Abstract
Numerous natural products available over the counter are commonly consumed by healthy, sub-healthy or ill people for the treatment and prevention of various chronic diseases. Among them, a few dietary polyphenols, including the curry compound curcumin, have been attracting the most attention from biomedical researchers and drug developers. Unlike many so-called “good drug candidates”, curcumin and several other dietary polyphenols do not have a single known therapeutic target or defined receptor. In addition, the bioavailability of these polyphenols is usually very low due to their poor absorption in the gut. These recently debated features have created enormous difficulties for drug developers. In this review, I do not discuss how to develop curcumin, other dietary polyphenols or their derivatives into pharmaceutical agents. Instead, I comment on how curcumin and dietary polyphenol research has enriched our knowledge of insulin signaling, including the presentation of my perspectives on how these studies will add to our understanding of the famous hepatic insulin function paradox.
Keywords: curcumin, dietary polyphenols, insulin resistance, type 2 diabetes, dietary intervention, ChREBP, Fgf21, hepatic insulin function paradox
Hepatic function of insulin and the paradox of hepatic insulin resistance
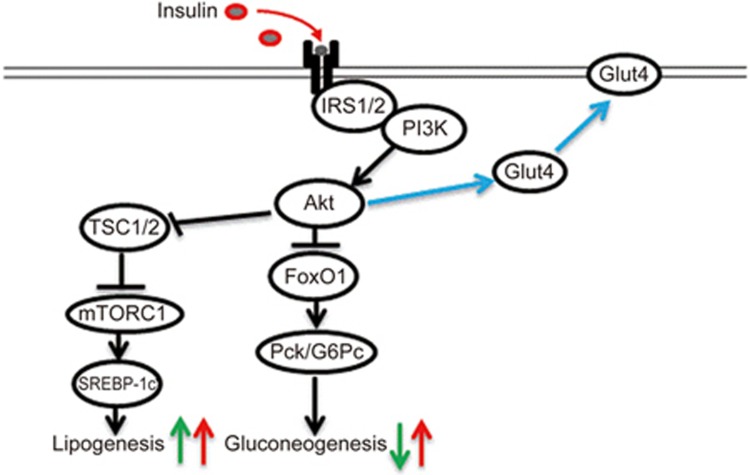
Insulin resistance is the common feature and the therapeutic target of type 2 diabetes (T2D) and its devastating vascular complications, as well as many other metabolic disorders. The liver is among the major organs that convey the function of insulin in maintaining metabolic homeostasis. Postprandial glucose elevation leads to the elevation of plasma insulin levels. Insulin exerts its glucose-lowering effect via a number of means, including the facilitation of glucose transport, the inhibition of hepatic gluconeogenesis and the stimulation of lipogenesis. As illustrated in Figure 1, in the liver, insulin exerts its regulatory effects via phosphoinositide 3-kinase (PI3K) mediated Akt (also known as protein kinase B, PKB) activation. Akt inactivates FoxO1, leading to the repression of the gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6pase). Akt can also inactivate tuberous sclerosis proteins 1 and 2 (TSC1/2) and hence release their repression of mTOR signaling, resulting in the activation of sterol regulatory element-binding protein 1c (SREBP-1c) and increased lipogenesis. SREBP-1c and carbohydrate-responsive element-binding protein (ChREBP) are the two well-defined lipogenic transcription factors1,2.
Figure 1.
A simplified illustration of hepatic insulin function and the paradox of hepatic insulin function. Insulin exerts its metabolic effects on glucose and lipid homeostasis via the common key molecule Akt. Postprandial insulin elevation leads to Akt activation (via IRS1/2 and PI3K). Insulin/Akt signaling inhibits the gluconeogenic enzymes (PEPCK and G6Pase as examples. They are encoded by the Pck and G6pc genes, respectively) and hence reduce gluconeogenesis. Akt can also activate mTORC1 and its target SREBP-1c (via TSC1/2 inactivation) and hence increase lipogenesis. Thus, physiologically, hepatic insulin signaling activation leads to increased lipogenesis and reduced gluconeogenesis (indicated by green arrows). Paradoxically, during insulin resistance, both gluconeogenesis and lipogenesis are elevated (indicated by red arrows). The stimulatory effect of Akt on Glut4 membrane translocation is also illustrated.
Paradoxically, subjects with insulin resistance show elevated hepatic gluconeogenesis, as well as lipogenesis, in contrast to the physiological scenario that insulin should exert completely opposite effects on lipogenesis and gluconeogenesis (Figure 1). This is known as the paradox of hepatic insulin function3,4,5. The exploration of mechanisms underlying this paradox for the past two decades has been advancing our knowledge of the pathogenesis of metabolic diseases in general and further investigation will have a great impact on refining the overall strategies in the prevention and treatment of T2D and other insulin resistance-related disorders. For example, one may wonder whether insulin treatment or the utilization of insulin signaling sensitization therapeutic agents will, in turn, exacerbate hepatic lipogenesis, resulting in non-alcoholic fatty liver diseases (NAFLD). Evidently, elevated de novo lipogenesis (DNL) was shown in both humans with IR and in the insulin resistance mouse model6,7,8,9.
It has been postulated by Brown and Goldstein that during insulin resistance, insulin loses its capability to repress gluconeogenesis but continues to stimulate lipogenesis, resulting in hyperglycemia and hyperlipidemia, as well as hepatic steatosis3,4. To test this “selective insulin resistant hypothesis”, great efforts have been made to identify the branch point in insulin signaling where hepatic glucose and lipid metabolism diverge. Li et al reported that in rat hepatocytes, insulin treatment increased the expression of the gene that encodes the lipogenic transcription factor SREBP1c and repressed the expression of the gene that encodes the gluconeogenic transcription factor PEPCK. Both of these effects require intact PI3K and Akt signaling pathways4. Sub-nanomolar concentrations of the mTORC1 inhibitor rapamycin, however, blocked the effect of insulin in stimulating SREBP1c but not the effect of insulin in repressing PEPCK. This, along with the lack of blockage with S6 kinase inhibition, allowed the authors to suggest that mTORC1 is an essential component in the insulin-regulated pathway for hepatic lipogenesis but not for gluconeogenesis4. Shimomura et alfound that chronic hyperinsulinemia led to reduced expression of the mRNA that encodes insulin receptor substrate 2 (IRS-2) and the development of insulin resistance. Furthermore, IRS-2 deficiency did not prevent stimulation by insulin of SREBP-1c production10. The differential effects of IRS-1 and IRS-2 on glucose and lipid metabolism, respectively, were also demonstrated with an siRNA-based gene silencing approach in human skeletal muscles11. Other investigations have led to the suggestion that insulin/mTORC regulated DNL can be independent of FoxO1 regulation12 and that Akt2 phosphorylation is required for some but not all Akt activities13.
Very recently, Vatner et al have reported that hepatic triglyceride synthesis can be stimulated by the substrate, independent of changes in hepatic insulin signaling5. The experiments were performed in normal or high fat diet (HFD) fed rats or in insulin receptor 2′-O-methoxyethyl chimeric antisense oligonucleotide-treated rats by infusing radioisotope labeled palmitate, followed by measuring the rate of fatty acid esterification into hepatic triglycerides. They found that the rate was dependent on plasma fatty acid infusion rates but appeared independent of changes in plasma insulin concentration or hepatocellular insulin signaling5. Taken together, these results obviate a paradox of selective insulin resistance, emphasizing the contribution of the lipogenic substrates.
Although these investigations have been advancing our understanding of hepatic functions of insulin in health and diseases, these investigations have not conceptually considered the existence of native compounds in our diet that can improve insulin signaling and prevent both hyperglycemia and dyslipidemia.
Dietary polyphenol and dietary polyphenol intervention
Nutrient sensing is known to affect insulin action in the liver and elsewhere, while dietary polyphenol intervention can effectively improve insulin signaling14,15. Curcumin, anthocyanin, and resveratrol are perhaps the three most heavily studied dietary polyphenols16. They are available over the counter in both western societies and Asian countries and are commonly utilized by healthy, sub-healthy or ill people for the treatment and prevention of various chronic diseases.
Anthocyanins (also known as anthocyans) are water-soluble vacuolar pigments of plants. Depending on the pH, anthocyanins may appear red, blue, or purple. Dietary plants that are rich in anthocyanins include blueberries, raspberries, black rice, and black soybeans. Resveratrol is a stilbenoid, a hydroxylated derivative of stilbenes. Resveratrol can be found in the skin of grapes, blueberries and raspberries.
The lipophilic polyphenol curcumin is the principal curcuminoid of turmeric. As a traditional medicine, turmeric has been utilized in numerous Asian countries for over 3000 years in the treatment of various diseases, including hepatitis, arthritis, a variety of skin disorders, rashes, and burn injuries16. In today's modern society, dietary supplements with turmeric rhizome or its extracts have been employed in the treatment of arthritis.
Curcumin, constituting approximately 2%–5% of turmeric, is the most studied component of turmeric. Intensive experimental studies have shown that it possesses antimicrobial, insecticidal, larvicidal, antimutagenic, cardioprotective, radioprotective, and anticancer activities. A number of investigations have also demonstrated that curcumin can improve insulin signaling13,17,18,19,20. Importantly, a recent clinical trial demonstrated that curcumin intervention in pre-diabetic human subjects lowered the number of individuals who eventually progressed to T2D18. Numerous in vitro investigations have shown that curcumin can regulate various signaling cascades in different cell lineages, while most previous in vivo studies have attributed the insulin signaling sensitizing and other beneficial effects of curcumin to their anti-inflammatory and anti-oxidation actions17,20,21,22,23,24,25,26. For example, Pan et al investigated the protective effects of the curcumin derivative C66 on diabetic cardiomyopathy. In the H9c2 cell line (a cardiomyocyte model) and in rat neonatal cardiomyocytes, C66 pretreatment reduced high glucose-induced overexpression of the inflammatory cytokines, possibly via the inactivation of nuclear factor-κB (NF-κB) and the inhibition of Jun NH2-terminal kinase (JNK) phosphorylation. In mice with type 1 diabetes, C66 administration decreased the levels of plasma and cardiac tumor necrosis factor-α (TNFα), which is associated with a decreased risk of cardiac cell death22. Weisberg et al assessed the effect of native curcumin intervention in HFD fed C57BL/6J mice and the leptin-deficient (ob/ob) obesity mouse model. In addition to improvements in glucose disposal and insulin tolerance, they demonstrated an effect of curcumin intervention in reducing macrophage infiltration in white adipose tissue, which is associated with decreased expression of hepatic nuclear factor-kappaB (NF-κB) activity and various markers of hepatic inflammation. They hence suggested that oral curcumin ingestion reversed many of the inflammatory and metabolic derangements associated with obesity and improved glycemic control in mouse models of T2D 17.
My team has also evaluated the long-term effect of curcumin intervention in HFD-fed C57BL/6J mice. We showed that concomitant curcumin interventions attenuated the effect of HFD on glucose disposal, body weight and fat gain, as well as the development of insulin resistance. Furthermore, curcumin inhibited hepatic lipogenic gene expression and blocked the effects of HFD on macrophage infiltration and the inflammatory pathways in white adipose tissue20. In a separate investigation, we fed C57BL/6J mice with HFD for 16 weeks to induce obesity and insulin resistance. The mice were then fed curcumin (via gavage) for 15 days. We found that this short-term curcumin treatment effectively ameliorated muscular oxidative stress by activation of the anti-oxidative Nrf2 signaling cascade27. A recent study by Hao et al demonstrated that in the mouse pancreatic MIN6 β-cell line, curcumin attenuated palmitate-induced cell death, possibly via the activation of cell survival Akt signaling and the inhibition of nuclear translocation of FoxO126.
Insulin signaling sensitization effect of curcumin can be independent of its anti-inflammation and body weight lowering effects
In addition to its anti-inflammatory and anti-oxidative properties, curcumin has been shown to target various other signaling cascades in different cell lineages that are involved in cell proliferation, cell apoptosis, cell adhesion, and other cellular events. These properties have been heavily assessed for exploring its potential utilization in cancer treatment28,29. In addition, a few recent studies have reported the potential effect of curcumin in promoting white adipocyte “browning”30,31. Furthermore, curcumin has been shown to repress hepatic gluconeogenic gene expression via AMPK activation32 and to activate the canonical Wnt signaling cascade in white adipose tissue to repress adipogenesis33,34,35. These effects, in the long run, may generate indirect effects on insulin sensitivity.
In 2015, my team proved the existence of the anti-inflammation and anti-oxidation effects of curcumin, independent of insulin sensitizing36. For this purpose, we have adopted a dexamethasone-induced insulin resistance mouse model37. Briefly, C57BL/6J mice fed a regular diet were administered daily intraperitoneal dexamethasone injections with or without daily curcumin gavage for 5 days. After a one-day rest, intraperitoneal insulin tolerance tests (IPITT) were performed on day 7, followed by an additional curcumin gavage on day 8. Mice were then sacrificed for serum and liver tissue collection on day 10. We defined this protocol as the “6-day curcumin intervention”. Dexamethasone injection induced insulin resistance while concomitant curcumin gavage improved insulin tolerance, indicating that insulin resistance attenuating effect of curcumin can be dissociated from its anti-inflammatory effects, and it may not necessarily be secondary to its bodyweight lowering effects36.
Dietary polyphenols regulate hepatic Fgf21 expression and curcumin intervention attenuates high-fat-diet-induced Fgf21 resistance
Fibroblast growth factor 21 (Fgf21) is a newly recognized hepatic hormone38,39,40,41. It is mainly produced and released by hepatocytes in response to fasting, although its expression has also been demonstrated in white adipose tissues and in pancreatic islets38,42,43. In both rodents and humans, Fgf21/FGF21 gene transcription is positively regulated by the nuclear receptor peroxisome proliferator-activated receptor α (PPARα), which is also the defined pharmacological target of the hypercholesterolemia drug fibrates44. During the adaptive starvation response period, the PPARα/Fgf21/PGC-1α axis can facilitate fatty acid oxidation, tri-carboxylic acid cycle flux and gluconeogenesis45. The insulin-sensitizing effect of Fgf21 has been well documented, and this hormone and its homologs have been intensively studied in pre-clinical studies and in clinical trials38,46,47,48,49,50,51,52,53,54.
Fgf21 expression and its circulation level in rodents can be robustly stimulated by a ketogenic diet39, while in human subjects, a ketogenic diet does not induce an appreciable elevation of circulating FGF21 levels55. Recently, a study showed that fructose ingestion acutely stimulated circulating FGF21 levels in human subjects 56. A few studies, including one conducted by our team, suggested a regulatory effect of dietary polyphenols, including resveratrol and curcumin, on hepatic Fgf21 expression36,57,58. We have also observed the stimulatory effect of anthocyanin on Fgf21 expression (unpublished). Recently, a study demonstrated that white pitaya (Hylocereus undatus) juice attenuated insulin resistance and hepatic steatosis in HFD-induced obese mice and this effect was associated with increased expression levels of Fgf21-related genes59. White pitaya juice is rich in various polyphenols, flavonoids, and vitamin C. Furthermore, dietary betaine supplementation has also been shown to increase plasma Fgf21 levels and is associated with improved glucose disposal and reduced hepatic steatosis in HFD-fed mice60.
Although Fgf21 was shown to exert a beneficial metabolic effect, in murine and human subjects with obesity or IR, serum Fgf21 levels were found to be elevated. Hence, Fisher and colleagues, as well as a number of other investigators, have suggested that obesity and IR represent Fgf21 resistant status50,61,62,63,64,65,66. Indeed, Fisher et al found that when obese mice (either after HFD consumption or due to a genetic defect) were treated with human recombinant FGF21, they showed an attenuated response to ERK1/2 phosphorylation and impaired induction of FGF21 target genes61
As stated above, we found that a “6-day curcumin intervention” attenuated dexamethasone-induced insulin resistance36. In conducting this study, we found that in the absence or presence of dexamethasone treatment, a curcumin intervention can increase hepatic Fgf21 mRNA and protein levels. We hence conducted further investigation into the role of curcumin in regulating Fgf21 both in vitro and in vivo. We showed that in mice on a low fat diet (LFD), daily curcumin gavage (4 or 8 days) increased serum and hepatic Fgf21 levels. Using the quantitative chromatin immunoprecipitation (ChIP) approach, we observed increased PPARα interactions with the Fgf21 promoter after curcumin treatment. HFD feeding also increased serum and hepatic Fgf21 levels, while the increase was attenuated by a 12 week concomitant curcumin intervention in association with partial restoration of hepatic expression of the genes that encode the Fgf21 receptor and co-receptor (FGFR1 and βKlotho), and a battery of lipolysis genes58. Importantly, hepatocytes from mice on a HFD showed an attenuated response to ex vivo recombinant FGF21 treatment, and the attenuation was effectively abolished by concomitant curcumin intervention58.
Both curcumin and insulin stimulate hepatic ChREBP expression
As illustrated in Figure 1, SREBP-1c and ChREBP are the two key lipogenic transcription factors1,2. Although the function of ChREBP is known to be controlled by glucose-induced nuclear translocation1,67, our team has demonstrated previously that insulin can stimulate ChREBP gene transcription68. Early investigations showed that ChREBP deletion led to reduced lipogenesis69 while ob/ob;ChREBP−/− mice exhibited lower expression of mRNAs for all hepatic lipogenic enzymes, decreased fatty acid synthesis and normalized plasma free fatty acid and triglyceride levels, as well as reduced weight gain and decreased adiposity70. These observations led to a suggestion that ChREBP inhibition may serve as a novel therapeutic tool for metabolic syndromes70. A few recent studies, however, have presented a completely opposing view. First, although hepatic over-expression of active ChREBP induced lipogenic programs, the mice with ChREBP over-expression remained insulin sensitive71 and, when placed on a HFD, showed improved insulin and glucose tolerance, despite the persistence of diet-induced hepatic steatosis. Thus, increased hepatic ChREBP expression can dissociate hepatic steatosis from IR, with improvements in both lipid and glucose homeostasis71. Second, a second ChREBP isoform, ChREBPβ, that predicts insulin sensitivity, has been identified in white adipose tissue, and the loss of adipose-ChREBP was shown to be sufficient to cause insulin resistance in mice72,73. Third, FA binding protein 4 (FABP4)-Cre mediated expression of constitutively active ChREBP in mice was shown to improve insulin sensitivity and glucose tolerance in response to HFD challenge74. Finally, very recent studies demonstrated that ChREBP protected the liver from fructose-induced injury75,76.
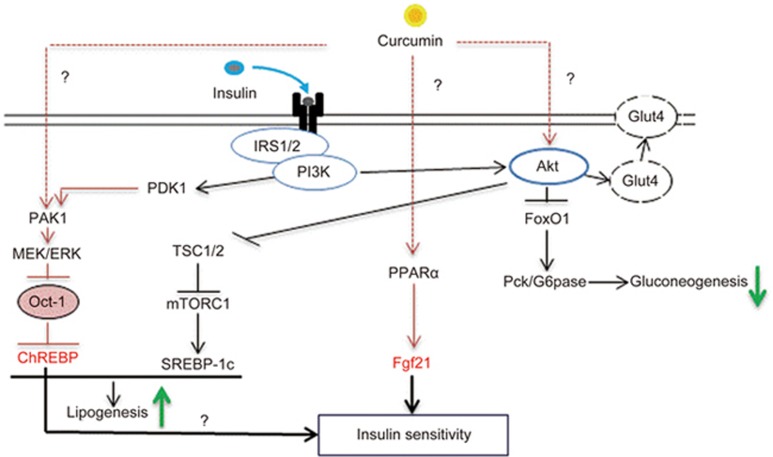
We recently found that both curcumin and insulin stimulated ChREBPα transcription via Akt-independent activation of MEK/ERK signaling, leading to the inactivation of the transcriptional repressor Oct-177. Further exploration revealed the involvement of p21-activated protein kinase 1 (Pak1). Pak1−/− mice are glucose and insulin intolerant, as demonstrated previously by my team and by other investigators78,79,80,81. Importantly, Pak1−/− mice showed reduced ChREBPα protein levels and an ∼70% reduction of ChREBPα and ChREBPβ mRNA levels77. Furthermore, these mice exhibited a reduced body fat volume. Indeed, Pak1−/− hepatocytes lack the normal ChREBP response to in vitro insulin or curcumin treatment77. We thus discovered a novel Pak1/MEK/ERK/Oct-1 signaling cascade that mediated insulin or curcumin-induced ChREBP expression. It needs to be noted that this novel signaling cascade (Figure 2) revealed by our study regulates ChREBPα but not ChREBPβ transcription, as there is no functional Oct-1 binding motif on the ChREBPβ promoter. Nevertheless, ChREBPβ expression is known to be positively regulated by ChREBPα because its promoter contains the binding motif for ChREBPα72.
Figure 2.
Summary of hepatic effects of curcumin intervention. Curcumin has been shown to repress glucose production in hepatocytes. Furthermore, curcumin treatment stimulates Fgf21 expression via a PPARα dependent mechanism. In addition, curcumin intervention attenuates HFD induced Fgf21 and insulin resistance (not illustrated in this figure). Finally, both insulin and curcumin can stimulate ChREBPα transcription via inactivating the transcriptional repressor Oct-1, which is involved in Akt-independent PAK1/MEK/ERK activation. It remains unknown mechanistically how curcumin can activate Akt, PPARα and PAK1 and hence regulate gluconeogenesis, as well as Fgf21 and ChREBP expression (indicated with the question marks). We speculate that ChREBP and Fgf21 form a network whereby the liver exerts its regulatory effects on energy homeostasis in response to nutrient sensing and dietary intervention.
Summary and perspectives
As mentioned above, curcumin (and many other dietary polyphenols) can target multiple organs or cell lineages without a known receptor or a defined target. This, along with other chemistry features of curcumin, has generated enormous difficulties in the detailed dissection of mechanistic pathways underlying its bio-medical functions. Indeed, these features have been causing some confusion among drug developers and have been heavily debated recently82,83,84,85. However, investigations presented by numerous investigators, including our team, suggest that the implications of dietary polyphenol research are beyond the scope of drug development. Utilizing curcumin as a tool, scientists have now linked dietary curcumin consumption with many functions of insulin signaling. The insulin signaling improvement effect of curcumin (and possibly other dietary polyphenols) can be attributed, in the long term, to its anti-inflammation, anti-oxidation and body weight lowering effects. The body weight lowering effect of this compound can be attributed to not only the attenuation of adipose tissue macrophage infiltration20 but also to its potential adipocyte “browning” effect, leading to an increase in energy expenditure30.
As illustrated in Figure 2, we have demonstrated that Fgf21 and ChREBP are two novel hepatic targets of curcumin; both of them are known as positive regulators of hepatic insulin signaling. Curiously, Fgf21 is a “starvation” hormone, and its circulating level is elevated after fasting, while ChREBP activity is facilitated by hyperglycemia and its production is likely stimulated by hyperinsulinemia. At this stage, the connection between these two hepatic metabolic regulators is largely unknown. I speculate that Fgf21 and ChREBP form the two “arms” of a yet to be fully recognized “large” network whereby the liver exerts its regulatory effects on energy homeostasis, such that impairment of either one of the “arms” will lead to the loss of response to curcumin intervention or to dietary intervention in general.
The stimulation of hepatic Fgf21 expression has been shown with curcumin, two other dietary polyphenols36,57,58, and white pitaya or dietary betaine supplementation59,60. It is necessary to determine whether ChREBP expression can be regulated by anthocyanin, resveratrol, and other dietary polyphenols. More importantly, it is urgent to investigate whether the simultaneous regulation of hepatic Fgf21 and ChREBP expression is a common feature of dietary polyphenols. It is also important to investigate whether Fgf21 signaling restoration by dietary polyphenol intervention is a pre-requirement for improving insulin signaling. If yes, the investigation will lead to a therapeutic approach for improving hepatic insulin signaling from a novel angle. Furthermore, detailed investigations are needed to explore whether a defect in either one of the two “arms” will lead to the loss of response of the subject to dietary polyphenol intervention for insulin signaling improvement. These investigations will likely bring about a “concept shift” in the metabolic field, adding to our understanding of the hepatic insulin function paradox.
Acknowledgments
The author thanks the Canadian Institute of Health Research (CIHR) and Banting and Best Diabetes Centre (BBDC) for supporting the curcumin research in his laboratory.
References
- Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 2007; 27: 179–92. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 2008; 7: 95–6. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A 2010; 107: 3441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci U S A 2015; 112: 1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003; 77: 43–50. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014; 146: 726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, et al. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 2003; 52: 1081–9. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 2000; 6: 77–86. [PubMed] [Google Scholar]
- Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, et al. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 2006; 4: 89–96. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med 2013; 19: 1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 2012; 7: e52013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alappat L, Awad AB. Curcumin and obesity: evidence and mechanisms. Nutr Rev 2010; 68: 729–38. [DOI] [PubMed] [Google Scholar]
- Munir KM, Chandrasekaran S, Gao F, Quon MJ. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: therapeutic implications for diabetes and its cardiovascular complications. Am J Physiol Endocrinol Metab 2013; 305: E679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors 2013; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008; 149: 3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012; 35: 2121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Larson-Meyer DE, Liebman M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am J Clin Nutr 2008; 87: 1262–7. [DOI] [PubMed] [Google Scholar]
- Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 2012; 7: e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekollu SK, Thomas R, O'Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes 2011; 60: 2928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes 2014; 63: 3497–511. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, et al. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 2006; 1122: 56–64. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu Y, Wu HL, Li YB, Li YH, Guo JB, et al. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur J Pharmacol 2008; 578: 43–50. [DOI] [PubMed] [Google Scholar]
- Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 2012; 8: 812–25. [DOI] [PubMed] [Google Scholar]
- Hao F, Kang J, Cao Y, Fan S, Yang H, An Y, et al. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic beta-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis 2015; 20: 1420–32. [DOI] [PubMed] [Google Scholar]
- He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes 2012; 3: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang J, Han J, Pan X, Cao Y, Guo H, et al. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha1-antitrypsin in lung cancer. Mol Oncol 2012; 6: 405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Liang Y, Jiang B, Li X, Xun H, Sun J, et al. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep 2016; 35: 2651–6. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun 2015; 466: 247–53. [DOI] [PubMed] [Google Scholar]
- Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem 2016; 27: 193–202. [DOI] [PubMed] [Google Scholar]
- Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun 2009; 388: 377–82. [DOI] [PubMed] [Google Scholar]
- Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr 2009; 139: 919–25. [DOI] [PubMed] [Google Scholar]
- Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am J Physiol Cell Physiol 2010; 298: C1510–6. [DOI] [PubMed] [Google Scholar]
- Tian L, Song Z, Shao W, Du WW, Zhao LR, Zeng K, et al. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis 2017; 8: e2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Zeng K, Shao W, Yang BB, Fantus IG, Weng J, et al. Short-term curcumin gavage sensitizes insulin signaling in dexamethasone-treated C57BL/6 mice. J Nutr 2015; 145: 2300–7. [DOI] [PubMed] [Google Scholar]
- Patel R, Bookout AL, Magomedova L, Owen BM, Consiglio GP, Shimizu M, et al. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol Endocrinol 2014: me20141259. [DOI] [PMC free article] [PubMed]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415–25. [DOI] [PubMed] [Google Scholar]
- Reitman ML. FGF21: a missing link in the biology of fasting. Cell Metab 2007; 5: 405–7. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 2008; 8: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426–37. [DOI] [PubMed] [Google Scholar]
- Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–74. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 2009; 106: 10853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008; 149: 6018–27. [DOI] [PubMed] [Google Scholar]
- Lim GE, Xu M, Sun J, Jin T, Brubaker PL. The rho guanosine 5'-triphosphatase, cell division cycle 42, is required for insulin-induced actin remodeling and glucagon-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology 2009; 150: 5249–61. [DOI] [PubMed] [Google Scholar]
- Shao M, Yu L, Zhang F, Lu X, Li X, Cheng P, et al. Additive protection by LDR and FGF21 treatment against diabetic nephropathy in type 2 diabetes model. Am J Physiol Endocrinol Metab 2015; 309: E45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol 2013; 78: 489–96. [DOI] [PubMed] [Google Scholar]
- Bozic M, Guzman C, Benet M, Sanchez-Campos S, Garcia-Monzon C, Gari E, et al. Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis. J Hepatol 2016; 65: 748–57. [DOI] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013; 18: 333–40. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013; 17: 790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab 2013; 17: 779–89. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 2016; 23: 427–40. [DOI] [PubMed] [Google Scholar]
- Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 2009; 94: 3594–601. [DOI] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab 2015; 4: 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 2014; 146: 539–49 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Tian L, Patel R, Shao W, Song Z, Liu L, et al. Diet polyphenol curcumin stimulates Hepatic Fgf21 production and restores its sensitivity in high-fat-diet-fed male mice. Endocrinology 2017; 158: 277–92. [DOI] [PubMed] [Google Scholar]
- Song H, Zheng Z, Wu J, Lai J, Chu Q, Zheng X. White Pitaya (Hylocereus undatus) juice attenuates insulin resistance and hepatic steatosis in diet-induced obese mice. PLoS One 2016; 11: e0149670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribo S, et al. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes 2016; 65: 902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010; 59: 2781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, et al. High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care 2011; 34: 2113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–53. [DOI] [PubMed] [Google Scholar]
- Berti L, Irmler M, Zdichavsky M, Meile T, Bohm A, Stefan N, et al. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Mol Metab 2015; 4: 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusli F, Deelen J, Andriyani E, Boekschoten MV, Lute C, van den Akker EB, et al. Fibroblast growth factor 21 reflects liver fat accumulation and dysregulation of signalling pathways in the liver of C57BL/6J mice. Sci Rep 2016; 6: 30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab 2017; 26: 324–41. [DOI] [PubMed] [Google Scholar]
- Sirek AS, Liu L, Naples M, Adeli K, Ng DS, Jin T. Insulin stimulates the expression of carbohydrate response element binding protein (ChREBP) by attenuating the repressive effect of Pit-1, Oct-1/Oct-2, and Unc-86 homeodomain protein octamer transcription factor-1. Endocrinology 2009; 150: 3483–92. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A 2004; 101: 7281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab 2006; 291: E358–64. [DOI] [PubMed] [Google Scholar]
- Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest 2012; 122: 2176–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012; 484: 333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar A, Aryal P, Wen J, Syed I, Vazirani RP, Moraes-Vieira PM, et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Rep 2017; 21: 1021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuotio-Antar AM, Poungvarin N, Li M, Schupp M, Mohammad M, Gerard S, et al. FABP4-Cre mediated expression of constitutively active ChREBP protects against obesity, fatty liver, and insulin resistance. Endocrinology 2015; 156: 4020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tong X, VanDommelen K, Gupta N, Stamper K, Brady GF, et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J Clin Invest 2017; 127: 2855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Finck BN. ChREBP refines the hepatic response to fructose to protect the liver from injury. J Clin Invest 2017; 127: 2533–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Tian L, Sirek A, Shao W, Liu L, Chiang YT, et al. Pak1 mediates the stimulatory effect of insulin and curcumin on hepatic ChREBP expression. J Mol Cell Biol 2017; 9: 384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YA, Shao W, Xu XX, Chernoff J, Jin T. P21-activated protein kinase 1 (Pak1) mediates the cross talk between insulin and beta-catenin on proglucagon gene expression and its ablation affects glucose homeostasis in male C57BL/6 mice. Endocrinology 2013; 154: 77–88. [DOI] [PubMed] [Google Scholar]
- Chiang YT, Jin T. p21-Activated protein kinases and their emerging roles in glucose homeostasis. Am J Physiol Endocrinol Metab 2014; 306: E707–22. [DOI] [PubMed] [Google Scholar]
- Chiang YT, Ip W, Shao W, Song ZE, Chernoff J, Jin T. Activation of cAMP signaling attenuates impaired hepatic glucose disposal in aged male p21-activated protein kinase-1 knockout mice. Endocrinology 2014; 155: 2122–32. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 2011; 286: 41359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger M. Drug screening: Don't discount all curcumin trial data. Nature 2017; 543: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017; 541: 144–45. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem 2017; 60: 1620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Song Z, Weng J, Fantus IGD. Curcumin and other dietary polyphenols: potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am J Physiol Endocrinol Metab 2018; 314: E201–E205. [DOI] [PubMed] [Google Scholar]