Supplemental Digital Content is available in the text
Keywords: adherence, antiretroviral therapies, inverse probability of treatment weighting, marginal structural model, virologic outcome
Abstract
Many studies have estimated the association between the adherence to antiretroviral therapies and human immunodeficiency virus (HIV) patients’ virologic/immunologic outcomes. However, evidence is lacking on the causal effect of adherence on the outcomes. The goal of this study is to understand whether near perfect adherence is necessary to achieve optimal virologic outcome and also to investigate the effect of initial adherence to antiretroviral therapies on initial viral suppression by different regimens. A cohort study was conducted on HIV veterans initiating antiretroviral therapies in 1999 to 2015. The primary outcome was the first viral suppression occurred within 30 to 60 days since the index date. Multiple imputation was used to impute the missing value of virologic outcomes. The inverse probability of treatment weighting (IPTW) method was applied to estimate the viral suppression rate at each specific adherence category for each regimen category. Marginal structural models with IPTW were used to estimate the risk of viral suppression in lower-adherence categories in comparison to near-perfect adherence level ≥95%. Data showed that lower adherence caused lower viral suppression rate, with the association differentiated by the regimen. Patients on integrase strand transfer had the highest viral suppression rate, with patients on protease inhibitors having the lowest rate. Regardless of regimens, the viral suppression rate among patients at initial adherence of 75 to <95% was not statistically different from patients at adherence of ≥95%; however, the differences might be clinically significant.
1. Introduction
The guidelines recommend physicians delay initiating antiretroviral therapies (ARTs) among patients who would potentially have poor adherence, because suboptimal adherence is associated with a lot of problems, such as virologic failure, drug-resistance, lowered immunity, and increased morbidity and mortality.[1] However, there is controversy on whether near-perfect adherence (adherence ratio ≥95%) is necessary. Many studies found that the response of the human immunodeficiency virus (HIV) to ARTs appeared to be linear rather than having a threshold.[2–20] Some studies found patients at medium adherence level could still achieve viral suppression without developing drug resistance.[21–23]
However, these studies have limitations. They evaluated association between adherence and outcomes, not the causal effect of adherence on outcome. They also simply used a cumulative measure for adherence and an end point measure for viral load and T cell CD4 count without addressing time-dependent confounder bias. In addition, no study investigated the effect of early adherence on initial viral suppression, and it remained unclear on the association between early adherence and long-term adherent behavior.
Since little is known about whether near perfect adherence is necessary for patients to achieve optimal virologic outcome or if the association differs for different regimens, the goal of this study was to investigate the effect of initial adherence to ARTs on initial viral suppression by different regimens.
2. Methods
We used the Veterans Health Administration (VHA) data between January 1, 1999, and December 31, 2015 as the data source. A total of 10,274 patients with incident HIV infection were identified from the VHA databases, who initiated with unboosted protease inhibitor (PI), boosted PI, non-nucleoside reverse transcriptase inhibitor (NNRTI), or integrase strand transfer (INSTI)-based regimens. The University of Utah Institutional Review Board and the Salt Lake City VA Health Care System Office of Research and Development approved this study.
2.1. Initial adherence
Each patient was followed up to 60 days starting from the first fill date of base agent. Since a complete regimen should include 1 base agent plus at least 2 other nucleoside reverse transcriptase inhibitors (NRTIs), initial adherence was calculated for a complete regimen. The initial adherence was measured as a coverage ratio [called as initial coverage ratio of complete regimen (ICRCR)], with the formula listed as follow:
 |
If a patient did not have a second fill of base agent within 60 days since the first fill, then we assumed the patient discontinued the treatment, because all incident patients had 30 days of supply for the first fill of base agent. Base agent was an unboosted PI, boosted PI, NNRTI, or INSTI. The ICRCR was classified into 3 groups: high adherence of ≥95%, medium adherence of 75–<95%, and low adherence of <75%.
2.2. Outcome
The outcome was first viral suppression occurred within 30 to 60 days after the first fill of base agent. The HIV treatment guidelines did not provide a specific definition for viral suppression. But the guidelines recommended to target treatment goal of achieving undetectable level of viral load, defined as <400 copies/mL in the guidelines of 1999 and <50 copies/mL since 2000.[24] It was also common to use ≥200/mL copies to define viral failure.[24] In this study, viral suppression was defined as HIV-1 viral load <400 copies/mL if test year was 1999 and HIV-1 viral load <50 copies/mL if test year was 2000 or after.
2.3. Confounders and covariates
Antiretroviral regimens with their relevant characteristics, including efficacy, side effects, and barriers to drug resistance, were associated with initial adherence and were also risk factors related to virologic outcomes. However, they were not on the causal pathway between adherence and outcome. This indicated that regimens and their characteristics were important confounders, which made causality of initial adherence on virologic outcome complex. Therefore, this study was based on the subgroup of each specific regimen category, which negated the need to consider characteristics as a confounder.
As shown in Figure 1, confounders also included patient demographics (age, sex, and race/ethnicity), baseline HIV disease severity [baseline viral load, baseline CD4 count, and opportunistic infection, and acquired immunodeficiency syndrome (AIDS) defined], baseline overall health status [Deyo-adapted Charlson Comorbidity Index (CCI)[25]], and HIV health care utilizations [length of stay (LOS), viral load test frequency, CD4 count test frequency, number of HIV office visits] during exposure period.
Figure 1.
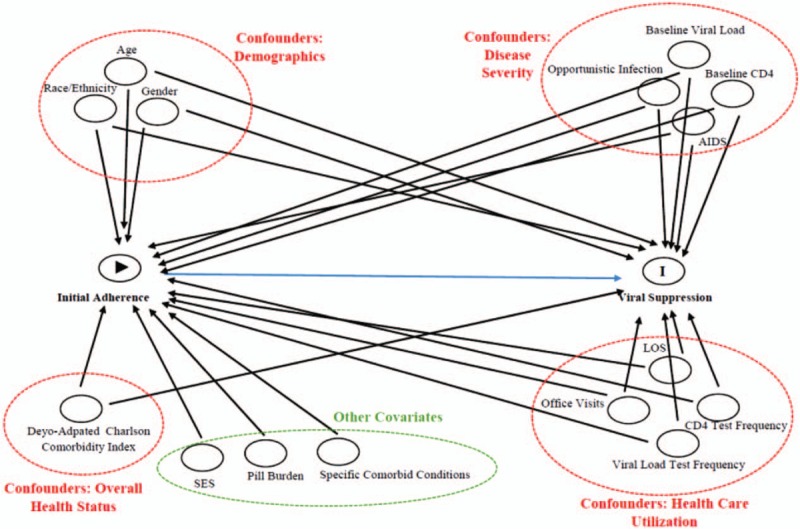
Directed acyclic graph for initial adherence and viral suppression. AIDS = acquired immunodeficiency syndrome, LOS = length of stay.
2.4. Imputation for missing data
Data were imputed for patients who had missing virologic outcomes via multiple imputation methods to impute log value with base 10 of absolute viral load. In order to maximize the accuracy for imputing outcome, imputation was completed for each specific initiated regimen category by comparing two different imputation methods including monotone regression and Markov chain Monte Carlo method.[26] The imputed outcome distribution derived from the 2 methods was compared with outcome distribution from the complete cases to identify the imputed data from 1 method which were more similar to the distribution of complete cases. The imputation model inputs were all variables that occurred before the outcome, including initiated pill burden, ICRCR, age, sex, race/ethnicity, socioeconomic status, baseline viral load, baseline CD4 count, Deyo-adapted CCI, AIDS, opportunistic infection, specific comorbid conditions, discontinuation indicator, switch indicator, time to switch, viral load test frequency, CD4 test frequency, LOS, HIV office visit frequency, death within 60 days after index date, and index year.
2.5. Inverse probability of treatment weighting
Inverse probability of treatment weighting (IPTW) method was used to address confounding bias in this study. The IPTW for each individual patient was calculated based on the following formula.[27–29] Both numerator and denominator were obtained by regimen-specific multinomial logistic regression models for predicting adherence.
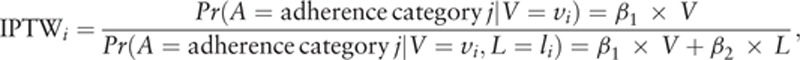 |
where i represents subject i; j is coverage ratio category: j = 1, 2, 3 with 1 = “≥95%,” 2 = “75–<95%,” 3 = “<75%,” and we use 1 as the reference group; A is initial coverage ratio category; a is observed initial coverage ratio; L is confounders; V is patient baseline characteristics except for confounders; and β is the coefficient estimate.
2.6. Marginal structural model
Viral suppression rate was calculated for each adherence group based on pseudo-population after weighting IPTW, and marginal structural models (MSMs) were calculated to estimate adherence effects on virologic outcomes. The steps were as follows: first, for each initiated regimen category, confounders between adherence groups were compared before and after applying IPTW via using absolute standardized difference estimate (0.1 as reference value). Second, for each initiated regimen category, viral suppression rate was calculated with 95% confidence interval for each adherence group after weighting IPTW. Third, for each initiated regimen category, adherence effect on virologic outcomes was estimated via MSMs models.[27–29]
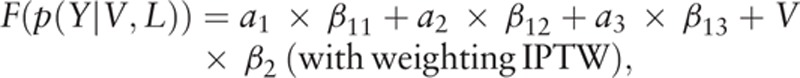 |
where Y is viral suppression outcome, V is baseline covariates, L is confounders, where a1 = 1 if ICRCR ≥95% and 0 otherwise, a2 = 1 if ICRCR 75% to <95% and 0 otherwise, a3 = 1 if ICRCR <75% and 0 otherwise, F is the function (logistic regression to estimate odds ratio in this study), and β is the coefficient estimate.
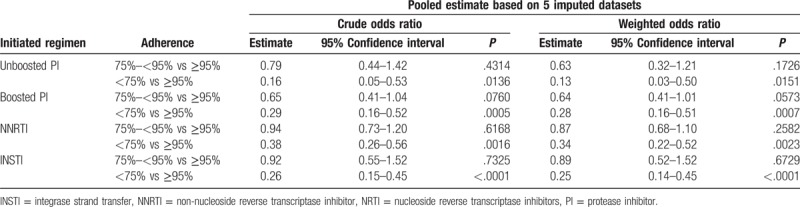
For each regimen, we calculated the crude odds ratios (ORs) of categorical ICRCR on viral suppression using univariate logistic regression, and the weighted ORs using marginal structured model.
For the statistical analyses, we set alpha level of 0.05 to define significance. All analyses were conducted in SAS version 9.2.
3. Results
3.1. Patient characteristics
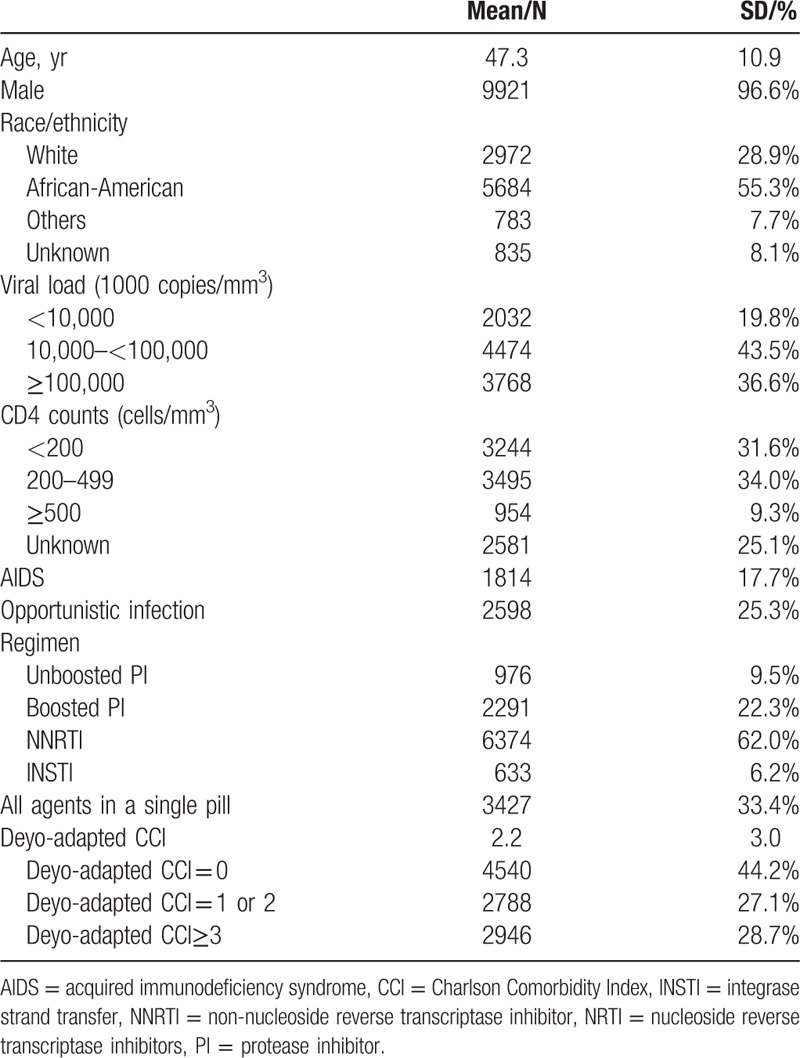
The cohort was relatively young with a mean age of 47.3 years old; the majority were younger than 65 years old at baseline. More than half were African-Americans, and approximately 29% were whites. There were 976 (9.5%), 2291 (22.3%), 6374 (62.0%), and 633 (6.2%) patients initiated on unboosted PIs, boosted PIs, NNRTIs, and INSTIs, respectively. Patient characteristics are shown in Table 1.
Table 1.
Patient baseline characteristics among human immunodeficiency virus antiretroviral-naïve veterans.
3.2. Missing outcome
There were 5955 (58.0%) patients who did not have records for virologic outcomes within 30 to 60 days of the index. We compared them to patients who did have virologic outcomes. We find that patients with missing outcomes were those who were younger, African-American, at lower baseline viral load and higher baseline CD4 counts, treated on PIs, healthier, and at lower adherence level.
In order to avoid selection bias, both patients with and without outcomes in the study were included. The outcome for patients who had missing value was imputed. The data distributions for viral load in log10 were also compared before and after imputation for each specific regimen category as shown in the Appendix I. The outcome distribution before and after imputation are very similar for each specific regimen category.
3.3. Absolute standardized differences
The absolute standardized differences for each confounder before and after weighting data by comparing patients at adherence 75% to <95% vs ≥95% and <75% vs ≥95% are shown in Appendix II. The confounders become balanced after IPTW weighting, except for both comparisons for INSTIs and adherence <75% vs ≥95% comparison for unboosted PIs.
3.4. Risk of viral suppression
In the MSM models, adherence had the biggest effect on viral suppression among patients on PI-based regimens. The results are shown in Table 2. Regardless of regimen, adherence at 75% to <95% did not have a statistical significant effect on viral suppression rate compared to adherence at ≥95%; however, these differences might still be clinically significant. For example, among pseudo-population initiated with unboosted PIs, patients with initial coverage ratio of ≥95% were 1.6 times (calculated as 1/0.63 = 1.6) more likely to achieve viral suppression in 30 to 60 days than those with coverage ratio of 75% to <95%; patients with initial coverage ratio of ≥95% were 7.7 times (calculated as 1/0.13 = 7.7) more likely to achieve viral suppression in 30 to 60 days than those with coverage ratio of <75%. In comparison, among pseudo-population initiated with INSTIs, patients with initial coverage ratio of ≥95% were 1.1 times (calculated as 1/0.89 = 1.1) more likely to achieve viral suppression in 30 to 60 days than those with coverage ratio of 75% to <95%; those with initial coverage ratio of ≥95% were 4 times (calculated as 1/0.25 = 4) more likely to achieve viral suppression in 30 to 60 days than those with coverage ratio of <75%.
Table 2.
Multilevel adherence effect estimates on viral suppression based on imputed data.
4. Discussion
The present study does not support the 95% threshold to be as important as suggested in the guidelines, because viral suppression rate among patients with adherence level of ≥75% was very similar to the rate among those with adherence level of ≥95%. In addition, although patients with medium adherence (75%–<95%) did not have a significantly reduced rate compared to patients with high adherence (≥95%), the differences between them were still clinical significant. Therefore, keeping patients’ adherence level as high as possible is important to maximize the possibility of achieving viral suppression.
Medium adherence was found to have the biggest effect on viral suppression rates among patients on unboosted PIs, followed next by boosted PIs. However, for patients on NNRTIs and INSTIs, there was an almost obvious difference in viral suppression rates between patients at medium adherence and at high adherence. These findings suggest adherence affected viral suppression rate variously by regimen class. For PI-based regimens, medium-level adherence would have significant effects on viral suppression; but for NNRTI- or INSTI-based regimens, adherence might not significantly influence viral suppression rate, until adherence is reduced to the lowest level. These findings are similar to what was reported in the literature that the 95% threshold might not be necessary and adherence works differently on viral suppression rate for various regimens.[12,14,15,30–33] However, all of the current findings suggest a 95% threshold for adherence is not necessary.
In the MSM models, patients at medium adherence had a significantly reduced rate of viral suppression for PI-based regimens, but not for NNRTI- or INSTI-based regimens. Across all regimens, low adherence was more consistently associated with a reduced viral suppression rate than high adherence.
Interestingly, patients on INSTIs had the highest viral suppression rate no matter what adherence level patients were at, followed by the patients on NNRTIs, and then those on PIs. For example, patients on INSTIs with adherence <75% still had a viral suppression rate of 20.7%, which was same as the rate for patients on NNRTIs with adherence ≥95% and higher than rate for the patients on PIs with adherence ≥95%. However, this may not indicate that INSTIs would be more potent than PIs and NNRTIs. It is because the patients initiated with different regimens had different characteristics, which made the adherence effects not comparable across the regimen categories. Noticeably, there was a limited sample size of patients on INSTI in this study. The patients at different adherence levels had significantly different characteristics even after the weighting. Patients at low adherence were more likely to be healthier than those at higher adherence. Therefore, the effect of adherence to INSTIs on viral suppression could be partially explained by the healthy user effect.
Our study applied an IPTW approach, a causal inference method to address confounding bias and to identify the causal effects of initial adherence to different HIV regimens on virologic outcomes among veterans with HIV-1. The present was a comprehensive study that investigated the various first-line regimens, including unboosted/boosted PI-, NNRTI-, and INSTI-based regimens. Different from previous studies, adherence effects were estimated via traditional relative risk estimate (i.e., OR), figures (Fig. 2) were also created to display viral suppression rates based on a pseudo-population after balancing confounders between comparison groups.
Figure 2.
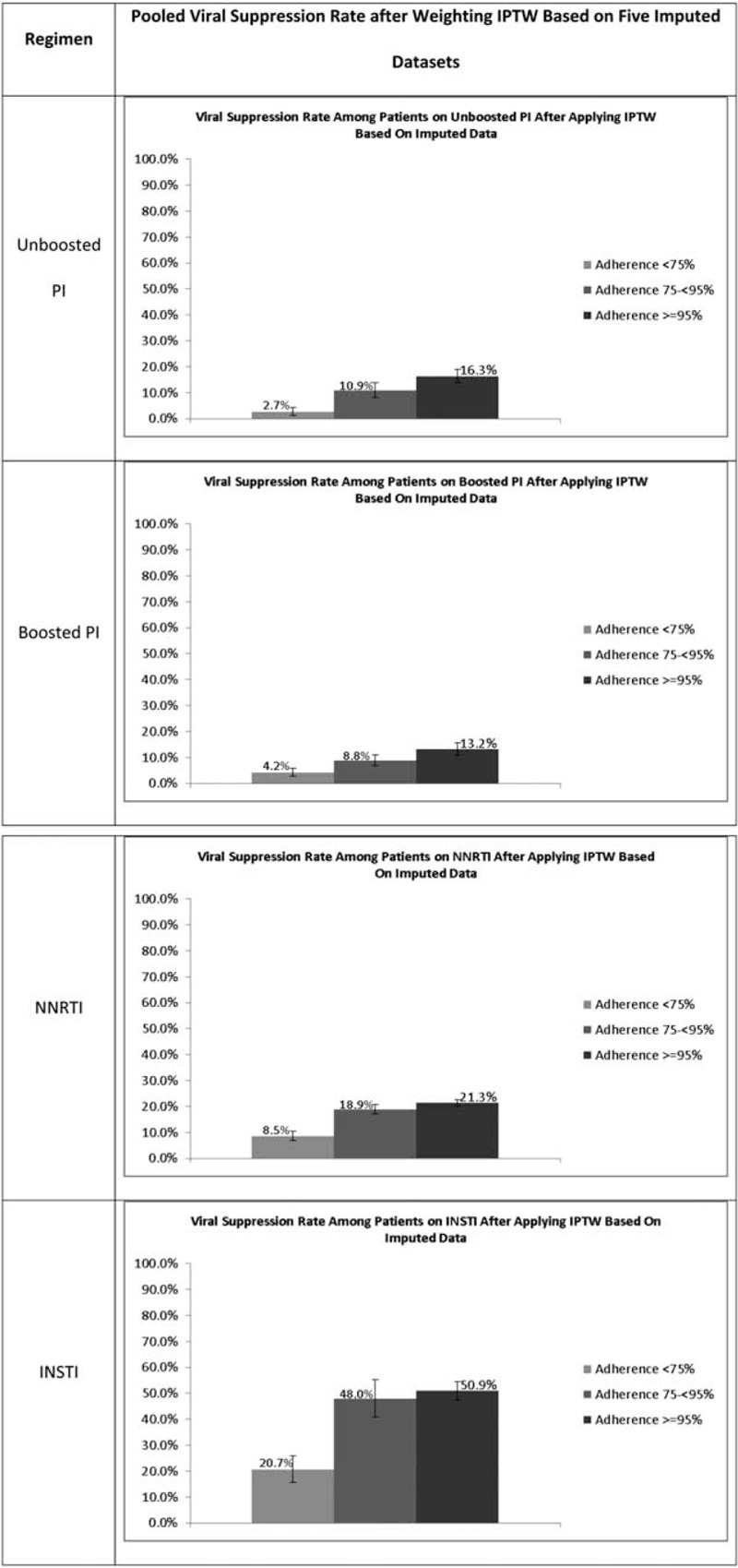
Viral suppression rate after applying IPTW based on imputed data. INSTI = integrase strand transfer, IPTW = inverse probability of treatment weighting, NNRTI = non-nucleoside reverse transcriptase inhibitor, PI = protease inhibitor.
This study did not simply exclude patients who had missing outcome similar to what previous studies have done, but applied imputation techniques to impute the outcome. The purpose of doing this was to avoid selection bias, which was especially true if patients who had missing outcomes were more likely to be those who had poor adherence. This has been confirmed when patient characteristics were compared between patients with or without missing outcomes.
However, there are still many questions need to be answered in future studies. It remains unknown if missing a scheduled HIV office visits potentially influences patient virologic outcomes. Further studies should investigate why patients on each specific regimen category had a lower adherence, including the reason that patients had a low adherence at baseline, and why so many patients who had a high initial adherence eventually moved to lower-adherence category. Finding-based interventions should be initiated among these patients. Future studies should apply more advanced technology, such as natural language process, to identify HIV labs and their values in medical notes to add more data and improve the data accuracy in our study. The present study only explored the effect of initial adherence on viral suppression, with the consideration of avoiding time-dependent confounding bias. Futures studies should explore the long-term effect with addressing time-dependent confounding bias, or the adherence effect on the time to event, or some other outcomes of interest such as immunologic outcomes, viral rebound, hospitalization caused by HIV/AIDS, death, drug resistance, or quality of life. More advanced methods should be applied to identify the causal effect of continuous adherence or delayed filling days on either categorical or continuous outcomes (i.e., viral load or CD4 counts). The study censored patients when they switched the initiated regimen. Future studies could apply dynamic treatment regimes approach to understand how the different treatment strategy combined with different starting adherence level would influence outcomes. Future studies also need to explore the adherence effects among treatment-experienced patients.
5. Conclusion
In summary, this study showed how initial adherence differently influenced the viral suppression rate across different regimens. No evidence shows 95% adherence threshold is necessary. Patients with medium adherence (75%–<95%) can achieve viral suppression with the rate not statistically significantly different from patients with high adherence.
Acknowledgments
The study was supported by the US Veterans Health Administration for their generous provision of the databases. The authors also appreciate Dr. Marlene Egger and Mr. Xiangyang Ye for their supports in statistical methodology.
Supplementary Material
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, ARTs = antiretroviral therapies, CCI = Charlson Comorbidity Index, HIV = human immunodeficiency virus, ICRCR = initial coverage ratio of complete regimen, INSTI = integrase strand transfer, IPTW = inverse probability of treatment weighting, LOS = length of stay, MSM = marginal structural models, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, OR = odds ratio, PI = protease inhibitor, SES = socioeconomic status, VHA = Veterans Health Administration.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015. Available at: http://aidsinfo.nih.gov/guidelines). Accessed February 13, 2016. [Google Scholar]
- [2].Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis 2001;33:1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 2002;17:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aziz N, Sokoloff A, Kornak J, et al. Time to viral load suppression in antiretroviral-naive and -experienced HIV-infected pregnant women on highly active antiretroviral therapy: implications for pregnant women presenting late in gestation. BJOG 2013;120:1534–47. [DOI] [PubMed] [Google Scholar]
- [5].Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS 2006;20:223–31. [DOI] [PubMed] [Google Scholar]
- [6].Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS 2007;21:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol 2004;57:1107–10. [DOI] [PubMed] [Google Scholar]
- [8].Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS 1999;13:1099–107. [DOI] [PubMed] [Google Scholar]
- [9].Julian FS, Martin P, Erickson SR. Validation of the special projects of national significance adherence tool in HIV/AIDS patients. Ann Pharmacother 2010;44:1003–9. [DOI] [PubMed] [Google Scholar]
- [10].Lima VD, Harrigan R, Murray M, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS 2008;22:2371–80. [DOI] [PubMed] [Google Scholar]
- [11].Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials 2007;8:282–92. [DOI] [PubMed] [Google Scholar]
- [12].Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 2005;40:158–63. [DOI] [PubMed] [Google Scholar]
- [13].Marconi VC, Wu B, Hampton J, et al. Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic. AIDS Patient Care STDS 2013;27:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Messou E, Chaix ML, Gabillard D, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d’Ivoire. J Acquir Immune Defic Syndr 2011;56:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30. [DOI] [PubMed] [Google Scholar]
- [16].Wagner JH, Justice AC, Chesney M, et al. Patient- and provider-reported adherence: toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol 2001;54(suppl 1):S91–8. [DOI] [PubMed] [Google Scholar]
- [17].Gross R, Yip B, Lo Re V, 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis 2006;194:1108–14. [DOI] [PubMed] [Google Scholar]
- [18].Hong SY, Jerger L, Jonas A, et al. Medication possession ratio associated with short-term virologic response in individuals initiating antiretroviral therapy in Namibia. PLoS One 2013;8:e56307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu H, Miller LG, Hays RD, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr 2006;41:315–22. [DOI] [PubMed] [Google Scholar]
- [20].Orrell C, Cohen K, Leisegang R, et al. Comparison of six methods to estimate adherence in an ART-naive cohort in a resource-poor setting: which best predicts virological and resistance outcomes? AIDS Res Ther 2017;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenblum M, Deeks SG, Van der Laan M, et al. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009;4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sethi AK, Celentano DD, Gange SJ, et al. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003;37:1112–8. [DOI] [PubMed] [Google Scholar]
- [23].Abara WE, Adekeye OA, Xu J, et al. Adherence to combination antiretroviral treatment and clinical outcomes in a Medicaid sample of older HIV-infected adults. AIDS Care 2017;29:441–8. [DOI] [PubMed] [Google Scholar]
- [24].Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2013. Available at: http://aidsinfo.nih.gov/guidelines). Accessed February 13, 2016. [Google Scholar]
- [25].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [26].Yuan Y. Multiple imputation using SAS software. J Stat Softw 2011;45:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–70. [DOI] [PubMed] [Google Scholar]
- [28].Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–25. [DOI] [PubMed] [Google Scholar]
- [29].Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- [30].Nachega JB, Hislop M, Dowdy DW, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007;146:564–73. [DOI] [PubMed] [Google Scholar]
- [31].Parienti JJ, Barrail-Tran A, Duval X, et al. Adherence profiles and therapeutic responses of treatment-naive HIV-infected patients starting boosted atazanavir-based therapy in the ANRS 134-COPHAR 3 trial. Antimicrob Agents Chemother 2013;57:2265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One 2012;7:e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ 2003;169:656–61. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


