate in pathogenesis of ASD. A case-control study, as a part of the CHARGE (Childhood Autism Risks from Genetics and Environment) study,[40] described a direct relationship between maternal autoantibodies and the risk of developing of autism.[41] The presence of autoantibodies to fetal brain proteins at 37 and 73 kDa occurred significantly more often among mothers of autism children, compared with 2 distinct control populations.[41] Further, the presence of autoantibodies correlated with the specific behaviors within autism, including expressive language and irritability.[42]
The present study should be treated with caution because of some limitations. First, the included case-control studies were commonly based on hospital. Only a few investigations recruited controls from possible source population. For example, Connolly et al[17] recruited control individuals from the birth records of Ohio state. Similarly, Krakowiak et al[19] selected controls from state birth files. However, the representativeness of state birth data was unknown. So more community-based studies are encouraged. Second, several included studies did not consider potential covariates when analyzing the association.[18,24,33] If these univariate studies were excluded, the overall estimated effect was still statistically significant (RR: 39%; 95% CI: 1.17–1.65), but relatively lower than the result given above (1.39 vs 1.48). It is noteworthy that some of the variables are strongly correlated to risk of ASD. For example, maternal obesity was related to an increased risk of ASD in offspring, according to recent meta-analytic studies.[43,44] Furthermore, the risk of ASD was greater when obesity and diabetes occurred concomitantly.[21] So studies that took potential covariates into account were more likely to reveal the true effect of maternal diabetes. To overcome this obstacle, we extracted the estimated effects that were adjusted by controlled variables. Third, the severity and type of maternal diabetes was ignored by the majority of the studies. Regarding to the effect of severity, 1 included study revealed that the risk of ASD conferred by mild diabetes (OR = 1.83, 95% CI = 1.53–2.19) was greater than that conferred by severe diabetes (OR = 1.64, 95% CI = 1.18–2.27).[23] Considering the number of mild diabetes cases (N = 10,076) was nearly 4 times larger than that of severe diabetes cases (N = 2566),[23] this result needs to be confirmed by further studies. Regarding the type of diabetes, there were only 2 studies clearly reported the recruited cases were diagnosed with type 2 DM.[1,29] Due to insufficient evidences we could not perform data synthesis, so we expect more studies reporting these characteristics and believe that introduction of biological markers that are capable to accurately describe severity, such as insulin and glucose level, would be of assistance.
In conclusion, convincing case-control studies suggest that maternal diabetes, especially GDM, is associated with an increased risk of ASD in offspring. Given the limited number of reliable evidences, more well-designed prospective studies, with standardized recruitment criteria, and rigorous diagnostic process, are needed to confirm this finding.
Footnotes
Abbreviations: ASDs = autism spectrum disorders, GDM = gestational diabetes mellitus, PDDs = pervasive developmental disorders, RR = relative risk.
The authors have no conflicts of interest to declare regarding this article.
References
- [1].Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA 2015;313:1425–34. [DOI] [PubMed] [Google Scholar]
- [2].Precenzano F, Ruberto M, Parisi L, et al. Executive functioning in preschool children affected by autism spectrum disorder: a pilot study. Acta Medica Mediterranea 2017;33:35. [Google Scholar]
- [3].Parisi L, Fortunato MR, Salerno M, et al. Sensory perception in preschool children affected by autism spectrum disorders: a pilot study. Acta Medica Mediterranea 2017;33:49. [Google Scholar]
- [4].Precenzano F, Ruberto M, Parisi L, et al. Sleep habits in children affected by autism spectrum diorders: a preliminary case-control study. Acta Medica Mediterranea 2017;33:405. [Google Scholar]
- [5].Parisi L, Salerno M, Maltese A, et al. Autonomic regulation in autism spectrum disorders. Acta Medica Mediterranea 2017;33:491. [Google Scholar]
- [6].Baxter AJ, Brugha TS, Erskine HE, et al. The epidemiology and global burden of autism spectrum disorders. Psychol Med 2015;45:601–13. [DOI] [PubMed] [Google Scholar]
- [7].Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012;5:160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Croen LA, Grether JK, Hoogstrate J, et al. The changing prevalence of autism in California. J Autism Dev Disord 2002;32:207–15. [DOI] [PubMed] [Google Scholar]
- [9].Parisi L, Di Filippo T, Roccella M. The child with autism spectrum disorders (ASDs): behavioral and neurobiological aspects. Acta Medica Mediterranea 2015;31:1187. [Google Scholar]
- [10].Lainhart JE. Brain imaging research in autism spectrum disorders: in search of neuropathology and health across the lifespan. Curr Opin Psychiatry 2015;28:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ornoy A, Weinstein-Fudim L, Ergaz Z. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD). Front Neurosci 2016;10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sullivan EL, Riper KM, Lockard R, et al. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav 2015;76:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bardenheier BH, Elixhauser A, Imperatore G, et al. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care 2013;36:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brimacombe M, Xue M, Parikh A. Familial risk factors in autism. J Child Neurol 2007;22:593–7. [DOI] [PubMed] [Google Scholar]
- [15].Buchmayer S, Johansson S, Johansson A, et al. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 2009;124:e817–25. [DOI] [PubMed] [Google Scholar]
- [16].Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal char vacteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–34. [PubMed] [Google Scholar]
- [17].Connolly N, Anixt J, Manning P, et al. Maternal metabolic risk factors for autism spectrum disorder—an analysis of electronic medical records and linked birth data. Autism Res 2016;9:829–37. [DOI] [PubMed] [Google Scholar]
- [18].Dodds L, Fell DB, Shea S, et al. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902. [DOI] [PubMed] [Google Scholar]
- [19].Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129:e1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leonard H, de Klerk N, Bourke J, et al. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol 2006;16:448–54. [DOI] [PubMed] [Google Scholar]
- [21].Li M, Fallin MD, Riley A, et al. The Association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137:e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lyall K, Pauls DL, Spiegelman D, et al. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Res 2012;5:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nahum SK, Friger M, Shohamvardi I, et al. Prenatal Exposure to Gestational Diabetes Mellitus as an Independent Risk Factor for Long- term Neuropsychiatric Morbidity of the Offspring. Am J Obstet Gynecol 2016;215:380.e1–7. [DOI] [PubMed] [Google Scholar]
- [24].Piven J, Simon J, Chase GA, et al. The etiology of autism: pre-, peri- and neonatal factors. J Am Acad Child Adolesc Psychiatry 1993;32:1256–63. [DOI] [PubMed] [Google Scholar]
- [25].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [26].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [28].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [29].Krakowiak P, Walker CK, Tancredi D, et al. Autism-specific maternal anti-fetal brain autoantibodies are associated with metabolic conditions. Autism Res 2017;10:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Field SS. Interaction of genes and nutritional factors in the etiology of autism and attention deficit/hyperactivity disorders: a case control study. Med Hypotheses 2014;82:654–61. [DOI] [PubMed] [Google Scholar]
- [31].Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology 2002;13:417–23. [DOI] [PubMed] [Google Scholar]
- [32].Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics 2001;107:E63. [DOI] [PubMed] [Google Scholar]
- [33].Elhameed MAA, Elbaky AEOA, Kamel EA. A controlled study of the risk factors and clinical picture of children with Autism in an Egyptian sample. Egyptian J Neurol Psychiat Neurosurg 2011;48:271–6. [Google Scholar]
- [34].Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009;195:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu G, Jing J, Bowers K, et al. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord 2014;44:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Krakowiak P, Walker CK, Tancredi DJ, et al. Maternal recall versus medical records of metabolic conditions from the prenatal period: a validation study. Matern Child Health J 2015;19:1925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jin Y, Wang G, Han SS, et al. Effects of oxidative stress on hyperglycaemia-induced brain malformations in a diabetes mouse model. Exp Cell Res 2016;347:201–11. [DOI] [PubMed] [Google Scholar]
- [38].De Felice A, Greco A, Calamandrei G, et al. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 2016;13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Felice A, Scattoni ML, Ricceri L, et al. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One 2015;10:e0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hertz-Picciotto I, Croen LA, Hansen R, et al. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 2006;114:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Braunschweig D, Ashwood P, Krakowiak P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology 2008;29:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Braunschweig D, Duncanson P, Boyce R, et al. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 2012;42:1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li YM, Ou JJ, Liu L, et al. Association between maternal obesity and autism spectrum disorder in offspring: a meta-analysis. J Autism Dev Disord 2016;46:95–102. [DOI] [PubMed] [Google Scholar]
- [44].Wang Y, Tang S, Xu S, et al. Maternal body mass index and risk of autism spectrum disorders in offspring: a meta-analysis. Sci Rep 2016;6:34248. [DOI] [PMC free article] [PubMed] [Google Scholar]


