Abstract
In this brief review we summarize the current fndings relative to the discovery of a small peptide ligand, phoenixin (PNX). Using a bioinformatic approach, two novel peptides PNX-14 and PNX-20 containing 14 and 20 amino acids, respectively, were isolated from diverse tissues including the brain, heart, lung and stomach. Mass spectrometry analysis identified a major and minor peak corresponding to PNX-14 and PNX-20, in rat or mouse spinal cord extracts. With the use of a rabbit polyclonal antiserum, phoenixin immunoreactivity (irPNX) was detected in discrete areas of the rodent brain including several hypothalamic subnuclei and dorsal motor nucleus of the vagus. In addition, irPNX was detected in a population of sensory ganglion cells including dorsal root ganglion, nodose ganglion and trigeminal ganglion, and in cell processes densely distributed to the superficial layers of the dorsal horn, nucleus of the solitary tract and spinal trigeminal tract. irPNX cell processes were also detected in the skin and myenteric plexus, suggesting a brain-gut and/or brain-skin connection. Pharmacological studies show that PNX-14 injected subcutaneously to the nape of the neck of mice provoked dose-dependent repetitive scratching bouts directed to the back of the neck with the hindpaws. Our result suggests that the peptide PNX-14 and/or PNX-20, may serve as one of the endogenous signal molecules transducing itch sensation. Additionally, results from other laboratories show that exogenous PNX may affect a number of diverse behaviors such as memory formation, depression, reproduction, food-intake and anxiolytic-like behaviors.
Keywords: neuropeptide, brain-gut-skin peptide, phoenixin, dorsal motor nucleus of the vagus, dorsal root ganglion, hypothalamus, myenteric plexus, nodose ganglion, itch sensation
Introduction
Completion of the Human Genome Project has yielded more than 700 genes that belong to the G-protein coupled receptor (GPCR) superfamily. Approximately half of these genes encode sensory receptors, a large number of which are predicted to be the targets of odorants. Of the remaining 360 receptors, the natural ligand has been identified for approximately 210 of them, leaving 150 orphan GPCRs with no known ligands1,2. Since the 1980s, several experimental and methodological advancements, including a high throughput screening of small molecules and peptide ligands, reverse pharmacology and application of bioinformatics to predict candidate ligands, have been developed to streamline the identification process. By utilizing the bioinformatics algorithm from information provided by the Human Genome Project, several previously unrecognized, secreted, highly conserved neuropeptides have been identified, one of which being neuronostatin with orphan receptor GPR107 as its cognate receptor3,4,5. Below is a brief account of the discovery of a second peptide ligand, phoenixin, which may target an orphan G-protein coupled receptor, GPR1736,7.
Phoenixin: isolation, structure and identification
Utilizing a similar strategy, two novel peptides: phoenixin-14 amide, referred to herein as phoenixin (PNX-14), and phoenixin-20 amide (PNX-20) from the rat brain as well as peripheral tissues such as the bovine heart and intestine were isolated and identified8. PNX-14 is identical among multiple species including human, rat, mouse, porcine, canine and Xenopus; whereas PNX-20 differs by one amino acid between the human and porcine or canine sequence8. The precursor for phoenixin is an uncharacterized protein encoded by the gene C4orf52 which contains a glycine residue that can undergo C-terminal amidation, and several conserved dibasic residues after glycine indicative of potential carboxypeptidase cleavage sites9. The most abundant peptide generated from C4orf52 is a 14-residue peptide, DVQPPGLKVWSDPF-amide, which is termed phoenixin-14 amide. An N-terminal extended peptide, AGIVQEDVQPPGLKVWSDPF-amide, termed phoenixin-20 amide, which is co-expressed with phoenixin-14 in tissue samples including the heart and hypothalamus8. Gene expression of C4orf52 has been reported in several human organs by the organism specific databases “GC04P025864” and “BioGPS gnf1h09115_at”. Serial Analysis of Gene Expression (SAGE) for C4orf52 also indicates that phoenixin precursor gene expression is relatively high in the spinal cord.
Phoenixin immunoreactivity in the brain and peripheral tissues
In our study, phoenixin peptide was chemically synthesized and the antibody directed against the synthetic peptide was raised in rabbits8. The polyclonal antiserum directed against phoenixin was then applied to the development of an enzyme immunoassay (EIA) to quantify the amount of immunoreactive phoenixin (irPNX) in various tissues of the rats. A lower level of irPNX was detected in peripheral tissues including the thymus, stomach, and spleen; the tissue with a high level of irPNX was the hypothalamus8. Accordingly, irPNX somata of varying intensity and number were present in following areas: the magnocellular and parvocellular regions of the paraventricular (PVN) and supraoptic nucleus. Labeled cells were also detected in the dorsal hypothalamus, zona incerta, ventromedial hypothalamus, lateral hypothalamus, and perifornical area. In the tuber cinereum, irPNX cells were present in the arcuate nucleus, ventromedial hypothalamus, perifornical nucleus and supraoptic retrochasmatic nucleus. Within the brainstem, irPNX cells were detected in several discrete areas including the substantia nigra pars reticulata, Edinger-Westphal nucleus, nucleus of the solitary tract and dorsal motor nucleus of the vagus. Lightly to moderately labeled cells were seen in the area postrema. irPNX was also observed in the median eminence. Within the pituitary, irPNX was detected in the anterior and posterior pituitary8. A recent double-labeling study reveals that over 50% of irPNX neurons in the hypothalamus were also nesfatin-1 positive, implying that the peptide may play a role in regulating the hypothalamic-pituitary-gonadal neuraxis10.
Insofar as the spinal cord and peripheral nervous system are concerned, irPNX cell processes are distributed to the superficial layers of the dorsal horn of all spinal segments11. Some of the dorsal root ganglion (DRG) cells are irPNX. The latter may give rise to irPNX fibers observed in the superficial layers of the dorsal horn; irPNX fibers are infrequently observed in deeper laminae. In addition to DRG cells, a population of trigeminal and nodose ganglion cells was irPNX with their cell processes projecting to the spinal trigeminal tract and nucleus of the solitary tract11. It is of interest to note that irPNX nerve fibers are detected underneath the skin of rodents. Further, subcutaneous injections of the retrograde fluorescent tracer Fluorogold labeled a population of DRG cells, some of which also contain irPNX, raising the possibility that the peptide released underneath the skin may act on sensory nerve fibers, transmitting sensory signal from the skin to the spinal cord12.
Brain-gut connection
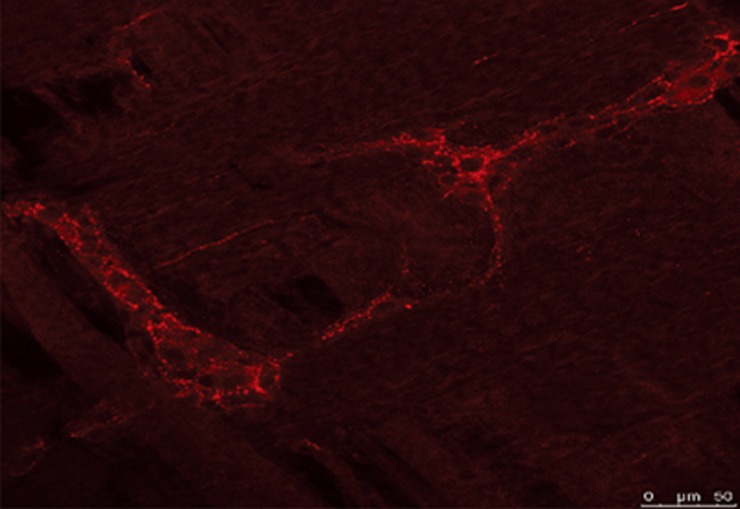
Peptides that occur within the gut, with few exceptions, are found to exist in the brain and/or spinal cord. Currently, more than 30 neuropeptides that exist in the gut, especially in the enteric nervous system and neuroendocrine cells, occur in the central nervous system as well; for example ghrelin, substance P, calcitonin gene related peptide, gastrin releasing peptide, enkephalin, cholecystokinin, and vasoactive intestinal polypeptide, to name a few. We explored here a possible brain-gut connection of irPNX by immunohistochemistry. Utilizing the same antibody, irPNX is conspicuously present in varicose cell processes surrounding myenteric ganglion cells of the rat; myenteric ganglion cells are not labeled (Figure 1). The origin of irPNX cell processes in the myenteric plexus has yet to be firmly established. The observation that irPNX is present in a population of dorsal motor vagal neurons suggests that irPNX cell processes in the myenteric plexus may originate from vagal neurons. Alternatively, they may arise from irPNX nodose ganglion cells11. Hence, our preliminary result supports the contention that the peptide phoenixin may serve as a common link connecting the gut to the spinal cord and/or brain.
Figure 1.
PNX-immunoreactive fibers in the rat myenteric plexus labeled with phoenixin antiserum conjugated to cy3 (red fluorescence, unpublished).
A signaling molecule in the nervous system
An abundance of phoenixin in the central and peripheral nervous systems suggests that the peptide may serve as a signaling molecule targeting multiple sites8,12,13,14. Because of a high expression of irPNX in the hypothalamus and pituitary, earlier studies explored the effect of phoenixin on reproduction-related tissues or cells. Exogenous PNX-14 acting on gonadotrophins was found to increase gonadotropin releasing hormone (GnRH) receptor expression and GnRH secretion, which in turn, enhanced the release of luteinizing hormone8. Moreover, in vivo siRNA knockdown of phoenixin resulted in a delayed appearance of oestrus and a reduced expression of GnRH receptors in the pituitary8. More recently, the orphan G-protein coupled receptor GPR173 has been proposed as the primary receptor candidate mediating the reproductive effect of phoenixin6,7. Reproduction is controlled by the hypothalamic-pituitary-gonadal axis which responds to cues from energy stores. The regulation of energy balance and reproduction is interlinked. Nesfatin-1 (an 82-amino acids hormone) has been reported to play a role in the regulation of energy stores via its anorectic actions and has also been implicated in gonadal function. Therefore, nesfatin-1 may play a role in the central control of reproduction15.
Notwithstanding a role in reproductive behaviors, the widespread distribution of phoenixin in various subnuclei of the hypothalamus suggests that the peptide may mediate a broad range of physiological and/or pathophysiological functions. Accordingly, administration of phoenixin by intracerebroventricular (i.c.v.) injections enhanced memory formation and retention as assayed by using novel object recognition and object location recognition tasks. The peptide also mitigated memory impairment caused by scopolamine and amyloid-β(1-42)16. In addition, phoenixin by i.c.v. injection exerted anxiolytic effects in mice17. In a more recent study, Schalla et al18 reported that phoenixin by i.c.v. injection, but not intraperitoneally, exerted a centrally mediated orexigenic response in rats. In obese men, plasma phoenixin levels were found to be negatively associated with anxiety, while other psychometric parameters were not noticeably impaired19.
Insofar as a biological role of phoenixin in the peripheral nervous system is concerned, several observations are consistent with the suggestion that the peptide may engage in transmitting itch sensation from the skin to the spinal cord or brainstem12. First, phoenixin is expressed in DRGs with their cell processes projecting centrally to the dorsal horn and peripherally to the skin, providing the neurochemical substrate for the transfer of sensory signals. Second, phoenixin upon subcutaneous injection to the nape of the neck provokes excessive scratching in mice. Third, phoenixin-induced scratching is attenuated by nalfurafine, the kappa opioid receptor agonist, which is a known agent effective against excessive scratching caused by several chemically diverse pruritogens20. Collectively, the results support the contention that phoenixin is one of the signaling molecules involved in bidirectional transfer of itch sensation in the mouse12.
Prospective
Market analysis in 2015 estimated that 30% of the drugs available in the global market target GPCRs. Viewed in this context, a clear and present need for information relative to novel GPCRs and their endogenous ligands is obvious. Phoenixin is a recently discovered peptide ligand that is reported to target the GPR1736,7. Similar to many peptide ligands, phoenixin is distributed broadly in the central and peripheral nervous systems with a particular concentration in the hypothalamus, spinal cord and myenteric plexus, establishing an anatomical as well a functional connection between the gut and brain. Results from our pharmacological study raise the possibility that phoenixin acting on superficial dorsal horn neurons may function as a pruritogen conveying itch information arising from the periphery, ascending to the dorsal horn and/or supraspinal structures. As a corollary, our study with phoenixin may help to unveil the biological bases of itch, and develop novel, efficacious anti-itch medications.
Acknowledgments
This work was partial supported from NIDA Grant DA13429 (Alan Cowan).
References
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2004; 85: 1159–204. [DOI] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol 2004; 44: 43–66. [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Tica AA, Yang J, Chang JK, Brailoiu E, et al. Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neurosci 2010; 166: 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GL, Klein C, et al. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem 2008; 283: 31949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Redlinger LL, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol 2012; 303: R941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LM, Tullock DVV, Mathews SK, Garcia-Gallanco D, Elias CF, Samson WK, et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol 2016; 311: R489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treen AK, Luo V, Belsham DD. Phoenixin activates immortalized GnRH and kisspeptin neurons through the novel receptor GPR173. Mol Endocrinol 2016; 30: 872–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Lyu RM, Hsueh AJ, Avsian-Kreetchmer O, Chang JK, Tullock CVV, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol 2013; 25: 206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD. Neuropeptides and other bioactive peptides: from discovery to function. Fricker LD, Devi L (Eds), Carboxypeptidase E, Chapter 3.5, 2012, Morgan & Claypool Life Sciences, Princeton, USA.
- Palasz A, Roiczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohisto-chemical study. Neurosci Lett 2015; 592: 17–21. [DOI] [PubMed] [Google Scholar]
- Lyu RM, Huang XF, Zhang Y, Dun SL, Luo JJ, Chang JK, et al. Phoenixin: a novel peptide in rodent sensory ganglia. Neurosci 2013; 250: 622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lyu RM, Chen YH, Dun SL, Chang JK, Dun NJ. Phoenixin: A candidate pruritogen in the mouse. Neurosci 2015; 301: 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz P, Scharner S, Friedrich T, Schalla M, Goebel-Stengel M, Rose M, et al. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophysical Res Comm 2017; 493: 195–201. [DOI] [PubMed] [Google Scholar]
- Yuan T, Sun Z, Zhao W, Wang T, Zhang J, Niu D. Phoenixin: a newly discovered peptide with multi-functions. Protein Pept Lett 2017; 24: 472–5. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang K, Song M, Li X, Luo L, Tian Y, et al. Role of Nesfatin-1 in the reproductive axis of male rat. Sci Rep 2016; 6: 32877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JH, He Z, Peng YL, Jin WD, Wang Z, Mu LY, et al. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res 2015; 1629: 298–308. [DOI] [PubMed] [Google Scholar]
- Jiang JH, He Z, Peng YL, Jin WD, Mu J, Xue HX, et al. Effects of phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res 2015; 286: 39–48. [DOI] [PubMed] [Google Scholar]
- Schalla M, Prinz P, Friedrich T, Scharner S, Kobelt P, Goebel-Stengel M, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017; 96: 53–60. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Weibert E, Ahnis A, Elbelt U, Rose M, Kiapp BF, et al. Phoenixin is negatively associated with anxiety in obese men. Peptides 2017; 88: 32–6. [DOI] [PubMed] [Google Scholar]
- Cowan A, Kehner GB, Inan S. Targeting itch with ligands selective for kappa opioid receptors. In: Cowan A, Yosipovitch G (Eds), Pharmacology of itch, 291–314, 2015, Springer, Heidelberg, Germany. [DOI] [PubMed]