Abstract
Salvia miltiorrhiza Burge (Danshen) is an eminent medicinal herb that possesses broad cardiovascular and cerebrovascular protective actions and has been used in Asian countries for many centuries. Accumulating evidence suggests that Danshen and its components prevent vascular diseases, in particular, atherosclerosis and cardiac diseases, including myocardial infarction, myocardial ischemia/reperfusion injury, arrhythmia, cardiac hypertrophy and cardiac fibrosis. The published literature indicates that lipophilic constituents (tanshinone I, tanshinone IIa, tanshinone IIb, cryptotanshinone, dihydrotanshinone, etc) as well as hydrophilic constituents (danshensu, salvianolic acid A and B, protocatechuic aldehyde, etc) contribute to the cardiovascular protective actions of Danshen, suggesting a potential synergism among these constituents. Herein, we provide a systematic up-to-date review on the cardiovascular actions and therapeutic potential of major pharmacologically active constituents of Danshen. These bioactive compounds will serve as excellent drug candidates in small-molecule cardiovascular drug discovery. This article also provides a scientific rationale for understanding the traditional use of Danshen in cardiovascular therapeutics.
Keywords: Salvia miltiorrhiza Burge, Danshen, cardiovascular diseases, herbal medicine, traditional Chinese medicine
Introduction
Danshen, the dried root of rhizome of Salvia miltiorrhiza Burge, has been widely used in Asian countries for treating cardiovascular diseases, including coronary heart disease, myocardial infarction (MI), angina pectoris and atherosclerosis1,2,3,4. Therefore, Danshen represents a traditional Chinese medicine (TCM) that has a relatively high safety profile. To date, the chemical constituents of Danshen have been well identified, including more than 30 lipophilic compounds that have a diterpene chinone structure (tanshinone I–VI, cryptotanshinone, isotanshinone I–II, Danshenol A etc) and more than 50 hydrophilic compounds that mainly have a phenolic acid structure (Danshensu, salvianolic acid A, salvianolic acid B, protocatechuic aldehyde, etc)1,5,6,7. More recently, Tasly Pharmaceuticals, Inc has completed a Phase III clinical trial to evaluate the safety and efficacy of Dantonic® (T89, also known as Compound Danshen Dripping Pills) in patients with chronic stable angina pectoris (ClinicalTrials.gov Identifier: NCT01659580). In this article, we provide a systematic and up-to-date overview of the pharmacological and therapeutic profile of bioactive compounds from Danshen in vascular diseases (atherosclerosis) and cardiac diseases (myocardial infarction, myocardial ischemia/reperfusion injury, arrhythmia, cardiac hypertrophy and cardiac fibrosis), with the aim of providing a scientific rationale for understanding the traditional use of Danshen in cardiovascular therapeutics.
The pathogenesis of atherosclerosis and the anti-atherosclerotic effects of Danshen
Key events in the pathogenesis of atherosclerosis
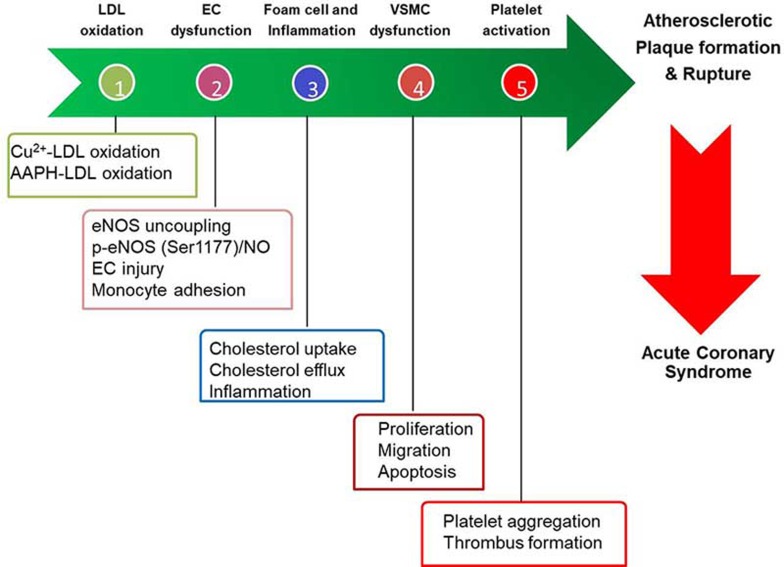
Atherosclerosis is a multifactorial, chronic inflammatory disease characterized by an inflammatory response, oxidative stress, and immune disorders8,9,10,11,12. Several diet-induced atherosclerotic animal models (such as ApoE−/− mice, LDLr−/− mice, and rabbits) have been widely used to study the pathogenesis of atherosclerosis and evaluate anti-atherosclerotic drugs13,14. There are several sequential and interrelated steps in the development of atherosclerosis (Figure 1). These critical steps have served as excellent models for evaluating atheroprotective drugs, which target one or more of these steps.
Figure 1.
Key cellular events in the pathogenesis of atherosclerosis.
(i) Low-density lipoprotein (LDL) oxidation: a high level of circulating LDL in the hypercholesterolemic microenvironment is prone to modification to form the modified LDL (mLDL). The major form of pathophysiologically mLDL is oxidized LDL (oxLDL), which activates endothelial cells and initiates the vicious cycle of atherosclerotic plaque progression15.
(ii) Endothelial dysfunction: the combination of multiple pro-atherogenic stimuli (such as oxLDL, high glucose, and homocysteine, among others) injures the integrity of the vascular endothelium, causes a leaky vessel and increases leukocyte (monocytes and neutrophils) adhesion to the diseased endothelium, impairs vasorelaxation, causes endothelial nitric oxide synthase (eNOS) uncoupling and reduces nitric oxide (NO) production16.
(iii) Vascular smooth muscle cell (VSMC) dysfunction: The injured vascular endothelium induces the phenotypic switch of VSMCs to proliferate and migrate from the media layer of blood vessels to form the neointima (or hyperplasia), the early form of atherosclerosis17.
(iv) Macrophage-derived foam cell formation and inflammation: Macrophages differentiated from circulating monocytes respond to local inflammatory cytokines or stimuli and are activated. Macrophages also avidly engulf modified LDL via membrane-located scavenger receptors (SR) [such as CD36, SR-A, lectin-like oxidized LDL receptor 1 (LOX-1)] to form foam cells, the hallmark of atherosclerosis18.
(v) Platelet activation and thrombus formation: After destabilization of atherosclerotic plaques, the plaques are susceptible to rupture, giving rise to platelet activation (adhesion and aggregation) and thrombus formation, which underlie the clinical presentation of atherothrombotic events19.
Anti-atherosclerotic effects of Danshen components
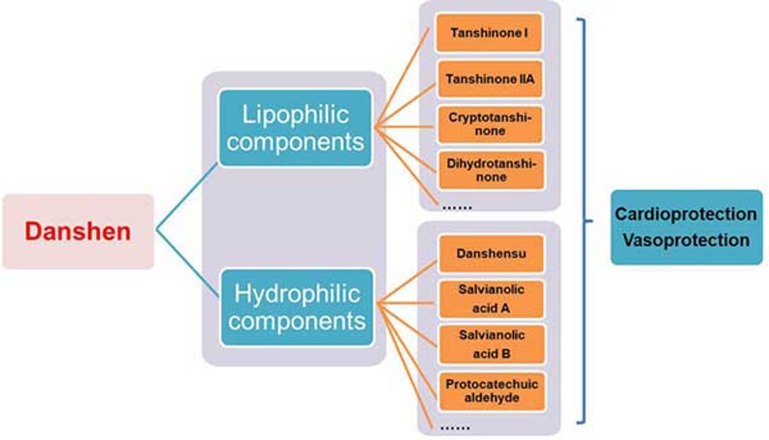
Danshen is a well-known multi-component and multi-targeting cardiovascular TCM, which can be used alone or together with other TCMs for cardiovascular therapy1,2,3,4 (Table 1). Both the lipophilic components (tanshinone I, tanshinone IIa, cryptotanshinone, and dihydrotanshinone, among others) and hydrophilic components (denshensu, salvianolic acid A, salvianolic acid B, and protocatechuic aldehyde, among others) from Danshen have protective effects in atherosclerotic vascular diseases, including atherosclerosis, calcification and aortic aneurysm formation1,2,3,4. In this section, we will review and discuss the anti-atherosclerotic effects and molecular mechanisms of individual major component (Table 2 and Supplementary Table S1) with the aim of providing a comprehensive understanding of the pharmacological effects of Danshen.
Table 1. Anti-atherosclerotic effects of TCM formula containing Danshen.
| Formula | Subjects or models | Effects and mechanisms | References |
|---|---|---|---|
| Cardiotonic Pill (Fufang Danshen Dripping Pill) | Rabbit+HCD+Ad-p53 ↓ICAM-1, ↓VCAM-1 ApoE−/− mice+HFD Hypercholesterolemic patients | ↓Plaque vulnerability ↓Lesion size, ↓ICAM-1 ↓ICAM-1, ↓E-selectin | 311 312 313 |
| Naoxintong | ApoE−/− mice+HFD Rabbit+HCD LDLr−/− mice+HFD | ↓Lesion size, vulnerability ↓MMP2, ↓TNFα, ↑SM22α ↓Lesion size, ↓iNOS/NO ↓Lesion size, ↓DC and Mφ content | 314 315 316 |
| Danshen-Gegen Injection Danhong Injection | Postmenopausal women with early hypercholesterolemia Rats+HFD Rabbits+HCD ApoE−/− mice +HFD LDLr−/− mice+HFD | ↓Carotid intima/media thickness ↓Hyperlipidemia, PPARα ↓Lesion size, iNOS, COX2, MDA ↓Lesion size, TNFα, IL-1β, IL-6↑ABCA1 | 317 318 319 320 |
Abbreviations: ABCA1, ATP-binding cassette transporter A1; ApoE−/−, ApoE deficient; COX2, cyclooxygenase 2; DC, dendritic cells; HCD, high cholesterol diet; HFD, high fat diet; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; iNOS, inducible nitric oxide (NO) synthase; LDLr, LDL receptor; Mφ, macrophage; MDA, malondialdehyde; MMP-2, matrix metalloproteinase 2; TNFα, tumor necrosis factor alpha; VCAM-1, vascular cellular adhesion molecule-1.
Table 2. Therapeutic benefits of bioactive components of Danshen in atherosclerotic vascular diseases.
| Compound | Animal Model | Effects and mechanisms | References |
|---|---|---|---|
| Tanshinone IIA | ApoE−/− mice+HFD | ↓Lesion size and instability, ↓CLIC1, ↓SRA, ↓CD36, ↓LOX1, ↓PPARγ, ↓CD68, ↓NF-κB, ↓MMP-9 | 28,29,30,31,32,38,39,41 |
| ApoE−/− (OVX) mice+HFD | ↓Lesion size, ↓NF-κB, ↓sICAM-1 ↓AP1, ↓E-selectin, ↓p-ERK1/2, ↓HDL, ↑SOD | 30 | |
| Rabbits+HCD | ↓Lesion size, ↓neointima, ↓CD40, ↓MMP-2/9, SOD, ↓MDA, ↓oxLDL, ↑GPx, ↓VCAM-1, ↓IL-1β | 33,34,35,36 27,321 | |
| Rats+HFD | ↓Hepatic lipid deposition ↓Aortic calcification, ↓ROS, ↓MDA, ↓oxLDL, ↑Cu/Zn-SOD | ||
| Rats+balloon injury | ↓Intimal hyperplasia, ↓PCNA | 25 | |
| Mice+carotid artery ligation | ↓Intimal hyperplasia, ↓PCNA | 26 | |
| Rats+ elastase perfusion | ↓AAA incidence, ↑elastin fibers, ↑VSMC content, ↓TLR4, ↓pNF-κB, ↓MyD-88, ↓MMP-2, ↓MMP-9, ↓MCP-1, ↓iNOS | 42,43 | |
| Cryptotanshinone | ApoE−/− mice+HFD | ↓Lesion size and instability, ↓IL-1β, ↓TNFα, ↓IL-6, ↓IL-17A ↓IFNγ, ↓MMP-9, ↓LOX1, ↓ROS | 80 |
| Dihydrotanshinone | ApoE−/− mice+HFD | ↓Lesion size, ↓TLR4, ↓NF-κB, ↓MyD88, ↓ROS, ↓LOX1, ↓NOX4 | 88 |
| Danshensu | Rats+ methionine-rich diet | ↓Lesion size, ↓Hcy, ↓TNFα, ↓ICAM-1, ↓ET1, ↑NO | 94 |
| Salvianolic acid A | ApoE−/− mice+HFD | ↓Lesion size, ↓CCL20, ↓CCR6 | 105 |
| ApoE−/− mice+HFD | ↓Aneurysm severity, ↓MMP-2/9 | 106 | |
| ↓Elastin fragmentation, ↓Macrophage infiltration | 109 | ||
| SHR | ↑Relaxation | 111 | |
| Rat+STZ+HFHS | ↓vWF, vasorelaxation, ↓MDA, ↓AGE | 109 | |
| Salvianolic acid B | Rabbits+HCD | ↓Lipid deposition, ↓neointimal formation, ↓LDL oxidation | 135 |
| ApoE−/− mice+HFD | ↓Neointimal formation, ↓foam cell, ↓MMP-2/9, ↓COX-2, ↓CD36 | 148,154,155 | |
| Rats+balloon injury | ↓Neointimal formation, ↓CXCR-4 | 152 | |
| Protocatechuic aldehyde | Rats+balloon injury | ↑Re-endothelization, ↓neointima, ↑GPER1, ↑CD31, ↓VCAM-1, ↓CD40 | 161 |
Abbreviations: AAA, abdominal aortic aneurysm; ABCA1, ATP binding cassette subfamily A member 1; AGE, advanced glycation endproducts; AP1, activator protein-1; CCL20, Chemokine (C-C motif) ligand 20; CCR6, C-C motif chemokine receptor 6; CD36, cluster of differentiation 36; CD40, cluster of differentiation 40; CLIC1, intracellular channel protein 1; COX2, cyclooxygenase 2; CXCR4, chemokine (C-X-C motif) receptor 4; ERK, extracellular signal–regulated kinases; GPER1, G-protein coupled estrogen receptor 1; GPx, glutathione peroxidase; HCD, high cholesterol diet; Hcy, homocysteine; HDL, high density lipoprotein; HFD, high fat diet; ICAM-1, intercellular adhesion molecule 1; IFNγ, interferon gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; LOX1, lectin-like oxidized low-density lipoprotein receptor-1; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MMP, matrix metalloproteinase; MyD88, Myeloid differentiation primary response gene 88; NF-κB, nuclear factor kappa B; NOX4, NADPH oxidase 4; oxLDL, oxidized LDL; OVX, ovariectomized; PCNA, Proliferating cell nuclear antigen; PPARγ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; SHR, spontaneously hypertensive rat; SOD, Superoxide dismutase; STAT3, signal transducer and activator of transcription 3; sCD40L, soluble CD40 ligand; sICAM1, soluble intercellular adhesion molecule 1; SRA, scavenger receptor A; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion protein 1; vWF, von Willebrand factor.
Major lipophilic components
Tanshinone I
The vasoprotective effects of tanshinone I are mainly observed in cultured cells. For example, in cultured vascular endothelial cells, tanshinone I has potent anti-angiogenic effects via blocking endothelial cell proliferation, migration and tube formation as well as vessel sprouting20. The molecular mechanism is related to the inhibition of basal as well as hypoxia-induced STAT3 phosphorylation at tyrosine 70520. This report suggests that tanshinone I could be a useful therapeutic agent in blocking tumor angiogenesis20. Tanshinone I also enhances endothelial integrity by stabilizing cell-cell junctions, thus preventing vascular leakage21. Lipopolysaccharide (LPS)-stimulated macrophage cell lines, such as RAW264.7, serve as an excellent in vitro model for evaluating anti-inflammatory compounds. Tanshinone I significantly inhibits LPS-induced cyclooxygenase-2 (COX-2)-mediated prostaglandin E2 (PGE2) production22 as well as IL-1223 production. The anti-inflammatory effects are mediated by the inhibitory effects on NF-κB and AP-1 activation23,24. Currently, there is no literature reporting the protective effects of tanshinone I against VSMC proliferation, migration, platelet activation and atherosclerosis development.
Tanshinone IIa
Tanshinone IIa is the most well studied bioactive lipophilic constituent of Danshen in cardiovascular medicine. Clinically, sodium sulfate derivatives of tanshinone IIa (STS) have long been used to treat patients with angina pectoris and coronary heart disease2. In experimental studies, tanshinone IIa has been shown to attenuate neointima hyperplasia25,26, atherosclerotic calcification27, diet-induced atherosclerosis28,29,30,31,32,33,34,35,36,37,38,39,40,41 and aortic aneurysm42,43. During the past decade, emerging evidence has suggested that tanshinone IIa modulates multiple key cellular events in vascular diseases, including LDL oxidation, monocyte-endothelial cell interactions, endothelial cell injury, eNOS-dependent vasorelaxation, proliferation, migration of smooth muscle cells, macrophage cholesterol uptake and efflux, and platelet activation1,2,3,4.
Inhibitory effects of Tanshinone IIa on LDL oxidation
In 2000, the preventative effects of tanshinone IIa on inhibiting LDL oxidation were comprehensively analyzed in vitro44. In both cell-free (Cu2+, peroxyl radical and peroxynitrite-mediated) and macrophage-derived oxidizing systems, tanshinone IIa potently inhibited LDL oxidation by scavenging peroxyl free radical and increasing LDL binding activity44, suggesting that it can block the initiation of atherosclerosis.
Protective effects of Tanshinone IIa on endothelial function
In endothelial cells, tanshinone IIa improves endothelial function through the following mechanisms. (1) Protecting endothelial cells against endothelial injury: Chronic oxidative stressors, such as H2O2 and methylglyoxal (MGO), trigger endothelial injury and subsequent atherogenic events, such as monocyte adhesion and transmigration. Tanshinone IIa has been shown to inhibit endothelial injury induced by H2O238,45,46,47,48 and MGO49 via its anti-oxidant, anti-inflammatory, and xenobiotic and endobiotic detoxification effects. Bi et al 48 designed and tested the endothelial protective effects of tanshinone IIa derivatives and found that several derivatives have increased efficacy against H2O2-induced injury via Nrf2 (nuclear factor (erythroid-derived 2)-like-2 factor) activation and superior water solubility. (2) Preventing inflammatory responses in endothelial cells and endothelial progenitor cells and preventing monocyte adhesion to diseased endothelium: Tanshinone IIa has potent anti-inflammatory effects by blocking the upregulation of pro-inflammatory mediators, such as tumor necrosis factor α (TNF-α), intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), monocyte chemotactic protein 1 (MCP-1), E-selectin, and interleukins (IL-8 & IL-1β), in response to pro-inflammatory stimuli50,51,52,53,54,55,56, thus reducing monocyte adhesion to endothelial cells50,54,56. (3) Regulation of vascular tone and vasorelaxation by increasing NO and decreasing endothelin-1 (ET-1): Several reports have revealed that tanshinone IIa increases the production of NO57,58,59 under different stress conditions in endothelial cells by increasing eNOS levels58,60,61 and eNOS phosphorylation at ser117761 while blocking eNOS ser1177 dephosphorylation61. Tanshinone IIa also regulates vascular tone via decreasing cyclic strain and TNFα-induced ET1 production62,63. In a model of chronic intermittent hypoxia, tanshinone IIa decreases the expression of ETA receptors while increasing that of ETB receptors, thereby dampening ET1 production and induced signaling64. (4) Prevention of eNOS uncoupling: Tanshinone IIa ameliorates eNOS uncoupling induced by multiple agents, such as high glucose. The underlying mechanism is linked to the upregulation of key components in the recoupling of eNOS including the following: ratios of eNOS dimer/monomer and tetrahydrobiopterin (BH4)/dihydrobiopterin (BH2), GTP cyclohydrolase I (GTPCH1), dihydrofolate reductase (DHFR) and heat shock protein 90 (HSP90)11,61,65.
Inhibitory effects of Tanshinone IIa on VSMC proliferation and migration
In VSMCs, tanshinone IIa inhibits the proliferation and migration of VSMCs by inhibiting the activation of ERK25 and PDK1 (3-phosphoinositide-dependent protein kinase 1)66 while activating the BKCa (large-conductance Ca2+-activated K+ channel)67, AMPK (adenosine 5′-monophosphate-activated protein kinase)68 and Nrf269 pathways. Tanshinone IIa also suppresses the apoptosis of VSMCs39, indicating its potential to reduce plaque vulnerability.
Inhibitory effects of Tanshinone IIa on foam cell formation
In macrophages, tanshinone IIa inhibits LPS-induced inflammation70,71,72, oxLDL-induced proliferation and macrophage migration39 and blocks scavenger receptor-mediated oxLDL uptake29,32,73 while promoting ATP-binding cassette transporters ABCA1 and ABCG1-mediated cholesterol efflux via the Nrf2/HO1 pathway32, thereby decreasing foam cell formation.
Inhibitory effects of Tanshinone IIa on platelet aggregation
In platelets, tanshinone IIa inhibits platelet aggregation and activation induced by collagen and ADP74. All the vasoprotective effects of tanshinone IIa contribute to its atheroprotective effects as observed in different animal models and in cultured cells.
Cryptotanshinone
We 75,76,77,78 and others79 have previously shown that cryptotanshinone is a neuroprotective compound in various models of neurodegenerative diseases in vitro and in vivo. However, the atheroprotective effects of cryptotanshinone have not been well recognized until very recently.
Endothelial protective effects of cryptotanshinone against atherosclerosis
Considering the potent anti-inflammatory effects of cryptotanshinone in various systems and participation of inflammation in all import phases of atherosclerosis, it is highly plausible that cryptotanshinone may also ameliorate atherosclerosis via its anti-inflammatory effects. Recently, we80 and several other independent groups81,82 have shown that cryptotanshinone shares some of the properties of tanshinone IIa in inhibiting inflammatory stimuli (such as TNFα- and oxLDL)-induced monocyte adhesion to endothelial cells, foam cell formation and platelet activation, thereby attenuating experimental atherosclerosis in ApoE−/− mice. Specifically, cryptotanshinone inhibits monocyte adhesion by suppressing the scavenger receptor LOX1-mediated pro-inflammatory response (ICAM-1 and VCAM-1 upregulation) in endothelial cells80. Because LOX1 functions as the upstream major receptor for oxLDL in endothelial cells18,83, LOX1 inhibition could be one major anti-atherosclerotic mechanism of cryptotanshinone. The endothelial protective effect of cryptotanshinone is mainly related to the attenuation of endothelial inflammation80. Therefore, the potential effects of cryptotanshinone on other critical aspects of endothelial function (such as eNOS phosphorylation and uncoupling) warrant further studies.
Effects of cryptotanshinone on VSMC proliferation and migration
Like tanshinone IIa, the inhibitory effects of cryptotanshinone on the proliferation and migration of VSMCs have also been reported84. The underlying mechanism is related to the inhibition of matrix metalloproteinase-9 (MMP-9) expression via the NF-κB (nuclear factor-kappa B) and AP1 (Activator protein 1) pathway84.
Anti-inflammatory effects of cryptotanshinone in macrophages
Although cryptotanshinone has minimal inhibitory effects against macrophage-derived foam cell formation85, a recent study has reported that cryptotanshinone displays superior anti-inflammatory effects in LPS-stimulated macrophages compared with tanshinone IIa86, confirming and extending our previous observation that cryptotanshinone inhibits the LPS-induced inflammatory response in murine macrophages by blocking activation of the NF-κB and MAPK (mitogen-activated protein kinase) pathways87. These findings also suggest the necessity to chemically modify cryptotanshinone to increase its therapeutic efficacy. Currently, there is no literature available regarding the thrombo-protective effects of cryptotanshinone in vitro and in vivo, which merit further studies in the future.
Dihydrotanshinone
A recent study88 from Chen's laboratory has shown that dihydrotanshinone attenuates diet-induced atherosclerosis in ApoE−/− mice. The underlying mechanism is related to blockade of the NOX4 (NADPH oxidase 4)/ROS (reactive oxygen species)/NF-κB/LOX-1 signaling pathway in LPS-stimulated human endothelial cells and subsequent oxLDL endocytosis and monocyte adhesion to endothelial cells88. Dihydrotanshinone also inhibits proliferation, migration and tube formation in endothelial cells, thereby inhibiting angiogenesis89. Currently, the regulatory effects of dihydrotanshinone on eNOS-derived NO production remain unknown. Based on a previous study90 showing that dihydrotanshinone has vasorelaxant activities in an aortic ring assay, it is plausible that dihydrotanshinone may have potential effects on NO production in the endothelium. In LPS-stimulated RAW264.7 macrophages, dihydrotanshinone significantly inhibits LPS induced production of COX2-mediated PGE2 as well as iNOS (inducible NO synthase)-dependent NO by blocking the activation of NF-κB and AP-122. Similarly, dihydrotanshinone also exhibits greater inhibitory effects against LPS-induced IL-12 production than tanshinone I and cryptotanshinone, without affecting IL-10 production23. In platelets, dihydrotanshinone functions as a potent thrombin inhibitor compared with tanshinone IIa and cryptotanshinone91. It also significantly inhibits collagen induced platelet aggregation (more potent than green tea component EGCG) by suppressing calcium mobilization and thromboxane B2 production92. The effects of dihydrotanshinone on VSMC pathophysiology and macrophage-derived foam cell formation warrant further studies.
Major hydrophilic components
Danshensu (or Salvianic acid A)
A high level of circulating homocysteine (Hcy) is a risk factor for cardiometabolic diseases, such as atherosclerosis and hyperhomocysteinemia93. In a rat model of hyperhomocysteinemia (by feeding rats with a methionine-rich diet), Danshensu decreases foam cell formation by reducing the expression of TNFα, ICAM-1, and ET-1 while increasing NO production, thus protecting the vascular endothelium from injury94. In cultured human endothelial cells challenged with Hcy (5 mmol/L), Danshensu represents the strongest component in the aqueous extract of Danshen that inhibits Hcy-induced injury95. Danshensu also prevents H2O2 induced endothelial cell injury by inhibiting CD4096 as well as TNFα-induced endothelial permeability by blocking VEGF (vascular endothelial growth factor) production and ERK activation97. In keeping with this function, an excellent study from Zhu's laboratory identified Danshensu as the major component of Danhong injection to exert endothelium-dependent vasodilation in an eNOS/NO-independent, but prostacyclin-dependent, manner98. This evidence provides mechanistic insight into the previously observed ability of Danshensu to dilate swine coronary artery99. In VSMCs, Danshensu has inhibitory effects on the proliferation of VSMCs by decreasing ET1 production while increasing NO production100. In an in vitro model of foam cell formation (RAW264.7 macrophages stimulated with oxLDL), Danshensu inhibits lipid accumulation and foam cell formation by decreasing CD36-dependent oxLDL uptake while promoting ABCA1- and ABCG1-dependent cholesterol efflux101, further extending a previous study that discovered Danshensu as a potential inhibitor of soluble CD36 binding to oxLDL and resultant oxLDL uptake102. In platelets, Danshensu displays excellent anti-platelet and anti-thrombotic activities in vivo by inhibiting COX2 and normalizing the ratio of thromboxane A2 (TXA2)/prostacyclin (PGI2)103 despite the low inhibitory effects on platelet aggregation observed in vitro 104. Currently, no literature is available regarding the protective effects of Danshensu in experimental animal models of atherosclerosis.
Salvianolic acid A
In vivo, salvianolic acid A (Sal-A) has recently been shown to inhibit diet-induced atherosclerosis105 and angiotension II (Ang II)-induced aortic aneurysm formation106 in ApoE−/− mice. It is one of the strongest anti-oxidant phenolic acids in Danshen due to its polyphenolic structure.
Inhibitory effects of Sal-A on LDL oxidation
In 2002, the effect of Sal-A on CuSO4-mediated LDL oxidation was investigated107. The authors observed that Sal-A could chelate Cu2+ and inhibit Cu2+-mediated LDL oxidation. As a result, Sal-A scavenges free radicals and decreases the endproduct of the lipid peroxidation- malondialdehyde (MDA)107.
Effects of Sal-A on endothelial dysfunction and vascular remodeling
Seminal studies from Du's laboratory108,109,110 and others111 have recently investigated the effects of Sal-A on endothelial dysfunction and vascular remodeling. The studies have revealed that Sal-A is not hypotensive, but it ameliorates hypertension and high-fat, high-sucrose diet-associated impairment of endothelium-dependent vasorelaxation in spontaneously hypertensive rats111 and diabetic rats109, respectively. In vitro, Sal-A increases endothelial barrier function in LPS-stimulated endothelial cells111. Multiple disease conditions, such as ischemia/reperfusion, impair NO production. Sal-A reverses the ischemia/reperfusion-induced decrease in NO bioavailability by decreasing MKP-3 (mitogen-activated protein kinase phosphatases 3)112. Sal-A also inhibits AGE (advanced glycation end products)-induced endothelial cell injury109. A more recent study has shown that Sal-A is a safe ET1 type A receptor (ETAR) antagonist in HEK293 cells overexpressing ETAR (IC50=5.7 μmol/L)113, suggesting that Sal-A could have therapeutic effects in hypertension-associated vascular remodeling. Sal-A does not affect basal endothelial cell proliferation and NO production, but it reduces Ang II-induced proliferation of human endothelial cells by inhibiting ROS generation as well as blocking the phosphorylation of Src and Akt114. Recent studies have shown that Sal-A represses TGF-β1 (transforming growth factor-β)- and hypoxia-induced endothelial-to-mesenchymal transition by activating Nrf2 and modulating Smads115,116. Sal-A also attenuates PDGF-BB (platelet-derived growth factor-BB)-induced proliferation and migration of VSMCs via the PDGFRβ/ERK108 and cAMP (cyclic adenosine monophosphate)/PKA (protein kinase A)/CREB (cAMP-response element binding protein) signaling pathways and shows efficacy in preventing neointimal hyperplasia110.
Effects of Sal-A on macrophages
In macrophages, Sal-A serves as an NF-κB inhibitor by targeting IKKβ (inhibitor of NF-κB kinase) as well as an activator of anti-oxidant HO-1, thereby suppressing LPS-induced upregulation of pro-inflammatory mediators (COX2, iNOS, TNFα and IL-6) and the generation of NO and PDE2117,118. Sal-A also attenuates Ang II-induced macrophage apoptosis by inhibiting the activation of Akt and NF-κB119, suggesting the occurrence of broad anti-inflammatory activities induced by multiple pro-inflammatory stimuli. It remains to be investigated whether Sal-A affects cholesterol uptake and efflux and resultant foam cell formation in macrophages.
Anti-thrombotic effects of Sal-A, its derivatives and preparations
In 1994, Yu et al120 evaluated the thrombo-protective effects of acetylsalvianolic acid A, a chemically modified derivative of Sal-A. The authors observed that acetyl-Sal-A could inhibit platelet aggregation induced by multiple pro-aggregative stimuli, including thrombin, collagen, ADP, and arachidonic acid, suggesting that acetyl-Sal-A has potent anti-thrombotic activities. Subsequent in vitro and in vivo studies have confirmed that Sal-A inhibits ADP and collagen-induced platelet aggregation and arterial thrombus formation in mice121,122,123,124. Salvianolic acids, in particular Sal-A and Sal-C, are core components of Danhong injection exerting anti-thrombotic activity125. The cardiovascular actions of salvianolic acids have recently been comprehensively reviewed elsewhere126.
Salvianolic acid B
Salvianolic acid B (Sal-B) and its derivative magnesium lithospermate B (also known as magnesium tanshinoate B) are commercially available and named Sal-B for simplicity hereafter.
Protective effects of Sal-B on endothelial function
In 2001, two research groups simultaneously reported that Sal-B improved endothelial function by decreasing TNFα-activated monocyte adhesion to endothelial cells127 as well as VEGF-triggered hyperpermeability in endothelial cells128, respectively. Subsequent studies have shown that Sal-B decreases TNFα-induced upregulation of PAI1 (plasminogen activator inhibitor-1), ICAM-1 and VCAM-1 by inhibiting NF-κB and AP1 activity as well as upregulating the anti-oxidant Nrf2/HO1 pathway129,130,131, underscoring its therapeutic effects in ameliorating inflammation by activating Nrf2 in vivo132. Sal-B modulates endothelial hemostasis by increasing tissue-type plasminogen activator (t-PA), anti-coagulant thromomodulin (TM), and eNOS-dependent NO production, while decreasing pro-thrombotic PAI1133,134. Sal-B also inhibits LDL oxidation135,136, extravasation137 and ensuing oxLDL-induced endothelial cell injury135 and apoptosis138. Sal-B also prevents oxidant H2O2-induced endothelial cell injury by activating the GRP78 (glucose regulated protein 78 kDa)/ATF6 (activating transcription factor 6) and PI3K (phosphoinositide 3-kinase) pathways139,140. In addition, Sal-B also improves endothelium-dependent vasorelaxation in diabetic rats with fluctuating blood glucose levels 141, as well as angiotensin II-infused mice142, by inhibiting AT1 receptor and NADPH oxidase-dependent ROS production, as well as restoring eNOS phosphorylation at Ser1177.
Inhibitory effects of Sal-B on VSMC proliferation and migration
In VSMCs, Sal-B attenuated the proliferation and migration of VSMCs (induced by PDGF-BB, serum, LPS and stromal cell-derived factor-1α (SDF-1α)) by cell cycle arrest and blocking CXCR4 as well as activating the Nrf2/HO1 pathway131,143,144. Another anti-proliferative mechanism of Sal-B is exerted by inhibiting TNFα-induced upregulation of MMP-2 expression and activity145.
Inhibitory effects of Sal-B on foam cell formation
In LPS-activated RAW264.7 macrophages, Sal-B inhibits iNOS-dependent NO production by activating the HO1 pathway146. Sal-B also reduces CD36-dependent oxLDL uptake while promoting cholesterol efflux via the PPARγ/LXRα/ABCA1 pathway147, thereby inhibiting foam cell formation102,147,148.
Inhibitory effects of Sal-B on platelet aggregation
In platelets, Sal-B significantly inhibits ADP and thrombin-induced platelet aggregation by reducing the release of soluble P-selectin and antagonizing the activity of phosphodiesterase (PDE) and P2Y12 receptor130,149,150. As a result, Sal-B reduces the adhesion of ADP-activated platelets to endothelial cells via the NF-κB-driven inflammatory response149 and limits LPS-induced disseminated intravascular coagulation in rabbits151.
The above-mentioned combined effects potentially contribute to the protective effects of Sal-B against neointimal hyperplasia135,152, angiotensin II-induced hypertension142, hyperglycemia/dyslipidemia153, and atherosclerosis development in ApoE−/− mice154,155.
Protocatechuic aldehyde
In 2004, Chan et al95 compared the efficacy of several components from the aqueous extract of Danshen in preventing Hcy-induced endothelial injury and observed that protocatechuic aldehyde also possesses protective effects, although it is less efficacious than danshensu. Subsequent studies have revealed that protocatechuic aldehyde inhibits LPS-induced endothelial cell injury and apoptosis by inhibiting caspase 3, thereby maintaining endothelial cell barrier integrity156. Protocatechuic aldehyde and its precursor compound 3-hydroxybenzaldehyde also inhibit TNFα-induced endothelial inflammation (ICAM-1 and VCAM-1 upregulation) and monocyte adhesion to endothelial cells by inhibiting the activation of JNK, AP1 and NF-κB157,158,159. In VSMCs, protocatechuic aldehyde and its precursor compound 3-hydroxybenzaldehyde show activity in attenuating PDGF-BB-stimulated migration and proliferation (via MAPK and PI3K/Akt pathways) of VSMCs and inhibiting platelet aggregation and the occurrence of neointimal hyperplasia as well as intravascular thrombosis in vivo159,160. A more recent study has identified GPER1 (G protein-coupled estrogen receptor-1) as the protective mechanism of protocatechuic aldehyde against endothelial dysfunction both in vitro and ex vivo 161. In TNFα-stimulated macrophages, protocatechuic aldehyde reduces HMGB1 (high mobility group box-1 protein) expression by blocking the activation of NF-κB, underscoring its protective effects against the inflammatory response associated with rat sepsis (induced by cecal ligation and puncture)162. Based on the protective effects mentioned above, protocatechuic aldehyde could potentially ameliorate experimental atherosclerosis in animal models, warranting further studies.
In addition to the above-described vasoprotection, bioactive constituents from Danshen also show prominent cardioprotective effects in several heart diseases. In the next section, we will provide an overview of the protective effects and mechanism of individual compounds in cardioprotection.
Cardioprotective effects of Danshen
Pathophysiology of heart diseases
Coronary heart disease is the leading cause of death and disability worldwide. The acute occlusion of the coronary artery commonly induced by atherosclerosis and plaque rupture subjects the myocardium to acute myocardial ischemia163. Ischemia of the heart resulting from oxygen and nutrient supply deprivation can lead to cardiomyocyte death and subsequently demarcate the area at risk of myocardial infarction164. Restoration of blood flow in the ischemic heart using either thrombolytic therapy or primary percutaneous coronary intervention induces additional cardiac damage, termed “myocardial ischemia-reperfusion injury”164,165. Chronically, the disturbance of cardiac homeostasis, implied by the loss of myocytes, inflammatory events and oxidative stress insult, leads to the development of pathological cardiac remodeling166. A prominent feature of the remodeling heart is cardiomyocyte hypertrophy167, which is due to the dysregulation of a number of cardiac transcription factors168,169. Extracellular matrix remodeling is also involved, which is characterized as fibrosis and activation of MMPs170. Cardiac remodeling is the key pathophysiological process leading to heart failure163,166,171.
Effects of Danshen components on heart diseases
A huge amount of experimental and clinical research have reported that Danshen, either the crude medicine or its preparations (Danshen injection, Danshen dripping pill, Danhong injection, and Danshen-Gegen decoction, among others), are favorable for the heart during pathological processes, such as myocardial ischemia, myocardial infarction, and reperfusion injury172,173,174,175,176,177,178,179,180. Danshen components, in particular the lipophilic tanshinone IIa and cryptotanshinone as well as the hydrophilic Danshensu, Sal-A and Sal-B, show potent beneficial effects on the heart. Most of these bioactive components protect the heart against acute ischemic injury due to their anti-oxidant, anti-inflammatory and anti-apoptotic properties. Additionally, some of them show favorable effects on pathological cardiac remodeling, reflecting their potential therapeutic promise in treating chronic heart diseases, such as heart failure. In the following section, we focus on the cardioprotective effects and mechanisms of the major Danshen components (Supplementary Table S2).
Major lipophilic components
Tanshinone IIa
Tanshinone IIa is one of the major components of lipophilic tanshinones in Danshen. Due to its poor absorption through the intestine, its sodium sulfate derivative STS has been developed to enhance the bioavailability181. The cardioprotective effects of tanshinone IIa and STS are discussed below with respect to their potent protective effects against acute cardiac ischemic injury, including myocardial infarction, myocardial I/R injury and arrhythmia, as well as chronic pathological cardiac remodeling, including cardiac hypertrophy and cardiac fibrosis.
Protective effects of Tanshinone IIa against ischemic injury of the heart
STS has been widely used in clinics for the treatment of coronary heart disease. Pharmacological studies have demonstrated that tanshinone IIa protects the heart against ischemic injury and would be a promising therapeutic agent in MI, myocardial I/R injury and arrhythmia.
MI is an orchestrated event that combines cardiomyocyte death (reflected as necrosis, apoptosis and autophagy), a massive inflammatory burst and ROS generation, in response to arrhythmic injury182. In animal models of MI, tanshinone IIa can reduce the MI size and preserve cardiac function183,184,185,186,187,188. These beneficial effects are not limited to the ability of tanshinone IIA to dilate the coronary artery and increase coronary blood flow but also to its anti-oxidant, anti-inflammatory, and anti-apoptotic effects on cardiomyocytes. The antioxidant effect of tanshinone IIa is attributed to the modulation of the redox-sensitive ERK/Nrf2/HO1 and AMPK/ACC (acetyl-coenzyme A carboxylase)/CPT1 (carnitine palmitoyltransterase-1) pathways185 and the stimulation of an electron transfer reaction in mitochondria189. Inflammation is critically involved in the pathogenesis of MI. In this regard, tanshinone IIa inhibits the activation of NF-κB, eventually attenuating the expression of the inflammatory mediators MCP1, TGF-β1 and TNFα and preventing macrophage infiltration into the infarcted myocardium184. Additionally, tanshinone IIa attenuates the formation of the NOD-like receptor (NLR) family, pyrin-domain containing 3 (NLRP3) inflammasome, which has been identified as a mediator of the inflammatory response in MI190, and subsequently prevents the downstream inflammatory cascades and lipid metabolism disorder183. Tanshinone IIa prevents cardiomyocyte apoptosis induced by oxidative stress191,192,193,194, hypoxia195,196, and oxygen-glucose deprivation/recovery197. The mechanisms underlying these anti-apoptotic effects involve the downregulation of caspase-3 and upregulation of the Bcl-2/Bax ratio via the PI3K/Akt-dependent192,195,198 or JNK/SAPK (stress-activated protein kinase)/MAPK signaling pathway194, as well as the regulation of microRNAs192,196,199,200. MicroRNAs are short, highly conserved, non-coding RNAs that regulate gene expression at the post-transcriptional level by inhibiting translation or promoting degradation of target mRNAs201. Tanshinone IIa upregulates the anti-apoptotic miR-133192,196 and miR-152-3p200, whereas it decreases the apoptotic miR-1199. All these observations have yielded promising results indicating that tanshinone IIa might be favorable for the treatment of MI. In addition to its benefits alone, tanshinone IIa also interacts with other agents or therapeutics in MI treatment. Combined therapy of tanshinone IIa and simvastatin reduces circulating inflammatory markers and improves symptoms of angina and blood stasis syndrome in post-MI patients202. Due to its ability to increase bone marrow mesenchymal stem cell (BMSC) engraftment in the ischemic myocardium, tanshinone IIa enhances the efficacy of BMSC transplantation treatment, which aims to confine myocardial damage and regenerate the myocardium in acute MI203,204. In contrast, tanshinone IIa can ameliorate the cardiotoxicity effect of adriamycin (also known as doxorubicin), an effective antineoplastic agent, mainly by preventing against cardiac apoptosis and lipid oxidation205,206,207,208.
Myocardial I/R injury refers to the damage to the heart caused by the restoration of coronary blood flow after an ischemic episode164,165. Treatment of tanshinone IIa, prior209,210,211,212,213 or after214,215 I/R injury, reduces the infarct size and ameliorates several consequences of myocardial I/R, including the myocardial zymogram, oxidative status, cardiac dysfunction and microstructure disorder. These observations have confirmed that tanshinone IIa is able to prevent and cure myocardial I/R injury. Optimization of the therapeutic time window for sodium tanshinone IIa sulfonate (8 mg/kg) resulted in 2 h to 4 h after reperfusion214. The underlying pathophysiology of myocardial I/R injury likely involves many factors, such as oxidative stress, intracellular calcium overload, altered cardiac energy metabolism, activation of cardiomyocyte apoptosis, and inflammatory responses164. Tanshinone IIa can decrease ROS production209,214, inhibit inflammation209,212,213, and protect cardiomyocytes against apoptosis211,213,216, potentially contributing to its beneficial effects on myocardial reperfusion injury.
During cardiac ischemia, arrhythmia commonly occurs, which might consequently lead to cardiac death. Tanshinone IIa decreases the incidence of arrhythmias induced by acute cardiac ischemia. This anti-arrhythmic effect is not fully understood. Shan et al reported that tanshinone IIa restored the diminished inward rectifying K+ (Kir) current and Kir2.1 protein level after MI in rat ventricular myocytes by suppressing miR-1199. Controversially, Sun et al have demonstrated that tanshinone IIa predominantly activates cardiac KCNQ1/KCNE1 K+ channels without affecting other K+ channels, including Kir, Kv1.5, or hERG (human ether-a-go-go-related gene)217. In addition to K+ channels, hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels have also been reported to be involved in the anti-arrhythmic effect of tanshinone IIA. The precise underlying mechanisms remain to be determined to draw more definite conclusions.
Protective effects of Tanshinone IIa against pathological cardiac remodeling
The protective effects of tanshinone IIa or STS against pathological cardiac remodeling are associated with its ameliorative effect against cardiac hypertrophy and cardiac fibrosis. The anti-hypertrophic properties of tanshinone IIa have been observed in spontaneously hypertensive rats 218,219, two-kidney one-clip hypertensive rats220, two-kidney two-clip hypertensive rats221, angiotensin II-infused rats222, and pressure-overloaded rats induced by transverse aortic constriction223. In most of these studies, favorable effects of tanshinone IIa have reflected the decrease in the ratio of left ventricular weight to body weight, and the decrease in cardiomyocyte size and diameter are independent of the alteration of systemic blood pressure218,220,221,222, thus eliminating the possibility that tanshinone IIa modulates cardiac hypertrophy by lowering blood pressure. The main drivers of pathological hypertrophy are neurohumoral mediators, particularly the renin–angiotensin system and the beta-adrenergic system224. Tanshinone IIa represses the hypertrophic process in response to hypertrophic stimuli, including angiotensin II222,225,226, isoproterenol (ISO)227, and insulin-like factor-II (IGF-II)228, suggesting a broad anti-hypertrophic effect of tanshinone IIa. The regulation of tanshinone IIa in cardiomyocyte hypertrophy involves multiple mechanisms: (1) tanshinone IIa suppresses intracellular signaling pathways that regulate expression of the cardiac genes encoding structural proteins or regulatory proteins, including MEK/ERK222, AP1 (c-jun/c-fos)225,226, calcineurin/NFAT3 (nuclear factor of activated T cells 3)227,228, and the Cys-C/Wnt signaling pathway219; (2) tanshinone IIa upregulates eNOS expression and promotes the phosphorylation of eNOS in the myocardium187,219; (3) tanshinone IIa activates silent information regulator 1 (SIRT1) to attenuate oxidative stress and inflammation involved in cardiac hypertrophy223; (4) tanshinone IIa diminishes NADPH oxidase-derived oxidative stress221. The anti-fibrotic effects of tanshinone IIa involve inhibition of myofibroblast proliferation229; prevention of the deposition of extracellular matrix (ECM) components, such as collagen and fibronectin230,231,232,233,234; and regulation of the balance between MMPs and tissue inhibitor of metalloproteinases (TIMPs)220,232,235,236. Mechanistically, these anti-fibrotic effects are mainly associated with the reduction of ROS production via the repression of NADPH oxidase221,230,236 and suppression of the typical fibrotic signaling pathway TGFβ1/Smad-2 or -3233,234. It has recently been reported that microRNAs are also involved in the regulation of tanshinone IIa in cardiac fibrosis. Tanshinone IIa upregulates the expression of miR-29b, which inhibits the synthesis of collagen through directly binding to its 3′ untranslated regions233. Taken together, these detailed studies suggest a promising effect of tanshinone IIa on attenuating pathological cardiac remodeling. Indeed, clinical studies provide evidence that STS treatment in patients with ST-segment elevation myocardial infarction, when used in combination with current therapies, may significantly reduce adverse left ventricular remodeling and potentially improve clinical outcomes237,238. Because of close association of cardiac remodeling with the development of heart failure, such experimental and clinical observations might suggest an emerging role of tanshinone IIa in chronic heart diseases, such as heart failure.
Cryptotanshinone
A limited number of reports regarding the cardioprotective effect of cryptotanshinone are available to date. We239 and others240 have previously reported that cryptotanshinone has protective effects against MI and myocardial I/R injury in vivo. In an acute MI experimental model induced by coronary artery ligation, cryptotanshinone dose-dependently ameliorated the disordered arrangement of myocardial tissues and accumulation of inflammatory cells239. In a rat model of myocardial I/R injury induced by occluding the left anterior descending coronary artery, pre-treatment of cryptotanshinone significantly reduced the infarct size and improved myocardial contractile dysfunction240. The underlying mechanisms were concluded to be the amelioration of microcirculatory disturbances through inhibition of endothelial inflammation. Unfortunately, the effects of cryptotanshinone on cardiac cells were not assessed in that study240. Jin et al reported that cryptotanshinone prevents cardiomyocyte apoptosis induced by hypoxia, potentially by modulating the mitochondrial apoptosis signaling pathway (referring to the regulation of mitochondrial hyperpolarization, cytochrome c release and caspase-3 activity) and expression of pro-apoptosis proteins195. In addition, a more recent study has revealed that cryptotanshinone improves mitochondrial function in cardiomyocytes by promoting mitochondrial biogenesis and ATP production and by suppressing the generation of free radicals241. These observations might at least partially explain the cardioprotective effect of cryptotanshinone on MI and myocardial I/R injury. Furthermore, the effect of cryptotanshinone against cardiac fibrosis has been investigated by our group239 and others242. The underlying mechanisms are mainly related to the suppression of MMP-2 production and NADPH oxidase-dependent ROS production239,242. The therapeutic potential of cryptotanshinone in the treatment of heart diseases must be further elucidated.
Tanshinone IV
Tanshinone IV and its water-soluble derivatives can recover cardiac contractility during hypoxia/reoxygenation injury by improving myocardial energy production and inhibiting calcium overloading243,244,245. These observations suggest the potential role of tanshinone IV against cardiac ischemia. In addition, tanshinone IV has been reported to prevent cardiomyocyte hypertrophy and cardiac fibrosis after stimulation by several humoral factors, including Ang II, ET1, IGF1 and the α-adrenoceptor agonist phenylephrine246,247. Further in vivo studies are still needed to assess the cardioprotective effects of tanshinone IV.
Major hydrophilic components
Danshensu
Protective effects of Danshensu against myocardial ischemia injury and I/R injury
In a rat model of myocardial ischemia injury induced by ISO, Danshensu can reverse changes in heart morphology and electrocardiographic patterns, and it can reduce the serum level of creatine kinase and lactate dehydrogenase, which are regarded as diagnostic marker enzymes for altered cardiac membrane integrity and/or permeability in MI248. In the rat MI model induced by left anterior descending coronary artery ligation, Danshensu can alleviate myocardial ischemia injury by potentiating post-ischemia neovascularization, probably by improving endothelial progenitor cell survival against hypoxia and accelerating proangiogenic functions249. By using the whole-cell patch-clamp techniques, Danshensu has been observed to inhibit the L-type calcium current, leading to a recovery of the augmented myocardial contractility that responds to myocardial ischemia injury248.
Additionally, Danshensu has been demonstrated to prevent myocardial I/R injury, which is related to its anti-apoptotic effects, by activating the PI3K/Akt and ERK1/2 signaling pathways250, as well as its antioxidant effects by activating the Akt/ERK/Nrf2/HO-1 signaling pathways251. A recent study using a coexpression network-based approach by integrating gene expression profile and protein-protein interaction data suggests that the protective effect of Danshensu in coronary heart disease is associated with sodium/hydrogen exchanger 3 (SLC9A3), prostaglandin G/H synthase 2 (PTGS2), oxidized low-density lipoprotein receptor 1 (OLR1), and fibrinogen gamma chain (FGG)252.
Protective effects of Danshensu against pathological cardiac remodeling
In pathological cardiac remodeling, Danshensu can diminish cardiac hypertrophy and cardiac fibrosis in response to spontaneous hypertension or β-adrenergic activation253,254. Danshensu also inhibits aldosterone-induced cardiomyocyte apoptosis by interfering with the p53 signaling pathway, suggesting that Danshensu is protective against heart failure caused by overactivation of the renin-angiotensin-aldosterone system255. Moreover, Danshensu is anti-arrhythmic, as implied by observations that Danshensu reduces the incidence of ventricular tachycardia and ventricular fibrillation253,254.
Cardioprotective effects of Danshensu derivatives and preparations
Although Danshensu has shown promising cardioprotective effects, its poor chemical stability, poor cellular permeability and low bioavailability have limited its therapeutic applications256. Thus, a series of novel derivatives of Danshensu have been developed. Pharmacological investigations have shown that these derivatives prevent myocardial ischemia injury in the heart, confirming their therapeutic potential in heart diseases256,257,258,259,260,261. Additionally, the combination of Danshensu and other agents, such as hydroxysafflor yellow A262, paeonol263,264, and puerarin265,266, shows synergistic cardioprotective effects, thus providing additional options for the clinical uses of Danshensu.
Salvianolic acid A
The predominant cardioprotective effects of Sal-A are to confine myocardial damage during the progression of MI and reperfusion injury. In MI models induced by either coronary artery ligation or ISO, Sal-A decreases the infarct size and improves systolic function post-MI267,268,269,270. One of the possible underlying mechanisms is suggested to be associated with its antioxidant properties. Sal-A is a potent free radical scavenger due to its polyphenolic structure271. Additionally, Sal-A improves cellular anti-oxidative defense against oxidative stress by elevating the activity of superoxide dismutase, catalase and glutathione peroxidase269. Moreover, Sal-A is able to maintain mitochondrial integrity and protect against mitochondrial respiratory function269. Considering these antioxidant properties together, Sal-A ameliorates oxidative stress-induced impairment of cellular functions and cell death in the myocardium. Another possible involved mechanism might be the ability of Sal-A to promote angiogenesis around the infarcted area268,272. Sal-A enhances the expression of pro-angiogenic factors, such as VEGF and VEGFR2, and elevates the numbers and function of endothelial progenitor cells (EPCs), leading to vasculogenesis and subsequently increasing the blood flow supply in the ischemic myocardium268. In addition to MI, Sal-A has also been shown to protect against myocardial I/R injury273,274,275,276,277. This protection is achieved by the reduction of myocardial cell apoptosis and damage induced by oxidative stress274,275,278, prevention of intracellular calcium overload by blocking L-type calcium current276, and inhibition of platelet aggregation and inflammation277.
Although comprehensive investigations of Sal-A in cardiac remodeling are not currently available, a study has revealed that Sal-A acts as a MMP-9 inhibitor to attenuate cardiac fibrosis in the spontaneously hypertensive rat279, shedding new light on the cardioprotective effects of Sal-A in pathological remodeling.
Salvianolic acid B
Protective effects of Sal-B against MI and I/R injury
Similarly to Sal-A, Sal-B has demonstrated cardioprotective effects on cardiac ischemic injury187,280,281,282 and reperfusion injury283,284,285,286,287,288.
During acute MI, Sal-B regulates multiple targets involved in cell apoptosis pathways, including the pivotal poly (ADP-ribose) polymerase-1 (PARP-1) and NF-κB signaling pathways282. In addition, Sal-B disrupts the interaction between p38 and TGFβ-activated protein kinase 1-binding protein 1 (TAB1), inhibiting the autophosphorylation of p38 and finally inhibiting TAB1/p38-mediated apoptosis signaling280. In addition to these anti-apoptotic effects, Sal-B inhibits voltage-dependent Ca2+ channels289 and the Ca2+-dependent cAMP and downstream PKA signaling281, which might also contribute to its anti-MI effects. Like tanshinone IIa, treatment with Sal-B could enhance BMSC transplantation290,291 and suppress the apoptosis of embryonic stem cell (ESC)-derived cardiomyocytes292, suggesting that Sal-B holds therapeutic potential in stem cell therapy for MI.
The predominant mechanism underlying the beneficial effect of Sal-B against myocardial I/R injury is associated with its anti-apoptotic properties283,287. This anti-apoptotic effect involves the regulation of relevant signaling pathways during myocardial I/R damage, including the PI3K/Akt-dependent287 and SAPK signaling pathways283. Additionally, the cardioprotective effects of Sal-B against myocardial I/R injury have also been attributed to its anti-oxidant and anti-inflammatory properties286,288,293. Moreover, Sal-B suppresses autophagy by upregulating miR-30a to improve cardiomyocyte viability during myocardial I/R damage285,294.
Protective effects of Sal-A against cardiac remodeling
Jiang et al have previously identified Sal-B as a MMP-9 inhibitor to prevent cardiac remodeling295. Our recent study has shown that Sal-B prevents cardiomyocyte hypertrophy by inhibiting PARP1296. These observations thus suggest the potential effects of Sal-B in the treatment of heart failure, which develops as an automatic response to pathological cardiac remodeling. In agreement with this notion, a recent study has demonstrated that Sal-B alleviates heart failure induced by pressure overload297. Therefore, Sal-B holds promise for cardioprotection against heart failure but requires confirmation in more experimental and clinical studies.
Protocatechuic aldehyde
An accumulating amount of research has shown that protocatechuic aldehyde exerts multiple biological activities, such as antioxidant, anti-inflammatory, anti-apoptosis and anti-proliferation in different tissues298,299. In the heart, protocatechuic aldehyde prevents myocardial I/R injury due to its anti-inflammatory, anti-apoptosis, and anti-platelet aggregation effects300; prevents against cardiomyocyte apoptosis induced by hypertension301; and ameliorates angina by decreasing fatty acid oxidation, which is beneficial for the ischemic heart by switching the energy substrate preference from fatty acids to glucose302. Moreover, protocatechuic aldehyde is regarded as a promising cardioprotective complementary medicine, as determined from observations that protocatechuic aldehyde improves cardiac function in streptozotocin-induced type 1 diabetic rats303 and prevents cardiotoxicity by exposure to the highly toxic environmental contaminant 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD)304.
Conclusions and perspectives
Danshen is a multi-component herbal medicine that benefits the cardiac and vascular system2. The eminent cardiovascular actions and therapeutic potential of the lipophilic and hydrophilic components have sparked broad research interest in the past decade. Understanding the pharmacological and therapeutic profiles of these constituents may broaden the potential clinical applications of these compounds in the treatment of cardiovascular diseases, and they may promote small-molecule cardiovascular drug discovery and development through the use of these compounds as important sources of lead compounds. Based on the broad cardiovascular protective profile of these bioactive constituents, it can be recognized that both lipophilic and hydrophilic components may function in concert, targeting different tissues and signaling pathways to achieve the versatile cardiovascular actions of Danshen in experimental animals and humans. However, the differential pharmacokinetic and pharmacodynamics properties of individual compounds remain a hurdle to the systematic evaluation of the cardiovascular efficacy of Danshen. In particular, tanshinone IIa305 and cryptotanshinone306 have relatively low oral bio-availability. Therefore, new formulation strategies and combination therapy that might maximize the beneficial actions and reduce the potential side effects would have great therapeutic potential in this regard307.
Although research investigating the cardiovascular effects of Danshen is expanding, many questions remain unaddressed. In the vascular system, although sodium tanshinone IIa sulfate is widely used in the clinic to treat patients with coronary artery disease, clinical studies addressing the efficacy of tanshinone IIa in patients with atherosclerosis merit further investigations. Additionally, understanding of the therapeutic basis of other bioactive components remains limited. In the cardiac system, although most of the Danshen components demonstrate promising therapeutic potential for the management of MI and myocardial I/R injury, investigations of their pharmacological actions on cardiac hypertrophy and cardiac fibrosis remain limited. The possible therapeutic role of Danshen components for the treatment of chronic heart diseases related to cardiac remodeling must be further elucidated. Future directions of cardiovascular research involving Danshen include the following: (1) use of the total synthesis of bioactive components of Danshen for the purpose of cardiovascular therapeutics as an alternative to obtaining purified compounds from the medicinal plant, such as the recently described synthesis of tanshinone I308; (2) use of a systems biology approach, such as RNA-sequencing309, or network-based pharmacological research310 to understand the gene regulation profile of each individual compound at the genome-wide level; and (3) elucidation of the therapeutic effects of Danshen components in cardiovascular aging, which is a common basis for all major cardiovascular and metabolic diseases. Overall, Danshen and its bioactive constituents represent an invaluable source for small-molecule cardiovascular drug discovery. Currently, Danshen and its preparations (such as Fufang Danshen Dripping Pill, Fufang Danshen injection, and Danhong injection, among others) have been widely used in China1,2,3,4. However, clinical applications of these Danshen preparations in other countries are still limited. Investigations of the cardiovascular effects and mechanisms of Danshen and its bioactive constituents may also broaden our understanding of Danshen and its preparations for therapeutic applications worldwide.
Figure 2.
The multi-component nature of Danshen in cardioprotection and vasoprotection.
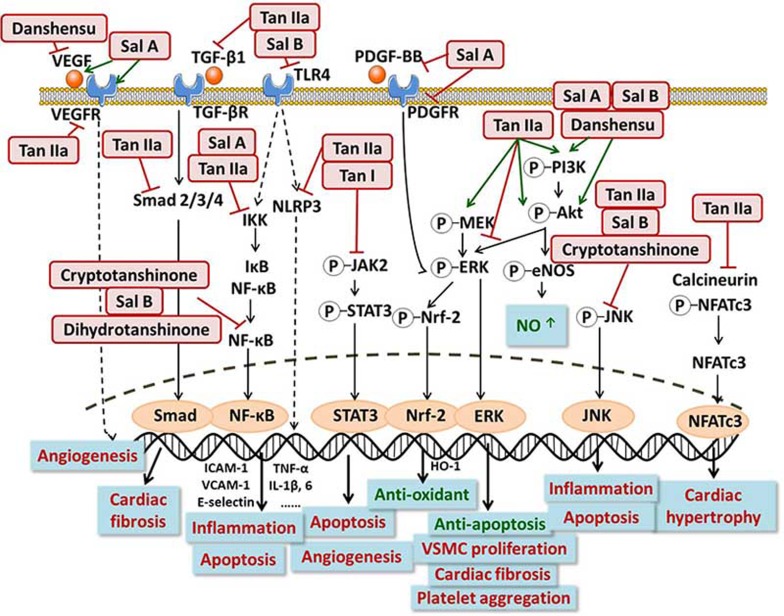
Figure 3.
The major signaling pathways involved in the cardiovascular effects of Danshen components.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No 81473205, 81400359, and 81673433), National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province), Major Project of Guangdong Provincial Department of Science and Technology (No 2015B020232009, 2014B020210003 and 2013B090700010), Major Project of Platform Construction Education Department of Guangdong Province (No 2014GKPT002), Guangdong Province Science and Technology Plan project-Public Research and Capacity Construction (2015(No 3)), Guangzhou Science and Technology Projects (No 201604020121 and 201509030006), and funding from Guangdong Provincial Engineering Laboratory of Druggability and New Drugs Evaluation, Guangzhou Key Laboratory of Druggability Assessment for Biologically Active Compounds, and 111 Project of China (No B16047).
Footnotes
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary Information
Anti-atherosclerotic mechanisms of bioactive components of Danshen
Cardioprotective mechanisms of bioactive components of Danshen
References
- Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005; 45: 1345–59. [DOI] [PubMed] [Google Scholar]
- Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 2012; 220: 3–10. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xu H. Anti-Inflammatory and immunomodulatory mechanism of tanshinone IIa for atherosclerosis. Evid Based Complement Alternat Med 2014; 2014: 267976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opin Ther Pat 2013; 23: 149–53. [DOI] [PubMed] [Google Scholar]
- Chen X, Guo J, Bao J, Lu J, Wang Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev 2014; 34: 768–94. [DOI] [PubMed] [Google Scholar]
- Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev 2007; 27: 133–48. [DOI] [PubMed] [Google Scholar]
- Zhao W, Feng H, Guo S, Han Y, Chen X. Danshenol A inhibits TNF-alpha-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep 2017; 7: 12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 2015; 116: 307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014; 114: 1867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473: 317–25. [DOI] [PubMed] [Google Scholar]
- Fang J, Little PJ, Xu S. Atheroprotective effects and molecular targets of tanshinones derived from herbal medicine Danshen. Med Res Rev 2017. [DOI] [PubMed]
- Xu S, Bai P, Little PJ, Liu P. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev 2014; 34: 644–75. [DOI] [PubMed] [Google Scholar]
- Kapourchali FR, Surendiran G, Chen L, Uitz E, Bahadori B, Moghadasian MH. Animal models of atherosclerosis. World J Clin Cases 2014; 2: 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentikainen MO, Oorni K, Ala-Korpela M, Kovanen PT. Modified LDL-trigger of atherosclerosis and inflammation in the arterial intima. J Intern Med 2000; 247: 359–70. [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atheros-clerosis. Circulation 2004; 109: III27–32. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 2015; 214: 33–50. [DOI] [PubMed] [Google Scholar]
- Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci 2013; 70: 2859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012; 1: 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li JX, Wang YQ, Miao ZH. Tanshinone I inhibits tumor angiogenesis by reducing Stat3 phosphorylation at Tyr705 and hypoxia-induced HIF-1alpha accumulation in both endothelial and tumor cells. Oncotarget 2015; 6: 16031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Zhou ZY, Li S, Huang XH, Tang JY, Hoi MPM, et al. Tanshinone I prevents atorvastatin-induced cerebral hemorrhage in zebrafish and stabilizes endothelial cell-cell adhesion by inhibiting VE-cadherin internalization and actin-myosin contractility. Pharmacol Res 2017; 128: 389–98. [DOI] [PubMed] [Google Scholar]
- Jeon SJ, Son KH, Kim YS, Choi YH, Kim HP. Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza bunge. Arch Pharm Res 2008; 31: 758–63. [DOI] [PubMed] [Google Scholar]
- Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology 2000; 49: 355–61. [DOI] [PubMed] [Google Scholar]
- Choi HS, Cho DI, Choi HK, Im SY, Ryu SY, Kim KM. Molecular mechanisms of inhibitory activities of tanshinones on lipopolysaccharide-induced nitric oxide generation in RAW 264.7 cells. Arch Pharm Res 2004; 27: 1233–7. [DOI] [PubMed] [Google Scholar]
- Li X, Du JR, Yu Y, Bai B, Zheng XY. Tanshinone IIa inhibits smooth muscle proliferation and intimal hyperplasia in the rat carotid balloon-injured model through inhibition of MAPK signaling pathway. J Ethnopharmacol 2010; 129: 273–9. [DOI] [PubMed] [Google Scholar]
- Du JR, Li X, Zhang R, Qian ZM. Tanshinone inhibits intimal hyperplasia in the ligated carotid artery in mice. J Ethnopharmacol 2005; 98: 319–22. [DOI] [PubMed] [Google Scholar]
- Tang F, Wu X, Wang T, Wang P, Li R, Zhang H, et al. Tanshinone II A attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vascul Pharmacol 2007; 46: 427–38. [DOI] [PubMed] [Google Scholar]
- Xu S, Little PJ, Lan T, Huang Y, Le K, Wu X, et al. Tanshinone II-A attenuates and stabilizes atherosclerotic plaques in apolipoprotein-E knockout mice fed a high cholesterol diet. Arch Biochem Biophys 2011; 515: 72–9. [DOI] [PubMed] [Google Scholar]
- Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J, Zhou XS, et al. Tanshinone IIa attenuates atherosclerosis in ApoE−/− mice through down-regulation of scavenger receptor expression. Eur J Pharmacol 2011; 650: 275–84. [DOI] [PubMed] [Google Scholar]
- Liu X, Guo CY, Ma XJ, Wu CF, Zhang Y, Sun MY, et al. Anti-inflammatory effects of tanshinone IIa on atherosclerostic vessels of ovariectomized ApoE mice are mediated by estrogen receptor activation and through the ERK signaling pathway. Cell Physiol Biochem 2015; 35: 1744–55. [DOI] [PubMed] [Google Scholar]
- Xu S, Liu Z, Huang Y, Chen J, Chen S, Shen X, et al. Effectiveness of combination therapy of atorvastatin and non lipid-modifying tanshinone IIa from Danshen in a mouse model of atherosclerosis. Int J Cardiol 2014; 174: 878–80. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang J, Huang E, Gao S, Li H, Lu J, et al. Tanshinone IIa suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J Lipid Res 2014; 55: 201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZY, Lin R, Yuan BX, Yang GD, Liu Y, Zhang H. Tanshinone IIa downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J Ethnopharmacol 2008; 115: 217–22. [DOI] [PubMed] [Google Scholar]
- Chen W, Tang F, Xie B, Chen S, Huang H, Liu P. Amelioration of atherosclerosis by tanshinone IIa in hyperlipidemic rabbits through attenuation of oxidative stress. Eur J Pharmacol 2012; 674: 359–64. [DOI] [PubMed] [Google Scholar]
- Zhang W, He H, Liu J, Wang J, Zhang S, Zhang S, et al. Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIa discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013; 34: 306–19. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Lin R, Yuan BX, Liu Y, Zhang H. Tanshinone IIa inhibits atherosclerotic plaque formation by down-regulating MMP-2 and MMP-9 expression in rabbits fed a high-fat diet. Life Sci 2007; 81: 1339–45. [DOI] [PubMed] [Google Scholar]
- Xuan Y, Gao Y, Huang H, Wang X, Cai Y, Luan QX. Tanshinone IIa attenuates atherosclerosis in apolipoprotein E knockout mice infected with Porphyromonas gingivalis. Inflammation 2017; (7347): 1–12. [DOI] [PubMed] [Google Scholar]
- Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z, et al. Tanshinone IIa sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol 2017; 815: 427–36. [DOI] [PubMed] [Google Scholar]
- Wang B, Ge Z, Cheng Z, Zhao Z. Tanshinone IIa suppresses the progression of atherosclerosis by inhibiting the apoptosis of vascular smooth muscle cells and the proliferation and migration of macrophages induced by ox-LDL. Biol Open 2017; 6: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Tong L, Zhang L, Li H, Wan Y, Zhang T. Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-kappaB activation in apolipoprotein-E-deficient mice. Mol Med Rep 2016; 14: 4983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Tong L, Zhang L, Li H, Wan Y, Zhang T. Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-kappaB activation in apolipoprotein-E-deficient mice. Mol Med Rep 2016; 14: 4983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang T, Ran F, Qiao Q, Liu Z, Liu CJ. Tanshinone IIa attenuates elastase-induced AAA in rats via inhibition of MyD88-dependent TLR-4 signaling. Vasa 2014; 43: 39–46. [DOI] [PubMed] [Google Scholar]
- Shang T, Liu Z, Zhou M, Zarins CK, Xu C, Liu CJ. Inhibition of experimental abdominal aortic aneurysm in a rat model by way of tanshinone IIA. J Surg Res 2012; 178: 1029–37. [DOI] [PubMed] [Google Scholar]
- Niu XL, Ichimori K, Yang X, Hirota Y, Hoshiai K, Li M, et al. Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radic Res 2000; 33: 305–12. [DOI] [PubMed] [Google Scholar]
- Lin R, Wang WR, Liu JT, Yang GD, Han CJ. Protective effect of tanshinone IIa on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J Ethnopharmacol 2006; 108: 217–22. [DOI] [PubMed] [Google Scholar]
- Chan P, Chen YC, Lin LJ, Cheng TH, Anzai K, Chen YH, et al. Tanshinone IIa attenuates H2O2-induced injury in human umbilical vein endothelial cells. Am J Chin Med 2012; 40: 1307–19. [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen Z, Ma Z, Tan H, Xiao C, Tang X, et al. Tanshinone IIa protects endothelial cells from H2O2-induced injuries via PXR activation. Biomol Ther (Seoul) 2017; 25: 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Zhang K, He L, Gao B, Gu Q, Li X, et al. Synthesis and biological evaluation of tanshinone IIa derivatives as novel endothelial protective agents. Future Med Chem 2017; 9: 1073–85. [DOI] [PubMed] [Google Scholar]
- Ren B, Zhang YX, Zhou HX, Sun FW, Zhang ZF, Wei Z, et al. Tanshinone IIa prevents the loss of nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and iNOS in the MPTP model of Parkinson's disease. J Neurol Sci 2015; 348: 142–52. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chu CF, Wang CN, Wu HT, Bi KW, Pang JH, et al. The anti-atherosclerotic effect of tanshinone IIa is associated with the inhibition of TNF-alpha-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 2014; 21: 207–16. [DOI] [PubMed] [Google Scholar]
- Tang C, Xue HL, Bai CL, Fu R. Regulation of adhesion molecules expression in TNF-alpha-stimulated brain microvascular endothelial cells by tanshinone IIA: involvement of NF-kappaB and ROS generation. Phytother Res 2011; 25: 376–80. [DOI] [PubMed] [Google Scholar]
- Nizamutdinova IT, Kim YM, Jin H, Son KH, Lee JH, Chang KC, et al. Tanshinone IIa inhibits TNF-alpha-mediated induction of VCAM-1 but not ICAM-1 through the regulation of GATA-6 and IRF-1. Int Immunopharmacol 2012; 14: 650–7. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Cheng TH, Shih NL, Liu JC, Chen JJ, Hong HJ, et al. Tanshinone IIa induces heme oxygenase 1 expression and inhibits cyclic strain-induced interleukin 8 expression in vascular endothelial cells. Am J Chin Med 2016; 44: 377–88. [DOI] [PubMed] [Google Scholar]
- Jiang KY, Ruan CG, Gu ZL, Zhou WY, Guo CY. Effects of tanshinone II-A sulfonate on adhesion molecule expression of endothelial cells and platelets in vitro. Zhongguo Yao Li Xue Bao 1998; 19: 47–50. [PubMed] [Google Scholar]
- Cheng J, Chen T, Li P, Wen J, Pang N, Zhang L, et al. Sodium tanshinone IIa sulfonate prevents lipopolysaccharide-induced inflammation via suppressing nuclear factor-kappaB signaling pathway in human umbilical vein endothelial cells. Can J Physiol Pharmacol 2017; 96: 26–31. [DOI] [PubMed] [Google Scholar]
- Yang JX, Pan YY, Ge JH, Chen B, Mao W, Qiu YG, et al. Tanshinone IIa attenuates TNF-alpha-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-kappaB. Cell Physiol Biochem 2016; 40: 195–206. [DOI] [PubMed] [Google Scholar]
- Huang KJ, Wang H, Xie WZ, Zhang HS. Investigation of the effect of tanshinone IIa on nitric oxide production in human vascular endothelial cells by fluorescence imaging. Spectrochim Acta A Mol Biomol Spectrosc 2007; 68: 1180–6. [DOI] [PubMed] [Google Scholar]
- Fan G, Zhu Y, Guo H, Wang X, Wang H, Gao X. Direct vasorelaxation by a novel phytoestrogen tanshinone IIa is mediated by nongenomic action of estrogen receptor through endothelial nitric oxide synthase activation and calcium mobilization. J Cardiovasc Pharmacol 2011; 57: 340–7. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Zhao Y, Li J. Sodium tanshinone IIa sulfonate suppresses heat stress-induced endothelial cell apoptosis by promoting NO production through upregulating the PI3K/AKT/eNOS pathway. Mol Med Rep 2017; 16: 1612–8. [DOI] [PubMed] [Google Scholar]
- Kim DD, Sanchez FA, Duran RG, Kanetaka T, Duran WN. Endothelial nitric oxide synthase is a molecular vascular target for the Chinese herb Danshen in hypertension. Am J Physiol Heart Circ Physiol 2007; 292: H2131–7. [DOI] [PubMed] [Google Scholar]
- Li YH, Xu Q, Xu WH, Guo XH, Zhang S, Chen YD. Mechanisms of protection against diabetes-induced impairment of endothelium-dependent vasorelaxation by tanshinone IIA. Biochim Biophys Acta 2015; 1850: 813–23. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Hsu FL, Tsai SC, Lin CH, Liu JC, Chen JJ, et al. Tanshinone IIa attenuates cyclic strain-induced endothelin-1 expression in human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol 2012; 39: 63–8. [DOI] [PubMed] [Google Scholar]
- Tang C, Wu AH, Xue HL, Wang YJ. Tanshinone IIa inhibits endothelin-1 production in TNF-alpha-induced brain microvascular endothelial cells through suppression of endothelin-converting enzyme-1 synthesis. Acta Pharmacol Sin 2007; 28: 1116–22. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo QH, Chang Y, Zhao YS, Li AY, Ji ES. Tanshinone IIa ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc Pathol 2017; 31: 47–53. [DOI] [PubMed] [Google Scholar]
- Zhou ZW, Xie XL, Zhou SF, Li CG. Mechanism of reversal of high glucose-induced endothelial nitric oxide synthase uncoupling by tanshinone IIa in human endothelial cell line EA.hy926. Eur J Pharmacol 2012; 697: 97–105. [DOI] [PubMed] [Google Scholar]
- Yu ZL, Wang JN, Wu XH, Xie HJ, Han Y, Guan YT, et al. Tanshinone IIa prevents rat basilar artery smooth muscle cells proliferation by inactivation of PDK1 during the development of hypertension. J Cardiovasc Pharmacol Ther 2015; 20: 563–71. [DOI] [PubMed] [Google Scholar]
- Tan XQ, Cheng XL, Yang Y, Yan L, Gu JL, Li H, et al. Tanshinone II-A sodium sulfonate (DS-201) enhances human BKCa channel activity by selectively targeting the pore-forming alpha subunit. Acta Pharmacol Sin 2014; 35: 1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WY, Yan H, Wang XB, Gui YZ, Gao F, Tang XL, et al. Sodium tanshinone IIa silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS One 2014; 9: e94957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HS, Wang SQ. Nrf2 is involved in the effect of tanshinone IIa on intracellular redox status in human aortic smooth muscle cells. Biochem Pharmacol 2007; 73: 1358–66. [DOI] [PubMed] [Google Scholar]
- Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH, Park R, et al. Tanshinone IIa from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-alpha, IL-1beta and IL-6 in activated RAW 264.7 cells. Planta Med 2003; 69: 1057–9. [DOI] [PubMed] [Google Scholar]
- Chen TH, Hsu YT, Chen CH, Kao SH, Lee HM. Tanshinone IIa from Salvia miltiorrhiza induces heme oxygenase-1 expression and inhibits lipopolysaccharide-induced nitric oxide expression in RAW 264.7 cells. Mitochondrion 2007; 7: 101–5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang ZY, Huang SA, Cui L. Tanshinone IIa down-regulates the expression of MMP-12 and TF in RAW 264.7 cells. Nan Fang Yi Ke Da Xue Xue Bao 2009; 29: 1317–20. [PubMed] [Google Scholar]
- Xu S, Liu Z, Huang Y, Le K, Tang F, Huang H, et al. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-kappaB activation. Transl Res 2012; 160: 114–24. [DOI] [PubMed] [Google Scholar]
- Maione F, De Feo V, Caiazzo E, De Martino L, Cicala C, Mascolo N. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J Ethnopharmacol 2014; 155: 1236–42. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zheng W, Pi R, Mei Z, Bao Y, Gao J, et al. Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Exp Brain Res 2009; 193: 109–18. [DOI] [PubMed] [Google Scholar]
- Mei Z, Zhang F, Tao L, Zheng W, Cao Y, Wang Z, et al. Cryptotanshinone, a compound from Salvia miltiorrhiza modulates amyloid precursor protein metabolism and attenuates beta-amyloid deposition through upregulating alpha-secretase in vivo and in vitro. Neurosci Lett 2009; 452: 90–5. [DOI] [PubMed] [Google Scholar]
- Mei Z, Yan P, Situ B, Mou Y, Liu P. Cryptotanshinione inhibits beta-amyloid aggregation and protects damage from beta-amyloid in SH-SY5Y cells. Neurochem Res 2012; 37: 622–8. [DOI] [PubMed] [Google Scholar]
- Mei Z, Situ B, Tan X, Zheng S, Zhang F, Yan P, et al. Cryptotanshinione upregulates alpha-secretase by activation PI3K pathway in cortical neurons. Brain Res 2010; 1348: 165–73. [DOI] [PubMed] [Google Scholar]
- Wong KK, Ho MT, Lin HQ, Lau KF, Rudd JA, Chung RC, et al. Cryptotanshinone, an acetylcholinesterase inhibitor from Salvia miltiorrhiza, ameliorates scopolamine-induced amnesia in Morris water maze task. Planta Med 2010; 76: 228–34. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu S, Huang X, Wang J, Gao S, Li H, et al. Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: role of lectin-like oxidized LDL receptor-1 (LOX-1). Br J Pharmacol 2015; 172: 5661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Ng CT, Fong LY, Bakar NA, Hussain NH, Ang KP, et al. Cryptotanshinone inhibits TNF-alpha-induced early atherogenic events in vitro. J Physiol Sci 2016; 66: 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wu C, Chen X. Cryptotanshinone inhibits oxidized LDL-induced adhesion molecule expression via ROS dependent NF-kappaB pathways. Cell Adh Migr 2016; 10: 248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997; 386: 73–7. [DOI] [PubMed] [Google Scholar]
- Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, et al. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem Pharmacol 2006; 72: 1680–9. [DOI] [PubMed] [Google Scholar]
- Cheng X, Zhang DL, Li XB, Ye JT, Shi L, Huang ZS, et al. Syntheses of diacyltanshinol derivatives and their suppressive effects on macrophage foam cell formation by reducing oxidized LDL uptake. Bioorg Chem 2014; 52: 24–30. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhang D, Lou H, Sun L, Ji J. Evaluation of the anti-inflammatory activities of tanshinones isolated from Salvia miltiorrhiza var. alba roots in THP-1 macrophages. J Ethnopharmacol 2016; 188: 193–9. [DOI] [PubMed] [Google Scholar]
- Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH, et al. Crypto-tanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-kappaB and MAPK signaling pathways. Inflammation 2011; 34: 111–8. [DOI] [PubMed] [Google Scholar]
- Zhao W, Li C, Gao H, Wu Q, Shi J, Chen X. Dihydrotanshinone I attenuates atherosclerosis in ApoE-deficient mice: role of NOX4/NF-kappaB mediated lectin-like oxidized LDL receptor-1 (LOX-1) of the endothelium. Front Pharmacol 2016; 7: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W, Chen F, Bai L, Zhang P, Qin W. Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2008; 40: 1–6. [DOI] [PubMed] [Google Scholar]
- Lam FF, Yeung JH, Chan KM, Or PM. Dihydrotanshinone, a lipophilic component of Salvia miltiorrhiza (danshen), relaxes rat coronary artery by inhibition of calcium channels. J Ethnopharmacol 2008; 119: 318–21. [DOI] [PubMed] [Google Scholar]
- Lu J, Song HP, Li P, Zhou P, Dong X, Chen J. Screening of direct thrombin inhibitors from Radix Salviae Miltiorrhizae by a peak fractionation approach. J Pharm Biomed Anal 2015; 109: 85–90. [DOI] [PubMed] [Google Scholar]
- Park JW, Lee SH, Yang MK, Lee JJ, Song MJ, Ryu SY, et al. 15,16-Dihydrotanshinone I, a major component from Salvia miltiorrhiza Bunge (Dansham), inhibits rabbit platelet aggregation by suppressing intracellular calcium mobilization. Arch Pharm Res 2008; 31: 47–53. [DOI] [PubMed] [Google Scholar]
- Yang F, Tan HM, Wang H. Hyperhomocysteinemia and atherosclerosis. Sheng Li Xue Bao 2005; 57: 103–14. [PubMed] [Google Scholar]
- Yang RX, Huang SY, Yan FF, Lu XT, Xing YF, Liu Y, et al. Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol Sin 2010; 31: 1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci 2004; 75: 3157–71. [DOI] [PubMed] [Google Scholar]
- Yang GD, Zhang H, Lin R, Wang WR, Shi XL, Liu Y, et al. Down-regulation of CD40 gene expression and inhibition of apoptosis with Danshensu in endothelial cells. Basic Clin Pharmacol Toxicol 2009; 104: 87–92. [DOI] [PubMed] [Google Scholar]
- Ding M, Ye TX, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int Immunopharmacol 2005; 5: 1641–51. [DOI] [PubMed] [Google Scholar]
- Wang D, Fan G, Wang Y, Liu H, Wang B, Dong J, et al. Vascular reactivity screen of Chinese medicine danhong injection identifies Danshensu as a NO-independent but PGI2-mediated relaxation factor. J Cardiovasc Pharmacol 2013; 62: 457–65. [DOI] [PubMed] [Google Scholar]
- Dong ZT, Jiang WD. Effect of danshensu on isolated swine coronary artery perfusion preparation (author's transl). Yao Xue Xue Bao 1982; 17: 226–8. [PubMed] [Google Scholar]
- Wu L, Li X, Li Y, Wang L, Tang Y, Xue M. Proliferative inhibition of danxiongfang and its active ingredients on rat vascular smooth muscle cell and protective effect on the VSMC damage induced by hydrogen peroxide. J Ethnopharmacol 2009; 126: 197–206. [DOI] [PubMed] [Google Scholar]
- Gao H, Li L, Li L, Gong B, Dong P, Fordjour PA, et al. Danshensu promotes cholesterol efflux in RAW264.7 macrophages. Lipids 2016; 51: 1083–92. [DOI] [PubMed] [Google Scholar]
- Wang L, Bao Y, Yang Y, Wu Y, Chen X, Si S, et al. Discovery of antagonists for human scavenger receptor CD36 via an ELISA-like high-throughput screening assay. J Biomol Screen 2010; 15: 239–50. [DOI] [PubMed] [Google Scholar]
- Yu C, Qi D, Lian W, Li QZ, Li HJ, Fan HY. Effects of danshensu on platelet aggregation and thrombosis: in vivo arteriovenous shunt and venous thrombosis models in rats. PLoS One 2014; 9: e110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang N, Ma J, Zhu Y, Wang M, Wang X, et al. A platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. J Pharm Biomed Anal 2016; 117: 178–83. [DOI] [PubMed] [Google Scholar]
- Zhang XC, Chen JQ, Li B. Salvianolic acid A suppresses CCL-20 expression in TNF-alpha-treated macrophages and ApoE-deficient mice. J Cardiovasc Pharmacol 2014; 64: 318–25. [DOI] [PubMed] [Google Scholar]
- Zhang T, Xu J, Li D, Chen J, Shen X, Xu F, et al. Salvianolic acid A, a matrix metalloproteinase-9 inhibitor of Salvia miltiorrhiza, attenuates aortic aneurysm formation in apolipoprotein E-deficient mice. Phytomedicine 2014; 21: 1137–45. [DOI] [PubMed] [Google Scholar]
- Liu YL and Liu GT. Inhibition of human low-density lipoprotein oxidation by salvianolic acid-A. Yao Xue Xue Bao 2002; 37: 81–5. [PubMed] [Google Scholar]
- Sun L, Zhao R, Zhang L, Zhang T, Xin W, Lan X, et al. Salvianolic acid A inhibits PDGF-BB induced vascular smooth muscle cell migration and proliferation while does not constrain endothelial cell proliferation and nitric oxide biosynthesis. Molecules 2012; 17: 3333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Qiang GF, Zhang L, Zhu XM, Wang SB, Sun L, et al. Salvianolic acid A protects against vascular endothelial dysfunction in high-fat diet fed and streptozotocin-induced diabetic rats. J Asian Nat Prod Res 2011; 13: 884–94. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhao R, Zhang L, Zhang W, He G, Yang S, et al. Prevention of vascular smooth muscle cell proliferation and injury-induced neointimal hyperplasia by CREB-mediated p21 induction: An insight from a plant polyphenol. Biochem Pharmacol 2016; 103: 40–52. [DOI] [PubMed] [Google Scholar]
- Teng F, Yin Y, Cui Y, Deng Y, Li D, Cho K, et al. Salvianolic acid A inhibits endothelial dysfunction and vascular remodeling in spontaneously hypertensive rats. Life Sci 2016; 144: 86–93. [DOI] [PubMed] [Google Scholar]
- Yang D, Xie P, Liu Z. Ischemia/reperfusion-induced MKP-3 impairs endothelial NO formation via inactivation of ERK1/2 pathway. PLoS One 2012; 7: e42076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang S, Yu Y, Sun S, Zhang Y, Zhang Y, et al. Salvianolic acid A, as a novel ETA receptor antagonist, shows inhibitory effects on tumor in Vitro. Int J Mol Sci 2016; 17: pii: E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LL, Li DY, Zhang YB, Zhu MY, Chen D, Xu TD. Salvianolic acid A inhibits angiotensin II-induced proliferation of human umbilical vein endothelial cells by attenuating the production of ROS. Acta Pharmacol Sin 2012; 33: 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yuan T, Zhang H, Yan Y, Wang D, Fang L, et al. Activation of Nrf2 attenuates pulmonary vascular remodeling via inhibiting endothelial-to-mesenchymal transition: an insight from a plant polyphenol. Int J Biol Sci 2017; 13: 1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Chen Y, Zhang H, Fang L, Du G. Salvianolic acid A, a component of Salvia miltiorrhiza, attenuates endothelial-mesenchymal transition of HPAECs induced by hypoxia. Am J Chin Med 2017; 45: 1185–200. [DOI] [PubMed] [Google Scholar]
- Oh KS, Oh BK, Mun J, Seo HW, Lee BH. Salvianolic acid A suppress lipopolysaccharide-induced NF-kappaB signaling pathway by targeting IKKbeta. Int Immunopharmacol 2011; 11: 1901–6. [DOI] [PubMed] [Google Scholar]
- Huang J, Qin Y, Liu B, Li GY, Ouyang L, Wang JH. In silico analysis and experimental validation of molecular mechanisms of salvianolic acid A-inhibited LPS-stimulated inflammation, in RAW264.7 macrophages. Cell Prolif 2013; 46: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu T, Du Y, Pan D, Wu W, Zhu H, et al. Salvianolic acid A attenuates cell apoptosis, oxidative stress, Akt and NF-kappaB activation in angiotensin-II induced murine peritoneal macrophages. Curr Pharm Biotechnol 2016; 17: 283–90. [DOI] [PubMed] [Google Scholar]
- Yu WG, Xu LN. Effect of acetylsalvianolic acid A on platelet function. Yao Xue Xue Bao 1994; 29: 412–6. [PubMed] [Google Scholar]
- Tang MK, Ren DC, Zhang JT, Du GH. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine 2002; 9: 405–9. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wu WY, Liu AH, Deng SS, Bi KS, Liu X, et al. Interaction of salvianolic acids and notoginsengnosides in inhibition of ADP-induced platelet aggregation. Am J Chin Med 2008; 36: 313–28. [DOI] [PubMed] [Google Scholar]
- Huang ZS, Zeng CL, Zhu LJ, Jiang L, Li N, et al. Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost 2010; 8: 1383–93. [DOI] [PubMed] [Google Scholar]
- Fan HY, Fu FH, Yang MY, Xu H, Zhang AH, Liu K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res 2010; 126: e17–22. [DOI] [PubMed] [Google Scholar]
- Zhao T, Chang L, Zhang B, Lu M, Wang X, Orgah JO, et al. Specific combination of salvianolic acids as core active ingredients of Danhong injection for treatment of arterial thrombosis and its derived dry Gangrene. Front Pharmacol 2017; 8: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JH, Hong CY. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci 2011; 18: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem 2001; 82: 512–21. [DOI] [PubMed] [Google Scholar]
- Qui Y, Rui YC, Zhang L, Li TJ, Zhang WD. VEGF induced hyperpermeability in bovine aortic endothelial cell and inhibitory effect of salvianolic acid B. Acta Pharmacol Sin 2001; 22: 117–20. [PubMed] [Google Scholar]
- Zhou Z, Liu Y, Miao AD, Wang SQ. Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem 2005; 96: 109–16. [DOI] [PubMed] [Google Scholar]
- Stumpf C, Fan Q, Hintermann C, Raaz D, Kurfurst I, Losert S, et al. Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am J Chin Med 2013; 41: 1065–77. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Seo M, Lee EJ. Salvianolic acid B inhibits atherogenesis of vascular cells through induction of Nrf2-dependent heme oxygenase-1. Curr Med Chem 2014; 21: 3095–106. [DOI] [PubMed] [Google Scholar]
- Wang B, Sun J, Shi Y, Le G. Salvianolic acid B inhibits high-fat diet-induced inflammation by activating the Nrf2 pathway. J Food Sci 2017; 82: 1953–60. [DOI] [PubMed] [Google Scholar]
- Shi CS, Huang HC, Wu HL, Kuo CH, Chang BI, Shiao MS, et al. Salvianolic acid B modulates hemostasis properties of human umbilical vein endothelial cells. Thromb Res 2007; 119: 769–75. [DOI] [PubMed] [Google Scholar]
- O K, Cheung F, Sung FL, Zhu DY, Siow YL. Effect of magnesium tanshinoate B on the production of nitric oxide in endothelial cells. Mol Cell Biochem 2000; 207: 35–9. [DOI] [PubMed] [Google Scholar]
- Yang TL, Lin FY, Chen YH, Chiu JJ, Shiao MS, Tsai CS, et al. Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J Sci Food Agric 2011; 91: 134–41. [DOI] [PubMed] [Google Scholar]
- O K, Lynn EG, Vazhappilly R, Au-Yeung KK, Zhu DY, Siow YL. Magnesium tanshinoate B (MTB) inhibits low density lipoprotein oxidation. Life Sci 2001; 68: 903–12. [DOI] [PubMed] [Google Scholar]
- Ba J, Peng H, Chen Y, Gao Y. Effects and mechanism analysis of vascular endothelial growth factor and salvianolic acid B on 125I-low density lipoprotein permeability of the rabbit aortary endothelial cells. Cell Biochem Biophys 2014; 70: 1533–8. [DOI] [PubMed] [Google Scholar]
- Chen HM, Luo H, Zeng WB, Liu B, Huang JC, Liu M, et al. Salvianolic acid B attenuates oxidized low-density lipoprotein-induced endothelial cell apoptosis through inhibition of oxidative stress, p53, and caspase-3 pathways. Chin J Integr Med 2017. doi: 10.1007/s11655-016-2645-4. [DOI] [PubMed]
- Wu HL, Li YH, Lin YH, Wang R, Li YB, Tie L, et al. Salvianolic acid B protects human endothelial cells from oxidative stress damage: a possible protective role of glucose-regulated protein 78 induction. Cardiovasc Res 2009; 81: 148–58. [DOI] [PubMed] [Google Scholar]
- Liu CL, Xie LX, Li M, Durairajan SS, Goto S, Huang JD. Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PLoS One 2007; 2: e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Tao S, Zheng S, Zhao M, Zhu Y, Yang J, et al. Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis. Eur J Pharmacol 2016; 791: 308–15. [DOI] [PubMed] [Google Scholar]
- Ling WC, Liu J, Lau CW, Murugan DD, Mustafa MR, Huang Y. Treatment with salvianolic acid B restores endothelial function in angiotensin II-induced hypertensive mice. Biochem Pharmacol 2017; 136: 76–85. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang WY, Wang YP. Inhibitory effects of lithospermic acid on proliferation and migration of rat vascular smooth muscle cells. Acta Pharmacol Sin 2009; 30: 1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Lim EY, Kim JM, Jung M, Lee HC, Seo M, et al. Nonmuscle myosin heavy chain and histone H3 are intracellular binding partners of lithospermic acid B and mediate its antiproliferative effect on VSMCs. Curr Med Chem 2012; 19: 1731–7. [DOI] [PubMed] [Google Scholar]
- Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol 2006; 41: 138–48. [DOI] [PubMed] [Google Scholar]
- Joe Y, Zheng M, Kim HJ, Kim S, Uddin MJ, Park C, et al. Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase–1 and arginase activities. J Pharmacol Exp Ther 2012; 341: 850–8. [DOI] [PubMed] [Google Scholar]
- Yue J, Li B, Jing Q, Guan Q. Salvianolic acid B accelerated ABCA1-dependent cholesterol efflux by targeting PPAR-gamma and LXRalpha. Biochem Biophys Res Commun 2015; 462: 233–8. [DOI] [PubMed] [Google Scholar]
- Bao Y, Wang L, Xu Y, Yang Y, Wang L, Si S, et al. Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 2012; 223: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Zhong A, Bu X, Ma H, Li W, Xu X, et al. Salvianolic acid B inhibits platelets-mediated inflammatory response in vascular endothelial cells. Thromb Res 2015; 135: 137–45. [DOI] [PubMed] [Google Scholar]
- Liu L, Li J, Zhang Y, Zhang S, Ye J, Wen Z, et al. Salvianolic acid B inhibits platelets as a P2Y12 antagonist and PDE inhibitor: evidence from clinic to laboratory. Thromb Res 2014; 134: 866–76. [DOI] [PubMed] [Google Scholar]
- Wu Z, Li JN, Bai ZQ, Lin X. Antagonism by salvianolic acid B of lipopolysaccharide-induced disseminated intravascular coagulation in rabbits. Clin Exp Pharmacol Physiol 2014; 41: 502–8. [DOI] [PubMed] [Google Scholar]
- Pan CH, Chen CW, Sheu MJ, Wu CH. Salvianolic acid B inhibits SDF-1alpha-stimulated cell proliferation and migration of vascular smooth muscle cells by suppressing CXCR4 receptor. Vascul Pharmacol 2012; 56: 98–105. [DOI] [PubMed] [Google Scholar]
- Huang MQ, Zhou CJ, Zhang YP, Zhang XQ, Xu W, Lin J, et al. Salvianolic acid B ameliorates hyperglycemia and dyslipidemia in db/db mice through the AMPK pathway. Cell Physiol Biochem 2016; 40: 933–43. [DOI] [PubMed] [Google Scholar]
- Chen YL, Hu CS, Lin FY, Chen YH, Sheu LM, Ku HH, et al. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem 2006; 98: 618–31. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Lee IT, Chen YH, Lin FY, Sheu LM, Ku HH, et al. Salvianolic acid B attenuates MMP-2 and MMP-9 expression in vivo in apolipoprotein-E-deficient mouse aorta and in vitro in LPS-treated human aortic smooth muscle cells. J Cell Biochem 2007; 100: 372–84. [DOI] [PubMed] [Google Scholar]
- Xing YL, Zhou Z. Agula, Zhong ZY, Ma YJ, Zhao YL, et al. Protocatechuic aldehyde inhibits lipopolysaccharide-induced human umbilical vein endothelial cell apoptosis via regulation of caspase-3. Phytother Res 2012; 26: 1334–41. [DOI] [PubMed] [Google Scholar]
- Tong YF, Liu Y, Hu ZX, Li ZC, A A. Protocatechuic aldehyde inhibits TNF-alpha-induced fibronectin expression in human umbilical vein endothelial cells via a c-Jun N-terminal kinase dependent pathway. Exp Ther Med 2016; 11: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Liu Y, Miao AD, Wang SQ. Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur J Pharmacol 2005; 513: 1–8. [DOI] [PubMed] [Google Scholar]
- Kong BS, Im SJ, Lee YJ, Cho YH, Do YR, Byun JW, et al. Vasculoprotective effects of 3-hydroxybenzaldehyde against VSMCs proliferation and ECs inflammation. PLoS One 2016; 11: e0149394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CY, Ku CR, Cho YH, Lee EJ. Protocatechuic aldehyde inhibits migration and proliferation of vascular smooth muscle cells and intravascular thrombosis. Biochem Biophys Res Commun 2012; 423: 116–21. [DOI] [PubMed] [Google Scholar]
- Kong BS, Cho YH, Lee EJ. G protein-coupled estrogen receptor-1 is involved in the protective effect of protocatechuic aldehyde against endothelial dysfunction. PLoS One 2014; 9: e113242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jiang WL, Zhang SP, Zhu HB, Hou J. Protocatechuic aldehyde protects against experimental sepsis in vitro and in vivo. Basic Clin Pharmacol Toxicol 2012; 110: 384–9. [DOI] [PubMed] [Google Scholar]
- Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014; 383: 1933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013; 123: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart 2016; 102: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Dent MR, Tappia PS, Sethi R, Barta J, Goyal RK. Subcellular remodeling as a viable target for the treatment of congestive heart failure. J Cardiovasc Pharmacol Ther 2006; 11: 31–45. [DOI] [PubMed] [Google Scholar]
- Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature 2008; 451: 919–28. [DOI] [PubMed] [Google Scholar]
- Kohli S, Ahuja S, Rani V. Transcription factors in heart: promising therapeutic targets in cardiac hypertrophy. Curr Cardiol Rev 2011; 7: 262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res 2003; 92: 1079–88. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Spinale FG. Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med 2001; 33: 623–34. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 2000; 35: 569–82. [DOI] [PubMed] [Google Scholar]
- Chang PN, Mao JC, Huang SH, Ning L, Wang ZJ, On T, et al. Analysis of cardioprotective effects using purified Salvia miltiorrhiza extract on isolated rat hearts. J Pharmacol Sci 2006; 101: 245–9. [DOI] [PubMed] [Google Scholar]
- Han B, Zhang X, Zhang Q, Zhao G, Wei J, Ma S, et al. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch Cardiovasc Dis 2011; 104: 313–24. [DOI] [PubMed] [Google Scholar]
- Han S, Zheng Z, Ren D. Effect of Salvia miltiorrhiza on left ventricular hypertrophy and cardiac aldosterone in spontaneously hypertensive rats. J Huazhong Univ Sci Technolog Med Sci 2002; 22: 302–4. [DOI] [PubMed] [Google Scholar]
- Jia Y, Huang F, Zhang S, Leung SW. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int J Cardiol 2012; 157: 330–40. [DOI] [PubMed] [Google Scholar]
- Liu B, Du Y, Cong L, Jia X, Yang G. Danshen (Salvia miltiorrhiza) compounds improve the biochemical indices of the patients with coronary heart disease. Evid Based Complement Alternat Med 2016; 2016: 9781715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lu Z. Effect of Salvia miltiorrhiza on coronary collateral circulation in dogs with experimental acute myocardial infarction. J Tongji Med Univ 1999; 19: 40–1, 69. [DOI] [PubMed] [Google Scholar]
- Sun J, Huang SH, Tan BK, Whiteman M, Zhu YC, Wu YJ, et al. Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sci 2005; 76: 2849–60. [DOI] [PubMed] [Google Scholar]
- Wu T, Ni J, Wu J. Danshen (Chinese medicinal herb) preparations for acute myocardial infarction. Cochrane Database Syst Rev 2008; (2): CD004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, He LF, Li YJ, Shen Y, Chao RB, Du JR. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol 2012; 139: 440–6. [DOI] [PubMed] [Google Scholar]
- Shang Q, Xu H, Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med 2012; 2012: 716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F, Carbone F, Schindler TH. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J 2016; 37: 1268–83. [DOI] [PubMed] [Google Scholar]
- Hu Q, Wei B, Wei L, Hua K, Yu X, Li H, et al. Sodium tanshinone IIa sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in Beagle dogs through NLRP3 inflammasome. Int J Cardiol 2015; 196: 183–92. [DOI] [PubMed] [Google Scholar]
- Ren ZH, Tong YH, Xu W, Ma J, Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine 2010; 17: 212–8. [DOI] [PubMed] [Google Scholar]
- Wei B, You MG, Ling JJ, Wei LL, Wang K, Li WW, et al. Regulation of antioxidant system, lipids and fatty acid beta-oxidation contributes to the cardioprotective effect of sodium tanshinone IIa sulphonate in isoproterenol-induced myocardial infarction in rats. Atherosclerosis 2013; 230: 148–56. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang J, Wu LM. Cardioprotective effects of tanshinone IIa on myocardial ischemia injury in rats. Pharmazie 2009; 64: 332–6. [PubMed] [Google Scholar]
- Pan C, Lou L, Huo Y, Singh G, Chen M, Zhang D, et al. Salvianolic acid B and tanshinone IIa attenuate myocardial ischemia injury in mice by NO production through multiple pathways. Ther Adv Cardiovasc Dis 2011; 5: 99–111. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang L, Chu W, Wang B, Zhang J, Zhao M, et al. Tanshinone IIa inhibits miR-1 expression through p38 MAPK signal pathway in post-infarction rat cardiomyocytes. Cell Physiol Biochem 2010; 26: 991–8. [DOI] [PubMed] [Google Scholar]
- Zhou G, Jiang W, Zhao Y, Ma G, Xin W, Yin J, et al. Sodium tanshinone IIa sulfonate mediates electron transfer reaction in rat heart mitochondria. Biochem Pharmacol 2003; 65: 51–7. [DOI] [PubMed] [Google Scholar]
- Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J 2014; 55: 101–5. [DOI] [PubMed] [Google Scholar]
- Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIa protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 2007; 568: 213–21. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue S, et al. Tanshinone IIa protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Exp Ther Med 2016; 12: 1147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Jia YH, Li J, Li LJ, Zhou FH. Study of anti-myocardial cell oxidative stress action and effect of tanshinone IIa on prohibitin expression. J Tradit Chin Med 2010; 30: 259–64. [DOI] [PubMed] [Google Scholar]
- Yang R, Liu A, Ma X, Li L, Su D, Liu J. Sodium tanshinone IIa sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol 2008; 51: 396–401. [DOI] [PubMed] [Google Scholar]
- Jin HJ, Xie XL, Ye JM, Li CG. TanshinoneIIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells. PLoS One 2013; 8: e51720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wu Y, Li Y, Xu C, Li X, Zhu D, et al. Tanshinone IIa improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell Physiol Biochem 2012; 30: 843–52. [DOI] [PubMed] [Google Scholar]
- Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin YL, et al. Sodium tanshinone IIa silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-kappaB/TNF-alpha pathway. Br J Pharmacol 2013; 169: 1058–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HJ, Liu JC, Cheng TH, Chan P. Tanshinone IIa attenuates angiotensin II-induced apoptosis via Akt pathway in neonatal rat cardiomyocytes. Acta Pharmacol Sin 2010; 31: 1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan H, Li X, Pan Z, Zhang L, Cai B, Zhang Y, et al. Tanshinone IIa protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br J Pharmacol 2009; 158: 1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Y, Sheng C, Yang C, Chen L, Sun J. Tanshinone IIa inhibits apoptosis in the myocardium by inducing microRNA-152-3p expression and thereby downregulating PTEN. Am J Transl Res 2016; 8: 3124–32. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cai J. The role of microRNAs in heart failure. Biochim Biophys Acta 2017; 1863: 2019–30. [DOI] [PubMed] [Google Scholar]
- Shang Q, Wang H, Li S, Xu H. The effect of sodium tanshinone iia sulfate and simvastatin on elevated serum levels of inflammatory markers in patients with coronary heart disease: a study protocol for a randomized controlled trial. Evid Based Complement Alternat Med 2013; 2013: 756519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Xu W, Han H, Chen Y, Yang J, Qiao H, et al. Tanshinone IIa increases recruitment of bone marrow mesenchymal stem cells to infarct region via up-regulating stromal cell-derived factor-1/CXC chemokine receptor 4 axis in a myocardial ischemia model. Phytomedicine 2011; 18: 443–50. [DOI] [PubMed] [Google Scholar]
- Xie J, Wang H, Song T, Wang Z, Li F, Ma J, et al. Tanshinone IIa and astragaloside IV promote the migration of mesenchymal stem cells by up-regulation of CXCR4. Protoplasma 2013; 250: 521–30. [DOI] [PubMed] [Google Scholar]
- Jiang B, Zhang L, Wang Y, Li M, Wu W, Guan S, et al. Tanshinone IIa sodium sulfonate protects against cardiotoxicity induced by doxorubicin in vitro and in vivo. Food Chem Toxicol 2009; 47: 1538–44. [DOI] [PubMed] [Google Scholar]
- Gao J, Yang G, Pi R, Li R, Wang P, Zhang H, et al. Tanshinone IIa protects neonatal rat cardiomyocytes from adriamycin-induced apoptosis. Transl Res 2008; 151: 79–87. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Liu JC, Chen PY, Chen JJ, Chan P, Cheng TH. Tanshinone IIa prevents doxorubicin-induced cardiomyocyte apoptosis through Akt-dependent pathway. Int J Cardiol 2012; 157: 174–9. [DOI] [PubMed] [Google Scholar]
- Zhou GY, Zhao BL, Hou JW, Ma GE, Xin WJ. Protective effects of sodium tanshinone IIa sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacol Res 1999; 40: 487–91. [DOI] [PubMed] [Google Scholar]
- Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F, et al. Protective effects of tanshinone IIa on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharm Biol 2015; 53: 1752–8. [DOI] [PubMed] [Google Scholar]
- Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIa protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother 2016; 84: 106–14. [DOI] [PubMed] [Google Scholar]
- Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y, Guo JP, et al. Sodium tanshinone IIa sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin 2013; 34: 1386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wei L, Sun D, Cao F, Gao H, Zhao L, et al. Tanshinone IIa pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes Obes Metab 2010; 12: 316–22. [DOI] [PubMed] [Google Scholar]
- Pan Y, Qian JX, Lu SQ, Chen JW, Zhao XD, Jiang Y, et al. Protective effects of tanshinone IIa sodium sulfonate on ischemia-reperfusion-induced myocardial injury in rats. Iran J Basic Med Sci 2017; 20: 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIa sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis 2014; 235: 318–27. [DOI] [PubMed] [Google Scholar]
- Yuan X, Jing S, Wu L, Chen L, Fang J. Pharmacological postconditioning with tanshinone IIa attenuates myocardial ischemia-reperfusion injury in rats by activating the phosphatidylinositol 3-kinase pathway. Exp Ther Med 2014; 8: 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MQ, Tu JF, Chen H, Shen Y, Pang LX, Yang XH, et al. Janus kinase/signal transducer and activator of transcription inhibitors enhance the protective effect mediated by tanshinone IIa from hypoxic/ischemic injury in cardiac myocytes. Mol Med Rep 2015; 11: 3115–21. [DOI] [PubMed] [Google Scholar]
- Sun DD, Wang HC, Wang XB, Luo Y, Jin ZX, Li ZC, et al. Tanshinone IIA: a new activator of human cardiac KCNQ1/KCNE1 (IKs) potassium channels. Eur J Pharmacol 2008; 590: 317–21. [DOI] [PubMed] [Google Scholar]
- Jiang FL, Leo S, Wang XG, Li H, Gong LY, Kuang Y, et al. Effect of tanshinone IIa on cardiomyocyte hypertrophy and apoptosis in spontaneously hypertensive rats. Exp Ther Med 2013; 6: 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chen HW, Pi LJ, Wang J, Zhan DQ. Protective effect of tanshinone IIa against cardiac hypertrophy in spontaneously hypertensive rats through inhibiting the Cys-C/Wnt signaling pathway. Oncotarget 2017; 8: 10161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H, Han B, Yu T, Peng Z. The complex regulation of tanshinone IIa in rats with hypertension-induced left ventricular hypertrophy. PLoS One 2014; 9: e92216. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang P, Wu X, Bao Y, Fang J, Zhou S, Gao J, et al. Tanshinone IIa prevents cardiac remodeling through attenuating NAD (P)H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie 2011; 66: 517–24. [PubMed] [Google Scholar]
- Yang L, Zou X, Liang Q, Chen H, Feng J, Yan L, et al. Sodium tanshinone IIa sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med 2007; 39: 65–73. [DOI] [PubMed] [Google Scholar]
- Feng J, Li S, Chen H. Tanshinone IIa inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: possible role of silent information regulator 1. Eur J Pharmacol 2016; 791: 632–9. [DOI] [PubMed] [Google Scholar]
- Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res 2010; 61: 269–80. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ouyang X, Komatsu K, Nakamura N, Hattori M, Baba A, et al. Sodium tanshinone IIa sulfonate derived from Danshen (Salvia miltiorrhiza) attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac cells. Biochem Pharmacol 2002; 64: 745–9. [DOI] [PubMed] [Google Scholar]
- Zhou D, Liang Q, He X, Zhan C. Changes of c-fos and c-jun mRNA expression in angiotensin II-induced cardiomyocyte hypertrophy and effects of sodium tanshinone IIa sulfonate. J Huazhong Univ Sci Technolog Med Sci 2008; 28: 531–4. [DOI] [PubMed] [Google Scholar]
- Tan X, Li J, Wang X, Chen N, Cai B, Wang G, Shan H, et al. Tanshinone IIa protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway. Int J Biol Sci 2011; 7: 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YS, Wang HF, Pai PY, Jong GP, Lai CH, Chung LC, et al. Tanshinone IIa prevents leu27IGF-II-induced cardiomyocyte hypertrophy mediated by estrogen receptor and subsequent Akt activation. Am J Chin Med 2015; 43: 1567–91. [DOI] [PubMed] [Google Scholar]
- Chan P, Liu JC, Lin LJ, Chen PY, Cheng TH, Lin JG, et al. Tanshinone IIa inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts. Am J Chin Med 2011; 39: 381–94. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhou S, Xu L, Lu Y, Yuan X, Zhang H, et al. Hydrogen peroxide-mediated oxidative stress and collagen synthesis in cardiac fibroblasts: blockade by tanshinone IIA. J Ethnopharmacol 2013; 145: 152–61. [DOI] [PubMed] [Google Scholar]
- Mao S, Li W, Qa'aty N, Vincent M, Zhang M, Hinek A. Tanshinone IIa inhibits angiotensin II induced extracellular matrix remodeling in human cardiac fibroblasts--Implications for treatment of pathologic cardiac remodeling. Int J Cardiol 2016; 202: 110–7. [DOI] [PubMed] [Google Scholar]
- Mao S, Wang Y, Zhang M, Hinek A. Phytoestrogen, tanshinone IIa diminishes collagen deposition and stimulates new elastogenesis in cultures of human cardiac fibroblasts. Exp Cell Res 2014; 323: 189–97. [DOI] [PubMed] [Google Scholar]
- Yang F, Li P, Li H, Shi Q, Li S, Zhao L. microRNA-29b mediates the antifibrotic effect of tanshinone IIa in postinfarct cardiac remodeling. J Cardiovasc Pharmacol 2015; 65: 456–64. [DOI] [PubMed] [Google Scholar]
- Zhan CY, Tang JH, Zhou DX, Li ZH. Effects of tanshinone IIa on the transforming growth factor beta1/Smad signaling pathway in rat cardiac fibroblasts. Indian J Pharmacol 2014; 46: 633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Xu SW, Wang P, Tang FT, Zhou SG, Gao J, et al. Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine 2010; 18: 58–64. [DOI] [PubMed] [Google Scholar]
- Yang L, Zou XJ, Gao X, Chen H, Luo JL, Wang ZH, et al. Sodium tanshinone IIa sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro. Exp Mol Med 2009; 41: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Li X, Wang L, Yang PC, Zhang M. Rationale and design of sodium tanshinone IIa sulfonate in left ventricular remodeling secondary to acute myocardial infarction (STAMP-REMODELING) trial: a randomized controlled study. Cardiovasc Drugs Ther 2015; 29: 535–42. [DOI] [PubMed] [Google Scholar]
- Mao S, Wang L, Zhao X, Shang H, Zhang M, Hinek A. Sodium tanshinone IIa sulfonate for reduction of periprocedural myocardial injury during percutaneous coronary intervention (STAMP trial): rationale and design. Int J Cardiol 2015; 182: 329–33. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li H, Yue Z, Guo J, Xu S, Xu J, et al. Cryptotanshinone attenuates cardiac fibrosis via downregulation of COX-2, NOX-2, and NOX-4. J Cardiovasc Pharmacol 2014; 64: 28–37. [DOI] [PubMed] [Google Scholar]
- Jin YC, Kim CW, Kim YM, Nizamutdinova IT, Ha YM, Kim HJ, et al. Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur J Pharmacol 2009; 614: 91–7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen L, Li F, Wang H, Yao Y, Shu J, et al. Cryptotanshinone protects against adriamycin-induced mitochondrial dysfunction in cardiomyocytes. Pharm Biol 2016; 54: 237–42. [DOI] [PubMed] [Google Scholar]
- Ma S, Yang D, Wang K, Tang B, Li D, Yang Y. Cryptotanshinone attenuates isoprenaline-induced cardiac fibrosis in mice associated with upregulation and activation of matrix metalloproteinase-2. Mol Med Rep 2012; 6: 145–50. [DOI] [PubMed] [Google Scholar]
- Takeo S, Tanonaka K, Hirai K, Kawaguchi K, Ogawa M, Yagi A, et al. Beneficial effect of tan-shen, an extract from the root of Salvia, on post-hypoxic recovery of cardiac contractile force. Biochem Pharmacol 1990; 40: 1137–43. [DOI] [PubMed] [Google Scholar]
- Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med 1989; 55: 51–4. [DOI] [PubMed] [Google Scholar]
- Yagi A, Okamura N, Tanonaka K, Takeo S. Effects of tanshinone VI derivatives on post-hypoxic contractile dysfunction of perfused rat hearts. Planta Med 1994; 60: 405–9. [DOI] [PubMed] [Google Scholar]
- Arino T, Tanonaka K, Kawahara Y, Maki T, Takagi N, Yagi A, et al. Effects of tanshinone VI on phosphorylation of ERK and Akt in isolated cardiomyocytes and cardiac fibroblasts. Eur J Pharmacol 2008; 580: 298–305. [DOI] [PubMed] [Google Scholar]
- Maki T, Kawahara Y, Tanonaka K, Yagi A, Takeo S. Effects of tanshinone VI on the hypertrophy of cardiac myocytes and fibrosis of cardiac fibroblasts of neonatal rats. Planta Med 2002; 68: 1103–7. [DOI] [PubMed] [Google Scholar]
- Song Q, Chu X, Zhang X, Bao Y, Zhang Y, Guo H, et al. Mechanisms underlying the cardioprotective effect of salvianic acid A against isoproterenol-induced myocardial ischemia injury in rats: possible involvement of L-type calcium channels and myocardial contractility. J Ethnopharmacol 2016; 189: 157–64. [DOI] [PubMed] [Google Scholar]
- Yin Y, Duan J, Guo C, Wei G, Wang Y, Guan Y, et al. Danshensu accelerates angiogenesis after myocardial infarction in rats and promotes the functions of endothelial progenitor cells through SDF-1alpha/CXCR4 axis. Eur J Pharmacol 2017; 814: 274–82. [DOI] [PubMed] [Google Scholar]
- Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan W, et al. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol 2013; 699: 219–26. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang L, Akinyi M, Li Y, Duan Z, Zhu Y, et al. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int J Clin Exp Med 2015; 8: 14793–804. [PMC free article] [PubMed] [Google Scholar]
- Huo M, Wang Z, Wu D, Zhang Y, Qiao Y. Using coexpression protein interaction network analysis to identify mechanisms of danshensu affecting patients with coronary heart disease. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang M, Chen C, Le X, Sun S, Yin Y. Cardiovascular protection with danshensu in spontaneously hypertensive rats. Biol Pharm Bull 2011; 34: 1596–601. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang M, Le X, Meng J, Huang L, Yu P, et al. Antioxidant and cardioprotective effects of Danshensu (3-(3,4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine 2011; 18: 1024–30. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang R, Li X, Zheng X. Danshensu attenuates aldosterone-induced cardiomyocytes injury through interfering p53 pathway. Mol Med Rep 2017; 16: 4994–5000. [DOI] [PubMed] [Google Scholar]
- Cui G, Shan L, Hung M, Lei S, Choi I, Zhang Z, et al. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol 2013; 168: 1349–59. [DOI] [PubMed] [Google Scholar]
- Chen YC, Cao WW, Cao Y, Zhang L, Chang BB, Yang WL, et al. Using neural networks to determine the contribution of danshensu to its multiple cardiovascular activities in acute myocardial infarction rats. J Ethnopharmacol 2011; 138: 126–34. [DOI] [PubMed] [Google Scholar]
- Cui Q, Chen Y, Zhang M, Shan L, Sun Y, Yu P, et al. Design, synthesis, and preliminary cardioprotective effect evaluation of danshensu derivatives. Chem Biol Drug Des 2014; 84: 282–91. [DOI] [PubMed] [Google Scholar]
- Dong C, Wang Y, Zhu YZ. Asymmetric synthesis and biological evaluation of Danshensu derivatives as anti-myocardial ischemia drug candidates. Bioorg Med Chem 2009; 17: 3499–507. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Chan JY, Shan L, Cui G, Cui Q, et al. A novel Danshensu derivative prevents cardiac dysfunction and improves the chemotherapeutic efficacy of doxorubicin in breast cancer cells. J Cell Biochem 2016; 117: 94–105. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu H, Luo J, Deng H, Yu P, Zhang Z, et al. A novel Danshensu-tetramethylpyrazine conjugate DT-010 provides cardioprotection through the PGC-1alpha/Nrf2/HO-1 pathway. Biol Pharm Bull 2017; 40: 1490–8. [DOI] [PubMed] [Google Scholar]
- Hu T, Wei G, Xi M, Yan J, Wu X, Wang Y, et al. Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. Int J Mol Med 2016; 38: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Song F, Duan LR, Sheng JJ, Xie YH, Yang Q, et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep 2016; 6: 23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xie YH, Yang Q, Wang SW, Zhang BL, Wang JB, et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One 2012; 7: e48872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 2007; 14: 652–8. [DOI] [PubMed] [Google Scholar]
- Wu L, Qiao H, Li Y, Li L. Cardioprotective effects of the combined use of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytother Res 2007; 21: 751–6. [DOI] [PubMed] [Google Scholar]
- He H, Li X, Wang H, Zhang W, Jiang H, Wang S, et al. Effects of salvianolic acid A on plasma and tissue dimethylarginine levels in a rat model of myocardial infarction. J Cardiovasc Pharmacol 2013; 61: 482–8. [DOI] [PubMed] [Google Scholar]
- Li YJ, Duan CL, Liu JX. Salvianolic acid A promotes the acceleration of neovascularization in the ischemic rat myocardium and the functions of endothelial progenitor cells. J Ethnopharmacol 2014; 151: 218–27. [DOI] [PubMed] [Google Scholar]
- Wang SB, Tian S, Yang F, Yang HG, Yang XY, Du GH. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol 2009; 615: 125–32. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Jiang M, Zhu Y, Hu L, Fan G, et al. Differential cardioprotective effects of salvianolic acid and tanshinone on acute myocardial infarction are mediated by unique signaling pathways. J Ethnopharmacol 2011; 135: 662–71. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Wang ZB, Xu JX. Effect of salvianic acid A on lipid peroxidation and membrane permeability in mitochondria. J Ethnopharmacol 2005; 97: 441–5. [DOI] [PubMed] [Google Scholar]
- Yu LJ, Zhang KJ, Zhu JZ, Zheng Q, Bao XY, Thapa S, et al. Salvianolic acid exerts cardioprotection through promoting angiogenesis in animal models of acute myocardial infarction: preclinical evidence. Oxid Med Cell Longev 2017; 2017: 8192383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu T, Li D, Pan D, Wu P, Luo Y, et al. JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid A intervention. Am J Transl Res 2016; 8: 2534–48. [PMC free article] [PubMed] [Google Scholar]
- Fan H, Yang L, Fu F, Xu H, Meng Q, Zhu H, et al. Cardioprotective effects of salvianolic acid a on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med 2012; 2012: 508938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Li D, Fang F, Chen D, Qi L, Zhang R, et al. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacol 2011; 58: 535–42. [DOI] [PubMed] [Google Scholar]
- Wang B, Liu JX, Meng HX, Lin CR. Blocking effect of salvianolic acid A on calcium channels in isolated rat ventricular myocytes. Chin J Integr Med 2012; 18: 366–70. [DOI] [PubMed] [Google Scholar]
- Yuan X, Xiang Y, Zhu N, Zhao X, Ye S, Zhong P, et al. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp Ther Med 2017; 14: 961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Fan JP, Liu JX, Liang LN. Salvianolic acid A protects neonatal cardiomyocytes against hypoxia/reoxygenation-induced injury by preserving mitochondrial function and activating Akt/GSK-3beta signals. Chin J Integr Med 2017. Doi: 10.1007/s11655-016-2747-z. [DOI] [PubMed]
- Jiang B, Li D, Deng Y, Teng F, Chen J, Xue S, et al. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS One 2013; 8: e59621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du CS, Yang RF, Song SW, Wang YP, Kang JH, Zhang R, et al. Magnesium lithospermate b protects cardiomyocytes from ischemic injury via inhibition of TAB1-p38 apoptosis signaling. Front Pharmacol 2010; 1: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zheng Y, Liu X, Liang X, Ngai S, Li T, et al. Metabolomic profiles of myocardial ischemia under treatment with salvianolic acid B. Chin Med 2012; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Deng Y, Feng L, Li D, Chen X, Ma C, et al. Cardio-protection of salvianolic acid B through inhibition of apoptosis network. PLoS One 2011; 6: e24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Yeung KK, Zhu DY, O K, Siow YL. Inhibition of stress-activated protein kinase in the ischemic/reperfused heart: role of magnesium tanshinoate B in preventing apoptosis. Biochem Pharmacol 2001; 62: 483–93. [DOI] [PubMed] [Google Scholar]
- Fung KP, Zeng LH, Wu J, Wong HN, Lee CM, Hon PM, et al. Demonstration of the myocardial salvage effect of lithospermic acid B isolated from the aqueous extract of Salvia miltiorrhiza. Life Sci 1993; 52: PL239–44. [DOI] [PubMed] [Google Scholar]
- Li D, Wang J, Hou J, Fu J, Liu J, Lin R. Salvianolic acid B induced upregulation of miR-30a protects cardiac myocytes from ischemia/reperfusion injury. BMC Complement Altern Med 2016; 16: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan W, Wei G, Zhou D, Zhu Y, Guo C, Wang Y, et al. Magnesium lithospermate B reduces myocardial ischemia/reperfusion injury in rats via regulating the inflammation response. Pharm Biol 2013; 51: 1355–62. [DOI] [PubMed] [Google Scholar]
- Quan W, Wu B, Bai Y, Zhang X, Yin J, Xi M, et al. Magnesium lithospermate B improves myocardial function and prevents simulated ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis through Akt-dependent pathway. J Ethnopharmacol 2014; 151: 714–21. [DOI] [PubMed] [Google Scholar]
- Quan W, Yin Y, Xi M, Zhou D, Zhu Y, Guan Y, et al. Antioxidant properties of magnesium lithospermate B contribute to the cardioprotection against myocardial ischemia/reperfusion injury in vivo and in vitro. J Tradit Chin Med 2013; 33: 85–91. [DOI] [PubMed] [Google Scholar]
- Wang W, Hu GY, Wang YP. Selective modulation of L-type calcium current by magnesium lithospermate B in guinea-pig ventricular myocytes. Life Sci 2006; 78: 2989–97. [DOI] [PubMed] [Google Scholar]
- Guo HD, Cui GH, Tian JX, Lu PP, Zhu QC, Lv R, et al. Transplantation of salvianolic acid B pretreated mesenchymal stem cells improves cardiac function in rats with myocardial infarction through angiogenesis and paracrine mechanisms. Int J Cardiol 2014; 177: 538–42. [DOI] [PubMed] [Google Scholar]
- Lv Y, Gao CW, Liu B, Wang HY, Wang HP. BMP-2 combined with salvianolic acid B promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Kaohsiung J Med Sci 2017; 33: 477–85. [DOI] [PubMed] [Google Scholar]
- Huang CY, Chen SY, Fu RH, Huang YC, Shyu WC, Lin SZ, et al. Differentiation of embryonic stem cells into cardiomyocytes used to investigate the cardioprotective effect of salvianolic acid B through BNIP3 involved pathway. Cell Transplant 2015; 24: 561–71. [DOI] [PubMed] [Google Scholar]
- Sun B, Li C, Zuo L, Liu P. Protection of SAL B with H9C2 cells. Pharm Biol 2016; 54: 889–95. [DOI] [PubMed] [Google Scholar]
- Han X, Liu JX, Li XZ. Salvianolic acid B inhibits autophagy and protects starving cardiac myocytes. Acta Pharmacol Sin 2011; 32: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Chen J, Xu L, Gao Z, Deng Y, Wang Y, et al. Salvianolic acid B functioned as a competitive inhibitor of matrix metalloproteinase-9 and efficiently prevented cardiac remodeling. BMC Pharmacol 2010; 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Ye J, Gao S, Fang W, Li H, Geng B, et al. Salvianolic acid B protects cardiomyocytes from angiotensin II-induced hypertrophy via inhibition of PARP-1. Biochem Biophys Res Commun 2014; 444: 346–53. [DOI] [PubMed] [Google Scholar]
- Yu J, Chen R, Tan Y, Wu J, Qi J, Zhang M, et al. Salvianolic acid B alleviates heart failure by inactivating ERK1/2/gata4 signaling pathway after pressure overload in mice. PLoS One 2016; 11: e0166560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella R, Santangelo C, D'archivio M, Li Volti G, Giovannini C, Galvano F. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem 2012; 19: 2901–17. [DOI] [PubMed] [Google Scholar]
- Semaming Y, Pannengpetch P, Chattipakorn SC, Chattipakorn N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid Based Complement Alternat Med 2015; 2015: 593902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Liu JX, Dong W, Li P, Li L, Lin CR, et al. Cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J Pharmacol Sci 2014; 125: 176–83. [DOI] [PubMed] [Google Scholar]
- Deng JS, Lee SD, Kuo WW, Fan MJ, Lin YM, Hu WS, et al. Anti-apoptotic and pro-survival effect of protocatechuic acid on hypertensive hearts. Chem Biol Interact 2014; 209: 77–84. [DOI] [PubMed] [Google Scholar]
- Cao YG, Zhang L, Ma C, Chang BB, Chen YC, Tang YQ, et al. Metabolism of protocatechuic acid influences fatty acid oxidation in rat heart: new anti-angina mechanism implication. Biochem Pharmacol 2009; 77: 1096–104. [DOI] [PubMed] [Google Scholar]
- Semaming Y, Kumfu S, Pannangpetch P, Chattipakorn SC, Chattipakorn N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J Endocrinol 2014; 223: 13–23. [DOI] [PubMed] [Google Scholar]
- Ciftci O, Disli OM, Timurkaan N. Protective effects of protocatechuic acid on TCDD-induced oxidative and histopathological damage in the heart tissue of rats. Toxicol Ind Health 2013; 29: 806–11. [DOI] [PubMed] [Google Scholar]
- Yu XY, Lin SG, Zhou ZW, Chen X, Liang J, Liu PQ, et al. Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza Bunge. Curr Drug Metab 2007; 8: 325–40. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang M, Guan S, Bi HC, Pan Y, Duan W, et al. A mechanistic study of the intestinal absorption of cryptotanshinone, the major active constituent of Salvia miltiorrhiza. J Pharmacol Exp Ther 2006; 317: 1285–94. [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhang W, Chen Z, Shi Z, He C, Chen M. Recent insights into the biological activities and drug delivery systems of tanshinones. Int J Nanomedicine 2016; 11: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Ma WC, Mao SJ, Wu Y, Jin H. Total synthesis of tanshinone I. J Nat Prod 2017; 80: 1697–700. [DOI] [PubMed] [Google Scholar]
- Xu S. Transcriptome profiling in systems vascular medicine. Front Pharmacol 2017; 8: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Cui MC. Systematic understanding of the mechanism of salvianolic acid A via computational target fishing. Molecules 2017; 22: pii: E644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li X, Li C, Rong Y, Xiao Y, Xu X, et al. Chinese herbal cardiotonic pill stabilizes vulnerable plaques in rabbits by decreasing the expression of adhesion molecules. J Cardiovasc Pharmacol 2016; 68: 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Dai A, Guo Z, Komesaroff PA. A preparation of herbal medicine Salvia miltiorrhiza reduces expression of intercellular adhesion molecule-1 and development of atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol 2008; 51: 38–44. [DOI] [PubMed] [Google Scholar]
- O'brien KA, Ling S, Abbas E, Dai A, Zhang J, Wang WC, et al. A chinese herbal preparation containing radix salviae miltiorrhizae, radix notoginseng and borneolum syntheticum reduces circulating adhesion molecules. Evid Based Complement Alternat Med 2011; 2011: 790784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sun L, Li Y, Ma C, Yang J, Zhang W, et al. NaoXinTong inhibits the advanced atherosclerosis and enhances the plaque stability in apolipoprotein E deficient mice. J Cardiovasc Pharmacol 2016; 67: 203–11. [DOI] [PubMed] [Google Scholar]
- Zhong XN, Wang HH, Lu ZQ, Dai YQ, Huang JH, Qiu W, et al. Effects of Naoxintong on atherosclerosis and inducible nitric oxide synthase expression in atherosclerotic rabbit. Chin Med J (Engl) 2013; 126: 1166–70. [PubMed] [Google Scholar]
- Zhao J, Zhu H, Wang S, Ma X, Liu X, Wang C, et al. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Curr Pharm Des 2013; 19: 5891–6. [DOI] [PubMed] [Google Scholar]
- Kwok T, Leung PC, Lam C, Ho S, Wong CK, Cheng KF, et al. A randomized placebo controlled trial of an innovative herbal formula in the prevention of atherosclerosis in postmenopausal women with borderline hypercholesterolemia. Complement Ther Med 2014; 22: 473–80. [DOI] [PubMed] [Google Scholar]
- Chen J, Deng J, Zhang Y, Yang J, He Y, Fu W, et al. Lipid-lowering effects of Danhong injection on hyperlipidemia rats. J Ethnopharmacol 2014; 154: 437–42. [DOI] [PubMed] [Google Scholar]
- Fu TT, Wang CJ, Min CY, Huang XH. Effects of danhong injection on experimental atherosclerosis rabbit model and its mechanism. Zhong Yao Cai 2009; 32: 1720–2. [PubMed] [Google Scholar]
- Chen Y, Liu M, Zhao T, Zhao B, Jia L, Zhu Y, et al. Danhong injection inhibits the development of atherosclerosis in both ApoE−/− and Ldlr−/− mice. J Cardiovasc Pharmacol 2014; 63: 441–52. [DOI] [PubMed] [Google Scholar]
- Jia LQ, Zhang N, Xu Y, Chen WN, Zhu ML, Song N, et al. Tanshinone IIa affects the HDL subfractions distribution not serum lipid levels: Involving in intake and efflux of cholesterol. Arch Biochem Biophys 2016; 592: 50–9. [DOI] [PubMed] [Google Scholar]
- Xing Y, Tu J, Zheng L, Guo L, Xi T. Anti-angiogenic effect of tanshinone IIa involves inhibition of the VEGF/VEGFR2 pathway in vascular endothelial cells. Oncol Rep 2015; 33: 163–70. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Yang RC, Wu HT, Pang JH, Huang ST. Anti-angiogenic effect of tanshinone IIa involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cells. Cancer Lett 2011; 310: 198–206. [DOI] [PubMed] [Google Scholar]
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci 2006; 27: 297–309. [DOI] [PubMed] [Google Scholar]
- Jia LQ, Yang GL, Ren L, Chen WN, Feng JY, Cao Y, et al. Tanshinone IIa reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA.hy926 cells. J Ethnopharmacol 2012; 143: 100–8. [DOI] [PubMed] [Google Scholar]
- Li FQ, Zeng DK, Jia CL, Zhou P, Yin L, Zhang B, et al. The effects of sodium tanshinone IIa sulfonate pretreatment on high glucose-induced expression of fractalkine and apoptosis in human umbilical vein endothelial cells. Int J Clin Exp Med 2015; 8: 5279–86. [PMC free article] [PubMed] [Google Scholar]
- Jia LQ, Feng JY, Yang GL, Chen WN, Chen Y. Effect of tanshinone II A on TLR4 and TNF-alpha of endothelial cells induced by LPS. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011; 27: 733–5. [PubMed] [Google Scholar]
- Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIa inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol 2006; 542: 1–7. [DOI] [PubMed] [Google Scholar]
- Li W, Li J, Ashok M, Wu R, Chen D, Yang L, et al. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol 2007; 178: 3856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol 2009; 113: 275–80. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li JH, et al. Tanshinone IIa attenuates seawater aspiration-induced lung injury by inhibiting macrophage migration inhibitory factor. Biol Pharm Bull 2011; 34: 1052–7. [DOI] [PubMed] [Google Scholar]
- Xu M, Cao F, Liu L, Zhang B, Wang Y, Dong H, et al. Tanshinone IIA-induced attenuation of lung injury in endotoxemic mice is associated with reduction of hypoxia-inducible factor 1alpha expression. Am J Respir Cell Mol Biol 2011; 45: 1028–35. [DOI] [PubMed] [Google Scholar]
- Li YI, Elmer G, Leboeuf RC. Tanshinone IIa reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase. Life Sci 2008; 83: 557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gao X, Zhang B. Tanshinone: an inhibitor of proliferation of vascular smooth muscle cells. J Ethnopharmacol 2005; 99: 93–8. [DOI] [PubMed] [Google Scholar]
- Jin UH, Suh SJ, Chang HW, Son JK, Lee SH, Son KH, et al. Tanshinone IIa from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem 2008; 104: 15–26. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cai F, Li PY, Li ML, Chen J, Chen GL, et al. Activation of high conductance Ca2+-activated K+ channels by sodium tanshinoneII-A sulfonate (DS-201) in porcine coronary artery smooth muscle cells. Eur J Pharmacol 2008; 598: 9–15. [DOI] [PubMed] [Google Scholar]
- Maione F, Cantone V, Chini MG, De Feo V, Mascolo N, Bifulco G. Molecular mechanism of tanshinone IIa and cryptotanshinone in platelet anti-aggregating effects: an integrated study of pharmacology and computational analysis. Fitoterapia 2015; 100: 174–8. [DOI] [PubMed] [Google Scholar]
- Shi C, Zhu X, Wang J, Long D. Tanshinone IIa promotes non-amyloidogenic processing of amyloid precursor protein in platelets via estrogen receptor signaling to phosphatidylinositol 3-kinase/Akt. Biomed Rep 2014; 2: 500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIa inhibits angiogenesisin vitro. Exp Mol Med 2005; 37: 133–7. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhuang Q, Mao W, Xu XM, Wang LH, Wang HB. Inhibitory effect of cryptotanshinone on angiogenesis and Wnt/beta-catenin signaling pathway in human umbilical vein endothelial cells. Chin J Integr Med 2014; 20: 743–50. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang SQ, Liu Y, Miao AD. Cryptotanshinone inhibits endothelin-1 expression and stimulates nitric oxide production in human vascular endothelial cells. Biochim Biophys Acta 2006; 1760: 1–9. [DOI] [PubMed] [Google Scholar]
- Ang KP, Tan HK, Selvaraja M, Kadir AA, Somchit MN, Akim AM, et al. Cryptotanshinone attenuates in vitro oxLDL-induced pre-lesional atherosclerotic events. Planta Med 2011; 77: 1782–7. [DOI] [PubMed] [Google Scholar]
- Ran X, Zhao W, Li W, Shi J, Chen X. Cryptotanshinone inhibits TNF-alpha-induced LOX-1 expression by suppressing reactive oxygen species (ROS) formation in endothelial cells. Korean J Physiol Pharmacol 2016; 20: 347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Yin LL, Ji XQ, Zhu XZ. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol 2006; 549: 166–72. [DOI] [PubMed] [Google Scholar]
- Li X, Lian LH, Bai T, Wu YL, Wan Y, Xie WX, et al. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int Immunopharmacol 2011; 11: 1871–6. [DOI] [PubMed] [Google Scholar]
- Oche B, Chen L, Ma YK, Yang Y, Li CX, Geng X, et al. Cryptotanshinone and wogonin up-regulate eNOS in vascular endothelial cells via ERalpha and down-regulate iNOS in LPS stimulated vascular smooth muscle cells via ERbeta. Arch Pharm Res 2016; 39: 249–58. [DOI] [PubMed] [Google Scholar]
- Ding M, Yuan YJ. Study on the mechanisms of an extract of Salvia miltiorrhiza on the regulation of permeability of endothelial cells exposed to tumour necrosis factor-alpha. J Pharm Pharmacol 2007; 59: 1027–33. [DOI] [PubMed] [Google Scholar]
- Wang S, Wu D, Liu L, Cui J, Qiao WL, Sun H, et al. Danshensu delays the senescence of rat aortic endothelial cells via activation of SIRT1-SOD pathway. Sheng Li Xue Bao 2014; 66: 575–82. [PubMed] [Google Scholar]
- Pan CS, Liu YH, Liu YY, Zhang Y, He K, Yang XY, et al. Salvianolic acid B ameliorates lipopolysaccharide-induced albumin leakage from rat mesenteric venules through src-regulated transcelluar pathway and paracellular pathway. PLoS One 2015; 10: e0126640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LX, Durairajan SS, Lu JH, Liu CL, Kum WF, Wang Y, et al. The effect of salvianolic acid B combined with laminar shear stress on TNF-alpha-stimulated adhesion molecule expression in human aortic endothelial cells. Clin Hemorheol Microcirc 2010; 44: 245–58. [DOI] [PubMed] [Google Scholar]
- Fung KP, Wu J, Zeng LH, Wong HN, Lee CM, Hon PM, et al. Lithospermic acid B as an antioxidant-based protector of cultured ventricular myocytes and aortic endothelial cells of rabbits. Life Sci 1993; 53: PL189–93. [DOI] [PubMed] [Google Scholar]
- Ma C, Yao Y, Yue QX, Zhou XW, Yang PY, Wu WY, et al. Differential proteomic analysis of platelets suggested possible signal cascades network in platelets treated with salvianolic acid B. PLoS One 2011; 6: e14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Lin R, Liu JT, Liu Y, Zhang H. Protection of vascular endothelial cells from ox-LDL induced injury by protocatechualdehyde. Zhong Yao Cai 2007; 30: 1541–4. [PubMed] [Google Scholar]
- Liang Q, Yang L, Wang Z, Huang S, Li S, Yang G. Tanshinone IIa selectively enhances hyperpolarization-activated cyclic nucleotide-modulated (HCN) channel instantaneous current. J Pharmacol Sci 2009; 110: 381–8. [DOI] [PubMed] [Google Scholar]
- Gu J, Li HL, Wu HY, Gu M, Li YD, Wang XG, et al. Sodium tanshinone IIa sulfonate attenuates radiation-induced fibrosis damage in cardiac fibroblasts. J Asian Nat Prod Res 2014; 16: 941–52. [DOI] [PubMed] [Google Scholar]
- Wei Y, Xu M, Ren Y, Lu G, Xu Y, Song Y, et al. The cardioprotection of dihydrotanshinone I against myocardial ischemia-reperfusion injury via inhibition of arachidonic acid omega-hydroxylase. Can J Physiol Pharmacol 2016; 94: 1267–75. [DOI] [PubMed] [Google Scholar]
- Jiang B, Zhang L, Li M, Wu W, Yang M, Wang J, et al. Salvianolic acids prevent acute doxorubicin cardiotoxicity in mice through suppression of oxidative stress. Food Chem Toxicol 2008; 46: 1510–5. [DOI] [PubMed] [Google Scholar]
- Lin TJ, Liu GT, Liu Y, Xu GZ. Protection by salvianolic acid A against adriamycin toxicity on rat heart mitochondria. Free Radic Biol Med 1992; 12: 347–51. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Guo LL, Wu GJ, Liu RH. Salvianolic acid B inhibits the TLR4-NFkappaB-TNFalpha pathway and attenuates neonatal rat cardiomyocyte injury induced by lipopolysaccharide. Chin J Integr Med 2011; 17: 775–9. [DOI] [PubMed] [Google Scholar]
- Wang M, Sun G, Wu P, Chen R, Yao F, Qin M, et al. Salvianolic acid B prevents arsenic trioxide-induced cardiotoxicity in vivo and enhances its anticancer activity in vitro. Evid Based Complement Alternat Med 2013; 2013: 759483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sun GB, Sun X, Wang HW, Meng XB, Qin M, et al. Cardioprotective effect of salvianolic acid B against arsenic trioxide-induced injury in cardiac H9c2 cells via the PI3K/Akt signal pathway. Toxicol Lett 2013; 216: 100–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-atherosclerotic mechanisms of bioactive components of Danshen
Cardioprotective mechanisms of bioactive components of Danshen