Abstract
ATP-sensitive potassium (KATP) channels are ubiquitously expressed on the plasma membrane of cells in multiple organs, including the heart, pancreas and brain. KATP channels play important roles in controlling and regulating cellular functions in response to metabolic state, which are inhibited by ATP and activated by Mg-ADP, allowing the cell to couple cellular metabolic state (ATP/ADP ratio) to electrical activity of the cell membrane. KATP channels mediate insulin secretion in pancreatic islet beta cells, and controlling vascular tone. Under pathophysiological conditions, KATP channels play cytoprotective role in cardiac myocytes and neurons during ischemia and/or hypoxia. KATP channel is a hetero-octameric complex, consisting of four pore-forming Kir6.x and four regulatory sulfonylurea receptor SURx subunits. These subunits are differentially expressed in various cell types, thus determining the sensitivity of the cells to specific channel modifiers. Sulfonylurea class of antidiabetic drugs blocks KATP channels, which are neuroprotective in stroke, can be one of the high stoke risk factors for diabetic patients. In this review, we discussed the potential effects of KATP channel blockers when used under pathological conditions related to diabetics and cerebral ischemic stroke.
Keywords: potassium channels, KATP channels, KATP channel blockers, sulfonylurea, stroke, diabetes
Introduction
Stroke and diabetes are currently the most common causes of death and the leading causes of chronic disability in the world. Diabetes is associated with higher risk of stroke. Both stroke and diabetes cause significant social and economic impacts worldwide. Thus, further understanding of stroke in diabetes can help to prevent occurrences and develop new therapeutic targets, which are priorities for stroke research.
Stroke is characterized by inadequate oxygen, blood and nutrient supply to the brain due to a vascular event, either a cerebrovascular clot or rupture. There are three main types of stroke, ischemic (commonly caused by vessel blockage), hemorrhagic (caused by vessel rupture) and transient ischemic attack (caused by temporary vessel blockage). Poor blood flow or bleeding in the brain due to stroke can result in neuronal death and rapid loss of cognitive and physical functions, which may be permanent. In ischemic stroke, recombinant tissue plasminogen activator (rtPA) can be used during a limited window of time immediately following the stroke insults to dissolve the blood clot and reduce the severity of the stroke damage in the brain, however, there is currently no other effective treatment for stroke. Therefore, taking prophylactic measures is the most effective strategy.
Diabetes mellitus is a group of metabolic disorders with persistent hyperglycemia that may be fatal if not managed appropriately. There are three main types of diabetes including type 1, type 2 and gestational diabetes. Persistent hyperglycemia in diabetes mellitus is caused by hypoinsulinemia and/or insulin insensitivity as a result of pancreatic beta-cell failure or periphery insulin resistance, diabetes is as a result of both genetic and environmental factors1. Serious long-term diabetic complications include stroke, heart disease, foot ulcers, nephropathy, retinopathy and neuropathy. Diabetes and its common comorbidities include hypertension, high blood cholesterol, atherosclerosis, atrial fibrillation and obesity, all of which independently contribute to increasing the risk for stroke2. As many as 43% of patients admitted for acute ischemic stroke have undiagnosed diabetes3. Diabetes is considered as a major independent risk factor for stroke that is consistently observed in multiple racial backgrounds4,5,6. Both ischemic and hemorrhagic stroke risk are demonstrated in diabetics7,8, however this review is focused on ischemic stroke which is more common in diabetics.
Diabetes and cerebral ischemic stroke
Diabetes increases stroke risk through a multitude of different mechanisms including high HbA1c, microvascular complications and low HDL cholesterol9. Under Medicare in the United States, one stroke event costs $22,657 initially and up to $2488 per month thereafter for up to a year10. A projected 439 million individuals will suffer from diabetes by 2030 and with it carry dramatically higher risks for limb amputations, vision loss, heart disease and stroke complications11. Without an effective treatment to reverse stroke damage, prevention remains the best option. Diabetes induces changes in all aspects of the neurovascular unit, increasing vascular disease risk and impairing functional recovery from ischemic events. Presently, the most effective preventative measures are intensive blood glucose, blood pressure and blood lipid control12,13,14. Hyperglycemia and hypoinsulinemia are detrimental to brain function. Acute and long-term complications can be minimized with adequate glycemic control through diet and exercise, insulin injections and/or oral medications. Diabetes and hyperglycemia cause more severe stroke outcomes15,16,17,18, and therefore glycemic control is extremely important for stroke prevention. The correlation between hyperglycemia, diabetes, increased stroke risk and poorer post-stroke outcomes is very well established19,20,21,22,23,24,25,26,27.
Neuron
Diabetes is associated with many different types of neuropathy. Prolonged hyperglycemia causes periphery (impaired sensation in extremities), autonomic (disrupted autonomic control), proximal (pain and weakness in limbs) and focal neuropathy (sudden weakness of one nerve). Most diabetic neuropathies are closely linked to microvascular injury, however there are various suggested mechanisms forming a direct link from hyperglycemia, hypoinsulinemia and insulin resistance to nerve damage. High levels of glucose can cause excessive influx of sugar alcohols, excessive free radical stress, loss of cytoskeletal proteins, and lack of up-regulation of axon repair proteins upon nerve injury28,29,30,31,32. In the case of diabetic stroke, hyperglycemia overloads anaerobic energy production causing stress on neurons and can exacerbate any calcium imbalances and ROS accumulation therefore leading to increased cell death upon ischemic injury33. Further, stroke in diabetes induces epigenetic down-regulation of neuron-specific enolase and neuronal nitric oxide synthase as compared to non-diabetic stroke34. Neuron-specific enolase is implicated in synapse formation and its release into serum is a biomarker for stroke35,36. Post-ischemic hyperglycemia enhances sodium-glucose transporter 1 and exacerbates neuronal damage33. Hence, strict glycemic control in diabetes reduces the incidence of diabetic neuropathy.
Cerebrovasculature and endothelia
The vasculature is essential to neuronal function as it is responsible for delivery of nutrients and removal of metabolites. Any impairment/damage to vasculature due to diabetes can have detrimental effects on neurological health especially in the event of ischemic injury. Prolonged hyperglycemia induces vascular changes, ranging from microvascular (retinopathy) to macrovascular (atherosclerosis) and leads to hypoperfusion/hypoxia. Diabetes leads to endothelial dysfunction causing poor structural integrity of vessel walls, arterial stiffening causing increase risk for vessel damage and systemic inflammation ultimately leading to atherosclerosis (risk factor for stroke) and stroke19. Hyperglycemia reduces available NO vasodilator, reducing perfusion to brain, intensifying inflammatory response and edema further increasing cell death post-stroke34,37. Further, STZ-diabetes induces S-glutathionylation of Kir6.1, reducing number of functional KATP channels, impairing vasodilation in heart, kidney and mesenteric rings. Similar studies have not been done to confirm effects in cerebrovasculature38,39. In ischemia conditions, intranasal insulin injections have been proven beneficial for acute events40,41. In addition, hyperglycemia induces down-regulation of microRNA223 and -146a leading to platelet activation and increased risk for stroke in diabetic patients42. Another diabetic complication, ketoacidosis, increases stroke risk and is known to induce acute cerebral infarction43,44,45,46. Diabetic ketoacidosis causes systemic inflammation disrupting vascular endothelia structure and tight-junction function, coagulopathy, increased hemorrhagic and thrombotic risk and impaired cerebral autoregulation45,46,47,48. When diabetic ketoacidosis is complicated with hypertension and/or hyperlipidemia (commonly present in diabetic patients), stroke risk is further increased43,44. Diabetes induces pathological neovascularization contributing to retinopathy, however diabetes can impair neovascularization and cause regression in other vascular beds like the brain49. Typically, angiogenic genes are unregulated with stroke shortly after the event as angiogenesis after stroke greatly improves functional recovery50,51. In diabetic condition after stroke, neoangiogenesis is impaired but improved with more intensive glucose control and blood pressure control52,53,54,55,56. In conclusion, diabetes induces vascular changes that are conducive of stroke events and poorer stroke recovery.
Glial cells
Glial cells are the most abundant cell type in the brain; the three main types are astrocytes, oligodendrocytes and microglial cells. Although they do not directly participate in synaptic signaling they have important supportive functions like maintaining the necessary chemical environment for proper signaling, myelination of axons to assist axon potential conductance and mediating response to brain injury. As compared to the non-diabetic stroke model mice, the diabetic stroke model showed epigenetic down-regulation of connexin-43, GFAP and CD11b in glial cells34. Connexin-43 is a component of astrocyte gap-junction, essential for gap-junction structure and function. In stroke, connexin-43 expression and translocation is disrupted and over-expression can stabilize astrocytes, rescue astrocytes from stroke's detrimental effect and promote neuronal recovery57. GFAP promotes axonal remodeling and motor behavioral recovery post stroke and is important in maintaining blood brain barrier properties and white matter vascularization58,59,60. This is consistent with reports that the blood brain barrier has compromised permeability under diabetic condition61. CD11b is a well-established proinflammatory cytotoxicity and phagocytosis marker62. In stroke condition it is usually up-regulated for microglial activation63. CD11b down-regulation in diabetic stroke is difficult to interpret without more spatioresolution as targeted phagocytosis may assist in early synaptic remodeling and containment of injury64. Additionally, the role of microglial in stroke is complex in that microglial activation can result in a range of phenotypes both pro- and anti-inflammatory and phagocytosis can attenuate inflammation but also cause more neuronal damage by phagocytosis of viable neurons65,66. Therefore, the role of microglial in stroke in presence of diabetes needs to be further studied. Glutamate uptake by astrocytic glutamate transporters is important to maintain a low extracellular concentration to avoid excitotoxicity and neuronal damage. In the case of neuronal injury by stroke, glutamate is exocytosed at great quantities causing excitotoxicity, ion imbalance and neuronal death. STZ-diabetic mice show no change in glutamate transporter (GLT-1 and GLAST) levels despite others reporting decrease in glutamate uptake in STZ-diabetic mice indicating a possible decrease in functionality of protein67. This suggests that although diabetes and prolonged hyperglycemia does not affect the number of glutamate transporter, it may be impairing transporter function. Diabetes also reduces oligodendrocyte progenitor cell proliferation and survival under chronic ischemia which both correlated with more severe white matter injury68. Diabetes results in more demyelination during stroke and less remyelination in the recovery of the ischemic penumbra69. In conclusion, the diabetic condition can impair glial function in turn compromising neuronal health and impair glial reaction to ischemic injury thereby exacerbating stroke injury.
KATP channels
Potassium channels are ubiquitously expressed ion channels, present across essentially all cell types70. Opening of K+ channel leads to an efflux of K+ ions, hyperpolarizing the cell. Adenosine triphosphate (ATP)-sensitive K+ (KATP) channels conduct weak inward rectifier potassium current and belong to the Kir superfamily of K+ channels. KATP channels are composed of 4 pore-forming subunits (Kir6.1 or Kir6.2 encoded by KCNJ8 and KCNJ11, respectively) and 4 regulatory sulfonylurea receptor SUR ATP-binding cassettes subunits (subfamily C: SUR1, SUR2A or SUR2B). KATP channels are inhibited by ATP and activated by Mg-ADP, allowing the cell to couple cellular metabolic state (ATP/ADP ratio) to electrical activity of the cell membrane. In pancreatic beta cells Kir6.2/SUR1 are the major subunits expressed, in cardiac myocytes Kir6.2/SUR2A subunits, in smooth muscles SUR2B, in adipose tissue Kir6.1/SUR2B, and in the brain neurons mostly Kir6.2/SUR1 while in astrocytes only Kir6.1/SUR1 and 271,72,73,74,75. KATP channels were first described in isolated ventricular myocytes of the guinea pig76, and have been studied for their role in diseases from diabetes and hyperinsulinemia to cardiac arrhythmias and cardiovascular disease. KATP channels mediate insulin secretion in pancreatic islet beta cells, and controlling vascular tone77. Under pathophysiological conditions, KATP channels play cytoprotective role in cardiac myocytes and neurons during ischemia and/or hypoxia78,79,80,81.
Neuroprotective effect of KATP channels in stroke
In a stroke or an ischemic event, there is a shortage of oxygen and/or nutrient delivery and hence reduction of cellular ATP. Therefore, KATP channels are activated by the rise in ADP/ATP ratio. This increase in KATP channel activity and hyperpolarization during an ischemic event is thought to be important for protecting the cells from cell death and excitotoxicity82,83. In ischemic conditions, activation of KATP channels underlie many cardioprotective mechanisms78. Alpha-lipoic acid, diosgenin, estrogen, atorvastatin, vitamin C and angiotensin III have all been implicated as therapeutic agents for purpose of cardioprotection and suggested to function via KATP channels84,85,86,87,88,89,90. Aside from these cytoprotective agents, KATP channels are implicated in ischemic preconditioning in the heart91. Ischemic preconditioning is when one or several intermittent periods of ischemia disconcertingly results in protection against tissue damage by a subsequent and sustained ischemic injury79. KATP channel activation prior to ischemic event mimics the effects of ischemic preconditioning78,80,92. Similarly, in the brain KATP channels play a role in ischemic tolerance in stroke, conferring neuroprotection81. In diabetic brain, expression of Kir6.2 was significantly reduced, however, whether SUR1 expression was affected remained inconclusive93.
Neuronal KATP channels
KATP pore forming subunits Kir6.1 and Kir6.2, as well as their regulatory subunits SUR1 and 2B, are expressed at high levels in the brain (cortical and hippocampal areas)73,74,81,94,95. Neuronal KATP channels play an important role in regulating neuronal excitability and spontaneous firing in neurons including: cholinergic basal forebrain neurons, expiratory neurons, entorhinal layer 3 cortical neurons, substantia nigra neurons, thalamocortical neurons96,97,98,99,100. Neuronal KATP channels also play a critical role in glucose homeostasis at the hypothalamic level by regulating the secretion of glucagon and catecholamines101. In neuronal monocultures, pretreatment with diazoxide, a of KATP channel opener, induced delayed preconditioning against oxygen glucose deprivation (OGD) and reduced cell death. These effects of diazoxide were suggested via inhibition of succinate dehydrogenase not mitochondrial KATP channel102. Hippocampal neuron culture studies suggest that diazoxide decreases neuron apoptosis by preventing cytochrome c release, increasing Bcl-2 release and inhibiting Bax association with mitochondria103. In a study comparing KATP channel blocker and activator, blocker increased neuronal death in OGD of cultures while activator conferred neuroprotection104. Activation of KATP channels is neuroprotective in both focal and global ischemia in vivo models, and the in vitro results suggest these effects are mediated at least in part by neuronal KATP channels81,94,95,105.
Glial KATP channels
Astrocytes can provide protection in the event of ischemic events by supporting blood brain barrier integrity, reducing glutamate excitotoxicty and donation of mitochondria to neurons during recovery106. Glutamate uptake by astrocytic glutamate transporters maintains low extracellular concentration to avoid excitotoxicity. Selective activation of mitochondrial KATP channels in astrocytes increases glutamate uptake in culture which could confer an protective advantage107. However, there has not been in vivo confirmation of these findings. In astrocyte monocultures, the channel opener diazoxide pretreatment induced delayed preconditioning against oxygen glucose deprivation (OGD) blocking cell death as did in neuronal cultures suggesting that the protective effects observed in vivo may be in part due to astrocytic KATP channels108. In primary microglia cultures, KATP channel opener can prevent rotenone-induced microglia activation and neuroinflammation. In BV2 microglia cell line, the channel blocker glibenclamide increased reactive microglia, phagocytic capacity and TNFα release in response to pro-inflammatory signalling109,110. Activated microglia at early phases of stroke was correlated with neuroprotection110. Currently, it is not clear whether Kir6.x channel subunits are affected by diabetes, however hyperglycemia can reduce expression and function of astrocytic ATP-sensitive Kir4.1 channels in parallel with a decrease in glial glutamate level, suggesting a role of astrocytic potassium channels in poor stroke prognoses111. Since diabetes induced S-glutathionylation of Kir6.1 is likely not limited to vasculature, the reduction of functional Kir6.1 subunit containing KATP channels in diabetic condition could exacerbate ischemic stroke-induced brain damage. Astrocytes and oligodendrocytes ubiquitously express Kir6.1 and SUR1 which are activated under ischemic condition, however the specific function and/or expression of the glial channels in diabetes have not been thoroughly studied112,113,114.
Vascular KATP channels
KATP channels are expressed in vascular smooth muscle115,116, likely Kir6.1 and SUR2B subunits117,118. Vasodilators (e.g. adenosine, calcitonin gene-related peptide and beta-agonists) and –constrictors (angiotensin II, endothelin-I and vasopressin) increase or decrease KATP channel activity, respectively, via PKC pathways115,119,120,121. KATP channels in the vasculature regulate vascular tone and blood flow to all organs including the brain. Vascular muscle KATP channel activation causes vasodilation by controlling arterial diameter122. In healthy volunteers gilbenclamide (SUR class KATP blocker) blocked while diazoxide (KATP channel opener) mimicked endothelial ischemic preconditioning in humans123. Before an ischemic event, KATP mediated ischemic preconditioning of endothelial and during an ischemia event, vascular smooth muscle KATP activation may be favourable as vasodilation could increase perfusion to the tissue and be therapeutic. In pathological conditions like hypertension (a stroke risk factor), the vasodilation response to KATP channels is impaired at large cerebral arteries and microvessels (much like with KATP channel blocker) and may predispose brain to ischemia and stroke124. Therefore blockade of vascular KATP channels can worsen hypertension and reduces blood flow which may predispose tissues to larger infarctions in the event of stroke125. In STZ diabetic model Kir6.1 S-glutathionylation reduces number of functional KATP channels, impairing vasodilation in heart, kidney and mesenteric rings38,39. Whether the neuroprotective effects of KATP channel activators are through neuronal, glial and/or vascular channels is not fully understood. Because glial cells and the vasculature play an important role in stroke pathobiology, understanding the role of KATP channels in glial and endothelial cells could further explain the detrimental effects of KATP channel blocking in ischemic stroke and the neuroprotective effects of activation.
KATP channels in in vivo stroke models
Middle cerebral artery occlusion (MCAO) of rodents is a commonly used animal model for focal stroke. KATP channel opener diazoxide reduced neuronal damage after MCAO126 and also induced delayed preconditioning against transient focal cerebral ischemia and reduced infarction volumes127 in rats. Similarly, activation of mitochondrial KATP channels by BMS-191095 reduced total infarction volume in rats undergone MCAO127. Consistent with these observations, KATP channel blocker tolbutamide increased infarction volume and neurological deficits in MCAO model in mice, while KATP channel opener provided neuroprotection104. A separate study using 5-hydroxydecanoate as antagonist and diazoxide as agonist in MCAO rat model showed these effects were conserved128. These findings were further confirmed in genetic knockout mouse model, indicating endogenous cortical KATP channel activation provides protection against cerebral ischemic stroke induced infarction and neurological deficits129,130. In transgenic mice overexpressing Kir6.2 channel, the animals exhibited strong neuroprotection against hypoxic-ischemic injury131. There are some conflicting accounts from studies using glibenclamide and glyburide, the second generation of sulfonylurea class KATP channel blocker. Used alone or in combination with hypothermia, glibenclamide improved neurological outcome after MCAO in rats and 30 day survival were improved132,133,134. SUR1 subunit can couple with non-selective cation channel, transient receptor potential melastin 4 (TRPM4) channel, which is involved in development of cerebral edema in brain injury135,136,137. It is possible that glibenclamide affects the SUR1-TRPM4 complexes, thus reduced cerebral edema and swelling following stroke138,139,140,141. Overall, animal studies suggest KATP channel openers reduce and the channel blockers increase brain damage. Further studies are required to understand the mechanisms underlying the differential effects of sulfonylurea class KATP channel blockers on stroke severity.
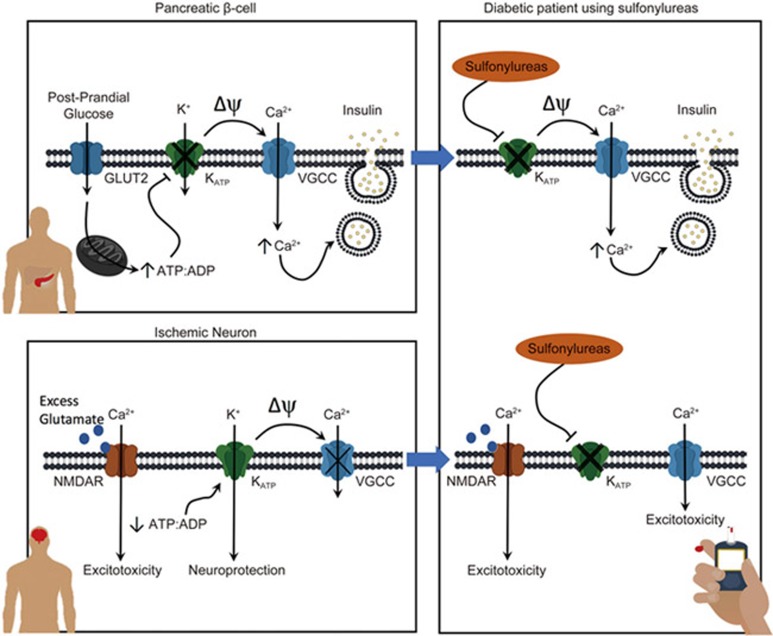
There is abundant evidence that KATP channel activity is neuroprotective in ischemic events. Not surprisingly, there are a plethora of patents involving KATP channels and neuroprotection142. Activation of KATP channels hyperpolarizes neurons, which can prevent excitotoxicity, stabilize membrane potential, reduce ionic imbalance and protect neurons from ischemia-induced death129. A schematic diagram is shown in Figure 1.
Figure 1.
KATP channels are neuroprotective and sulfonylurea use can exacerbate ischemia-induced brain damage. In the pancreatic β, KATP channels serve as a metabolic sensor to post-prandial glucose metabolism. The closure of KATP channels depolarizes cell membrane, activates voltagegated calcium channels (VGCCs) and thus leads to insulin release. In the diabetic patients, sulfonylureas can be used to block KATP channels and increase insulin release. In an ischemic neuron, the reduction in ATP: ADP ratio opens KATP channels, lowering membrane potential and stabilizing the membrane, thus reducing cell excitotoxicity. In other words, the opening of KATP channels is neuroprotective. In the diabetic patients using sulfonylureas, the neuroprotective effects of KATP channels neuroprotective effects are abolished.
KATP channel blockers in diabetes treatment
A prime example of KATP channels coupling metabolism to electrical activity is in pancreatic beta cells. Glucose metabolism causes depolarization of the cell linking to insulin secretion. When glucose enters via GLUT2 transporter and it is metabolized by glucokinase resulting in increase in ATP/ADP ratio. ATP induces KATP channel closure causing beta cell depolarization, voltage-gated calcium channel activation and leading to calcium-dependent insulin release (Figure 1). SUR subunit facilitates KATP current via its ADP-binding. SUR1 paired potassium channel are highly sensitive to sulfonylurea inhibition and diazoxide activation143. In special circumstances, PIP2 can uncouple Kir6.2 from sulfonylurea bound SUR1, producing SU-independent current71. Mutations that alter KATP channel activity are commonly seen in patients with neonatal diabetes, hyperinsulinemia and developmental delay-epilepsy-neonatal diabetes (DEND syndrome)144,145,146,147. Specifically, mutations in SUR subunit are associated with diabetes148. KATP channels and SUR modulatory subunits act as key drug targets for diabetes hyperglycemic control. SUR subunit renders KATP channels sensitive to sulfonylureas. Sulfonylureas, KATP channel blockers, are the oldest class of hyperglycemic controlling drugs. Sulfonylureas reduce MgADP binding and efficacy of ADP-induced opening, and results in closure of KATP channel149. Effectively, sulfonylureas block KATP channel activity and induce insulin release (Figure 1).
KATP channels are a major drug target in type 2 and neonatal diabetes. Closure of KATP results in depolarization and insulin secretion in pancreatic β cells (Figure 1). SUR blockers can be categorized into two sets, drugs that block both SUR1 and SUR2: gilbenclamide, glimepiride, repaglinide, meglitinide and those that are SUR1 specific: tolbutamide, ngliclazide and nateglinde150. Gliclazide and tolbutamide inhibition is readily reversible while gilbenclamide, glimepiride and repaglinide exhibit a much slower reversibility. Gilbenclamide binds to SUR1 at two sites, thus perhaps rendering slow dissociation. This is in line with the similar structure between gilbenclamide and glimepiride150. Sulfonylurea class anti-diabetic drugs and its derivatives are used in diabetes mellitus to stimulate insulin release and control blood glucose. Clinically, they are classified into three generations: the second and third generation sulfonylureas are generally safer (i.e. lower risk of hypoglycemia, cardiovascular events) than the first generation. Sulfonylureas have potent glucose lowering effects and newer oral antidiabetics (including metformin, thiazolidinediones, exenatides, and symlins) show lower risk of inducing hypoglycemia, thus are a popular choice in western-healthcare. However first generation of sulfonylureas remains the key in diabetes care in developing countries151,152.
Cerebrovascular safety of SUR blocking anti-diabetic drugs and future directions
The American College of Physicians (ACP) in clinical practice guideline updates for oral pharmacological treatment of T2D states that sulfonylureas are associated with weight gain and more episodes of hypoglycemia than metformin153. However, there is low quality and inconsistent evidence to suggest sulfonylureas alone or metformin combination treatment increases cardiovascular risks/all-cause mortality as compared to metformin treatment of T2D. A recent meta-analysis of sulfonylurea treatment of diabetes and stroke risk summarizes 17 randomized controlled trials concluded with high confidence that sulfonylureas monotherapy or combination therapy increases the number of stroke events in diabetic patients as compared to comparator drug or placebo group104,154. Since then there have been other reviews on cardiovascular events and anti-diabetic agents, however no new analysis focusing on stroke and sulfonylureas155,156,157,158,159. In line with the ACP, other independent reviews including Cochrane review of 301 clinical trials conclude that sulfonylurea safety in treatment of diabetes is still unclear157,158,160. As for stroke risk, studies of effects of sulfonylureas on stroke severity and recovery are incomplete161,162,163,164. Despite new evidences emerging, due to many conflicting accounts in both animal and human studies, cerebrovascular safety of sulfonylurea remains controversial161,163,165,166,167,168.
Many sources could contribute to the heterogeneity among the studies. For instance, the wide variety of sulfonylureas has been used in studies or prescribed in clinics (different generations, short, intermediate or long acting). Clinical/epidemiological studies that indicate the specific sulfonylurea subgroup analysis are insufficient. Sulfonylurea subgroup analysis by generation has been employed in terms of evaluating risk of hypoglycemia however cardiovascular and cerebrovascular risk are newer areas in comparison169. Although there has been no report comparing the types of sulfonylurea, one meta-analysis that excluded studies using first generation sulfonylureas found no appreciable increase in all-cause mortality, stroke or myocardial infarction with prescribed second or third generation sulfonylureas168. To move forward, new studies in relationship between sulfonylurea use and cerebrovascular mortality, as well as all-cause mortality should specify individual sulfonylureas used by each participant.
Sulfonylureas target a fundamental step of insulin secretion and are effective in treating diabetes with diverse genetic causes, thus are useful where genetic testing is not readily available170. The heterogeneity of their effects on stroke might be in part related to genetic polymorphisms at the cytochrome P450 2C9 (CYP2C9) gene, encoding the enzyme that primarily metabolizes sulfonylureas171 or at the ABCC gene sites. Individual differences affecting how the body processes sulfonylureas to how the sulfonylureas act on the targets remained to have a large impact. In line with these possibilities, efforts could be made in pharmacogenetics to determine patients with CYP2C9 mutations (CYP2C9*3/*3) which prolong effects of sulfonylureas in the body171. This poses a great challenge as the areas that have fast growing diabetic populations and tend toward sulfonylureas are unlikely to have access to genetic screening before treatment. In developing countries with limited access to genetic testing and limited resource, affordable and reliable treatments like sulfonylureas are highly valuable. According to the Association of Physicians of India, sulfonylureas are prescribed as their first line for non-obese diabetic patients by most of doctors172. Given the role of KATP channels in neuroprotection, there is a concern for the safety of sulfonylureas usage in this population with increased risk of stroke.
The pharmacokinetic and pharmacodynamic profiles of each sulfonylureas are different. Prescribing sulfonylureas with lower permeability to the brain or shorter half-life could mitigate their effects on stroke risk while achieving insulin and glycemic targets. Further, sulfonylureas display almost complete serum protein binding (90%–99%) once absorbed and their clearance is hindered by renal impairment which is common in diabetics. Only tolbutamide has been studied for its ability to across the blood-brain-barrier and have a minimal serum accumulation173,174. Under diabetic or stroke conditions, the blood-brain-barrier integrity is damaged and its permeability to drugs is altered175, and thus detailed understanding of the levels of individual sulfonylureas in brain under these pathological/pathophysiological conditions should be further explored.
Conclusion
KATP channels play important roles both in physiologic and pathophysiologic settings, from insulin secretion to cyto/neuroprotection. Activation of KATP channels in ischemia and/or hypoxia can provide neuroprotection to stroke and hypoxia. Diabetes is one of the major risk factors for stroke and leads to more severe stroke outcomes particularly if hyperglycemic management is inadequate. Sulfonylurea class of antidiabetic drugs blocks KATP channels which are neuroprotective in stroke, and can be one of the high stoke risk factors for diabetic patients. The first generation of sulfonylurea is currently less used in clinics because of their potential side effects, however remains the first line of diabetic treatment in third world countries. Further studies are needed to verify whether the long term use of the KATP channel blockers would increase the vulnerability of the brain to ischemic/hypoxic insult. As the incidence of diabetes increases, to fully and safely capitalize on sulfonylureas, focus could be made on finding effective ways to stratify the population into well-defined risk groups so that sulfonylureas can be used safely. Until the risks are clear, the data warrant caution when recommending sulfonylurea as treatment especially if patients display high risk for stroke.
Abbreviation
KATP channel, adenosine triphosphate-sensitive K+ channel; ABCC, ATP-binding cassette transporter sub-family C; KCNJ, Potassium Voltage-Gated Channel Subfamily J; Kir, Inward-rectifer potassium channel; OGD, oxygen-glucose deprivation; SUR, sulfonylurea receptor; rtPA, recombinant tissue plasminogen activator; TRPM4, transient receptor potential melastatin 4; GLUT2, glucose transporter 2; VGCC, voltage-gated calcium channel; MCAO, middle cerebral artery occlusion; GFAP, glial fibrillary acidic protein; ROS, reactive oxygen species; STZ, streptozotocin; GLT-1, glutamate transporter 1; GLAST, glutamate aspartate transporter; ACP, American College of Physicians; CYP2C9, cytochrome P450 2C9.
Acknowledgments
VYS holds an NSERC Canada Graduate Scholarship. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants, to Zhong-ping FENG (RGPIN-2014-06471) and to Hong-shuo SUN (RGPIN-2016-04574).
References
- Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. In: Handbook of clinical neurology. 2014; 126: 211–22. [DOI] [PubMed] [Google Scholar]
- Al Kasab S, Cassarly C, Le NA, Martin R, Brinley J, Chimowitz MI, et al. Postprandial clearance of oxidized low-density lipoprotein in patients with stroke due to atherosclerosis. J Stroke Cerebrovasc Dis 2017; 26: 488–93. [DOI] [PubMed] [Google Scholar]
- Vancheri F, Curcio M, Burgio A, Salvaggio S, Gruttadauria G, Lunetta MC, et al. Impaired glucose metabolism in patients with acute stroke and no previous diagnosis of diabetes mellitus. QJM. Oxford University Press 2005; 98: 871–8. [DOI] [PubMed] [Google Scholar]
- Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke 2013; 44: 1500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wen X, Li W, Li X, Wang Y, Lu W. Risk factors for stroke in the chinese population: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2017; 26: 509–17. [DOI] [PubMed] [Google Scholar]
- Melgaard L, Gorst-Rasmussen A, Søgaard P, Rasmussen LH, Lip GYH, Larsen TB. Diabetes mellitus and risk of ischemic stroke in patients with heart failure and no atrial fibrillation. Int J Cardiol 2016; 209: 1–6. [DOI] [PubMed] [Google Scholar]
- Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project. Stroke 2003; 34: 688–94. [DOI] [PubMed] [Google Scholar]
- The Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375: 2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, et al. Incidence and risk factors for stroke in type 2 diabetic patients: the DAI Study. Stroke 2007; 38: 1154–60. [DOI] [PubMed] [Google Scholar]
- Johnston SS, Sheehan JJ, Shah M, Cappell K, Princic N, Smith D, et al. Cardiovascular event costs in patients with type 2 diabetes mellitus. J Med Econ 2015; 18: 1032–40. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011; 123: 933–44. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361: 2005–16.12814710 [Google Scholar]
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–72. [DOI] [PubMed] [Google Scholar]
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziomalos K, Dimitriou P, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM, et al. Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metabolism 2017; 67: 99–105. [DOI] [PubMed] [Google Scholar]
- Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, et al. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 2013; 44: 1915–23. [DOI] [PubMed] [Google Scholar]
- Gofir A, Mulyono B, Sutarni S. Hyperglycemia as a prognosis predictor of length of stay and functional outcomes in patients with acute ischemic stroke. Int J Neurosci 2017; 127: 923–9. [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia—ischemia in the diabetic mouse. J Cereb Blood Flow Metab 2007; 27: 710–8. [DOI] [PubMed] [Google Scholar]
- Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 2016; 351: 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, et al. Diabetes mellitus and risk of stroke and its subtypes among Japanese: the Japan Public Health Center Study. Stroke 2011; 42: 2611–4. [DOI] [PubMed] [Google Scholar]
- Laing SP, Swerdlow AJ, Carpenter LM, Slater SD, Burden AC, Botha JL, et al. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke 2003; 34: 418–21. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the nurses' health study. Diabetes Care 2007; 30: 1730–5. [DOI] [PubMed] [Google Scholar]
- McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomised, controlled trial of insulin for acute post-stroke hyperglycemia. Ann Neurol 2010; 67: 570–8. [DOI] [PubMed] [Google Scholar]
- Ntaios G, Egli M, Faouzi M, Michel P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010; 41: 2366–70. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–32. [DOI] [PubMed] [Google Scholar]
- Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke 2004; 35: 363–4. [DOI] [PubMed] [Google Scholar]
- Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 1999; 353: 376–7. [DOI] [PubMed] [Google Scholar]
- Mohiuddin L, Tomlinson DR. Impaired molecular regenerative responses in sensory neurones of diabetic rats: gene expression changes in dorsal root ganglia after sciatic nerve crush. Diabetes 1997; 46: 2057–62. [DOI] [PubMed] [Google Scholar]
- Maeda K, Fernyhough P, Tomlinson DR. Regenerating sensory neurones of diabetic rats express reduced levels of mRNA for GAP-43, gamma-preprotachykinin and the nerve growth factor receptors, trkA and p75NGFR. Brain Res Mol Brain Res 1996; 37: 166–74. [DOI] [PubMed] [Google Scholar]
- Yagihashi S, Kamijo M, Watanabe K. Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol 1990; 136: 1365–73. [PMC free article] [PubMed] [Google Scholar]
- West IC. Radicals and oxidative stress in diabetes. Diabetic Medicine. Blackwell Science Ltd; 2000; 17:171–80. [DOI] [PubMed]
- Greene DA, Lattimer SA, Sima AAF. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med 1987; 316: 599–606. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Ogihara S, Harada S, Tokuyama S. Activation of cerebral sodium-glucose transporter type 1 function mediated by post-ischemic hyperglycemia exacerbates the development of cerebral ischemia. Neuroscience 2015; 310: 674–85. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Tyagi N. Diabetic stroke severity: epigenetic remodeling and neuronal, glial, and vascular dysfunction. Diabetes 2015; 64: 4260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis 2005; 20: 213–9. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Marangos PJ, Connolly SM, Morest DK. Synapse formation is related to the onset of neuron-specific enolase immunoreactivity in the avian auditory and vestibular systems. Dev Neurosci 1982; 5: 298–307. [DOI] [PubMed] [Google Scholar]
- Li D, Huang B, Liu J, Li L, Li X. Decreased brain KATP channel contributes to exacerbating ischemic brain injury and the failure of neuroprotection by sevoflurane post-conditioning in diabetic rats. Arai K, editor. PLoS One 2013; 8: e73334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular KATP channels by S-glutathionylation. J Biol Chem 2010; 285: 38641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cui N, Yang Y, Trower TC, Wei YM, Wu Y, et al. Impairment of the vascular KATP channel imposes fatal susceptibility to experimental diabetes due to multi-organ injuries. J Cell Physiol 2015; 230: 2915–26. [DOI] [PubMed] [Google Scholar]
- Lioutas VA, Alfaro-Martinez F, Bedoya F, Chung CC, Pimentel DA, Novak V. Intranasal insulin and insulin-like growth factor 1 as neuroprotectants in acute ischemic stroke. Transl Stroke Res 2015; 6: 264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntaios G, Papavasileiou V, Bargiota A, Makaritsis K, Michel P. Intravenous insulin treatment in acute stroke: a systematic review and meta-analysis of randomized controlled trials. Int J Stroke 2014; 9: 489–93. [DOI] [PubMed] [Google Scholar]
- Duan X, Zhan Q, Song B, Zeng S, Zhou J, Long Y, et al. Detection of platelet microRNA expression in patients with diabetes mellitus with or without ischemic stroke. J Diabetes Complications 2014; 28: 705–10. [DOI] [PubMed] [Google Scholar]
- Chen Y, Weng S, Yang C, Wang J, Tien K. Long-term risk of stroke in type 2 diabetes patients with diabetic ketoacidosis: a population-based, propensity score-matched, longitudinal follow-up study. Diabetes Metab 2017; 43: 223–8. [DOI] [PubMed] [Google Scholar]
- Jovanovic A, Stolic R, Markovic Jovanovic S, Rasic D, Peric V. Stroke and diabetic ketoacidosis–some diagnostic and therapeutic considerations. Vasc Health Risk Manag 2014; 10: 201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JR, Morrison G, Fraser DD. Diabetic ketoacidosis-associated stroke in children and youth. Stroke Res Treat 2011; 2011: 219706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M, Patterson EK, Cepinskas G, Clarson C, Omatsu T, Fraser DD. Dynamic regulation of plasma matrix metalloproteinases in human diabetic ketoacidosis. Pediatr Res 2016; 79: 295–300. [DOI] [PubMed] [Google Scholar]
- Ma L, Roberts JS, Pihoker C, Richards TL, Shaw DWW, Marro KI, et al. Transcranial Doppler–based assessment of cerebral autoregulation in critically Ill children during diabetic ketoacidosis treatment*. Pediatr Crit Care Med 2014; 15: 742–9. [DOI] [PubMed] [Google Scholar]
- Tasker RC, Acerini CL. Cerebral edema in children with diabetic ketoacidosis: vasogenic rather than cellular? Pediatr Diabetes 2014; 15: 261–70. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes 2011; 60: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 2003; 23: 166–80. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke 1997; 28: 564–73. [DOI] [PubMed] [Google Scholar]
- Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab 2014; 34: 553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis 2011; 43: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther 2009; 330: 532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol 2007; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 2013; 44: 2875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Yu X, Feng L. Connexin 43 stabilizes astrocytes in a stroke-like milieu to facilitate neuronal recovery. Acta Pharmacol Sin 2015; 36: 928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Stanness KA, Eliasson C, Betsholtz C, Janigro D. Impaired induction of blood-brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia 1998; 22: 390–400. [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, et al. Beneficial effects of GFAP/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 2014; 62: 2022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 1996; 17: 607–15. [DOI] [PubMed] [Google Scholar]
- Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003; 74:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics 2010; 7: 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation 2013; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 2009; 29: 3974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol 2013; 5:73–90. [PMC free article] [PubMed] [Google Scholar]
- Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci U S A 1996; 93: 14833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman E, Judd R, Hoe L, Dennis J, Posner P. Effects of diabetes mellitus on astrocyte GFAP and glutamate transporters in the CNS. Glia 2004; 48: 166–78. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Tanaka R, Shimada Y, Yamashiro K, Liu M, Mitome-Mishima Y, et al. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience 2015; 289: 214–23. [DOI] [PubMed] [Google Scholar]
- Jing L, He Q, Zhang JZ, Li PA. Temporal profile of astrocytes and changes of oligodendrocyte-based myelin following middle cerebral artery occlusion in diabetic and non-diabetic rats. Int J Biol Sci 2011; 9: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. An overview of the potassium channel family. Genome Biol 2000; 1: REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Wu J-X, Ding D, Cheng J, Gao N, Chen L. Structure of a pancreatic ATP-sensitive potassium channel. Cell 2017; 168: 101–110. e10. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol 2006; 577: 1053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomzig, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, et al. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane KATP channels. Mol Cell Neurosci 2001; 18: 671–90. [DOI] [PubMed] [Google Scholar]
- Thomzig, Laube G, Prüss H, Veh RW. Pore-forming subunits of KATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol 2005; 484: 313–30. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Kis B, Szabó C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res 2003; 994: 27–36. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 1983; 305: 147–8. [DOI] [PubMed] [Google Scholar]
- Akrouh A, Halcomb SE, Nichols CG, Sala-Rabanal M. Molecular biology of K(ATP) channels and implications for health and disease. IUBMB Life 2009; 61: 971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol 2000; 32: 677–95. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124–36. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 1999; 84: 973–9. [DOI] [PubMed] [Google Scholar]
- Sun H, Xu B, Chen W, Xiao A, Turlova E, Alibraham A, et al. Neuronal KATP channels mediate hypoxic preconditioning and reduce subsequent neonatal hypoxic-ischemic brain injury. Exp Neurol 2015; 263:161–71. [DOI] [PubMed] [Google Scholar]
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, et al. Targeting mitochondrial ATP-sensitive potassium channels—a novel approach to neuroprotection. Brain Res Rev 2004; 46: 282–94. [DOI] [PubMed] [Google Scholar]
- Sun, Feng Z. Neuroprotective role of ATP-sensitive potassium channels in cerebral ischemia. Acta Pharmacol Sin 2013; 34: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Knutelska J, Bednarski M, Nowiński L, Zygmunt M, Bilska-Wilkosz A, et al. Alpha lipoic acid protects the heart against myocardial post ischemia–reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol Reports 2014; 66: 499–504. [DOI] [PubMed] [Google Scholar]
- Badalzadeh R, Yousefi B, Tajaddini A, Ahmadian N. Diosgenin-induced protection against myocardial ischaemia-reperfusion injury is mediated by mitochondrial KATP channels in a rat model. Perfusion 2015; 30: 565–71. [DOI] [PubMed] [Google Scholar]
- Gao J, Xu D, Sabat G, Valdivia H, Xu W, Shi NQ. Disrupting KATP channels diminishes the estrogen-mediated protection in female mutant mice during ischemia-reperfusion. Clin Proteomics 2014; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Cui W, Zhang H, Gao H, Li X, Wang Y, et al. Pre-treatment of a single high-dose of atorvastatin provided cardioprotection in different ischaemia/reperfusion models via activating mitochondrial KATP channel. Eur J Pharmacol 2015; 751: 89–98. [DOI] [PubMed] [Google Scholar]
- Hao J, Li WW, Du H, Zhao ZF, Liu F, Lu JC, et al. Role of vitamin c in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem Pharm Bull (Tokyo) 2016; 64: 548–57. [DOI] [PubMed] [Google Scholar]
- Park BM, Gao S, Cha SA, Park BH, Kim SH. Cardioprotective effects of angiotensin III against ischemic injury via the AT2 receptor and KATP channels. Physiol Rep 2013; 1: e00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S, Giebisch G. ATP-sensitive potassium channels in physiology, pathophysiology, and pharmacology. Curr Opin Nephrol Hypertens 1992; 1: 21–33. [DOI] [PubMed] [Google Scholar]
- Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, et al. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. AJP Hear Circ Physiol 2004; 288: H1252–6. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Myocardial KATP channels in preconditioning. Circ Res 2000; 87: 845–55. [DOI] [PubMed] [Google Scholar]
- Acosta-Martinez M, Levine JE. Regulation of KATP channel subunit gene expression by hyperglycemia in the mediobasal hypothalamus of female rats. AJP Endocrinol Metab 2007; 292: E1801–7. [DOI] [PubMed] [Google Scholar]
- Sun, Feng ZP, Miki T, Seino S, French RJ. Enhanced neuronal damage after ischemic insults in mice lacking Kir6.2-containing ATP-sensitive K+ channels. J Neurophysiol 2006r 7; 95: 2590–601. [DOI] [PubMed] [Google Scholar]
- Sun HS, Feng ZP, Barber PA, Buchan AM, French RJ. Kir6.2-containing ATP-sensitive potassium channels protect cortical neurons from ischemic/anoxic injury in vitro and in vivo. Neuroscience 2007; 144: 1509–15. [DOI] [PubMed] [Google Scholar]
- Allen TGJ, Brown DA. Modulation of the excitability of cholinergic basal forebrain neurones by KATP channels. J Physiol 2004; 554: 353–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. J Physiol 1996; 494: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemak MS, Voloshanenko O, Draguhn A, Egorov A V. KATP channels modulate intrinsic firing activity of immature entorhinal cortex layer III neurons. Front Cell Neurosci 2014; 8: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A, Birnbaumer L, Yellen G. Metabolism regulates the spontaneous firing of substantia nigra pars reticulata neurons via KATP and nonselective cation channels. J Neurosci 2014; 34: 16336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten MR, Anderson MP. Self-regulation of adult thalamocortical neurons. J Neurophysiol 2015; 114: 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky GJ, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 1998; 47: 995–1005. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem 2004; 87: 969–80. [DOI] [PubMed] [Google Scholar]
- Liu, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of bax translocation and cytochrome c release. J Cereb Blood Flow Metab 2002; 22: 431–43. [DOI] [PubMed] [Google Scholar]
- Liu R, Wang H, Xu B, Chen W, Turlova E, Dong N, et al. Cerebrovascular safety of sulfonylureas: the role of KATP channels in neuroprotection and the risk of stroke in patients with type 2 diabetes. Diabetes 2016; 65: 2795–809. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Bertaina V, Widmann C, Lazdunski M. K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc Natl Acad Sci U S A 1993; 90: 9431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016; 535: 551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XL, Zeng XN, Zhou F, Dai CP, Ding JH, Hu G. KATP channel openers facilitate glutamate uptake by gluts in rat primary cultured astrocytes. Neuropsychopharmacology 2008; 33: 1336–42. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija D. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res 2003; 73: 206–14. [DOI] [PubMed] [Google Scholar]
- Zhou F, Yao HH, Wu JY, Ding JH, Sun T, Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med 2008; 12: 1559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, et al. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia–ischemia in rats. Exp Neurol 2012; 235: 282–96. [DOI] [PubMed] [Google Scholar]
- Rivera-Aponte DE, Méndez-González MP, Rivera-Pagán AF, Kucheryavykh YV, Kucheryavykh LY, Skatchkov SN, et al. Hyperglycemia reduces functional expression of astrocytic Kir4.1 channels and glial glutamate uptake. Neuroscience 2015; 310: 216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, et al. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane KATP channels. Mol Cell Neurosci 2001; 18: 671–90. [DOI] [PubMed] [Google Scholar]
- Zhou M, Tanaka O, Suzuki M, Sekiguchi M, Takata K, Kawahara K, et al. Localization of pore-forming subunit of the ATP-sensitive K+-channel, Kir6.2, in rat brain neurons and glial cells. Mol Brain Res 2002; 101: 23–32. [DOI] [PubMed] [Google Scholar]
- Zhou M, Tanaka O, Sekiguchi M, Sakabe K, Anzai M, Izumida I, et al. Localization of the ATP-sensitive potassium channel subunit (Kir6.1/uK(ATP)-1) in rat brain. Brain Res Mol Brain Res 1999; 74: 15–25. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77: 1165–232. [DOI] [PubMed] [Google Scholar]
- Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol 2006; 572: 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br J Pharmacol 1996; 118: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ U, Hambrock A, Artunc F, Löffler-Walz C, Horio Y, Kurachi Y, et al. Coexpression with the inward rectifier K+ channel Kir6.1 increases the affinity of the vascular sulfonylurea receptor SUR2B for glibenclamide. Mol Pharmacol 1999; 56: 955–61. [PubMed] [Google Scholar]
- Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A 1995; 92: 12441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Nakaya Y. Activation of ATP-Sensitive K+ channels by cyclic AMP-dependent protein kinase in cultured smooth muscle cells of porcine coronary artery. Biochem Biophys Res Commun 1993; 193: 240–7. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, City T. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res Cardiol 1995; 90: 332–6. [DOI] [PubMed] [Google Scholar]
- Cole WC, Clément-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol 2003; 14: 94–103. [DOI] [PubMed] [Google Scholar]
- Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation 2004; 110: 2077–82. [DOI] [PubMed] [Google Scholar]
- Pinheiro JM, Malik AB. KATP-channel activation causes marked vasodilation in the hypertensive neonatal pig lung. Am J Physiol 1992; 263: H1532–6. [DOI] [PubMed] [Google Scholar]
- Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012; 157: 601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoKATP opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am J Physiol 2002; 283: H1005–11. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gáspár T. Katakam P V., Busija DW. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res 2007; 1168: 106–11. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pan S, Zheng X, Wan Q. Cytomembrane ATP-sensitive K+channels in neurovascular unit targets of ischemic stroke in the recovery period. Exp Ther Med 2016; 12: 1055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Feng Z-P, Barber PA, Buchan AM, French RJ. Kir6.2-containing ATP-sensitive potassium channels protect cortical neurons from ischemic/anoxic injury in vitro and in vivo. Neuroscience 2007; 144: 1509–15. [DOI] [PubMed] [Google Scholar]
- Dong YF, Wang LX, Huang X, Cao WJ, Lu M, Ding JH, et al. Kir6.1 knockdown aggravates cerebral ischemia/reperfusion-induced neural injury in mice. CNS Neurosci Ther 2013; 19: 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héron-Milhavet L, Xue-jun Y, Vannucci SJ, Wood TL, Willing LB, Stannard B, et al. Protection against hypoxic–ischemic injury in transgenic mice overexpressing Kir6.2 channel pore in forebrain. Mol Cell Neurosci 2004; 25: 585–93. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Jolkkonen J, Mahy N, Rodríguez MJ. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2013; 33: 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhu SZ, Hu YF, Gu Y, Wang SN, Lin ZZ, et al. Glibenclamide enhances the effects of delayed hypothermia after experimental stroke in rats. Brain Res 2016; 1643: 113–22. [DOI] [PubMed] [Google Scholar]
- Abdallah DM, Nassar NN, Abd-El-Salam RM. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain Res 2011; 1385: 257–62. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov K V, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat Med 2006; 12: 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Puccio AM, Okonkwo DO, Zusman BE. Park S-Y, Wallisch J, et al. ABCC8 Single nucleotide polymorphisms are associated with cerebral edema in severe TBI. Neurocrit Care 2017; 26: 213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Puccio AM, Chou SH, Chang CC, Wallisch JS, Molyneaux BJ, et al. Sulfonylurea receptor-1. Crit Care Med 2017; 45: e255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali B, Ishrat T, Atif F, Hua F, Stein DG, Sayeed I. Glibenclamide administration attenuates infarct volume, hemispheric swelling, and functional impairments following permanent focal cerebral ischemia in rats. Stroke Res Treat 2012; 2012: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, Battey TWK, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care 2014; 20: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016; 15: 1160–9. [DOI] [PubMed] [Google Scholar]
- Tosun C, Kurland DB, Mehta R, Castellani RJ, deJong JL, Kwon MS, et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 2013; 44: 3522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SI, Smith PJ. Patents related to therapeutic activation of KATP and K2P potassium channels for neuroprotection: ischemic/hypoxic/anoxic injury and general anesthetics. Expert Opin Ther Pat 2009; 19: 433–60. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/Kir6.x KATP channels. Annu Rev Physiol; 1998; 60: 667–87. [DOI] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 2005; 54: 2645–54. [DOI] [PubMed] [Google Scholar]
- Lin YW, Bushman JD, Yan FF, Haidar S, MacMullen C, Ganguly A, et al. Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism. J Biol Chem 2008; 283: 9146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D mutation in Kir6.2 ( KCNJ11 ) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab 2008; 93: 1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Friedman JB, Nichols CG. Diabetes induced by gain-of-function mutations in the Kir6.1 subunit of the KATP channel. J Gen Physiol 2017; 149: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Sulfonylurea receptor-associated channels: involvement in disease and therapeutic implications. Neurology 2017; 88: 314–21. [DOI] [PubMed] [Google Scholar]
- de Wet H, Proks P. Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochem Soc Trans 2015; 43: 901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Pharmacological modulation of KATP channels. Biochem Soc Trans 2002; 30: 333–9. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–9. [DOI] [PubMed] [Google Scholar]
- Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of clinical endocrinologists and American College of endocrinology on the comprehensive type 2 diabetes management algorithm -- 2016 Executive summary. Endocr Pract 2016; 22: 84–113. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med 2017; 166: 279–90. [DOI] [PubMed] [Google Scholar]
- Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes, Obes Metab 2013; 15: 938–53. [DOI] [PubMed] [Google Scholar]
- Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nyström T, Norhammar A. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabetes Res Clin Pract 2016; 117: 39–47. [DOI] [PubMed] [Google Scholar]
- Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia 2016; 59: 1692–701. [DOI] [PubMed] [Google Scholar]
- Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes. JAMA 2016; 316: 313. [DOI] [PubMed] [Google Scholar]
- Cheng JWM, Badreldin HA, Patel DK, Bhatt SH. Current medical research and opinion antidiabetic agents and cardiovascular outcomes in patients with heart diseases antidiabetic agents and cardiovascular outcomes in patients with heart diseases. Curr Med Res Opin 2017; 33: 985–92. [DOI] [PubMed] [Google Scholar]
- Xu J, Rajaratnam R. Cardiovascular safety of non-insulin pharmacotherapy for type 2 diabetes. Cardiovasc Diabetol 2017; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pladevall M, Riera-Guardia N, Margulis A V, Varas-Lorenzo C, Calingaert B, Perez-Gutthann S. Cardiovascular risk associated with the use of glitazones, metformin and sufonylureas: meta-analysis of published observational studies. BMC Cardiovasc Disord 2016; 16: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G, Goyal N, Iftikhar S, Zand R, Chang JJ, Elijovich L, et al. Sulfonylurea pretreatment and in-hospital use does not impact acute ischemic strokes (ais) outcomes following intravenous thrombolysis. J Stroke Cerebrovasc Dis 2017; 26: 795–800. [DOI] [PubMed] [Google Scholar]
- Weih M, Amberger N, Wegener S, Dirnagl U, Reuter T, Einhäupl K. Sulfonylurea drugs do not influence initial stroke severity and in-hospital outcome in stroke patients with diabetes. Stroke 2001; 32: 2029–32. [PubMed] [Google Scholar]
- Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke 2007; 38: 2526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favilla CG, Mullen MT, Ali M, Higgins P, Kasner SE. Virtual international stroke trials archive (VISTA) collaboration. Sulfonylurea use before stroke does not influence outcome. Stroke 2011; 42: 710–5. [DOI] [PubMed] [Google Scholar]
- Li G, Xu X, Wang D, Wang J, Wang Y, Yu J. Microglial activation during acute cerebral infarction in the presence of diabetes mellitus. Neurol Sci 2011; 32: 1075–9. [DOI] [PubMed] [Google Scholar]
- Floyd JS, Wiggins KL, Christiansen M, Dublin S, Longstreth WT, Smith NL, et al. Case-control study of oral glucose-lowering drugs in combination with long-acting insulin and the risks of incident myocardial infarction and incident stroke. Pharmacoepidemiol Drug Saf 2016; 25: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol 2012; 72: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rados DV, Pinto LC, Remonti LR, Leitão CB, Gross JL. The association between sulfonylurea use and all-cause and cardiovascular mortality: a meta-analysis with trial sequential analysis of randomized clinical trials. Lehman R, editor. PLoS Med 2016; 13: e1001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44: 751–5. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Dateki S, Hirose M, Satomura K, Sawada H, Mizuno H, et al. Molecular and clinical features of KATP-channel neonatal diabetes mellitus in Japan. Pediatr Diabetes 2017; 18: 532–9. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmöller J. Effect of genetic polymorphisms in cytochrome P450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs. Clin Pharmacokinet 2005; 44: 1209–25. [DOI] [PubMed] [Google Scholar]
- Sadikot SM, Singh V. Managing diabetes in India: paradigms in care–outcomes and analysis in a comprehensive, clinical practice survey of Indian physicians. J Indian Med Assoc 2011; 109: 839–42, 844–8. [PubMed] [Google Scholar]
- Koyabu N, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, et al. Tolbutamide uptake via pH- and membrane-potential-dependent transport mechanism in mouse brain capillary endothelial cell line. Drug Metab Pharmacokinet 2004; 19: 270–9. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Murakami H, Koyabu N, Matsuo H, Naito M, Tsuruo T, et al. Efflux transport of tolbutamide across the blood-brain barrier. J Pharm Pharmacol 1998; 50: 1027–33. [DOI] [PubMed] [Google Scholar]
- Pooja Naik LC, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil 2014; 2: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]