Abstract
Background:
Increasing evidence has suggested that rs2279744 is associated with rs117039649 polymorphism, which can increase the risk of gynecological cancers, including cervical, ovarian, breast, and endometrial cancer. The results are inconsistent so that we performed a meta-analysis of current literature to clarify the impacts of these polymorphisms on gynecological cancer.
Methods:
Eligible articles were identified through an exhaustive search of relevant databases including PubMed, Embase, Web of science, Springer Link, Chinese National Knowledge Infrastructure (CNKI), and Weipu database for the period up to July 2016. Data about the association between single nucleotide polymorphisms (SNPs) and cancer risk were refined from the selected articles as well as other information about cases and controls, and all of them were extracted by 2 independent researchers and pooled odds ratio with 95% confidence interval was calculated.
Results:
This analysis included 24 articles, 27 case–control studies of rs2279744 polymorphism and 3 case–control studies of rs117039649 polymorphism. Significant association with the risk of gynecological cancer was observed for both SNPs. Subgroup analysis by ethnicity and cancer type (cervical, ovarian, breast, and endometrial) also showed a positive relationship between rs2279744 polymorphism and gynecological cancer risk in Caucasian; and there was also a notable association between rs2279744 polymorphism and cervical cancer.
Conclusions:
We found that rs2279744 (SNP309) and rs117039649 (SNP285) were both associated with the risk of gynecological cancers. Subgroup analysis showed that rs2279744 (SNP309) was associated with the risk of gynecological cancers in Caucasian and Asian according to the ethnicity and cancer type, especially for endometrial cancer.
Keywords: gynecological cancer, meta-analysis, polymorphism, rs117039649, rs2279744
1. Introduction
It is well known that the gynecological cancer is the leading cause of cancer-related death in women. And the major cancer types include cervical cancer (CC), ovarian cancer (OC), endometrial cancer (EC), and breast cancer (BC). The BC is the most common cancer, which can be affected by both environmental and genetic factors. However, the mechanism remains unknown.[1,2] We thought that BC must have some resemblance in estrogen regulation with the above 3 types. Hence, here we considered it as gynecological cancer to study together[3]. CC is the 3rd-leading cause of death in women’ neoplasis worldwide, and the morbidity of CC has increased recently.[4] It has been reported that human papillomavirus is an important cause for CC.[5,6]
OC is most commonly seen among women who died from gynecological malignancies in China, while EC often occurs in well-developed countries and is also influenced by environmental factors.[7–9] In general, gynecological cancers are threatening the health and lives of women all over the world. However, the therapies for them were absent now. Therefore, further understanding of the mechanism and new method for diagnosis and treatment from genetic perspective are of great significance.
Recent studies have shown that the morbidity of gynecological oncology was controlled by the heredity of genes,[10] and studying on genes to the gynecological oncology is beneficial to analysis the internal mechanism for them. In recent years, the gene fiMDM2 (murine double minute 2), as a proto-oncogene, was found to be an important regulator of P53 through multifarious pathways.[11] Several studies have found that over expression of MDM2 gene can result in excessive inactivation of p53, which can enable damaged cells to escape the cell-cycle checkpoint control and become cancerous.[12,13] Meanwhile, MDM2 results in the degradation of P53 through E3 ubiquitin-ligating enzyme, which decreases the function of P53,[14,15] and leads to the onset and development of various diseases, including cancer.[16,17]
It has been reported that genetic polymorphisms play an important role in gynecological cancer.[18] Research has shown that a T to G change at nucleotide 309 (SNP309) in the first intron of MDM2 gene (rs2279744) increases the affinity of the promoter to the transcription activator Sp1, which leads to the high level of MDM2 mRNA and protein expression that weakens the P53 pathway.[19] It has been reported that MDM2 SNP309 genetic polymorphism could predispose the patient to sporadic cancer risk.[20] For instance, Hong et al[21] found that the MDM2 309GG genotype was associated with an increased risk of esophageal squamous cell carcinoma. Meanwhile, the 309G allele has also been associated with early diagnosis of estrogen receptor-positive BC.[22] In addition, another functional single nucleotide polymorphisms (SNPs) at nucleotide 285 G>C (rs117039649) was also identified in the promoter region located 24 bps from SNP309 in Caucasian,[23] It has been reported that the presence of the 285C allele correlated with a decreased cancer risk for breast, ovarian and EC in patients who harbored 309G allele,[22] which suggested that it may function as a neutralizer to the effect of SNP309 in MDM2.
Several studies reported that the polymorphism of rs2279744 or rs117039649 may be associated with the increased susceptibility to gynecological cancers, and the published articles of meta-analysis nearly included all the types of gynecological cancers, but most of them are single studies, including single type of locus or cancer.[7,24,25] None of the articles analyzed the relationship between the polymorphisms of these 2 SNPs and the overall risk of gynecological cancer. In addition, the studies of specific type of cancer also reported conflicting results, such as the analysis of CC by Meissner et al[26] and Roszak et al.[27] The signal path diagram was shown in Fig. 1.
Figure 1.
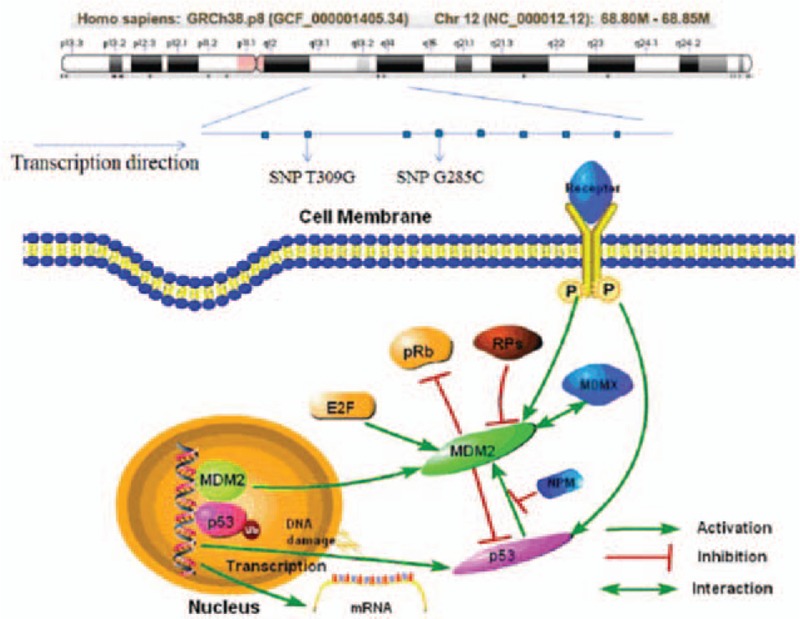
Gene structure and signal path of MDM2. E2F = transcription factor E2F, MDM2 = murine double minute 2, NPM = nucleophosmin, P = phosphorylation, pRb = pro retinoblastoma, Rps = ribosomal protein S, Ub = ubiquitination.
In our meta-analysis, we aimed to detect the association between rs2279744 or rs117039649 polymorphism and the overall gynecological cancer risk. Meanwhile, the subgroup analysis based on race and cancer types can also help elucidate this association in subgroup of patients and for individual type of cancer.
2. Materials and methods
2.1. Publication research and inclusion criteria
We searched several databases, including PubMed, Embase, Web of science, Springer Link, CNKI and Weipu databases, for genetic association studies of the MDM2 T309G, G285C polymorphisms and gynecological cancer. The keywords used were “MDM2 T309G or MDM2 G285C or rs2279744 or rs117039649” combined with “gynecological cancer or gynecological tumor or gynecology,” “GWAS or SNP or polymorphism or allele.” All databases were searched from their inception to July, 2016. Only papers published in English or Chinese with English abstract were considered.
Papers are eligible if they meet the following criteria: publication should be a case–control study with all required data elements available, publication evaluated the association between MDM2 T309G or MDM2 G285C polymorphism with the risk of CC, OC, BC, or EC, publication is a human study. The paper screening was conducted independently by 2 investigators. The same criteria were applied when assessed the quality and data extraction of the publications. And publication bias of all articles was analyzed by the funnel plots, Begg test Pr > |t| value >0.05 indicating no publication bias existed. In addition, we have to claim that no ethical approval and patient consent are required in this paper, because all analyses were based on previous published studies in this article, which does not involve using of tissue, blood, urine, genetic material samples and survey scales.
2.2. Quality assessment and data extraction
The quality of the identified studies was assessed according to the New Castle–Ottawa Quality assessment Scale (NOS), which measures the quality of a study based on 10 criteria. Publication with a total score of 8 to 9 points were considered to be high-quality, 6 to 7 points were considered moderate quality; and 5 points or lower to be low quality. Two reviewers independently extracted the data using a standard extraction form that includes the following: the first author's name, year of publication; the country of the study, ethnicity of the study subjects, age of the cases and controls, numbers of the cases and controls, cancer type, genotyping methods for MDM2 T309G or MDM2 G285C, and the genotypes’ frequency. If a study contained more than 1 cancer type or ethnicity, genotype data were extracted separately according to cancer type or ethnicity for subgroup analysis.
2.3. Statistical analysis
We used the goodness-of-fit test to assess the Hardy–Weinberg equilibrium (HWE). Summary odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated for each polymorphism in different genetic models, including recessive genotype, dominant genotype, heterozygous genotype, homozygote genotype, and allele genotype to assess the association between MDM2 T309G or MDM2 G285C polymorphism and the risk of gynecological cancer. Cochran Q test and Higgins (I2) test were used to assess the degree of heterogeneity between studies. The pooled ORs were calculated using a fixed-effects (P > .05 or I2 < 50%) or random-effects model (P < .05 or I2 > 50%) based on the level of heterogeneity. The publication bias was also evaluated using the Begg funnel plots (Pr > |z|) and Egger test (Pr > |t|). All data were analyzed using STATA 12.0 software.
3. Results
3.1. Characteristics and quality of the publications
A total of 24 articles were included in this meta-analysis. The selection process was summarized in Fig. 2, and the characteristics of the 24 studies are showed in Table 1.
Figure 2.
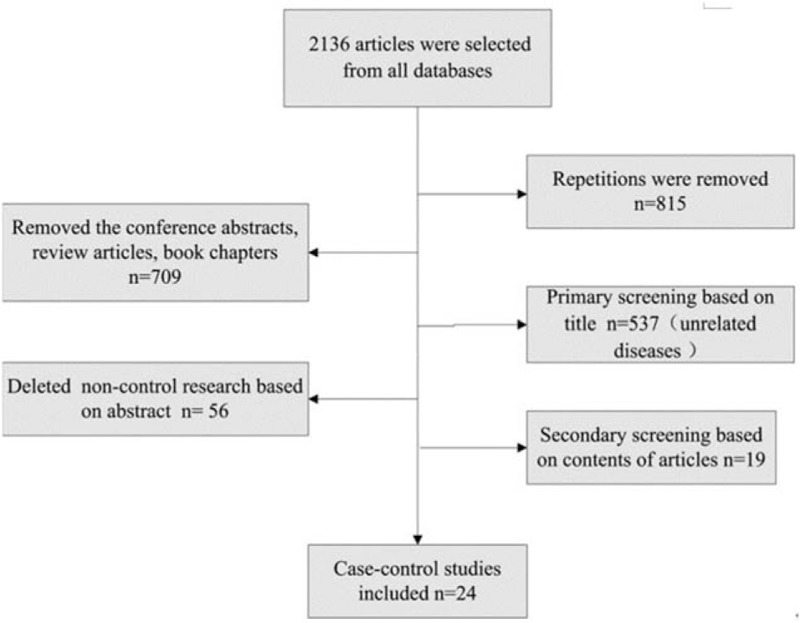
Process of articles selection. n = 24 articles were selected eventually.
Table 1.
Characteristics of the studies included in the meta-analysis.
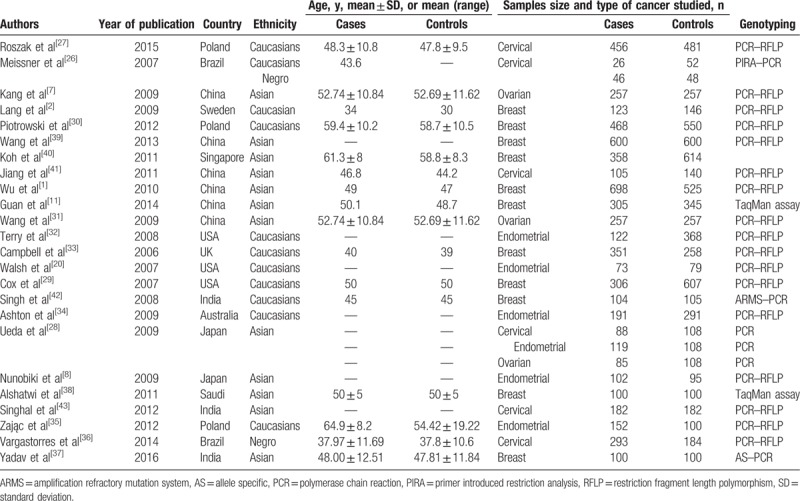
Our analysis included 27 case–control studies (1 article reported the study of 2 ethnicity groups,[26] another reported the study of 3 different types of cancer.[28]). All studied rs2279744. Of them, 12 studies were conducted among Caucasians, 14 studies were among Asians and 2 in African American. Only 3 papers reported rs117039649, all in Caucasians. The majority of the studies were not deviated from HWE, only 6 showed genetic disequilibrium (P < .05) (Table 2). According to NOS criteria, 2 publications[28,29] were considered high quality (8 or 9 points), 12[1,7,8,30–38] were moderate quality (6 or 7 points), and 10[2,11,20,26,27,39–43] low quality (5 or lower points) (Table 3).
Table 2.
Genotype and allele distribution of MDM2 polymorphisms in cases and controls.
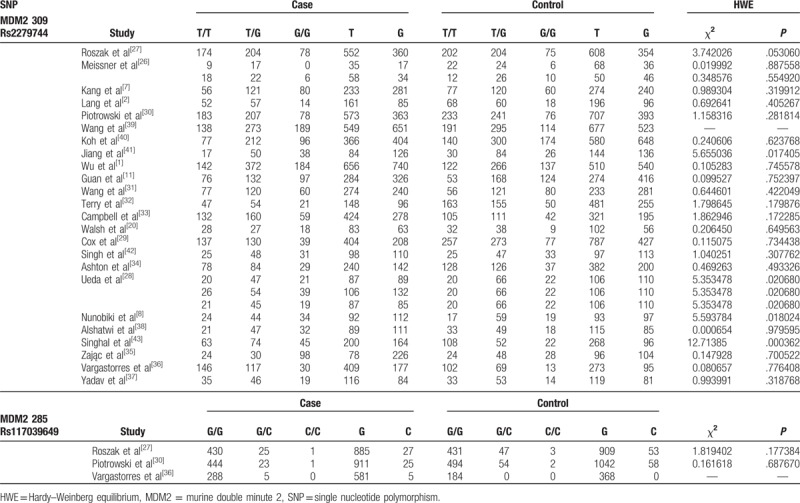
Table 3.
Quality of articles included in the analysis.
3.2. Analysis of relationship between SNPs and cancer risk
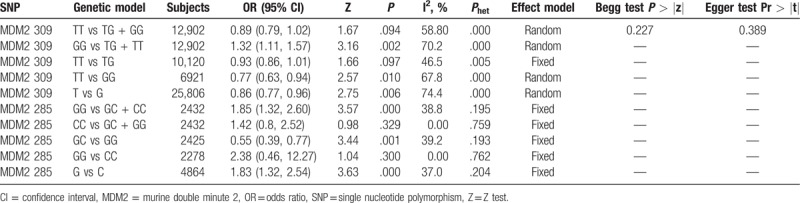
3.2.1. Association between rs2279744 or rs117039649 polymorphism and gynecological cancer risk
The meta-analysis of rs2279744 polymorphism included 6094 cases and 6808 controls from 27 case–control studies. Three studies containing 1217 cases and 1215 were included for the analysis of rs117039649 polymorphism. The genotypes of rs2279744 polymorphism are listed in Table 2. Based on the heterogeneity test (Phet < .05), 4 genetic models were analyzed using random-effect models: TT vs TG + GG, GG vs TG + TT, TT vs GG, and T vs G; and TT vs TG model was analyzed using a fixed-effect model. The ORs for GG vs TG + TT, TT vs GG, and T vs G genetic models were 1.32 (95% CI: 1.11, 1.57, P < .05), 0.77 (95% CI: 0.63, 0.94, P < .05), and 0.86 (95% CI: 0.77, 0.96, P < .05) (Fig. 3A–C), respectively. The data indicated that TT and T (compare with GG, G) genotype is associated with a reduced risk of gynecological cancer while GG genotype (compared with TG + TT) showed an increased risk of gynecological cancer. No significant association between MDM2 T309G polymorphism and gynecological cancer risk was observed in the TT vs TG + GG (OR = 0.89, 95% CI: 0.79, 1.02, P > .05) and TT vs TG (OR = 0.93, 95% CI: 0.86, 1.01, P > .05) models.
Figure 3.
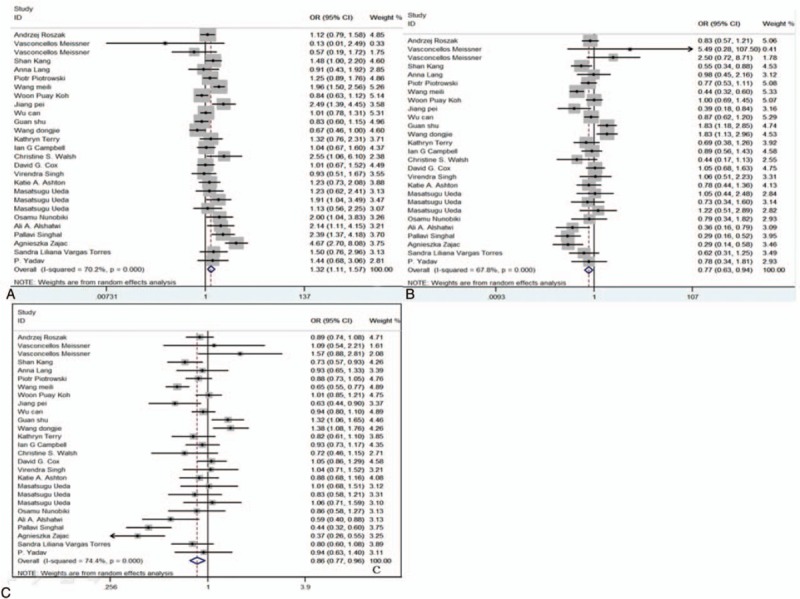
Forest plots of the association between SNP309 polymorphism and risk of gynecological cancers. Left to 1 (solid line in the middle) indicates SNP is associated with a reduced risk of cancer; Right to 1: SNP is associated with an increased risk of cancer. (A) Model GG vs TG + TT; (B) model TT vs GG; (C) model T vs G. CI = confidence interval, OR = odds ratio, SNP = single nucleotide polymorphism.
For rs117039649 polymorphism, the heterogeneity was insignificant (Phet > .05). Thus, the fixed-effects model was used and the pooled ORs for GG vs GC + CC, GC vs GG, and G vs C genetic models were 1.85 (95% CI: 1.32, 2.60, P < .05), 0.55 (95% CI: 0.39, 0.77, P < .05), and 1.83 (95% CI: 1.32, 2.54, P < .05), respectively (Fig. 4A–C), suggesting that GG or G genotype might be associated with an increased risk of gynecological cancer, compared with GC + CC or C, while GC showed a reduced risk when compared with GG.
Figure 4.
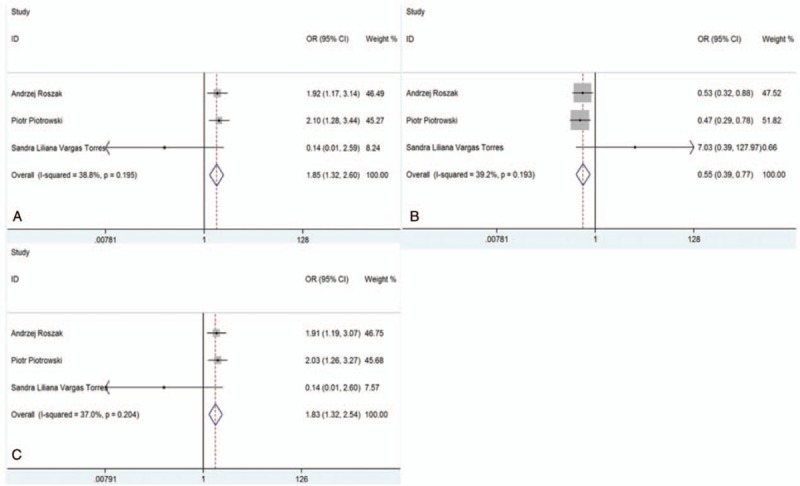
Forest plots of the association between SNP285 polymorphism and risk of gynecological cancers. (A) Model GG vs GC + CC; (B) model GC vs GG; and (C) model G vs C.
3.2.2. Subgroup analysis
3.2.2.1. The association between rs2279744 and rs117039649 polymorphism and gynecological cancer risk by ethnicity
For rs2279744, all 3 subgroups (Caucasian, Asian, and African American) were analyzed. In Caucasian, 3 genetic models including TT vs TG + GG (OR = 0.89, 95% CI: 0.79, 0.99, P < .05) (Fig. 5A), TT vs GG (OR = 0.78, 95% CI: 0.63, 0.96, P < .05) (Fig. 5B), and T vs G (OR = 0.86, 95% CI: 0.75, 0.98, P < .05) (Fig. 5C) were indicated to be significant, suggesting that TT or T polymorphism may be protective for gynecological cancer. In Asian, an increased risk was observed in the GG vs TG + TT model (OR = 1.36, 95% CI: 1.07, 1.74, P < .05) (Fig. 5D). However, we did not observe significant association between rs2279744 polymorphism and gynecological cancer risk in African American. These results indicated that rs2279744 polymorphism may be associated with gynecological cancer risk in Caucasian and Asian.
Figure 5.
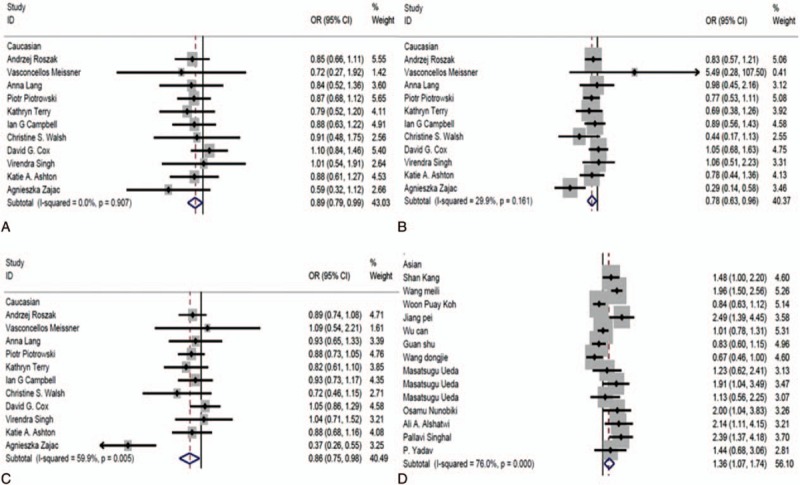
The association between SNP309 polymorphism and risk of gynecological cancer by ethnicity. (A) Model TT vs TG + GG in Caucasian; (B) model TT vs GG in Caucasian; (C) model T vs G in Caucasian; and (D) model GG vs TG + TT in Asian.
3.2.2.2. The association between rs2279744 or rs117039649 polymorphism and the types of gynecological cancer
Five genetic models were used to investigate the association between rs2279744 polymorphism and the types of gynecological cancer. Our findings indicated that 3 genetic models, including GG vs TG + TT (OR = 2.02, 95% CI: 1.31, 3.11, P < .05) (Fig. 6A), TT vs GG (OR = 0.60, 95% CI: 0.43, 0.83, P < .05) (Fig. 6B), and T vs G (OR = 0.73, 95% CI: 0.56, 0.94, P < .05) (Fig. 6C), had a significant association with the risk of EC, suggesting that TT or T polymorphism might be associated with a decreased risk of EC. No evidence was observed in relation to other 3 types of cancers. We did not conduct the subgroup analysis because of data limitation. All the data are summarized in Table 4.
Figure 6.
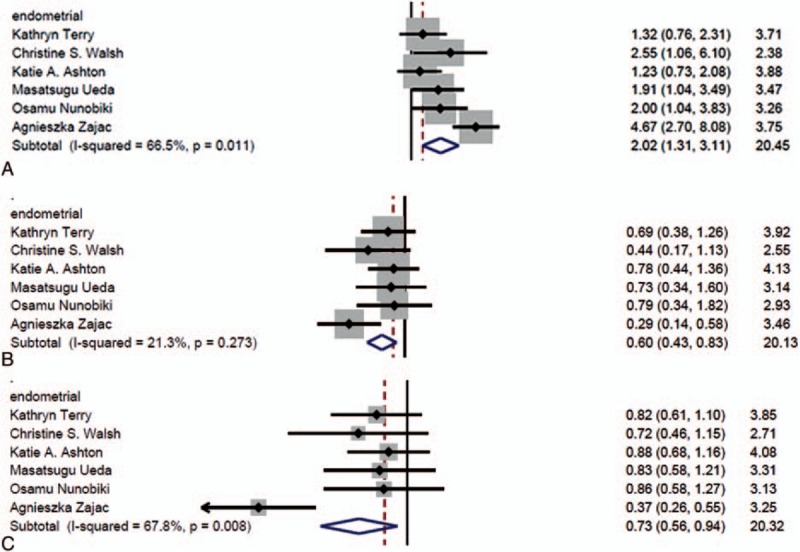
The association between SNP309 polymorphism and risk of gynecological cancer by type. (A) Model GG vs TG + TT in; (B) model TT vs GG; and (C) model T vs G.
Table 4.
Results of meta-analysis.
3.3. Publication bias
Funnel plots were symmetric in all genetic models, and Begg test did not show significant publication bias (P > .05) (Fig. 7), indicating that we random selected the articles with positive and negative outcomes together to a certain extent.
Figure 7.
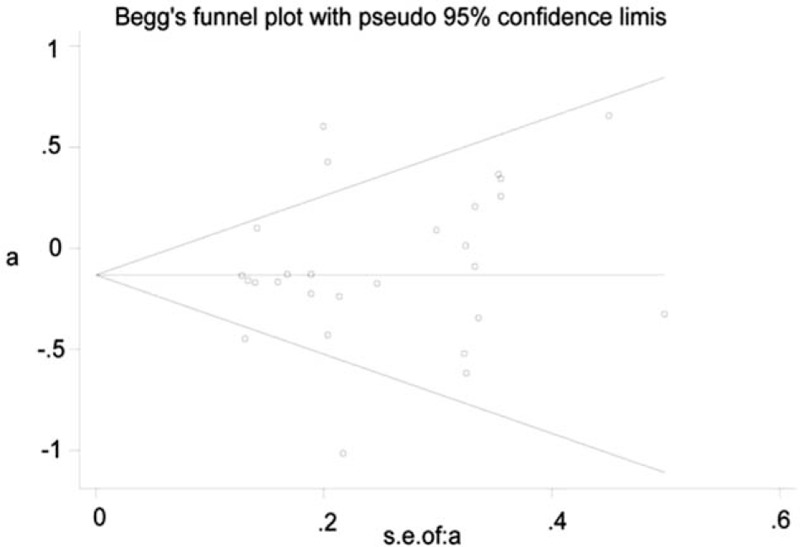
Funnel plots of all articles included in the analysis. a = log OR, s.e. of a = se (log OR).
4. Discussion
It has been reported that Sp1 binding to the MDM2 P2 promoter could be influenced by the polymorphism of SNPT309G, resulting in a high-level expression of MDM2 protein.[19] However, MDM2 SNPG285C allele may play a reverse role to SNP309.[25] The status of 309G is associated with an early diagnosis and tumor formation in Li–Fraumeni syndrome and several malignancies according to recent reports.[44,45] Interestingly, the association was only observed in women. So we aimed to explore the association between the polymorphisms at these 2 locus and the risk of gynecological cancers in this analysis.
Recently, Xue et al[23] published a meta-analysis of the association between MDM2 T309G polymorphism and EC, but they only studied the SNPT309G with the EC and the analysis was based on the search of 2 databases. In our meta-analysis, we first analyzed the association between SNPs of MDM2 and the risk of gynecological cancer as a whole. Five genetic models were considered including dominant, recessive, homozygote, heterozygous, and allele genotypes. We found that TT and T allele were associated with a reduced risk of gynecological cancer when compared with GG and G allele in SNP309, suggesting that SNPT309G was a risky mutation to women. Meanwhile, SNPG285C performs an opposite role. When compared with GC + CC, an increased risk for gynecological cancer was observed for GG polymorphism, the same was for G vs C. However, GC was associated with a reduced risk of gynecological cancer when compared with GG. Although only 3 articles were included in the analysis of SNPG285C, the number of subjects was still large: 1117 cases and 1221 controls. Hence, the results should be relatively reliable. Interestingly, we also confirmed that SNP309G and 285C, as 2 locus in MDM2 promoter, had an opposing effects in Caucasians regarding the risk of gynecological cancer. One similar point was observed for both SNPs: the GG and G alleles were all associated with increased risk of gynecological cancer when compared with their corresponding variant alleles. In other words, GG or G allele seems play a protective role in these 2 SNPs. We also speculated that patients have a good prognosis when harbor SNP285C even with SNP309G. In summary, we reported the overall effects of SNPT309G/G285C on the risk of gynecological cancers. To our best knowledge, this has not been reported so far.
Subgroup analysis was conducted in 2 ways. First, we assessed the association between MDM2 T309G polymorphism and gynecological cancer risk by ethnicity. We found that TT or T allele was associated with a decreased cancer risk in dominate, heterozygote and allele models in Caucasian. While in Asian, compared with TG + TT, GG genotype was associated with a significantly increased risk of gynecological cancer. The results were consistent with our overall analysis. No significant association was found in African American, which may be due to the low number of articles (2 articles). Second, we evaluated the association between MDM2 T309G polymorphism and 4 types of gynecological cancer, respectively. Previous articles of meta-analysis were conducted for breast, cervical, ovarian, and ECs individually. Our study found that GG genotype was associated with an increased risk of endometrial tumor, but not breast tumor,[44,46,47] ovarian or cervical tumor. In addition, we found that TT or T allele genotype (compared with GG or G allele) has a significant association with the risk of EC. In a recent report by Xue et al,[24] a significant association between MDM2 T309G polymorphism and EC in the recessive model was identified, which is consistent with our results. However, we also found that SNPT309G is associated with a reduced endometrial risk in TT vs GG and T vs G models (Fig. 6B and C). Of note, the results of previous studies were conflicting. Kang et al reported that SNPT390G polymorphism of MDM2 reduced the risk of ovarian tumor in Chinese,[7] while Knappskog et al concluded that the SNP309 G increased the risk of OC.[48] A subsequent meta-analysis[25] also identified a significant association in Asian population. However, in our analysis, we did not find a significant relationship between G allele or GG genotype and ovarian tumor.
BC was common. From our subgroup analysis, we did not find its relationship with SNP309G. One reason may be that the effects may vary by ethnicity. Our analysis has several limitations. Six articles were considered to have HWE disequilibrium, which could have an influence on our results. Only 2 articles included analysis based on African American, which restricted our analysis in this population. The number of reports studied SNP285C polymorphism was small, limits our conclusion in this analysis. More researches are needed in the future, to address the limitations of this analysis.
5. Conclusion
We found that rs2279744 (SNP309) and rs117039649 (SNP285) were both associated with the risk of gynecological cancers. Subgroup analysis showed that rs2279744 (SNP309) was associated with the risk of gynecological cancers in Caucasian and Asian according to the ethnicity and cancer type, especially for EC.
Footnotes
Abbreviations: BC = breast cancer, CC = cervical cancer, CI = confidence interval, CNKI = Chinese National Knowledge Infrastructure, EC = endometrial cancer, HWE = Hardy–Weinberg equilibrium, MDM2 = murine double minute 2, NOS = New Castle–Ottawa Quality assessment Scale, OC = ovarian cancer, OR = odds ratio, SNP = single nucleotide polymorphism.
JZ carried out the entire procedure including the literature search, data extraction, performed the statistical analysis, drafted the manuscript, and revised submitted the manuscript. ZZ conceived of the study, coordinated and participated in the entire process of drafting and revised the manuscript. YZ contributed to statistical analysis and revision the manuscript. JZ and YZ contributed to the revisions of the manuscript. All authors have contributed significantly. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Wu C, Xu Y, Ouyang T, et al. Association between MDM2 SNP309 polymorphism and breast cancer risk in Chinese women. Chinese J Clin Oncol 2010;37:131–3. [Google Scholar]
- [2].Lång A, Palmebäck WP, Wingren S. The significance of MDM2 SNP309 and p53 Arg72Pro in young women with breast cancer. Oncol Rep 2009;22:575–9. [DOI] [PubMed] [Google Scholar]
- [3].Gnerlich JL, Deshpande AD, Jeffe DB, et al. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 2011;18:1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- [5].Hausen HZ. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta 1996;1288:F55–78. [DOI] [PubMed] [Google Scholar]
- [6].Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kang S, Wang DJ, Li WS, et al. Association of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer in Chinese women. Int J Gynecol Cancer 2009;19:572–7. [DOI] [PubMed] [Google Scholar]
- [8].Nunobiki O, Ueda M, Yamamoto M, et al. Polymorphisms of p53 codon 72 and MDM2 promoter 309 and the risk of endometrial cancer. Hum Cell 2009;22:101–6. [DOI] [PubMed] [Google Scholar]
- [9].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA A Cancer J Clin 2009;59:225–49. [DOI] [PubMed] [Google Scholar]
- [10].Rosenthal AN, Side LE. Inherited Gene Mutations in Gynecological Oncology. 2015;London:Springer, 283–294. [Google Scholar]
- [11].Guan S, Cao Y, Wang YY, et al. The effects of MDM2 SNP309 and p53 Arg72Pro on the breast cancer risk in Northeast Chinese women. J China Med Univ 2014;43:205–8. [Google Scholar]
- [12].Chen J, Wu X, Lin J, et al. MDM-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol 1996;16:2445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 2007;6:909–23. [DOI] [PubMed] [Google Scholar]
- [14].Wang X, Jiang X. Mdm2 and MdmX partner to regulate p53. FEBS Lett 2012;586:1390–6. [DOI] [PubMed] [Google Scholar]
- [15].Wang X. p53 regulation: teamwork between RING domains of Mdm2 and MdmX. Cell Cycle 2011;10:4225–9. [DOI] [PubMed] [Google Scholar]
- [16].Zhao Y, Yu H, Hu W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys Sin 2014;46:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eischen CM, Lozano G. The Mdm network and its regulation of p53 activities: a rheostat of cancer risk. Hum Mutat 2014;35:728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meyer LA, Westin SN, Lu KH, et al. Genetic polymorphisms and endometrial cancer risk. Expert Rev Anticancer Ther 2008;8:1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004;119:591–602. [DOI] [PubMed] [Google Scholar]
- [20].Walsh CS, Miller CW, Karlan BY, et al. Association between a functional single nucleotide polymorphism in the MDM2 gene and sporadic endometrial cancer risk. Gynecol Oncol 2007;104:660–4. [DOI] [PubMed] [Google Scholar]
- [21].Hong Y, Miao X, Zhang X, et al. The role of P53 and MDM2 polymorphisms in the risk of esophageal squamous cell carcinoma. Cancer Res 2006;65:14–5. [DOI] [PubMed] [Google Scholar]
- [22].Karni-Schmidt O, Lokshin M, Prives C. The roles of MDM2 and MDMX in cancer. Annu Rev Pathol 2016;11:617–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Knappskog S, Lønning PE. Letter to the editor MDM2 SNP309 and risk of endometrial cancer. Polish J Pathol 2013;1:69–72. [DOI] [PubMed] [Google Scholar]
- [24].Xue ZW, Zhu XL, Teng Y. Relationship between murine double minute 2 (MDM2) T309G polymorphism and endometrial cancer risk: a meta-analysis. Med Sci Monit 2016;22:3186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ma YY, Guan TP, Yao HB, et al. The MDM2 309T>G polymorphism and ovarian cancer risk: a meta-analysis of 1534 cases and 2211 controls. PLoS ONE 2013;8:e55019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meissner RDV, Barbosa RNF, Galvão TM. No association between SNP309 promoter polymorphism in the MDM2 and cervical cancer in a study from northeastern Brazil. Cancer Detect Prev 2007;31:371–4. [DOI] [PubMed] [Google Scholar]
- [27].Roszak A, Misztal M, Sowińska A, et al. Murine double-minute 2 homolog single nucleotide polymorphisms 285 and 309 in cervical carcinogenesis. Mol Diagn Ther 2015;19:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ueda M, Yamamoto M, Nunobiki O, et al. Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum Cell 2009;22:49–54. [DOI] [PubMed] [Google Scholar]
- [29].Cox DG, Deer D, Guo Q, et al. The p53 Arg72Pro and MDM2-309 polymorphisms and risk of breast cancer in the nurses’ health studies. Cancer Causes Control 2007;18:621–5. [DOI] [PubMed] [Google Scholar]
- [30].Piotrowski P, Lianeri M, Rubis B, et al. Murine double minute clone 2309T/G and 285G/C promoter single nucleotide polymorphism as a risk factor for breast cancer: a Polish experience. Int J Biol Markers 2012;27:e105–10. [DOI] [PubMed] [Google Scholar]
- [31].Wang DJ, Li Y, Zhou RM, et al. Association of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer. Tumor 2009;19:892–7. [DOI] [PubMed] [Google Scholar]
- [32].Terry K, Mcgrath M, Lee I, et al. MDM2 SNP309 is associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2008;17:983–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Campbell IG, Eccles DM, Choong DYH. No association of the MDM2 SNP309 polymorphism with risk of breast or ovarian cancer. Cancer Lett 2006;240:195–7. [DOI] [PubMed] [Google Scholar]
- [34].Ashton KA, Proietto A, Otton G, et al. Polymorphisms in TP53 and MDM2 combined are associated with high grade endometrial cancer. Gynecol Oncol 2009;113:109–14. [DOI] [PubMed] [Google Scholar]
- [35].Zając A, Stachowiak G, Pertyński T, et al. Association between MDM2 SNP309 polymorphism and endometrial cancer risk in Polish women. Polish J Pathol 2012;63:278–83. [DOI] [PubMed] [Google Scholar]
- [36].Vargastorres SL, Portari EA, Klumb EM, et al. Effects of MDM2 promoter polymorphisms on the development of cervical neoplasia in a Southeastern Brazilian population. Biomarkers 2014;19:637–45. [DOI] [PubMed] [Google Scholar]
- [37].Yadav P, Masroor M, Tanwer K, et al. Clinical significance of TP53 (R72P) and MDM2 (T309G) polymorphisms in breast cancer patients. Clin Transl Oncol 2016;18:728–34. [DOI] [PubMed] [Google Scholar]
- [38].Alshatwi AA, Hasan TN, Shafi G, et al. A single-nucleotide polymorphism in the TP53 and MDM-2 gene modifies breast cancer risk in an ethnic Arab population. Fundam Clin Pharmacol 2012;26:438–43. [DOI] [PubMed] [Google Scholar]
- [39].Wang ML, Xu YX, Qian J, et al. Association of murine double minute 2 and P53 polymorphisms with breast cancer susceptibility. Zhonghua Yu Fang Yi Xue Za Zhi 2013;47:124–8. [PubMed] [Google Scholar]
- [40].Koh WP, Van DBD, Jin A, et al. Combined effects of MDM2 SNP309 and TP53 R72P polymorphisms, and soy isoflavones on breast cancer risk among Chinese women in Singapore. Breast Cancer Res Treat 2011;130:1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang P, Liu JW, Li W, et al. Correlation between MDM2 gene SNP309 polymorphisms and cervical cancer. Chinese J Clin Oncol 2011;38:1–4. [Google Scholar]
- [42].Singh V, Rastogi N, Mathur N, et al. Association of polymorphism in MDM-2 and p53 genes with breast cancer risk in Indian women. Ann Epidemiol 2008;18:48–57. [DOI] [PubMed] [Google Scholar]
- [43].Singhal P, Hussain S, Thakur N, et al. Association of MDM2 and p53 polymorphisms with the advancement of cervical carcinoma. DNA Cell Biol 2013;32:19–27. [DOI] [PubMed] [Google Scholar]
- [44].Bond GL, Hirshfield KM, Kirchhoff T, et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res 2006;66:5104–10. [DOI] [PubMed] [Google Scholar]
- [45].Bond GL, Menin C, Bertorelle R, et al. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet 2006;43:950–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li Y, Zhao H, Sun L, et al. MDM2 SNP309 is associated with endometrial cancer susceptibility: a meta-analysis. Hum Cell 2011;24:57–64. [DOI] [PubMed] [Google Scholar]
- [47].Liu GY, Jiang DK, Shen SQ, et al. MDM2 SNP309T>G polymorphism with hepatocellular carcinoma risk: a meta-analysis. Arch Med Res 2011;42:149–55. [DOI] [PubMed] [Google Scholar]
- [48].Knappskog S, Bjørnslett M, Myklebust LM, et al. The MDM2 promoter SNP285C/309G haplotype (1) diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer Cell 2011;19:273–82. [DOI] [PubMed] [Google Scholar]


