Supplemental Digital Content is available in the text
Keywords: BRCA, meta-analysis, mutation, ovarian cancer, survival
Abstract
Objective:
A meta-analysis was performed to determine if BRCA1/2 mutations are associated with improved overall survival (OS) and progression-free survival (PFS) in patients with ovarian cancer.
Research design and methods:
Studies of patients with primary or recurrent ovarian cancer that examined the relationship between BRCA1/2 mutation status and outcomes were included.
Main outcome measures:
The primary outcomes were OS and PFS of patients with and without BRCA1 and BRCA2 mutations. The secondary outcome was treatment response: complete response, partial response, and overall response.
Results:
Overall analysis revealed BRCA1/2 mutations were associated with improved OS [hazard ratio (HR) = 0.75; 95% confidence interval (CI): 0.64, 0.88; P < .001] and PFS (HR = 0.80; 95% CI: 0.64, 0.99; P = .039). BRCA1 mutations were significantly associated with improved OS (HR = 0.75) but not PFS, and BRCA2 mutations alone were not associated with either improved OS or PFS. The presence of BCRA1/2 mutations was associated with a better overall response rate, higher complete response rate, and lower partial response rate; however, BRCA1 or BRCA2 alone was not associated with overall response rate.
Conclusions:
BRCA1 mutations appear to be associated with improved OS in patients with ovarian cancer. However, the effect of BRCA1 mutations on PFS and BRCA2 mutations alone on OS and PFS is less clear.
1. Introduction
Ovarian cancer is a leading cause of death from gynecological malignancies, with 5-year survival rates of only 5% to 30% for patients with advanced disease despite cytoreductive surgery and platinum- and taxane-based chemotherapy.[1–4] Although the majority of ovarian cancer cases represent sporadic disease, it is now recognized that BRCA1 and BRCA2 mutations confer a genetic susceptibility to ovarian cancer, and females with mutations in these genes have a lifetime risk of 36% to 60% and 16% to 27%, respectively, of developing ovarian cancer.[5,6] Elective salpingo-oophorectomy after completion of childbearing can significantly reduce, though not completely eliminate the risk.[7]
Determining the most appropriate treatment for ovarian cancer includes an analysis of risk factors and disease characteristics. Interestingly, whereas BRCA1/2 mutations are associated with an increased risk of ovarian cancer, some studies have indicated that mutation carriers respond better to platinum-based chemotherapy and exhibit longer progression-free survival (PFS).[8–12] Other studies, however, have shown that the presence of BRCA1/2 mutations has no effect on response to chemotherapy and overall survival (OS) or PFS.[13–15] Differences in study results can be because of a multitude of factors including study design, patient population, other prognostic factors, degree of debulking surgery, age, and mutation characteristics. It has been postulated that an improved response to chemotherapy in patients with BRCA1/2 mutations may be because of inhibition of a DNA-repair pathway that sensitizes tumor cells to the DNA-damaging effects of chemotherapies.[16]
The purpose of this study was to perform an updated meta-analysis to determine if BRCA1/2 mutations are associated with improved OS and PFS in patients with ovarian cancer.
2. Methods
2.1. Literature search strategy and study selection
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines.[17] Medline, Cochrane, EMBASE, and Google Scholar databases were searched from inception until May 12, 2017 using the keywords: BRCA, BRCA1, BRCA2, ovarian cancer, overall survival, progression-free survival, prognosis, and response. Reference lists of relevant studies were hand-searched to identify additional potential articles of interest. Meta-analysis inclusion criteria were: prospective, retrospective, and cohort studies; patients with primary or recurrent ovarian cancer; examined the relationship between BRCA1/2 mutation status and OS, PFS, and treatment response to chemotherapy; and provided quantitative outcome data. Letters, comments, editorials, case reports, proceedings, personal communications, and one-arm studies were excluded.
The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of patients in each group, patient age, cancer stage, histopathological type, treatment, follow-up time, OS, PFS, and response rate to treatment.
The ethical approval and informed consent were not necessary, because meta-analysis does not involve human subjects and does not require IRB review.
2.2. Quality assessment
The Quality in Prognostic Studies (QUIPS) tool was used to assess the quality of the studies included in the meta-analysis.[18] Briefly, the tool assess bias in 6 domains; study participation, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis.
2.3. Outcome measures and data analysis
The primary outcomes for this meta-analysis were OS and PFS of patients with and without BRCA1 and BRCA2 mutations. The secondary outcome was treatment response; complete response (CR), partial response (PR), and overall response (ORR). Hazard ratios (HR) and 95% confidence intervals (CIs) were extracted for each individual study, and calculated for studies combined. If a HR with a 95% CI was not available, the HR and its variance was estimated using methods described by Parmar et al[19] and Williamson et al.[20] A HR < 1 indicated that BRCA1/2 mutations were associated with a longer OS or PFS. Treatment response rates were extracted for each individual study and odds ratios (ORs) were calculated for the studies combined. A χ2-based test of homogeneity was performed, and the inconsistency index (I2) and Q statistics were determined. A Q statistic value of P < .10 or an I2 > 50% were considered to indicate statistically significant heterogeneity. If significant heterogeneity was detected a random-effects model of analysis was used; otherwise, a fixed-effects model was employed. Pooled effects were calculated, and a 2-sided value of P < .05 was considered statistically significant. Publication bias was assessed by constructing funnel plots for OS and PFS and by Egger test. The absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution, and a value of P > .10 in Egger test. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
3. Results
3.1. Literature search
A flow diagram of study selection is shown in Figure 1. A total of 381 potentially relevant articles were identified in the database searches. After screening by title and abstract, 320 were excluded. Of the 61 remaining articles that underwent a full text review, 28 were excluded, the reasons for which are shown in Figure 1. Thus, ultimately 33 articles were included in the meta-analysis.[8–11,15,21–48]
Figure 1.
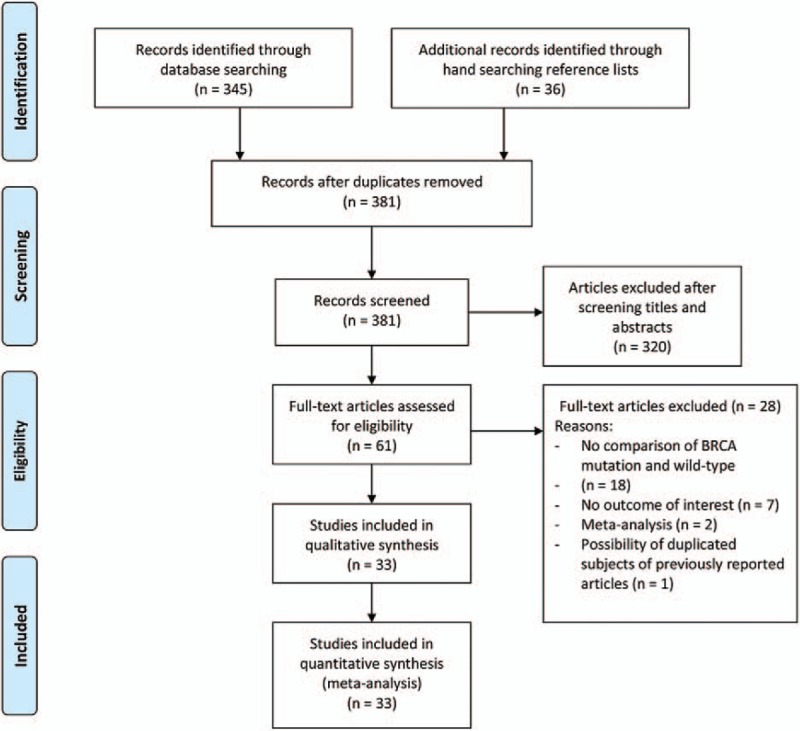
PRISMA flow diagram of study selection.
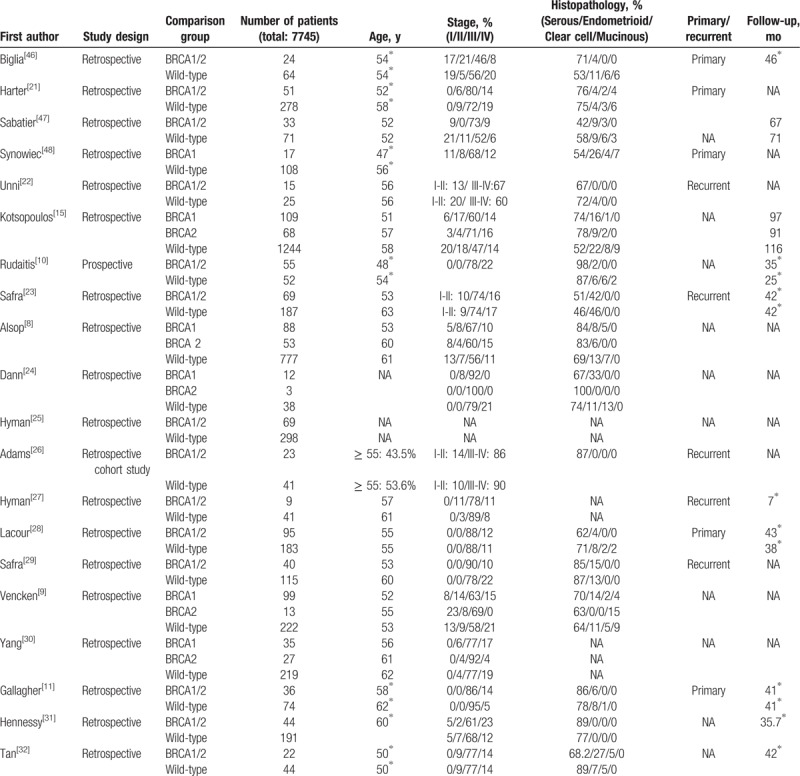
The basic characteristics of the 33 studies are summarized in Table 1 .[8–11,15,21–48] The 33 studies enrolled a total of 7745 patients with primary or recurrent ovarian cancer. One study was prospective, and the others were retrospective. Patient age ranged from 48 to 73 years, and more than 80% of patients had advanced stage disease (stage III or IV). The most common pathological type was serous carcinoma.
Table 1.
3.2. Meta-analysis of BRCA1/2 mutations
Twenty-three studies provided OS data and were included in the analysis. Heterogeneity was observed among the 23 studies (I2 = 74.83%, Q statistic = 86.37, P < .001); therefore, a random-effects model was used. The analysis revealed a significant OS advantage in ovarian cancer patients with BRCA1/2 mutations (HR = 0.75; 95% CI: 0.64, 0.88; P < .001) (Fig. 2A). In addition, primary ovarian cancer patients with BRCA1/2 mutations had significantly longer OS than noncarriers (HR = 0.513; 95% CI: 0.381, 0.689; P < .001) as did recurrent ovarian cancer patients (HR = 0.652; 95% CI: 0.530, 0.802; P < .001). However, BRCA1/2 mutations were not associated with OS in patients with advanced-stage ovarian cancer (HR = 0.743; 95% CI: 0.302, 1.828; P = .518) (Table 2).
Figure 2.
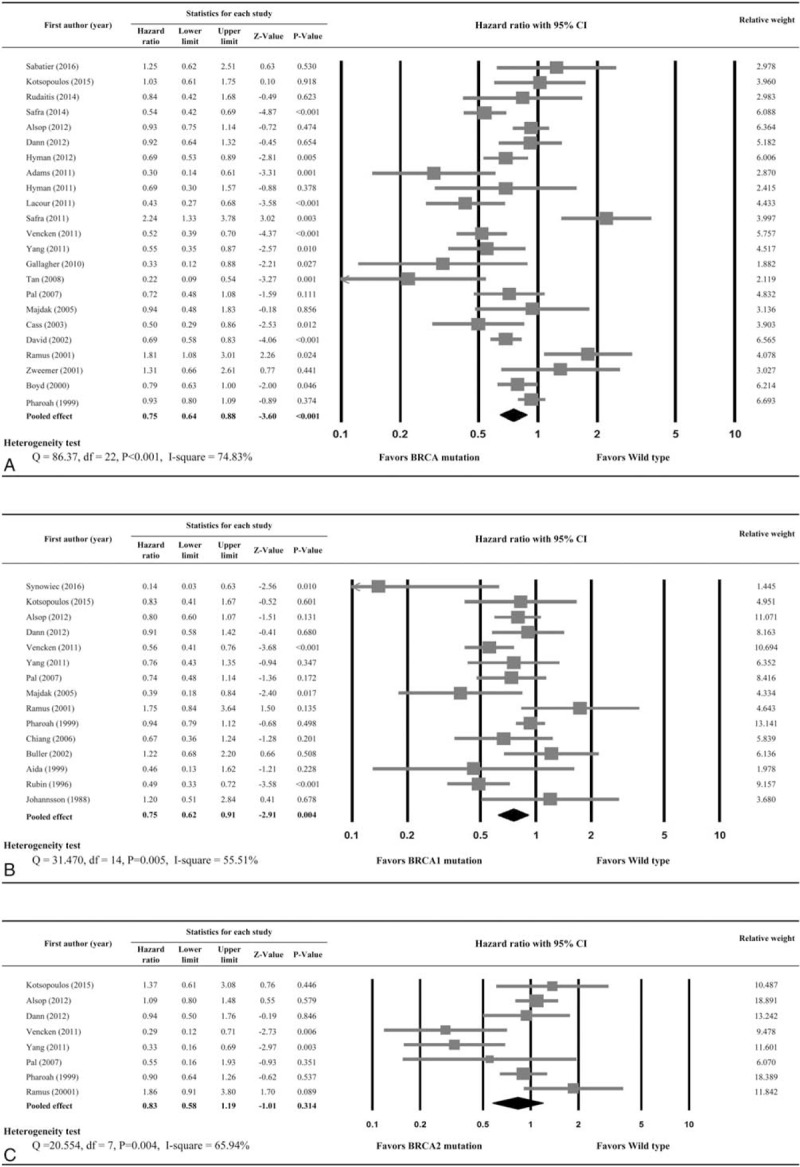
Forest plots of overall survival by (A) BRCA1/2 mutations, (B) BRCA1 mutations only, and (C) BRCA2 mutations only. Hazard ratios represent comparison of patients with BRCA mutations and wild-type gene.
Table 1 (Continued).
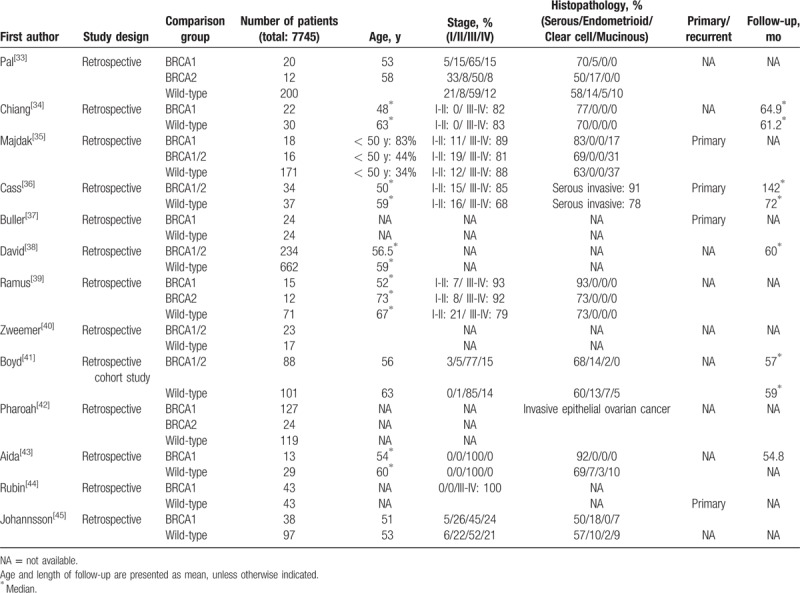
Fourteen studies provided data with respect to PFS. A random-effects model was used because significant heterogeneity was noted (I2 = 80.85%, Q statistic = 67.892, P < .001). The presence of BRCA1/2 mutations was associated with improved PFS (HR = 0.80; 95% CI: 0.64, 0.99; P = .039) (Fig. 3A).
Figure 3.
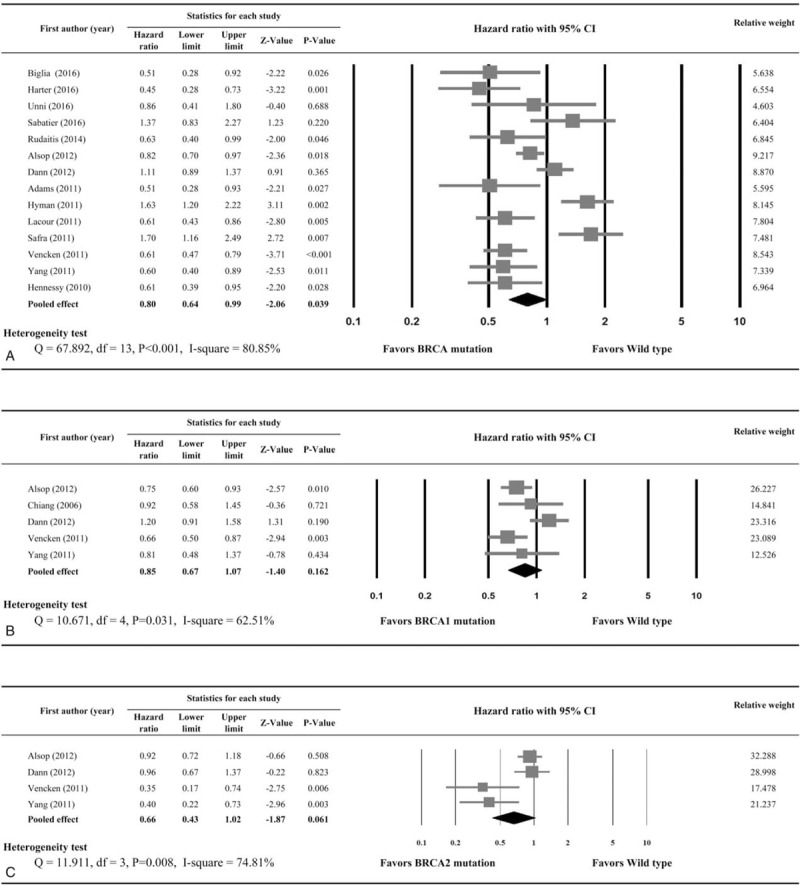
Forest plots of progression-free survival by (A) BRCA1/2 mutations, (B) BRCA1 mutations only, and (C) BRCA2 mutations only. Hazard ratios represent comparison of patients with BRCA mutations and wild-type gene.
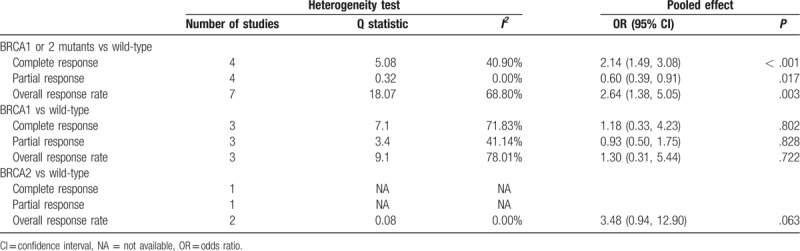
Results of the meta-analysis of treatment responses between patients with and without BRCA1 and BRCA2 mutations are shown in Table 3. Seven studies provided ORR data, and were included in the meta-analysis. A random-effects model of analysis was used because heterogeneity was present (I2 = 68.80%, Q statistic = 18.07). Ovarian cancer patients with BRCA1/2 mutations were more sensitive to treatment compared with wild-type patients (OR = 2.64; 95% CI: 1.38, 5.05; P = .003). Compared with patients with sporadic disease, BRCA-positive patients had a higher CR rate (OR = 2.14; 95% CI: 1.49, 3.08; P < .001), but lower PR rate (OR = 0.60; 95% CI: 0.39, 0.91; P = .017). The presence of BCRA1 or BCRA2 mutations (vs wild-type) did not affect the CR, PR, or ORR; however, only 3 or fewer studies provided data available for the analysis (Table 3). A summary of the treatments and the response rates reported in all of the studies included in the meta-analysis is shown in Supplemental Table 1.
Table 2.
Subgroup analysis of primary, recurrent, and advanced-stage disease.
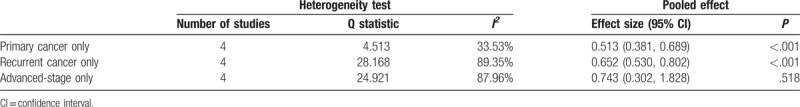
Table 3.
Meta-analysis for treatment response.
3.3. Meta-analysis of BRCA 1 or BRCA 2 mutations alone
BRCA1 mutation remained significantly associated with improved OS (n = 15 studies; HR = 0.75; 95% CI: 0.62, 0.91; P = .004) (Fig. 2B), but BRCA2 mutation alone was not associated with increased OS (n = 8 studies; HR = 0.83; 95% CI: 0.58, 1.19; P = .314) (Fig. 2C). BRCA1 mutation alone was not significantly associated with improved PFS (n = 5 studies; HR = 0.85; 95% CI: 0.67, 1.07; P = .162) (Fig. 3B), nor was BRCA2 mutation alone significantly associated with improved PFS (n = 4 studies; HR = 0.66; 95% CI: 0.43, 1.02; P = .061) (Fig. 3C).
Only 3 studies provided ORR data for BRCA1 mutations alone, and 2 studies for BRCA2 mutations alone. The analysis revealed that BRCA1 mutations alone and BRCA2 mutations alone were not associated with ORR (BRCA1 mutations alone: OR = 1.30, 95% CI: 0.31, 5.44, P = .722; BRCA2 mutations alone: OR = 3.48, 95% CI: 0.49, 12.90, P = .063) (Table 3).
3.4. Publication bias and quality assessment
Results of the analysis of publication bias are shown in Supplemental Figure 1. The funnel plots of OS and PFS had a symmetrical distribution, and Egger's test indicated no evidence of publication bias (P = .312 for OS, and P = .225 for PFS).
As shown in Supplemental Figure 2, all included studies had low risk of bias in study participation, study attrition, and analysis. However, 7 studies had high risk, and 1 study had unclear risk of bias in prognostic factor measurement, and the major limitation is in outcome measurement. Overall, the included studies had low risk of bias in study participation, study attrition and analysis, but relatively high risk of bias in prognostic factor and outcome measurement.
Upon review of the individual study results, it was apparent that 3 studies had a strong signal and low OR, and potentially may have had the following biases which may have overly influenced the results: Rubin (1996): outcome measure and confounding measure and account; Venchen (2001): prognostic factor measurement; and Majdak (2005): outcome measure. For this reason, we performed the meta-analysis with the exclusion of these 3 studies, and the results remained the same as indicated in Figure 2. The overall analysis excluding the 3 studies revealed BRCA1/2 mutations and BRCA1 mutations were associated with improved OS (HR = 0.76; 95% CI: 0.64, 0.90; P = .001 and HR = 0.88; 95% CI: 0.78, 1.00; P = .045, respectively), and BRCA2 mutations alone was not associated with OS (Supplemental Figure 3).
4. Discussion
The purpose of this study was to perform an updated meta-analysis examining the influence of BRCA1/2 mutations on OS and PFS in patients with ovarian cancer. The overall results indicated that the presence of BRCA1/2 mutations were associated with improved OS and PFS, but the improved OS was only seen in patients with primary and recurrent disease, not in those with advanced stage disease. BRCA1/2 mutations were associated with a better ORR, though BRCA-positive patients had a higher CR rate but lower PR rate than did patients with sporadic disease. When examined separately, BRCA1 mutations remained significantly associated with improved OS but not PFS, and BRCA2 mutations alone were not associated with either improved OS or PFS. In addition, neither BRCA1 nor BRCA2 mutations alone were associated with ORR, though these findings were limited by a very small number of studies.
BRCA1/2 function as tumor suppressor genes, and their proteins play an important role in repairing damaged DNA through homologous recombination.[16] The majority of BRCA1/2-associated carcinomas have deletions in the genes, resulting in deficiency of the gene protein.[16] Deficiency of the protein results in a carcinoma with a diminished capacity to repair DNA, which is the mechanism by which the mutations lead to an increased susceptibility to cancer.[49] Protein deficiency also results in sensitivity to platinum-based chemotherapy agents, presumably as a result of an inability to repair double-strand DNA breaks caused by chemotherapy,[50,51] and poly (ADP-ribose) polymerase (PARP) inhibitors that inhibit DNA repair mechanisms.[52–54]
Studies have generally shown that BRCA1/2 mutations are associated with an improved response to platinum-based chemotherapy.[9,12,32,33,36,41] However, how an improved response translates into survival benefits is unclear. Kotsopoulos et al[15] studied 1421 patients with epithelial ovarian cancer of whom 109 had BRCA1 mutations and 68 had BRCA2 mutations, and found that although mutation carriers exhibited an initial survival advantage, the presence of a mutation was not associated with survival status at 10 years. The study also reported that the strongest predictor of 10-year survival was no residual disease at resection.
The current analysis found that when examined separately BRCA1 mutations remained significantly associated with improved OS but not PFS, and BRCA2 mutations alone were not associated with either improved OS or PFS. A few studies have examined differences between BRCA genotypes. Liu et al[13] compared event-free survival (EFS) and OS between BRCA1 and BRCA2 patients, and found no difference in the 2 measures between the 2 genotypes, though there was a nonsignificant trend towards improved OS in BRCA2 patients with advanced-stage disease. A study by Yang et al[23] reported that an OS advantages was only seen in patients with BRCA2 mutations, and not those with BRCA1 mutations. Similarly, a study by Sun et al[55] suggested that the HR for OS for patients with BRCA2 mutations was lower than that for BRCA1 mutations. This may be because BRCA2 mutations result in more significant homologous recombination defects than BRCA1 mutations.[56]
Other meta-analyses have examined the influence of BRCA1/2 mutations on survival of patients with ovarian cancer. In 2015 Zhong et al[57] studied patients with ovarian and breast cancer and identified 14 studies examining ovarian cancer and 13 examining breast cancer. The analysis showed that both BRCA1 and BRCA1 mutations were associated with better OS and PFS regardless of tumor stage, grade, or histological subtype. With respect to breast cancer, BRCA1 mutation carriers had worse OS but similar PFS as compared with noncarriers, and BRCA2 was not associated with breast cancer prognosis. A recent meta-analysis by Xu et al[58] found that BRCA1 and BRCA2 mutations were associated with improved OS and PFS; however, that analysis did perform subgroup analysis based on disease stage or examine treatment response. Another meta-analysis of 34 evaluable studies showed that BRCA mutations was a favorable prognostic factor for OS (HR = 0.69, 95% CI: 0.61, 0.79, P < .001), and analysis of 18 evaluable studies showed that mutations were associated with longer PFS (HR = 0.69, 95% CI: 0.63, 0.76, P = .118).[55] When the studies were categorized into BRCA1/2 mutation and low protein/mRNA expression, both categories were found to be favorable prognostic factors, whereas BRCA promotor methylation was associated with a poorer prognosis (HR = 1.59, 95% CI: 0.72, 3.50, P = .077). There are some differences between our study and the aforementioned analysis by Sun et al[55] in that the prior study examined the role of BRCA status on prognosis of patients with epithelial ovarian cancer, and the BRCA status included BRCA mutation, BRCA methylation, BRCA1 promotor methylation, BRCA1 mRNA level, and BRCA1 protein expression by immunohistochemistry. The objective of the current was to only examine the role of BRCA mutation status with respect to the prognosis of patients with ovarian cancer. Differences in the study results may be because of the purposes of the studies, the study inclusion criteria, and/or the outcome measures examined.
There are a number of limitations to the current analysis. Not all of the included studies examined both BRCA1 and BRCA2, nor did they all examine OS and PFS. Although most of the included studies had a follow-up time > 36 months, many did not report follow-up length, and one study reported a follow-up of only 7 months.[27] Importantly, there was marked heterogeneity between studies with respect to treatment, disease stage, and histopathological cancer type. Levels of BRCA expression, promotor methylation, epigenetic alterations, histopathological type, and other risk factors may all affect response to therapy, and these were not taken into consideration in the analysis. The subgroup analyses of BRCA1 and 2 for OS and PFS contained a small number of studies, as did the analysis of ORR, and whereas most chemotherapies were platinum-based and some were not. The number of studies that analyzed BRCA1 alone and BRCA2 alone was markedly difference, and the reason may be that in ovarian cancer patients the BRCA1 mutation occurs at a higher frequency that the BRCA2 mutation.[8] The finding that BRCA1/2 was associated with ovarian cancer prognosis, whereas BRCA1 alone or BRCA2 alone was generally not is likely also because of the small numbers of studies that examined the 2 mutations individually (e.g., for analysis of OS, there were 15 articles included in BRCA1 subgroup, and 8 articles in BRCA2 subgroup; however, for analysis of PFS there were only 5 articles in BRCA1 subgroup and 4 articles in BRCA2 subgroup). It would have been valuable is other subgroup analyses could have been performed (e.g., retrospective vs prospective studies, promotor status, or geographical region); however, only 1 prospective study was included in the analysis, and data for other subgroup analyses were limited.
It should also be mentioned that many studies used in the analysis were published in 2011, and this may raise the concern of duplicate data; however, review of the articles excluded the possibility of duplicate patients. For example, Candido-dos-Reis et al[59] extracted unpublished data from 2 case-controlled study datasets, and extended survival-time data for 4314 patients from previously reported studies were used for comparison which might duplicate data from other studies and for this reason the study was not included in the analysis.
In conclusion, BRCA1/2 mutations were associated with improved OS and PFS, but the improved OS was only seen in patients with primary and recurrent disease, not in those with advanced state disease. BRCA1/2 mutations were associated with a better ORR, though BRCA-positive patients had a higher CR rate, but lower PR rate than did patients with sporadic disease. When examined separately, BRCA1 mutations remained significantly associated with improved OS, but not PFS, and BRCA2 mutations alone were not associated with either improved OS or PFS. In addition, neither BRCA1 nor BRCA2 mutations alone were associated with ORR, though these findings were limited by a small number of studies. Further elucidation of mutation characteristics and their effect on survival and response to therapy may lead to a more individualized approach to the treatment of ovarian cancer and improved outcomes.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CR = complete response, EFS = event-free survival, HR = hazard ratio, OR = odds ratio, ORR = overall response rate, OS = overall survival, PARP = poly (ADP-ribose) polymerase, PFS = progression-free survival, PR = partial response, QUIPS = Quality in Prognostic Studies.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol 2009;27:1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [3].Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621–7. [DOI] [PubMed] [Google Scholar]
- [4].Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194–200. [DOI] [PubMed] [Google Scholar]
- [5].Brose MS, Rebbeck TR, Calzone KA, et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 2002;94:1365–72. [DOI] [PubMed] [Google Scholar]
- [6].Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health 2014;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012;30:2654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vencken PM, Kriege M, Hoogwerf D, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol 2011;22:1346–52. [DOI] [PubMed] [Google Scholar]
- [10].Rudaitis V, Zvirblis T, Kanopiene D, et al. BRCA1/2 mutation status is an independent factor of improved survival for advanced (stage III-IV) ovarian cancer. Int J Gynecol Cancer 2014;24:1395–400. [DOI] [PubMed] [Google Scholar]
- [11].Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol 2011;22:1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chetrit A, Hirsh-Yechezkel G, Ben-David Y, et al. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol 2008;26:20–5. [DOI] [PubMed] [Google Scholar]
- [13].Liu J, Cristea MC, Frankel P, et al. Clinical characteristics and outcomes of BRCA-associated ovarian cancer: genotype and survival. Cancer Genet 2012;205:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Artioli G, Borgato L, Cappetta A, et al. Overall survival in BRCA-associated ovarian cancer: case-control study of an Italian series. Eur J Gynaecol Oncol 2010;31:658–61. [PubMed] [Google Scholar]
- [15].Kotsopoulos J, Rosen B, Fan I, et al. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol 2016;140:42–7. [DOI] [PubMed] [Google Scholar]
- [16].Muggia F, Safra T. ’BRCAness’ and its implications for platinum action in gynecologic cancer. Anticancer Res 2014;34:551–6. [PMC free article] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [18].Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- [19].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [20].Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337–51. [DOI] [PubMed] [Google Scholar]
- [21].Harter P, Johnson T, Berton-Rigaud D, et al. BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol Oncol 2016;140:443–9. [DOI] [PubMed] [Google Scholar]
- [22].Unni SK, Schauerhamer MB, Deka R, et al. BRCA testing, treatment patterns and survival in platinum-sensitive recurrent ovarian cancer - an observational cohort study. J Ovarian Res 2016;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Safra T, Rogowski O, Muggia FM. The effect of germ-line BRCA mutations on response to chemotherapy and outcome of recurrent ovarian cancer. Int J Gynecol Cancer 2014;24:488–95. [DOI] [PubMed] [Google Scholar]
- [24].Dann RB, DeLoia JA, Timms KM, et al. BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol 2012;125:677–82. [DOI] [PubMed] [Google Scholar]
- [25].Hyman DM, Long KC, Tanner EJ, et al. Outcomes of primary surgical cytoreduction in patients with BRCA-associated high-grade serous ovarian carcinoma. Gynecol Oncol 2012;126:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Adams SF, Marsh EB, Elmasri W, et al. A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol 2011;123:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hyman DM, Zhou Q, Arnold AG, et al. Topotecan in patients with BRCA-associated and sporadic platinum-resistant ovarian, fallopian tube, and primary peritoneal cancers. Gynecol Oncol 2011;123:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lacour RA, Westin SN, Meyer LA, et al. Improved survival in non-Ashkenazi Jewish ovarian cancer patients with BRCA1 and BRCA2 gene mutations. Gynecol Oncol 2011;121:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther 2011;10:2000–7. [DOI] [PubMed] [Google Scholar]
- [30].Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011;306:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 2010;28:3570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tan DS, Rothermundt C, Thomas K, et al. BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J CLin Oncol 2008;26:5530–6. [DOI] [PubMed] [Google Scholar]
- [33].Pal T, Permuth-Wey J, Kapoor R, et al. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer 2007;6:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chiang JW, Karlan BY, Cass L, et al. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol 2006;101:403–10. [DOI] [PubMed] [Google Scholar]
- [35].Majdak EJ, Debniak J, Milczek T, et al. Prognostic impact of BRCA1 pathogenic and BRCA1/BRCA2 unclassified variant mutations in patients with ovarian carcinoma. Cancer 2005;104:1004–12. [DOI] [PubMed] [Google Scholar]
- [36].Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 2003;97:2187–95. [DOI] [PubMed] [Google Scholar]
- [37].Buller RE, Shahin MS, Geisler JP, et al. Failure of BRCA1 dysfunction to alter ovarian cancer survival. Clin Cancer Res 2002;8:1196–202. [PubMed] [Google Scholar]
- [38].Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol 2002;20:463–6. [DOI] [PubMed] [Google Scholar]
- [39].Ramus SJ, Fishman A, Pharoah PD, et al. Ovarian cancer survival in Ashkenazi Jewish patients with BRCA1 and BRCA2 mutations. Eur J Surg Oncol 2001;27:278–81. [DOI] [PubMed] [Google Scholar]
- [40].Zweemer RP, Verheijen RH, Coebergh JW, et al. Survival analysis in familial ovarian cancer, a case control study. Eur J Obstet Gynecol Reprod Biol 2001;98:219–23. [DOI] [PubMed] [Google Scholar]
- [41].Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA 2000;283:2260–5. [DOI] [PubMed] [Google Scholar]
- [42].Pharoah PD, Easton DF, Stockton DL, et al. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. Cancer Res 1999;59:868–71. [PubMed] [Google Scholar]
- [43].Aida H, Takakuwa K, Nagata H, et al. Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res 1998;4:235–40. [PubMed] [Google Scholar]
- [44].Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med 1996;335:1413–6. [DOI] [PubMed] [Google Scholar]
- [45].Jóhannsson OT, Ranstam J, Borg A, et al. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol 1998;16:397–404. [DOI] [PubMed] [Google Scholar]
- [46].Biglia N, Sgandurra P, Bounous VE, et al. Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: analysis of prognostic factors and survival. Ecancermedicalscience 2016;10:639.doi: 10.3332/ecancer.2016.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sabatier R, Lavit E, Moretta J, et al. Ovarian cancer patients at high risk of BRCA mutation: the constitutional genetic characterization does not change prognosis. Fam Cancer 2016;15:497–506. [DOI] [PubMed] [Google Scholar]
- [48].Synowiec A, Wcisło G, Bodnar L, et al. Clinical features and outcomes of germline mutation BRCA1-linked versus sporadic ovarian cancer patients. Hered Cancer Clin Pract 2016;14:1.doi: 10.1186/s13053-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol 2009;4:461–87. [DOI] [PubMed] [Google Scholar]
- [50].Foulkes WD. BRCA1 and BRCA2: chemosensitivity, treatment outcomes, and prognosis. Fam Cancer 2006;5:135–42. [DOI] [PubMed] [Google Scholar]
- [51].Bhattacharyya A, Ear US, Koller BH, et al. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem 2000;275:23899–03. [DOI] [PubMed] [Google Scholar]
- [52].Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245–51. [DOI] [PubMed] [Google Scholar]
- [53].Gien LT, Mackay HJ. The emerging role of PARP inhibitors in the treatment of epithelial ovarian cancer. J Oncol 2010;2010:151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015;137:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One 2014;9:e95285.doi: 10.1371/journal.pone.0095285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Davies AA, Masson JY, McIlwraith MJ, et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 2001;7:273–82. [DOI] [PubMed] [Google Scholar]
- [57].Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 2015;21:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xu K, Yang S, Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget 2017;8:285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Candido-dos-Reis FJ, Song H, Goode EL, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res 2015;21:652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


