Abstract
Organ transplantation is the most effective therapy for patients with end-stage disease. Preservation solutions and techniques are crucial for donor organ quality, which is directly related to morbidity and survival after transplantation. Currently, static cold storage (SCS) is the standard method for organ preservation. However, preservation time with SCS is limited as prolonged cold storage increases the risk of early graft dysfunction that contributes to chronic complications. Furthermore, the growing demand for the use of marginal donor organs requires methods for organ assessment and repair. Machine perfusion has resurfaced and dominates current research on organ preservation. It is credited to its dynamic nature and physiological-like environment. The development of more sophisticated machine perfusion techniques and better perfusates may lead to organ repair/reconditioning. This review describes the history of organ preservation, summarizes the progresses that has been made to date, and discusses future directions for organ preservation.
Keywords: organ transplantation, organ preservation, static cold storage, machine perfusion, organ assessment, organ repair, ischemia-reperfusion injury
Introduction
Organ transplantation is the only effective therapy for patients with end-stage disease in many cases. A number of factors have contributed to the success of organ transplantation, including organ preservation, surgery, immunosuppressive medication, and post-transplantation care. A supply of high-quality donor organs is crucial to transplantation procedures; organ preservation has been described as “the supply line for organ transplantation”1. It allows time for preparation of the recipient, organization of staff and facilities, allocation and transportation of the organ, and laboratory tests2,3.
Static cold storage (SCS) offers a simple and effective way to preserve and transport organs and is the most commonly used method4. However, a number of limitations are associated with SCS, including tissue damage induced by prolonged hypothermic preservation, difficulty in assessing donor organ function and viability, inevitability of ischemia-reperfusion injury (IRI), and limited opportunity for organ repair. Recently, the growing use of marginal organs from extended criteria donors has led to an emergence of ex vivo lung perfusion (EVLP) to assess donor lung function5,6. In addition to being an excellent graft assessment tool, EVLP has also shown potential for enabling graft repair, reconditioning, and immunomodulation7, which inspired similar research and clinical applications in other organ systems8,9,10. The desire to extend preservation times has motivated research on optimal preservation solutions, temperatures, techniques, and therapeutic additives for organ repair and reconditioning11,12,13,14. By reviewing the history of organ perfusion and preservation, we noted that before the introduction of SCS in 1960s15, machine perfusion with plasma or blood-based solutions was the clinical method for preserving isolated organs16,17. Reevaluating the advantages and limitations of early organ perfusion/preservation may help with the development of new techniques/solutions that enable prolonged safe preservation and the repair of extended criteria donor organs to address the organ shortage issue. Theories, preservation techniques, preservation solutions, and clinical practices are discussed.
Past: a story of organ perfusion and preservation
Primitive concepts underwent a number of modifications over decades of scientific exploration to arrive at current practices in organ perfusion and preservation. It is essential to understand this history to be able to evaluate the future direction of this field of research.
Organ perfusion as a primitive preservation technique
Organ preservation developed from the primitive concept of extracorporeal circulation, which first emerged in 1812 in the monography of Cesar Julien Jean Le Gallois. He speculated that “if the place of the heart could be supplied by injection—and if, for the regular continuance of this injection, there could be furnished a quantity of arterial blood, whether natural or artificially formed, supposing such a formation possible—then life might be indefinitely maintained in any portion”18. In 1849, German scientist Carl Eduard Loebell described in his Dissertation Inaugurals the first perfusion experiments on isolated pig kidneys. He observed that the bright red arterial blood perfused through porcine kidneys became dark and viscous upon its course through the renal veins19. In 1885, Max von Frey and Max Gruber constructed the first closed artificial circulation system, which shares many similarities to today's organ perfusion systems20. In 1895, Jacobj created a double circulation apparatus, which used an isolated lung as an oxygenator and permitted organ perfusion for several hours19. These early studies led to the development of extracorporeal membrane oxygenation (ECMO) and the subsequent development of perfusion systems for organ preservation19,20,21,22,23,24.
From blood to chemically defined perfusion solution
Historically, blood was used as perfusate in early apparatuses. Primitive perfusion apparatuses required a large supply of blood to operate, whereby the volume of an animal's own blood was insufficient. People tried to substitute an animal's own blood with blood from a different animal species. The use of cross-species blood was toxic to the graft and led to its rapid decline25. Scientists then diluted the animal's own blood with normal saline or Ringer's solution. These methods led to the development of severe edema in organs, especially in the lung25. These early studies led to the realization of xenoimmunity and the development of transfusion solutions.
In 193726, Alexis Carrel perfused isolated cat thyroids in the Lindbergh apparatus with Tyrode's solution comprised of glucose, ions, and 40%–50% homologous serum. He found that the organs were viable for 3–21 days. However, cultivation over 6 days showed a tendency towards hyperplasia. In 196827, Hou et al cultured normal human placentas in a chemically defined culture medium. Placentas were kept viable for at least 14 days, but the stroma underwent great modification within 3 days. These studies demonstrated that organs or tissues were capable of surviving outside of the body for several days under normothermic conditions in culture medium. However, maintaining the normal histological morphology of cultured organs raised challenges, which slowed down organ culture research for several decades.
Temperature: from normothermic to hypothermic
Originally, organs were perfused at room temperature. In 1876, Bunge and Schmiedeberg added a water bath to the circuit to maintain perfusion blood at physiological temperatures19. Later, scientists began to speculate that the use of lower temperatures might attenuate organ damage during perfusion by abating cellular metabolism. In the 1960s, a number of experiments were performed with cooled diluted serum or heparinized blood, and kidney perfusion was extended from hours to days28,29. However, the use of cold blood also caused many problems, such as vascular spasm in kidney grafts30.
From dynamic to static modality
In the 1960s, kidneys were successfully preserved for 3–5 days by continuous perfusion with cooled, oxygenated blood or plasma28,29. However, this method required complex and costly equipment, which limited its availability and made the transportation of organs extremely difficult. In 1969, Collins GM was able to successfully preserve canine kidneys for 12 h by immersing them in iced saline solution, and he later further prolonged cold storage time to 30 h with Collins solution15,31. This simple method for organ preservation was more cost-efficient and convenient for organ transportation than its predecessors. The birth of SCS replaced dynamic perfusion methods and became the standard method of organ preservation.
Present: current practice and research on organ preservation
Preservation techniques (temperature, apparatus, perfusion setting, etc) and perfusion solutions are the major fields of research in organ preservation.
Static cold storage and preservation solutions
Since the 1960s, SCS has gradually become the gold standard method for organ preservation. SCS involves flushing the procured organ with preservation solution at 0–4 °C, then immersing it into preservation solution at the same temperature until transplantation. The hypothermic environment is responsible for decreasing cellular metabolism, and the preservation solution reduces cellular metabolism and provides cytoprotection.
Collins solution was the first preservation solution to enter the commercial market in 196915. It was used to preserve the kidney, heart, liver, and lung grafts. In 1980, Collins solution was modified via impermeant composition and improved chemical stability. The new solution was named a Euro-Collins solution, and it provided better protection during prolonged cold ischemia and was widely used2,32. The University of Wisconsin (UW) solution was introduced in the mid-1980s33 and continues to be used today for abdominal organ preservation34. These solutions are so-called intracellular fluid (ICF)-type solutions characterized by low Na+ and high K+ concentrations. ICF-type solutions were intended to prevent cellular edema by maintaining intracellular ion concentrations upon cold-induced dysfunction of Na+/K+ pumps35.
Adding amino acids to the preservation solution and using a histidine buffer system led to the development of histidine-tryptophan-ketoglutarate (HTK) solution, which is characterized by low K+ and low Na+ concentrations. It was originally developed for cardiac preservation, but it also achieved comparable patient survival for abdominal organ transplants36,37. Custodiol-N is a modified HTK solution with additional amino acids and chemicals. It is currently undergoing clinical trials; experimental studies showed promising reductions of hypoxic and cold-induced cell injury38,39. Celsior solution was originally made available in the 1990s as a heart preservation solution and was later also used for both thoracic and abdominal organ preservations40. Like HTK solution, Celsior solution also uses a histidine buffer and a low K+ concentration. However, its Na+ concentration is much higher. It shows equivalent performance to UW solution at a cheaper cost41,42.
The risk of hyperkalemia-induced pulmonary vasoconstriction led to the development of extracellular fluid (ECF)-type solutions, which have lower K+ and higher Na+ concentrations43. In the 1980s, an ECF-type solution called EP4 (or EP-TU) was introduced, which sustained a canine lung preservation model for as long as 96 h44. A low-potassium dextran glucose (LPDG) solution was developed and currently used as the gold standard for lung preservation43,45,46. ET-K solution was developed by optimizing the properties of sugar and electrolyte contents and by adding a protective component for pulmonary endothelium, which showed excellent postoperative lung graft performance47. ET-K and EP-TU solutions have been applied in clinical lung transplantation in Japan48,49. Table 1 summarizes information regarding the composition of popular cold preservation solutions.
Table 1. Composition of popular cold preservation solutions.
| Solutions | EC | UW | HTK (Custodiol) | Custodiol-N | Celsior | LPDG (Perfadex) | Ep4 (EP-TU) | ET-Kyoto | IGL-1 |
|---|---|---|---|---|---|---|---|---|---|
| K+ | 115 | 125 | 10 | 10 | 15 | 6 | 26 | 44 | 30 |
| Na+ | 10 | 25 | 15 | 16 | 100 | 138 | 141 | 100 | 125 |
| Cl− | 15 | 20 | 32 | 30 | 71 | 142 | 103 | – | – |
| Ca2+ | – | – | 0.015 | 0.02 | 0.25 | – | – | – | 0.03 |
| Mg2+ | – | 5 | 4 | 8 | 13 | 0.8 | 4 | 0 | 5 |
| Colloid/Impermeant | – | Pentafraction Lactobionate Raffinose | Mannitol | – | Lactobionate Mannitol | Dextran 40 | Dextran 40 | Pentafraction Trehalose | Lactobionate Raffinose Polyethylene glycol |
| Buffer | Phosphate Bicarbonate | Phosphate | Histidine | Histidine | Histidine | Phosphate THAM | Phosphate | Phosphate | Phosphate |
| Antioxidant | – | Glutathione Allopurinol | Mannitol Tryptophan α-ketoglutarate | Tryptophan α-Ketoglutarate | Glutathione Mannitol | – | – | – | Glutathione Allopurinol |
| Glucose | 180 | – | – | – | – | 5 | 10 | – | – |
| Amino acids | – | – | Histidine Tryptophan | Histidine N-acetylhistidine Glycine Alanine Tryptophan Arginine Aspartate | Histidine Glutamic acid | – | – | – | – |
| Others Sulfate, | – | Sulfate Adenosine | – | Sucrose | – | Sulfate | Sulfate | Nitroglycerin | |
| Deferoxamine LK 614 | Dibutyryl cAMP Gluconate | Adenosine | |||||||
| Reference | 41 | 41 | 38 | 38 | 41 | 43 | 48 | 49 | 41 |
Note: Values are expressed in mmol/liter unless indicated otherwise.
Ex vivo machine perfusion
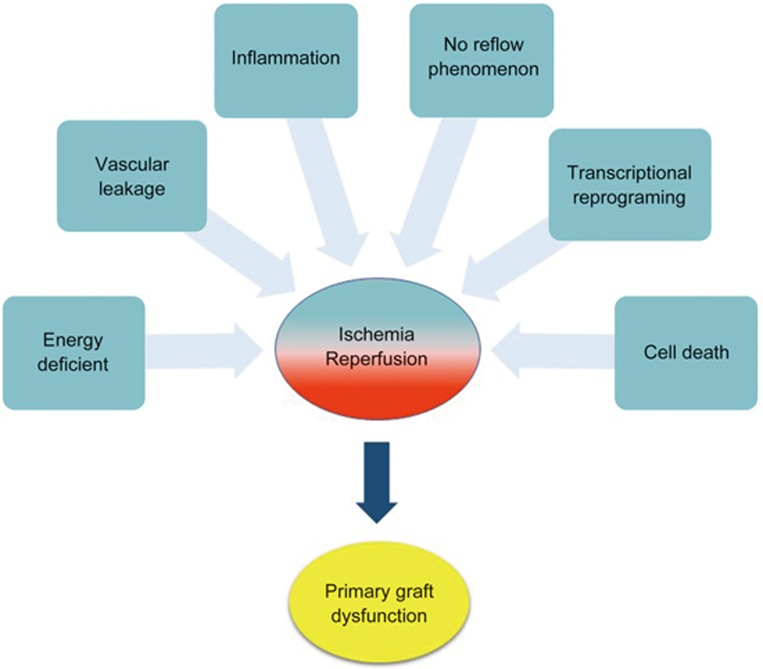
IR-induced injury increases the risk of early graft dysfunction and reduces long-term survival after transplantation50 (Figure 1). Meanwhile, the shortage of donor organs has led to the use of extended criteria donor (ECD) organs. Proper donor organ functional assessment and ex vivo repair/reconditioning of organs prior to transplantation has become necessary. Machine perfusion is a method involving organ perfusion with a controlled flow of perfusate. It facilitates the maintenance of organ microvasculature tone, provision of oxygen and nutrients in support of tissue metabolism, and removal of toxic metabolic waste.
Figure 1.
Biological processes induced during ischemia-reperfusion that may lead to primary graft dysfunction.
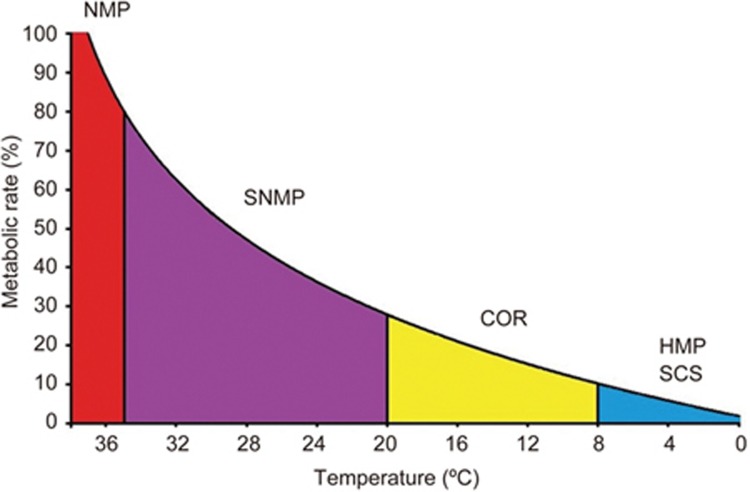
The cellular rate of respiration is proportional to the surrounding temperature51. For example, SCS at 0–4 °C reduces the metabolic rate of the organ to approximately 5% of its physiological level52,53. Different temperatures have been investigated for ex vivo machine perfusion, including normothermic machine perfusion (NMP) at 35–38 °C, subnormothermic machine perfusion (SNMP) at 20–34 °C, controlled oxygenated rewarming (COR) at 8–20 °C, and hypothermic machine perfusion (HMP) at 0–8 °C (Figure 2) (Table 2).
Figure 2.
Metabolic rate reduces with a decrease in temperature in humans. (SCS=static cold storage, HMP=hypothermic machine perfusion, COR=controlled oxygenated rewarming, SNMP=subnormothermic machine perfusion, and NMP=normothermic machine perfusion).
Table 2. Comparison of various methods used for preservation of organs.
| Methods | Temperature | Organ | Merits | Challenge | Clinical application | Reference |
|---|---|---|---|---|---|---|
| Cold static storage | 0–8 °C | Kidney, liver, lung, heart | Low cost, simple and easy to operate | Metabolite accumulation, not for organ function assessment | Kidney, liver, lung, heart | 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 |
| Hypothermic machine perfusion | 0–8 °C | Kidney, liver, lung, heart | Provides oxygen and metabolic substrates | Perfusion time is limited, not for organ assessment | Kidney, liver | 28,29,54,55,56,57,58 |
| Normothermic machine perfusion | 35–38 °C | Kidney, liver, lung, heart | Provides oxygen and essential substrates; maintains metabolic activity and viability; good for organ assessment and repair | Perfusion time is limited for organ regeneration | Kidney, liver, lung, heart | 8,10,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78 |
| Subnormothermic machine perfusion | 20–34 °C | Kidney, liver | Newly proposed technique | To be determined | – | 85,86,87,88,89 |
| Controlled oxygenated rewarming | 8–20 °C | Kidney, Liver | Slowly, gradually rise the perfusate temperature to mitigate ischemia reperfusion injury | To be determined | Liver | 90,91,92 |
Hypothermic machine perfusion
HMP (0–8 °C) is based on the concept that oxidative energy production by mitochondrial electron transport is sustained at hypothermic temperatures. HMP continuously provides metabolic substrates for the generation of ATP, which enables the graft to restore tissue energy. The first clinically available HMP device was developed by Folkert Belzer in 1960s28 and used to perform the first HMP-preserved human kidney transplant in 196816. Belzer et al achieved perfusion of the kidney with hypothermic, diluted plasma or blood for 3 days28. Humphries et al were able to extend kidney perfusion to 5 days29.
The 1990s saw the resurgence in interest in HMP for kidney preservation as both the demand for organs and reliance on ECD donors grew54. New HMP technology showed decreased rates of delayed graft function and improved outcomes in the case of marginal donors relative to SCS. By 2015, approximately 25% to 35% of all transplanted kidneys in the United States were preserved with HMP54.
Challenges arise in the use of HMP for liver preservation since the liver receives blood from both the portal vein and hepatic artery2. However, the first clinical trial of HMP-preserved liver grafts showed shorter hospital stays and reduced vascular and biliary complications as benefits55. Few studies on HMP in heart and lung transplants have been reported. Nakajima et al reported that short-term HMP (1–2 h) can improve lung tissue energy levels and ameliorate IRI by decreasing the production of reactive oxygen species in rat lungs56,57. Michel et al showed that HMP preserved the cellular structure of donor hearts better than SCS during prolonged ischemic times in pigs58. Additional research in the field of cardiac and lung HMP is required.
Normothermic machine perfusion
NMP (35–38 °C) is a method of perfusing organs under physiologic conditions to maintain metabolic activity and viability. NMP maintains donor organs at body temperature while providing oxygen and essential substrates. Historically, NMP was developed to assess organ function prior to transplantation59,60,61 and to preserve donor organs during distant procurement62,63. In 2001, Steen et al reintroduced the EVLP technique to evaluate lungs from donation after cardiac death (DCD)64. In 2007, they performed the first human transplantation of a rejected donor lung after assessment with EVLP65. Early studies were only able to achieve perfusion times of less than 6 h in large animal models66,67. In 2008, Cypel et al in Toronto modified the EVLP technique with low tidal volume ventilation, reduced perfusion rate and acellular perfusate, and extended perfusion time for up to 12 h in swine lungs with stable lung function68. The Toronto group conducted the first clinical trial successfully and reported excellent outcomes in 201169. They further reported extended clinical outcome data70,71, and the marginal donor lungs treated with EVLP showed comparable or even better results than regular lung transplants72.
The success of EVLP inspired many research groups worldwide to further investigate the role of NMP in other organ systems. Nicholson et al described that a short period (1 or 2 h) of NMP could restore function and replenish ATP after warm and cold ischemia in porcine kidneys8,73,74. The first clinical study on preserving kidney grafts with NMP was reported in 201175. A follow-up clinical study showed that the delayed graft function rate was significantly lower in the NMP group than in the SCS group in ECD kidney transplantation76. The first clinical trial on NMP in liver transplantation was reported in 2016; 16 donations after brain death livers and 4 DCD livers were transplanted after NMP. The results showed that 30-day graft survival was similar between the NMP and SCS groups, and the median peak aspartate aminotransferase level was significantly lower in the NMP group than in the SCS group77. Clinical studies have shown promising results with NMP in resuscitating marginal and declined donor livers9,78. In addition, NMP has been shown to be superior to SCS in preserving DCD hearts in dogs79. In pigs, DCD hearts reconditioned with NMP showed comparable function to brain death donor hearts80. Over a 2-year period in a clinical trial involving 159 cases of orthotopic heart transplantation, NMP showed higher recipient survival and lower incidences of primary graft dysfunction (PGD) and acute rejection than SCS10.
Several companies have now marketed a commercial portable machine to facilitate ex vivo machine perfusion, such as Organ Care System™ (TransMedics, USA) for the heart, lung, or liver and Organ Assist® device (Organ Assist, The Netherlands) for the lung, liver, or kidney. These devices can be used during organ transportation, which offers a platform for normothermic organ preservation immediately after procurement, monitoring and assessing graft function continuously11,81. These mobile devices have demonstrated encouraging results in clinical studies, which opens new avenues for organ preservation and transportation82,83,84.
Subnormothermic machine perfusion
Subnormothermic machine perfusion (SNMP, 20–34 °C) is a midway approach between HMP and NMP85. Although better preservation times were accomplished with NMP than with HMP, it was speculated that the cytoprotective benefits of reduced cellular metabolism under hypothermic temperatures could further improve organ preservation. Meanwhile, sufficient metabolism would be maintained for viability assessment and organ repair/reconditioning86. Although studies have shown that livers or kidneys perfused with SNMP are superior to grafts preserved under SCS87,88, a recent study showed that porcine kidneys preserved under SNMP were associated with higher indices of renal and tubular injury upon reperfusion than those preserved under NMP89. Therefore, whether SNMP should be developed in addition to NMP should be further determined.
Controlled oxygenated rewarming
Following cold ischemic preservation, the abrupt change in temperature from hypothermia to normothermia upon reperfusion may effectuate dysfunction of the mitochondria and pro-apoptotic signal transduction, which contributes to reperfusion-induced organ injury90. Hypothermic preservation is meant to protect the ischemic organ by reducing metabolism. However, ischemic redox dyshomeostasis leads to impairment of the mitochondrial membrane potential through mitochondrial transition pore opening. Mitochondrial damage can be further enhanced upon reperfusion91. COR (8–20 °C) is an alternative organ perfusion method involving a slow, gradual rise in the perfusate temperature. The period of COR is aimed to minimize injury to the graft and improve hepatocellular function upon reperfusion, offering gentle restitution of mitochondrial function91. Clinical studies have shown that COR is safely transferable to clinical practice in liver transplantation91. By the end of 2016, COR had been effectively applied in 15 human liver transplantations92. Minor and colleagues demonstrated that COR following SCS had better kidney function with mitigated activity of mitochondrial permeability transition pore opening, caspase 9 activation, and apoptosis in porcine kidneys90. It should be noted that during EVLP, lung perfusate was gradually warmed up during the first 30 min68. COR could be integrated into NMP.
Organ perfusate: from chemically defined solutions to blood/blood substitute
Perfusate composition is of central importance in maintaining stable organ function ex vivo. Blood-based perfusates were commonly used for organ perfusion before cell culture media were developed in the 1950s93,94. Due to its variable nature and associated technical and ethical concerns, the use of blood or blood products was gradually replaced by chemically defined solutions. For example, Steen Solution™, a chemically defined solution, has been widely used for EVLP and machine perfusion of other organs; it contains colloid components (human serum albumin and Dextran 40) to maintain oncotic pressure, physiological ion concentrations to regulate osmolality, and buffers to retain normal pH and glucose as an energy resource. However, the supplementation of additional nutrients, including red blood cells and other blood substitutes, to Steen Solution™ is under investigation to extend perfusion time.
Blood/blood substitutes
Studies have identified that blood-based perfusate is necessary during NMP to transport oxygen and meet metabolic demands, and it provides superior functional preservation in the case of kidney, liver and heart storage95,96,97,98. It is still disputed whether blood or red blood cells should be involved in EVLP. Some studies have highlighted the use of blood over acellular perfusates99, whereas others have observed spontaneous lung injury when using whole blood100. When looking over the studies that have currently achieved the longest perfusion times, it is interesting to see that perfusates used in these studies were either whole blood or blood-based solutions95,101,102,103,104. One study that provided pertinent evidence in support of the essentiality of blood in perfusate is a cross-circulation study. O'Neill et al connected a conventional EVLP circuit to the internal jugular vein of a recipient pig so that metabolic substrates and hormones from the recipient pig were available to the perfused lungs, whereas metabolic waste produced by the perfused lungs was cleared by the recipient; they effectively perfused the lungs with recipient autologous blood for 36 h without notable changes in physiological parameters105.
However, the use of blood-based perfusate is accompanied by a series of concerns, such as immune-mediated responses, hemolysis, thrombus formation, biochemical and humoral variations, and a risk of blood-borne infectious transmission106. Further development of an acellular perfusate is another major direction.
Nutrients
Currently, commonly used perfusates, such as Steen solution™ and Organ Care System (OCS) perfusate, use glucose as the only energy resource. However, during NMP, organs are perfused at body temperature. Glucose alone is not sufficient for organ metabolism. To prolong NMP for organ repair, the incorporation of more nutrients, such as amino acids, vitamins, lipids and others, should be considered. Amino acids are basic components of proteins and are essential nutrients for cell survival and proliferation. Vitamins can help cells use the provided chemical energy to process proteins, carbohydrates, and fats required for cellular metabolism107. Amino acids and vitamins have been used routinely in cell culture media93,94. Interestingly, cell culture media were used for organ culture to maintain isolated organs for days without serum or blood supplements27. In liver and kidney studies, amino acids and extra glucose have been added into perfusate during NMP, and this approach showed promising results in pigs108,109.
Fetal mouse lungs cultured in a medium without growth factors showed poorly developed airways and a lack of defined acinar structures110, which suggests that growth factors and hormones may also be required for organ rebuilding /regeneration.
To avoid the use of human blood products, interest has increased in acellular oxygen carriers, which have similar oxygen carrying capacity to human hemoglobin111. Initial studies on hemoglobin-based oxygen carriers have shown encouraging results, including enhanced oxygenation and improved allograft function of ex vivo perfused organs in normothermic/subnormothermic conditions106,112, which opens the door for blood substitution in future.
It is reasonable to conclude that an ideal perfusate should offer oxygen carrying capacity, oncotic properties, buffers to maintain physiological pH, metabolic substrates and physiological electrolyte levels, growth factors and hormones. A blood substitute designed to replace human blood in ex vivo machine perfusion will be a promising direction for prolonging the preservation of isolated organs.
Future perspectives: organ repair/reprogramming with ex vivo machine perfusion
Prolonged ex vivo machine perfusion & organ repair/reprogramming
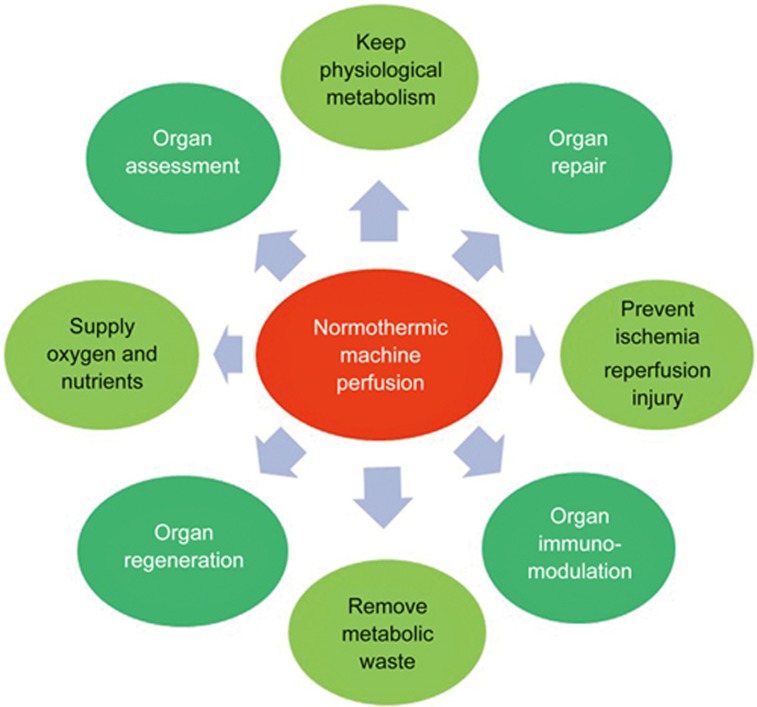
The incredible progress of organ preservation research over the past few decades has led to the booming success of clinical organ transplantation as a treatment for patients with end-stage disease. However, this demand has skyrocketed to a level that cannot be satisfied by the number of available donor organs. The use of ex vivo machine perfusion aspires to warranting the use of marginal donors by minimizing IRI and facilitating the repair/regeneration of suboptimal grafts in order to expand the donor pool and improve overall graft function after transplantation. For this purpose, prolonged ex vivo perfusion time is required (Figure 3).
Figure 3.
The advantage of the potential use of normothermic machine perfusion.
Organ repair
There has been an increasing number of studies focusing on the application of ex vivo machine perfusion for organ repair. EVLP is among the most active areas of study. A series of therapeutic strategies have been studied using EVLP for lung repair. For example, different drugs were delivered through perfusate to mitigate IRI113,114,115, therapeutic gases (NO, CO, H2) were inhaled during EVLP to reduce inflammatory response and lung edema116,117,118, mesenchymal stem cells were used to treat lung injury induced by endotoxins and infection119, and IL-10 gene therapy was developed to prevent IRI120,121. When the types of injury are clear, injury-specific treatments can be used during EVLP. For example, high-dose, broad-spectrum anti-microbial agents were added to perfusate to treat human donor lung infection122,123, lung lavage and surfactant replacement were used to treat acid aspiration-induced pig lung injury124,125,126, and pulmonary thrombolysis was performed to eliminate pulmonary embolism followed by successful lung transplantation127,128.
In kidney, Brasile et al delivered heme analog cobalt protoporphyrin during ex vivo kidney perfusion to reduce inflammatory and free radical injury by upregulating the protective gene hemoxygenase-1 in canines129. They also used growth factors to upregulate cellular processes to resuscitate and repair warm IRI in canines and in rejected human kidneys130. Hosgood et al delivered nitric oxide donors and carbon monoxide-releasing molecules during NMP, which enhanced renal flow and improved renal function in pigs131. Yang et al investigated the effect of adding erythropoietin to perfusate during NMP and found that erythropoietin promoted inflammatory cell apoptosis and drived inflammatory and apoptotic cells into tubular lumens, which led to inflammation clearance, renal protection, and tissue remodeling in a porcine model132.
In liver, studies on therapeutic medications during NMP to reduce IRI showed promising results in pigs and rats133,134,135. Goldaracena et al delivered an antiviral drug to perfusate during normothermic ex vivo liver perfusion and effectively induced Hepatitis C virus resistance after pig liver transplantation136.
Organ regeneration
In 2008, Ott et al reported the first whole organ engineering success. They used ex vivo machine perfusion as a platform, decellularized rat hearts by coronary perfusion with detergents in a Langendorff apparatus, then reseeded these constructs by perfusion with cardiac or endothelial cells; eight constructs were maintained for up to 28 days by coronary perfusion with a nutrient-rich medium in a bioreactor that simulated cardiac physiology. This study revolutionized the field of tissue engineering, kindled hope for possibility of whole organ engineering137. They also successfully created bioartificial rat lungs using a slightly modified approach and subsequently transplanted the regenerated left lungs orthotopically. The bioartificial lungs provided gas exchange in vivo for up to 6 h after extubation138. Using the same perfusion system, Ott's group further maintained the bioartificial rat lungs for up to 7 days with good function after implantation139. They later decellularized human and porcine lungs140, which brought the matrix to clinical scale. A similar perfusion method has also been used to create kidney and liver scaffolds in animals and in clinically rejected human organs141. Although there are still many challenges, the use of NMP alongside stem cells for organ engineering has received increasing interest.
Organ immunomodulation
Ex vivo machine perfusion has also provided a potential platform for organ immunomodulation. Miyoshi et al reported that ex vivo perfusion of canine pancreaticoduodenal allografts using class-II-specific monoclonal antibodies delays the onset of acute rejection142. Brasile et al treated canine kidney grafts with a bioengineered interface consisting of a nano-barrier membrane during NMP for 3 h. They found that untreated control dogs experienced a mean onset of rejection on day 6, whereas the mean onset of rejection was significantly delayed until day 30 in dogs in the treatment group143. Martens et al distributed multipotent adult progenitor cells in the airway during EVLP and observed a reduction in pro-inflammatory cytokines and neutrophils in bronchoalveolar lavage fluid, which is related to innate immune system modulation and may play an important role in reducing PGD after transplantation144.
Due to severe donor shortage from humans, xenotransplantation is gaining more attention. Ex vivo perfusion of porcine lungs with fresh human blood is used to study discordant pulmonary xenograft injury145,146. Pre-perfusion of donor organs with recipient serum147 and the delivery of targeted drugs have been attempted to prevent hyperacute rejection148. Ex vivo machine perfusion offers an effective platform to alleviate discordant xenograft rejection by removing the recipient's xenoreactive natural antibodies149. Studies on the recellularization of animal organ scaffolds by human liver stem/progenitor cells with ex vivo machine perfusion techniques are under investigation150,151.
Conclusion
Since the very first speculation on organ preservation made by Cesar Julien Jean Le Gallois over two centuries ago, tremendous progress has been made in this field of research. In the early days, organs were perfused with blood at physiological temperatures. The introduction of SCS in the 1960s revolutionized organ preservation. From then on, it became standard practice to statically preserve organs at hypothermic temperatures. With the recent demand to expand the organ donor pool, the currently accepted status of organ preservation is seeing a retrospective shift from SCS to theories inspired by early techniques, as these techniques provide great potential for improved graft preservation, viability assessment, and most importantly, repair/regeneration. The success of organ preservation with dynamic machine perfusion operating on the basis of blood-based perfusates at close-to-physiological temperatures has prompted further in-depth studies on organ preservation and repair/reconditioning. The need to prolong ex vivo machine perfusion time requires the optimization of current perfusates with the addition of essential components to meet metabolic needs. Prolonged ex vivo machine perfusion opens a door for organ repair and reprogramming, warranting further investigation of novel strategies to improve donor graft quality prior to transplantation.
Acknowledgments
Lei JING is a PhD candidate of the First Bethune Hospital of Jilin University and is supervised by Dr Li-ping PENG; she is supported by the First Bethune Hospital of Jilin University. This work was supported by research grants from the Canadian Institutes of Health Research (MOP-42546, MOP-119514, and PJT-148847) and an Ontario Research Fund-Research Excellence award.
References
- Southard J, Belzer F. Organ preservation. Annu Rev Med 1995; 46: 235–47. [DOI] [PubMed] [Google Scholar]
- Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez J V, Fuller BJ. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother 2011: 125–42. [DOI] [PMC free article] [PubMed]
- Catena F, Coccolini F, Montori G, Vallicelli C, Amaduzzi A, Ercolani G, et al. Kidney preservation: review of present and future perspective. Transplant Proc 2013; 45: 3170–7. [DOI] [PubMed] [Google Scholar]
- Liu WP, Humphries AL, Russell R, Stoddard LD, Moretz WH, Moretz WH. 48-hour storage of canine kidneys after brief perfusion with Collins' solution. Ann Surg 1971; 173: 748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg 2006; 81: 460–6. [DOI] [PubMed] [Google Scholar]
- Gao X, Liu W, Liu M. Ex vivo lung perfusion: scientific research and clinical application. Pract J Organ Transplant 2017; 5: 177–87. [Google Scholar]
- Cypel M, Keshavjee S. The clinical potential of ex vivo lung perfusion. Expert Rev Respir Med 2012; 6: 27–35. [DOI] [PubMed] [Google Scholar]
- Hosgood SA, Barlow AD, Yates PJ, Snoeijs MGJ, Van Heurn EL, Nicholson ML. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res 2011; 171: 283–90. [DOI] [PubMed] [Google Scholar]
- Perera T, Mergental H, Stephenson B, Roll GR, Cilliers H, Liang R, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl 2016; 22: 120–4. [DOI] [PubMed] [Google Scholar]
- Koerner MM, Ghodsizad A, Schulz U, Banayosy A El, Koerfer R, Tenderich G. Normothermic ex vivo allograft blood perfusion in clinical heart transplantation. Heart Surg Forum 2014; 17: E141–5. [DOI] [PubMed] [Google Scholar]
- Van Raemdonck D, Neyrinck A, Rega F, Devos T, Pirenne J. Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transpl 2013; 18: 24–33. [DOI] [PubMed] [Google Scholar]
- Mariscal A, Cypel M, Keshavjee S. Ex vivo lung perfusion: a key tool for translational science in the lungs. Curr Transplant Reports 2017; 4: 149–58. [Google Scholar]
- Hosgood SA, Van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int 2015; 28: 657–64. [DOI] [PubMed] [Google Scholar]
- Selten J, Schlegel A, de Jonge J, Dutkowski P. Hypo- and normothermic perfusion of the liver: which way to go? Best Pract Res Clin Gastroenterol 2017: 171–9. [DOI] [PubMed]
- Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation: initial perfusion and 30 hours' ice storage. Lancet 1969; 294: 1219–22. [DOI] [PubMed] [Google Scholar]
- Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med 1968; 278: 608–10. [DOI] [PubMed] [Google Scholar]
- Brettschneider L, Daloze PM, Huguet C, Porter KA, Groth CG, Kashiwagi N, et al. The use of combined preservation techniques for extended storage of orthotopic liver homografts. Surg Gynecol Obstet 1968; 126: 263–74. [PMC free article] [PubMed] [Google Scholar]
- Le Gallois M. Experiments on the principle of life, and particularly on the principle of the motions of the heart, and on the seat of this principle: including the report made to the first class of the Institute, upon the experiments relative to the mtions of the heart, Philadelphia: M Thomas; 1813.
- Boettcher W, Merkle F, Weitkemper HH. History of extracorporeal circulation: the conceptional and developmental period. J Extra Corpor Technol 2003; 35: 172–83. [PubMed] [Google Scholar]
- Okiljević B, Šušak S, Redžek A, Rosić M, Velicki L. Development of cardiopulmonary bypass–a historical review. Srp Arh Celok Lek 2016; 144: 670–5. [PubMed] [Google Scholar]
- Embley E, Martin C. The action of anaesthetic quantities of chloroform upon the blood vessels of the bowel and kidney; with an account of an artificial circulation apparatus. J Physiol 1905; 32: 147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly I de B, Thorpe WV. An isolated mammalian heart preparation capable of performing work for prolonged periods. J Physiol 1933; 79: 199–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowell OA. The histology of the isolated perfused lung. Q J Exp Physiol Cogn Med Sci 1943; 32: 203–12. [Google Scholar]
- Veith FJ, Hagstrom JW, Nehlsen SL, Karl RC, Deysine M. Functional, hemodynamic, and anatomic changes in isolated perfused dog lungs: the importance of perfusate characteristics. Ann Surg 1967; 165: 267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie TG. The perfusion of surviving organs. J Physiol 1903; 29: 266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel A. The culture of whole organs: I. technique of the culture of the thyroid gland. J Exp Med 1937; 65: 515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LT, Ewen SW, Beck JS. Histological, metabolic and histochemical studies on normal human placenta in organ culture. Br J Exp Pathol 1968; 49: 648–57. [PMC free article] [PubMed] [Google Scholar]
- Belzer FO, Ashby BS, Dunphy JE. 24-hour and 72-hour preservation of canine kidneys. Lancet 1967; 2: 536–8. [DOI] [PubMed] [Google Scholar]
- Humphries AL, Russell R, Stoddard LD, Moretz WH. Successful five-day kidney preservation. Perfusion with hypothermic, diluted plasma. Invest Urol 1968; 5: 609–18. [PubMed] [Google Scholar]
- Calne RY, Pegg DE, Pryse-Davies J, Brown FL. Renal preservation by ice-cooling: an experimental study relating to kidney transplantation from cadavers. Br Med J 1963; 2: 651–5. [PMC free article] [PubMed] [Google Scholar]
- Collins GM, Bravo-Shugarman M, Terasaki PI, Braf Z, Sheil AG, Williams G. Kidney preservation for transportation. IV. eight-thousand-mile international air transport. Aust N Z J Surg 1970; 40: 195–7. [DOI] [PubMed] [Google Scholar]
- Aydin G, Okiye SE, Zincke H. Successful 24-hour preservation of the ischemic canine kidney with Euro-Collins solution. J Urol 1982; 128: 1401–3. [DOI] [PubMed] [Google Scholar]
- Wahlberg JA, Southard JH, Belzer FO. Development of a cold storage solution for pancreas preservation. Cryobiology 1986; 23: 477–82. [DOI] [PubMed] [Google Scholar]
- Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front Biosci 2008; 13: 221–35. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kondo T. Preservation solution for lung transplantation. Gen Thorac Cardiovasc Surg 2009; 57: 635–9. [DOI] [PubMed] [Google Scholar]
- Schneeberger S, Biebl M, Steurer W, Hesse UJ, Troisi R, Langrehr JM, et al. A prospective randomized multicenter trial comparing histidine-tryptophane-ketoglutarate versus University of Wisconsin perfusion solution in clinical pancreas transplantation. Transpl Int 2009; 22: 217–24. [DOI] [PubMed] [Google Scholar]
- Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc 2006; 38: 3572–5. [DOI] [PubMed] [Google Scholar]
- Loganathan S, Radovits T, Hirschberg K, Korkmaz S, Koch A, Karck M, et al. Effects of Custodiol-N, a novel organ preservation solution, on ischemia/reperfusion injury. J Thorac Cardiovasc Surg 2010; 139: 1048–56. [DOI] [PubMed] [Google Scholar]
- Pizanis N, Petrov A, Heckmann J, Wiswedel I, Wohlschläger J, de Groot H, et al. A new preservation solution for lung transplantation: evaluation in a porcine transplantation model. J Hear Lung Transplant 2012; 31: 310–7. [DOI] [PubMed] [Google Scholar]
- Karam G, Compagnon P, Hourmant M, Despins P, Duveau D, Noury D, et al. A single solution for multiple organ procurement and preservation. Transpl Int 2005; 18: 657–63. [DOI] [PubMed] [Google Scholar]
- Voigt MR, DeLario GT. Perspectives on abdominal organ preservation solutions: a comparative literature review. Prog Transplant 2013; 23: 383–91. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JM, Morgan RD, Knight SR, Morris PJ. The effect of preservation solutions for storage of liver allografts on transplant outcomes. Ann Surg 2014; 260: 46–55. [DOI] [PubMed] [Google Scholar]
- Latchana N, Peck JR, Whitson B, Black SM. Preservation solutions for cardiac and pulmonary donor grafts: a review of the current literature. J Thorac Dis 2014; 6: 1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa M, Fujimura S, Kondo T, Ichinose T, Shiraishi Y, Nakada T. A study of preservation solution for 48- and 96-hour simple hypothermic storage of canine lung transplants. Tohoku J Exp Med 1989; 159: 205–14. [DOI] [PubMed] [Google Scholar]
- Keshavjee SH, Yamazaki F, Cardoso PF, McRitchie DI, Patterson GA, Cooper JD. A method for safe twelve-hour pulmonary preservation. J Thorac Cardiovasc Surg 1989; 98: 529–34. [PubMed] [Google Scholar]
- Date H, Matsumura A, Manchester JK, Obo H, Lima O, Cooper JM, et al. Evaluation of lung metabolism during successful twenty-four-hour canine lung preservation. J Thorac Cardiovasc Surg 1993; 105: 480–91. [PubMed] [Google Scholar]
- Ikeda M, Bando T, Yamada T, Sato M, Menjyu T, Aoyama A, et al. Clinical application of ET-Kyoto solution for lung transplantation. Surg Today 2015; 45: 439–43. [DOI] [PubMed] [Google Scholar]
- Okada Y, Matsumura Y, Date H, Bando T, Oto T, Sado T, et al. Clinical application of an extracellular phosphate-buffered solution (EP-TU) for lung preservation: preliminary results of a Japanese series. Surg Today 2012; 42: 152–6. [DOI] [PubMed] [Google Scholar]
- Omasa M, Hasegawa S, Bando T, Hanaoka N, Yoshimura T, Nakamura T, et al. Application of ET-Kyoto solution in clinical lung transplantation. Ann Thorac Surg 2004; 77: 338–9. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med 2011; 17: 1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WR, Peters RH, Zimmermann J. The effects of body size and temperature on metabolic rate of organisms. Can J Zool 1983; 61: 281–8. [Google Scholar]
- de Perrot M, Bonser RS, Dark J, Kelly RF, McGiffin D, Menza R, et al. Report of the ISHLT working group on primary lung graft dysfunction part III: donor-related risk factors and markers. J Heart Lung Transplant 2005; 24: 1460–7. [DOI] [PubMed] [Google Scholar]
- Weeder PD, van Rijn R, Porte RJ. Machine perfusion in liver transplantation as a tool to prevent non-anastomotic biliary strictures: rationale, current evidence and future directions. J Hepatol 2015; 63: 265–75. [DOI] [PubMed] [Google Scholar]
- Henry SD, Guarrera J V. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev 2012; 26: 163–75. [DOI] [PubMed] [Google Scholar]
- Guarrera J V, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant 2010; 10: 372–81. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Chen F, Yamada T, Sakamoto J, Osumi A, Fujinaga T, et al. Hypothermic machine perfusion ameliorates ischemia-reperfusion injury in rat lungs from non-heart-beating donors. Transplantation 2011; 92: 858–63. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Chen F, Okita K, Motoyama H, Hijiya K, Ohsumi A, et al. Reconditioning lungs donated after cardiac death using short-term hypothermic machine perfusion. Transplant J 2012; 94: 999–1004. [DOI] [PubMed] [Google Scholar]
- Michel SG, LaMuraglia II GM, Madariaga MLL, Titus JS, Selig MK, Farkash EA, et al. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann Transplant 2015; 20: 461–8. [DOI] [PubMed] [Google Scholar]
- Jirsch DW, Fisk RL, Couves CM. Ex vivo evaluation of stored lungs. Ann Thorac Surg 1970; 10: 163–8. [DOI] [PubMed] [Google Scholar]
- Carter JN, Green RD, Halasz NA, Collins GM. Ex vivo perfusion: a renal preservation model. J Surg Res 1981; 31: 55–60. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K, Shimada M, Takenaka K, Sugimachi K. Ex vivo perfusion for accurate assessment of liver graft viability in dogs. J Invest Surg 1990; 3: 261–6. [DOI] [PubMed] [Google Scholar]
- Hardesty RL, Griffith BP. Autoperfusion of the heart and lungs for preservation during distant procurement. J Thorac Cardiovasc Surg 1987; 93: 11–8. [PubMed] [Google Scholar]
- Kontos GJ Jr, Borkon AM, Adachi H, Baumgartner WA, Hutchins GM, Brawn J, et al. Successful extended cardiopulmonary preservation in the autoperfused working heart-lung preparation. Surgery 1987; 102: 269–76. [PubMed] [Google Scholar]
- Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet 2001; 357: 825–9. [DOI] [PubMed] [Google Scholar]
- Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg 2007; 83: 2191–4. [DOI] [PubMed] [Google Scholar]
- Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003; 76: 244–52; discussion 252. [DOI] [PubMed] [Google Scholar]
- Erasmus ME, Fernhout MH, Elstrodt JM, Rakhorst G. Normothermic ex vivo lung perfusion of non-heart-beating donor lungs in pigs: from pretransplant function analysis towards a 6-h machine preservation. Transpl Int 2006; 19: 589–93. [DOI] [PubMed] [Google Scholar]
- Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Hear Lung Transpl 2008; 27: 1319–25. [DOI] [PubMed] [Google Scholar]
- Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011; 364: 1431–40. [DOI] [PubMed] [Google Scholar]
- Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012; 144: 1200–7. [DOI] [PubMed] [Google Scholar]
- Tikkanen JM, Cypel M, Machuca TN, Azad S, Binnie M, Chow CW, et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J Heart Lung Transplant 2015; 34: 547–56. [DOI] [PubMed] [Google Scholar]
- Machuca TN, Mercier O, Collaud S, Tikkanen J, Krueger T, Yeung JC, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015; 15: 993–1002. [DOI] [PubMed] [Google Scholar]
- Bagul A, Hosgood SA, Kaushik M, Kay MD, Waller HL, Nicholson ML. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg 2008; 95: 111–8. [DOI] [PubMed] [Google Scholar]
- Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res 2013; 182: 153–60. [DOI] [PubMed] [Google Scholar]
- Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011; 92: 735–8. [DOI] [PubMed] [Google Scholar]
- Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013; 13: 1246–52. [DOI] [PubMed] [Google Scholar]
- Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MTPR, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant 2016; 16: 1779–87. [DOI] [PubMed] [Google Scholar]
- Mergental H, Perera MTPR, Laing RW, Muiesan P, Isaac JR, Smith A, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 2016; 16: 3235–45. [DOI] [PubMed] [Google Scholar]
- Repse S, Pepe S, Anderson J, McLean C, Rosenfeldt FL, Shimizu N. Cardiac reanimation for donor heart transplantation after cardiocirculatory death. J Heart Lung Transplant 2010; 29: 747–55. [DOI] [PubMed] [Google Scholar]
- Ali AA, White P, Xiang B, Lin HY, Tsui SS, Ashley E, et al. Hearts from DCD donors display acceptable biventricular function after heart transplantation in pigs. Am J Transplant 2011; 11: 1621–32. [DOI] [PubMed] [Google Scholar]
- Van Raemdonck D, Neyrinck A, Cypel M, Keshavjee S. Ex-vivo lung perfusion. Transpl Int 2015; 28: 643–56. [DOI] [PubMed] [Google Scholar]
- Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015; 385: 2577–84. [DOI] [PubMed] [Google Scholar]
- Zeriouh M, Sabashnikov A, Mohite PN, Zych B, Patil NP, García-Sáez D, et al. Utilization of the organ care system for bilateral lung transplantation: preliminary results of a comparative study. Interact Cardiovasc Thorac Surg 2016; 23: 351–7. [DOI] [PubMed] [Google Scholar]
- op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MWN, Gouw ASH, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant 2013; 13: 1327–35. [DOI] [PubMed] [Google Scholar]
- Berendsen TA, Bruinsma BG, Lee J, D'Andrea V, Liu Q, Izamis ML, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res 2012; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, García-Gil A, Adam R, et al. Emerging concepts in liver graft preservation. World J Gastroenterol 2015; 21: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolboom H, Izamis ML, Sharma N, Milwid JM, Uygun B, Berthiaume F, et al. Subnormothermic machine perfusion at both 20°C and 30°C recovers ischemic rat livers for successful transplantation. J Surg Res 2012; 175: 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer DP, Gallinat A, Swoboda S, Wohlschläger J, Rauen U, Paul A, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int 2014; 27: 1097–106. [DOI] [PubMed] [Google Scholar]
- Adams TD, Patel M, Hosgood SA, Nicholson ML. Lowering perfusate temperature from 37°C to 32°C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct 2017; 3: e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopp I, Reissberg E, Lüer B, Efferz P, Minor T. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci 2015; 8: 475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer DP, Mathé Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation. Transplantation 2016; 100: 147–52. [DOI] [PubMed] [Google Scholar]
- Hoyer DP, Paul A, Minor T. Prediction of hepatocellular preservation injury immediately before human liver transplantation by controlled oxygenated rewarming. Transplant Direct 2017; 3: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science 1955; 122: 501–14. [DOI] [PubMed] [Google Scholar]
- EAGLE H. The specific amino acid requirements of a mammalian cell (strain L) in tissue culture. J Biol Chem 1955; 214: 839–52. [PubMed] [Google Scholar]
- Liu Q, Nassar A, Farias K, Buccini L, Mangino MJ, Baldwin W, et al. Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death. Am J Transplant 2016; 16: 794–807. [DOI] [PubMed] [Google Scholar]
- Höchel J, Lehmann D, Fehrenberg C, Unger V, Groneberg DA, Grosse-Siestrup C. Effects of different perfusates on functional parameters of isolated perfused dog kidneys. Nephrol Dial Transplant 2003; 18: 1748–54. [DOI] [PubMed] [Google Scholar]
- Podesser BK, Hallström S, Schima H, Huber L, Weisser J, Kröner A, et al. The erythrocyte-perfused “working heart” model: hemodynamic and metabolic performance in comparison to crystalloid perfused hearts. J Pharmacol Toxicol Methods 1999; 41: 9–15. [DOI] [PubMed] [Google Scholar]
- White CW, Hasanally D, Mundt P, Li Y, Xiang B, Klein J, et al. A whole blood-based perfusate provides superior preservation of myocardial function during ex vivo heart perfusion. J Heart Lung Transplant 2015; 34: 113–21. [DOI] [PubMed] [Google Scholar]
- Kraft SA, Fujishima S, McGuire GP, Thompson JS, Raffin TA, Pearl RG. Effect of blood and albumin on pulmonary hypertension and edema in perfused rabbit lungs. J Appl Physiol 1995; 78: 499–504. [DOI] [PubMed] [Google Scholar]
- Pearse DB, Sylvester JT. Spontaneous injury in isolated sheep lungs: role of resident polymorphonuclear leukocytes. J Appl Physiol 1992; 72: 2475–81. [DOI] [PubMed] [Google Scholar]
- Loor G, Howard BT, Spratt JR, Mattison LM, Panoskaltsis-Mortari A, Brown RZ, et al. Prolonged EVLP using OCS lung: cellular and acellular perfusates. Transplantation 2017; 101: 2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt JR, Mattison LM, Iaizzo PA, Brown RZ, Helms H, Iles TL, et al. An experimental study of the recovery of injured porcine lungs with prolonged normothermic cellular ex vivo lung perfusion following donation after circulatory death. Transpl Int 2017; 30: 932–44. [DOI] [PubMed] [Google Scholar]
- Kaths JM, Cen JY, Chun YM, Echeverri J, Linares I, Ganesh S, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant 2017; 17: 957–69. [DOI] [PubMed] [Google Scholar]
- Trahanas JM, Witer LJ, Alghanem F, Bryner BS, Iyengar A, Hirschl JR, et al. Achieving 12 hour normothermic ex situ heart perfusion. ASAIO J 2016; 62: 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JD, Guenthart BA, Kim J, Chicotka S, Queen D, Fung K, et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng 2017; 1: 37. [Google Scholar]
- Laing RW, Bhogal RH, Wallace L, Boteon Y, Neil DAH, Smith A, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation 2017; 101: 2746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender DA. Nutritional biochemistry of the vitamins. Cambridge: cambridge University Press; 2003.
- Kaths JM, Echeverri J, Goldaracena N, Louis KS, Chun YM, Linares I, et al. Eight-hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation 2016; 100: 1862–70. [DOI] [PubMed] [Google Scholar]
- Zhang Z-B, Gao W, Liu L, Shi Y, Ma N, Shen ZY. Development and assessment of normothermic machine perfusion preservation for extracorporeal splitting of pig liver. Ann Transplant 2017; 22: 507–17. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Zgleszewski SE, Cilley RE, Blewett CJ, Krummel TM, Reisher SR, et al. Influence of epidermal growth factor and transforming growth factor beta-1 on patterns of fetal mouse lung branching morphogenesis in organ culture. Pediatr Pulmonol 1998; 25: 244–56. [DOI] [PubMed] [Google Scholar]
- Kocian R, Spahn DR. Haemoglobin, oxygen carriers and perioperative organ perfusion. Best Pract Res Clin Anaesthesiol 2008; 22: 63–80. [DOI] [PubMed] [Google Scholar]
- Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant 2015; 15: 381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg 2016; 151: 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerter ME, Sharma AK, Zhao Y, Charles EJ, Kron IL, Laubach VE. Attenuation of pulmonary ischemia-reperfusion injury by adenosine A2B receptor antagonism. Ann Thorac Surg 2016; 102: 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens A, Boada M, Vanaudenaerde BM, Verleden SE, Vos R, Verleden GM, et al. Steroids can reduce warm ischemic reperfusion injury in a porcine donation after circulatory death model with ex vivo lung perfusion evaluation. Transpl Int 2016; 29: 1237–46. [DOI] [PubMed] [Google Scholar]
- Dong BM, Abano JB, Egan TM. Nitric oxide ventilation of rat lungs from non-heart-beating donors improves posttransplant function. Am J Transplant 2009; 9: 2707–15. [DOI] [PubMed] [Google Scholar]
- Dong B, Stewart PW, Egan TM. Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non-heart-beating donors. J Thorac Cardiovasc Surg 2013; 146: 429–36. e1. [DOI] [PubMed] [Google Scholar]
- Noda K, Shigemura N, Tanaka Y, Bhama J, D'Cunha J, Kobayashi H, et al. Hydrogen preconditioning during ex vivo lung perfusion improves the quality of lung grafts in rats. Transplantation 2014; 98: 499–506. [DOI] [PubMed] [Google Scholar]
- Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013; 187: 751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009; 1: 4ra9. [DOI] [PubMed] [Google Scholar]
- Machuca TN, Cypel M, Bonato R, Yeung JC, Chun Y-M, Juvet S, et al. Safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy in a large animal lung transplant survival model. Hum Gene Ther 2017; 28: 757–65. [DOI] [PubMed] [Google Scholar]
- Andreasson A, Karamanou DM, Perry JD, Perry A, Özalp F, Butt T, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Hear Lung Transplant 2014; 33: 910–6. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Cypel M, Bonato R, Machuca TN, Iskender I, Hashimoto K, et al. Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant 2016; 16: 1229–37. [DOI] [PubMed] [Google Scholar]
- Inci I, Hillinger S, Arni S, Kaplan T, Inci D, Weder W. Reconditioning of an injured lung graft with intrabronchial surfactant instillation in an ex vivo lung perfusion system followed by transplantation. J Surg Res 2013; 184: 1143–9. [DOI] [PubMed] [Google Scholar]
- Khalifé-Hocquemiller T, Sage E, Dorfmuller P, Mussot S, Le Houérou D, Eddahibi S, et al. Exogenous surfactant attenuates lung injury from gastric-acid aspiration during ex vivo reconditioning in pigs. Transplant J 2014; 97: 413–8. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Liu M, Ohsumi A, Kalaf R, Iskender I, Hsin M, et al. Lung lavage and surfactant replacement during ex vivo lung perfusion for treatment of gastric acid aspiration-induced donor lung injury. J Heart Lung Transplant 2017; 36: 577–85. [DOI] [PubMed] [Google Scholar]
- Machuca TN, Hsin MK, Ott HC, Chen M, Hwang DM, Cypel M, et al. Injury-specific ex vivo treatment of the donor lung: pulmonary thrombolysis followed by successful lung transplantation. Am J Respir Crit Care Med 2013; 188: 878–80. [DOI] [PubMed] [Google Scholar]
- Inci I, Yamada Y, Hillinger S, Jungraithmayr W, Trinkwitz M, Weder W. Successful lung transplantation after donor lung reconditioning with urokinase in ex vivo lung perfusion system. Ann Thorac Surg 2014; 98: 1837–8. [DOI] [PubMed] [Google Scholar]
- Brasile L, Buelow R, Stubenitsky BM, Kootstra G. Induction of heme oxygenase-1 in kidneys during ex vivo warm perfusion. Transplantation 2003; 76: 1145–9. [DOI] [PubMed] [Google Scholar]
- Brasile L, Stubenitsky BM, Haisch CE, Kon M, Kootstra G. Repair of damaged organs in vitro. Am J Transplant 2005; 5: 300–6. [DOI] [PubMed] [Google Scholar]
- Hosgood SA, Bagul A, Kaushik M, Rimoldi J, Gadepalli RS, Nicholson ML. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg 2008; 95: 1060–7. [DOI] [PubMed] [Google Scholar]
- Yang B, Hosgood SA, Bagul A, Waller HL, Nicholson ML. Erythropoietin regulates apoptosis, inflammation and tissue remodelling via caspase-3 and IL-1β in isolated hemoperfused kidneys. Eur J Pharmacol 2011; 660: 420–30. [DOI] [PubMed] [Google Scholar]
- Monbaliu D, Vekemans K, Hoekstra H, Vaahtera L, Libbrecht L, Derveaux K, et al. Multifactorial biological modulation of warm ischemia reperfusion injury in liver transplantation from non-heart-beating donors eliminates primary nonfunction and reduces bile salt toxicity. Ann Surg 2009; 250: 808–17. [DOI] [PubMed] [Google Scholar]
- Maida K, Akamatsu Y, Hara Y, Tokodai K, Miyagi S, Kashiwadate T, et al. Short oxygenated warm perfusion with prostaglandin E1 administration before cold preservation as a novel resuscitation method for liver grafts from donors after cardiac death in a rat in vivo model. Transplantation 2016; 100: 1052–8. [DOI] [PubMed] [Google Scholar]
- Goldaracena N, Echeverri J, Spetzler VN, Kaths JM, Barbas AS, Louis KS, et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant 2016; 22: 1573–83. [DOI] [PubMed] [Google Scholar]
- Goldaracena N, Spetzler VN, Echeverri J, Kaths JM, Cherepanov V, Persson R, et al. Inducing hepatitis C virus resistance after pig liver transplantation-a proof of concept of liver graft modification using warm ex vivo perfusion. Am J Transplant 2017; 17: 970–8. [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008; 14: 213–21. [DOI] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16: 927–33. [DOI] [PubMed] [Google Scholar]
- Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 2011; 92: 998–1006. [DOI] [PubMed] [Google Scholar]
- Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant 2014; 33: 298–308. [DOI] [PubMed] [Google Scholar]
- Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization–a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 6526–9. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Sakagami K, Orita K. Ex vivo perfusion of canine pancreaticoduodenal allografts using class-II-specific monoclonal antibody delays the onset of acute rejection. Transpl Int, 1992, 5 Suppl 1: S516–20. [DOI] [PubMed] [Google Scholar]
- Brasile L, Glowacki P, Castracane J, Stubenitsky BM. Pretransplant kidney-specific treatment to eliminate the need for systemic immunosuppression. Transplantation 2010; 90: 1294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens A, Ordies S, Vanaudenaerde BM, Verleden SE, Vos R, Van Raemdonck DE, et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res Ther 2017; 8: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdorf L, Azimzadeh AM, Pierson RN. Xenogeneic lung transplantation models. Methods Mol Biol 2012: 169–89. [DOI] [PMC free article] [PubMed]
- Laird C, Burdorf L, Pierson RN. Lung xenotransplantation. Curr Opin Organ Transplant 2016; 21: 272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Tong C, Guo W, Pu R, Zhang G, Wang L, et al. Synergistic suppression of pre-perfusion of donor livers with recipient serum and cobra venom factor treatment on hyperacute rejection following liver xenotransplantation. Acta Cir Bras 2012; 27: 301–5. [DOI] [PubMed] [Google Scholar]
- Cantu E, Gaca JG, Palestrant D, Baig K, Lukes DJ, Gibson SE, et al. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction. Transplantation 2006; 81: 1157–64. [DOI] [PubMed] [Google Scholar]
- Azimzadeh A, Meyer C, Watier H, Beller JP, Chenard-Neu MP, Kieny R, et al. Removal of primate xenoreactive natural antibodies by extracorporeal perfusion of pig kidneys and livers. Transpl Immunol 1998; 6: 13–22. [DOI] [PubMed] [Google Scholar]
- Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res 2012; 0173: e11–25. [DOI] [PubMed] [Google Scholar]
- Navarro-Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A 2015; 21: 1929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]