Abstract
Activation of swelling-induced Cl− current (ICl,swell) during neonatal hypoxia-ischemia (HI) may induce brain damage. Hypoxic-ischemic brain injury causes chronic neurological morbidity in neonates as well as acute mortality. In this study, we investigated the role of ICl,swell in hypoxic-ischemic brain injury using a selective blocker, 4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl) oxybutyric acid (DCPIB). In primary cultured cortical neurons perfusion of a 30% hypotonic solution activated ICl,swell, which was completely blocked by the application of DCPIB (10 μmol/L). The role of ICl,swell in neonatal hypoxic-ischemic brain injury in vivo was evaluated in a modified neonatal hypoxic-ischemic brain injury model. Before receiving the ischemic insult, the mouse pups were injected with DCPIB (10 mg/kg, ip). We found that pretreatment with DCPIB significantly reduced the brain damage assessed using TTC staining, Nissl staining and whole brain imaging, and improved the sensorimotor and vestibular recovery outcomes evaluated in neurobehavioural tests (i.e. geotaxis reflex, and cliff avoidance reflex). These results show that DCPIB has neuroprotective effects on neonatal hypoxic-ischemic brain injury, and that the ICl,swell may serve as a therapeutic target for treatment of hypoxic-ischemic encephalopathy.
Keywords: neonatal stroke, neonatal hypoxic-ischemic brain injury, volume regulated anion channels, chloride channels, cortical neurons, whole-cell recording, DCPIB, neurobehavioural tests, neuroprotection
Introduction
Neonatal hypoxic-ischemic (HI) brain injury is a condition that occurs when the brain is deprived of adequate oxygen1,2. In infants and children, HI brain damage is a major cause of acute mortality and chronic neurological disability, such as hypoxic-ischemic encephalopathy (HIE)3. The leading cause of HI brain injury is birth asphyxia, which causes 23% of all neonatal deaths worldwide4,5. Although the prevalence of asphyxia in full-term newborn infants is ∼0.2% (2–4 per 1000), it reaches ∼60% in premature or low birth weight newborns. Mortality rate is high at ∼20% to 50% of those afflicted. Furthermore, survivors suffer from severe motor impairments and learning disabilities2,5. This is in part because HI brain injury often results in HIE and/or cerebral palsy, a generic term describing a group of perceptive, cognitive, and motor disorders resulting from damage to or abnormal development of the brain6. Children with cerebral palsy have affected neurological functions (eg vision, auditory, etc), and depending on the severity, early detection of cerebral palsy can be challenging6,7,8. Currently, there is no cure available; standard therapeutic strategies are aimed at assisting patients with cerebral palsy to develop into their full potential of independency7,8. However, lifelong rehabilitation is often necessary7,8. Therefore, survivors of infant HI brain injury suffer lasting neurological damage, which negatively influences their quality of life. Moreover, the lifetime cost to the healthcare system is ∼$1.5 million per person in Canada9. Thus, HI brain injury and HIE represent a serious socioeconomic burden, and there is urgent need to elucidate the underlying mechanisms in order to develop a more effective prevention and treatment. However, the mechanisms underlying neonatal HI remain ill-defined.
An important cellular role of the volume-regulated anion channels (VRACs), a major class of ubiquitously expressed Cl− channels, is the regulation of cell volume10. This is accomplished through the efflux of Cl− when the cell experiences hypo-osmotic swelling10,11. Consequently, VRACs mediate the swelling-induced Cl− current ICl,swell. This has major implications on cell volume homeostasis (ie to decrease cell volume during osmotic perturbations). VRACs are highly expressed in the brain, and the swelling-induced chloride current ICl,swell has been suggested to be involved in cerebral ischemia10,12. Under physiological conditions, hypo-osmotic swelling activates swelling-induced chloride current ICl,swell via mechanosensory mechanisms (that is still poorly understood), which results in Cl− efflux. This efflux is coupled with K+ efflux, which leads to the expulsion of water osmotically13. As a result, cell volume is reduced, thus retaining homeostasis. Nonetheless, it should be noted that the molecular identities of the channels underlying the swelling-induced Cl− current ICl,swell remain controversial13. Despite this, however, there is evidence demonstrating that 4-(2-butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid (DCPIB) is a selective antagonist that potently inhibits the swelling-induced Cl− current ICl,swell 14. Under pathological conditions, prolonged neuron overstimuation (eg by glutamate) results in excitotoxicity that causes excessive depolarization15. This, in turn, drives water inflow and consequently results in cell swelling15. Due to the persistent depolarization, there is influx of Cl− (rather than efflux) through the swelling-induced chloride current ICl,swell (ie, opposite phenomenon compared to the normal regulatory volume decrease mechanism under physiological conditions). Consequently, a positive feedback for further swelling is established, and ultimately necrotic neuronal death is induced.
Because swelling-induced Cl− current ICl,swell is normally inactive15, the rationale is that pharmacologically reducing swelling-induced Cl− current ICl,swell activity would not affect normal brain function. Thus, swelling-induced Cl− current ICl,swell is a suitable potential drug target for treatment of HI brain injury, and inhibition of swelling-induced Cl− current ICl,swell is a feasible therapeutic strategy. Previous publication described the role of the swelling-induced Cl− current ICl,swell in mediating adult stroke damage16. The authors found that pharmacologically inhibiting the swelling-induced Cl− current ICl,swell with DCPIB endowed neuroprotection by reducing infarction and improving neurobehavioural scores in a rat transient middle cerebral artery occlusion (MCAO) model when the drug was delivered intracisternally16. In our laboratory, we previously described the pharmacological role of DCPIB in reducing HI brain injury in vivo17, and suppressing glioblastoma cellular functions in vitro by inhibiting the swelling-induced Cl− current ICl,swell18. Additionally, we also reported that pharmacologically inhibiting the swelling-induced Cl− current ICl,swell in vitro with DCPIB decreased the hypotonic-inducing intracellular Cl− concentration, and reduced the oxygen and glucose deprivation (OGD)-induced cell death in PC12 cell line17. Moreover, we showed preliminary evidence that the swelling-induced Cl− current ICl,swell-dependent mechanism was involved partially in neonatal HI brain injury17. However, there is currently no in vivo behavioural assessment demonstrating whether inhibition of the swelling-induced Cl− current ICl,swell by DCPIB can confer neuroprotection at the level of neurobehavioural performance. Here, we show that: 1) in vitro, DCPIB inhibits the swelling-induced Cl− current ICl,swell in cortical neurons; and 2) in vivo, inhibiting the swelling-induced Cl− current ICl,swell with DCPIB treatment results in reduced brain damage, and improved short-term neurobehavioural performance following the HI insult. Thus, the swelling-induced Cl− current ICl,swell -mediated outcomes represent a non-glutamate stroke mechanism that plays critical role in both adult and neonatal variations of multiple brain diseases. There is broad implication because, ultimately, the goal of our work is contribution towards the development of effective therapeutic neuroprotective agents for reducing HI brain injury and its related HIE.
Materials and methods
Reagents
All reagents, unless specified otherwise, were from Sigma-Aldrich (Canada). DCPIB (Sigma catalogue # D4068) was first dissolved in 0.2% dimethyl sulfoxide (DMSO), and then diluted in 1% phosphate buffered saline (PBS) for the final concentration of 10 mg/kg.
Animals
CD1 timed-pregnant mice were purchased from Charles River Laboratories (Sherbrooke, Quebec, Canada). Each cage only housed one nursing female mouse and her litter. Food and water were readily accessible, and maintained in a room with an ambient temperature of 20±1°C and a 12:12 h light/dark cycle. Experiments involving animals followed the guidelines of the Canadian Council on Animal Care (CCAC) protocol. All animal experimental protocols were approved by the local Animal Care and Use Program Committee, Office of the Research Ethics at the University of Toronto.
Surgical preparation
Prior to surgery, mouse pups were anesthetised with isoflurane. The skin was cleaned with iodine followed by 75% alcohol before incision. A midline ventral incision was conducted at the anterior neck. DCPIB was given via intraperitoneal administration. A temperature control and heating blanket system (Harvard Apparatus) was used to monitor/maintain body temperature.
Hypoxic-ischemic brain injury model
Seven-day-old (P7) postnatal CD1 wild-type mouse pups of either sex were used. As previously described17,19, a modified Rice-Vannucci neonatal adaptation of the Levine procedure was used to induce cerebral HI injury in the mouse pups. Appropriate protocols were undertaken to minimize discomfort and pain of animals throughout the common carotid artery occlusion (CCAO) procedure. In brief, P7 mice (weighing ∼5 to 5.5 g) were anesthetised with 3% isoflurane for 3 min as induction, and then followed by 2% isoflurane for maintenance during the procedure. We used a stereo dissecting microscope containing ring lens illumination and fiber-optic bifurcation (SMZ-2B Nikon, Japan). After conducting the midline cervical incision (0.5 cm) and separating the muscles at the frontolateral neck, the right common carotid arteries become exposed. These were separated from the associated sympathetic and vagus nerves, and then occluded via bipolar electrical coagulation (Vetroson V-10 Bi-polar electrosurgical unit, Summit Hill Laboratories, Tinton Falls, NJ, USA). Animal body temperature was maintained with a homeothermic heating blanket. For each pup, the procedure took ∼5 min for each pup. After surgery, wounds were closed and the pups recovered in a 37 °C incubator for 10 min (or until regaining consciousness). Afterwards, pups were returned to their dam (at least 90 min) for further recovery.
To induce hypoxia, pups were placed into an airtight chamber (A-Chamber A-15274 with ProOx 110 Oxygen Controller/ E-720 Sensor, Biospherix, NY, USA), into which a humidified gas mixture (7.8% oxygen balanced with 92.2% nitrogen) was perfused. Flow of gas was constant for 60 min. A compact oxygen controller (ProOx 110 controller, Biospherix, NY, USA) with a compressed nitrogen gas source (Linde, Mississauga, ON, Canada) was used to regulate oxygen concentration. A homeothermic blanket control unit (K-017484 Harvard Apparatus, Massachusetts, USA) monitored and maintained animal body temperature at 36.5 °C. After exposure to hypoxia, pups recovered on a heating pad and then returned to their dam.
Drug administration
Mouse pups weighing ∼5 g were randomly assigned into sham group, control HI group or DCPIB-treated HI group. DCPIB (10 mg/kg) with 0.2% DMSO (DCPIB-treated HI group) or 0.2% DMSO alone (control HI group) was diluted in 1% PBS before intraperitoneal injection into the mouse pups. Drug injection was done ∼20 min before CCAO for all tests listed below except for the TTC test (∼1 h after CCAO). The total volume injected per mouse pup was 0.1 mL.
Neurobehavioral tests
Two neurobehavioral tests (ie geotaxis, cliff avoidance reaction) were used to assess the recovery outcomes of the mouse pups on Day 1, Day 3, and Day 7 after the HI insult. These tests are well-documented20, and were carried out as previously described21,22,23,24,25,26,27. In brief, geotaxis reflex is an automatic, stimulus-bound orientation movement. It is considered to be diagnostic of vestibular and/or proprioceptive function. Here, pups were placed head facing down in the middle of a plane inclined at an angle of 45°, and the latency time for the pup to rotate 180° (ie, head facing up) was recorded. Cliff avoidance reaction evaluates the integration of exteroceptive input and locomotor output. For this test, pups were placed on the edge of a platform, and the latency time for the pup to remove both paws from the edge by turning away from the cliff was recorded.
Measurement of infarct volume
TTC staining: 24 h after the HI episode, mouse pups were sacrificed and the brains were extracted and coronally cut into ∼1 mm sections, which were immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC) in 1% PBS at 37°C in the dark (20–30 min). TTC is a redox indicator that can indicate cellular respiration for differentiating between metabolically active and inactive tissues. After stained with TTC, brain slices were scanned and infarct areas were measured, as previously described24,25,26,27. An image analysis system (NIH ImageJ) was used to quantify the ipsilateral and contralateral hemisphere area for each section. Volumes were determined by summing the representative areas in all sections and then multiplying by slice thickness. Edema correction was conducted. Infarction volume was calculated with the following formula: CIV (%)=[CHV-(IHV-IV)]/CHV*100; where CIV is corrected infarct volume, CHV is contralateral hemisphere volume, IHV is ipsilateral hemisphere volume, and IV is infarct volume.
Nissl staining/whole brain imaging: Seven days after the HI episode, whole brain tissue was collected and imaged to reveal morphological changes between the groups, as previously described24,25,26,27. At this point, the infarct areas have undergone liquefactive necrosis. The severity of the brain damage was quantified. The brains were sliced into ∼100 μm coronal sections and stained with 1% Cresyl violet (Nissl) to indicate histological damage. Infarct area was imaged using ImageJ, and was calculated as follows: IV (%) = IV/CHV*100.
Primary mouse neuron culture
Primary cortical neurons were cultured from E16 CD1 mice, as previously described 28. In brief, cortices were isolated and then digested using 0.025% trypsin/EDTA (37 °C; 15 min). An Improved Neubauer hemocytometer was used to measure cell density. Cells were plated at 1.0×104 on poly-D-lysine coated glass coverslips (Sigma; 12 mm No 1 German Glass, Bellco cat. No 1943-10012). Neurons were maintained in serum-free culture Neurobasal medium supplemented with 1.8% B-27, 0.25% Glutamax, and 1% antibiotic-antimyocotic. Incubation of cells was in 5% CO2 at 37 °C.
Patch clamp electrophysiology
Recordings were carried out in the whole-cell configuration using the Axopatch 700B (Axon Instruments, Inc), similar to our previous studies18,29,30,31. The ramp protocol was from -100 to +100 mV (400 ms; -70 mV holding; 5 s interval at 2 kHz; digitized at 5 kHz). Data acquisition and analyses were via Clampex 9.2 and Clampfit 9.2. All experiments were performed at room temperature. The pipette (intracellular) solution contained (in mmol/L): 145 CsMSF, 8 NaCl, 55 KCl, 10 HEPES, 1 MgATP, and 10 EGTA (pH adjusted to 7.2 with CsOH). After filling with solution, pipette resistance was 8–13 MΩ. The bath (extracellular) solution contained (in mmol/L): 140 NaCl, 5 KCl, 2 CaCl2, 20 HEPES, and 10 glucose (pH adjusted to 7.4 with NaOH; osmolarity to ∼300 mOsm with sucrose).
Statistic analysis
GraphPad Prism 6 (GraphPad Software, Inc) was used for statistical analysis. The statistical significance between control and DCPIB-treatment group was assessed with the Student's t-test. In multiple groups, one-way ANOVA with Bonferroni multiple comparison test was used. Significance was defined as P<0.05. Data are shown as mean±SEM.
Results and discussion
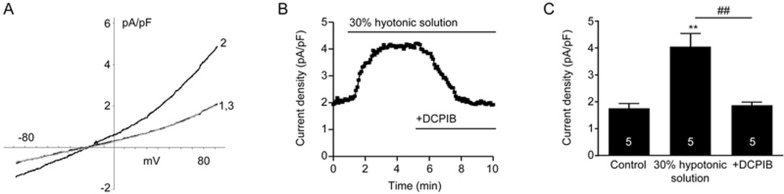
Since the molecular identities of the swelling-induced Cl− channels remain elusive, we first measured the electrophysiological properties of the swelling-induced Cl− current ICl,swell in primary neurons and pharmacologically verified with DCPIB (Figure 1). When exposed to 30% hypotonic solution, there was activation of swelling-induced Cl− current ICl,swell, with a reversal potential that is consistent with what we previously reported18. In the continued presence of the hypotonic solution, application of DCPIB (10 μmol/L) inhibited the activated current (Figure 1A and 1B). As shown in the summary graph (Figure 1C), perfusion of the hypotonic solution activated a swelling-induced Cl− current ICl,swell, which was fully inhibited by subsequent application of DCPIB (ie, hypotonic solution increased current density to 4.05±0.49 pA/pF from 1.76±0.17 pA/pF, P<0.01; DCPIB administration abolished this increase by reducing current density back to 1.87±0.11 pA/pF, P<0.01). Therefore, the swelling-induced Cl− current ICl,swell is present in primary neurons and sensitive to inhibition by DCPIB.
Figure 1.
In primary cortical neurons, the swelling-induced Cl− current ICl,swell is inhibited by DCPIB. (A) Current density-voltage trace where: 1 is bath solution; 2 is with perfusion of 30% hypotonic solution; and 3 is with application of DCPIB (10 μmol/L) in the continued presence of hypotonic solution. Holding potential was -70 mV. (B) Representative time course plotting the outward current at +80 mV. (C) Comparison summary chart of the outward current (at +80 mV) of: control bath solution, activation of swelling-induced Cl− current ICl,swell with hypotonic solution, and subsequent inhibition with DCPIB in the continued presence of hypotonic solution. **P<0.01 vs control. ##P<0.01 vs 30% hypotonic solution, respectively (n=5/group; Repeated-measure One-way ANOVA with Bonferonni multiple comparison test).
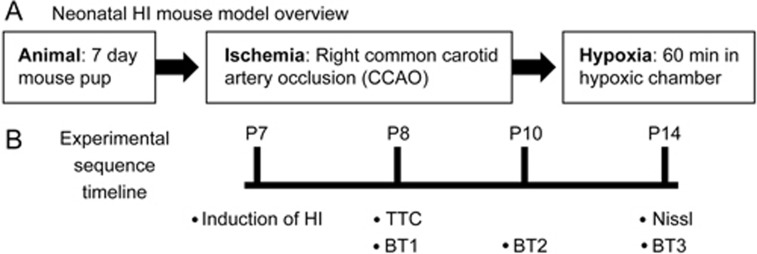
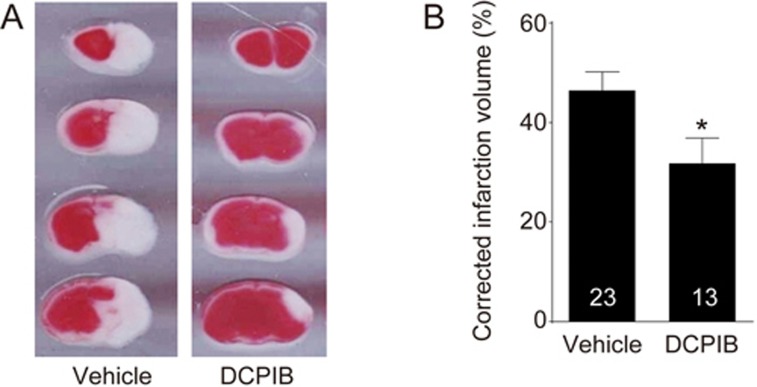
Next, we provide the first in vivo evidence demonstrating the neuroprotective effects of a selective swelling-induced Cl− current ICl,swell antagonist DCPIB on improving behavioural functional outcomes after HI brain injury. As shown in the schematic in Figure 2A, we used a well-documented mouse hypoxic-ischemic (HI) model (Rice-Vannucci method) with modifications17,19. The key events throughout the procedure have been noted on the timeline in Figure 2B. Our model of neonatal HI brain injury formed a brain infarct that is confined from the ipsilateral brain hemisphere to the common carotid occlusion, as shown from the TTC stain in Figure 3A (white section of the brain; extent of damage 24 h following HI). On the contrary, the contralateral hemisphere did not demonstrate brain injury (red section). This finding is consistent with our previous report17, noting the reliability of our HI model. The right hemispheric corrected infarct volume was 46.38%±3.76% (n=23) in animals treated with vehicle (control HI group; Figure 3B). When DCPIB was injected ∼1 h after common carotid artery occlusion (CCAO), the corrected infarct volume was significantly reduced to 31.75%±5.14% (DCPIB-treated HI group; P<0.05). This suggested that inhibition of the swelling-induced Cl− current ICl,swell with DCPIB can reduce the severity of brain damage.
Figure 2.
Schematic of the in vivo neonatal hypoxic-ischemic (HI) mouse model to examine the effects of DCPIB. (A) Overview of the process involved in developing the model, which contained two main parts: ischemia and hypoxia. First, using postnatal day 7 (P7) mice, ischemia was induced by isolation of the right common carotid artery and then subsequent ligation with a bipolar electrocoagulation device (common carotid artery occlusion; CCAO). Following recovery from the surgery, hypoxia was then induced by placing operated pups in a 37°C chamber perfused with a gas mixture of 7.5% oxygen and 92.5% nitrogen for 60 min. (B) Timelines of the experimental sequences for model development as well as the subsequent histological, morphological, and behavioural assessments. The timeline was carried out across three groups: sham, control HI, and DCPIB-treated HI. The “control HI group” represents HI-induced animals administered with vehicle only; “DCPIB-treated HI group” represents HI-induced animals treated with DCPIB. Infarct volume measurement with TTC was carried out 24 h after HI (ie, when pups were P8), whereas measurement with Nissl was 7 days after (ie when pups were P14). Neurobehavioural performance (BT; behavioural test) was assessed at three separate time points; at one, three, and seven days after HI (ie, when pups were P8, P10, and P14, respectively).
Figure 3.
Treatment with DCPIB reduced brain infarct volume of neonatal hypoxic-ischemic (HI) brain injury in vivo. (A) Representative 2,3,5-triphenyltetrazolium chloride (TTC) images of the infarcts of the coronal sections of P8 mouse pup brains treated with vehicle (control HI group) or 10 mg/kg DCPIB (DCPIB-treated HI group). (B) Column chart summarizing that animals in the DCPIB-treated HI group (n=13) had significantly reduced brain infarct volume compared to those in the control HI group (n=23; *P<0.05; Student's t-test).
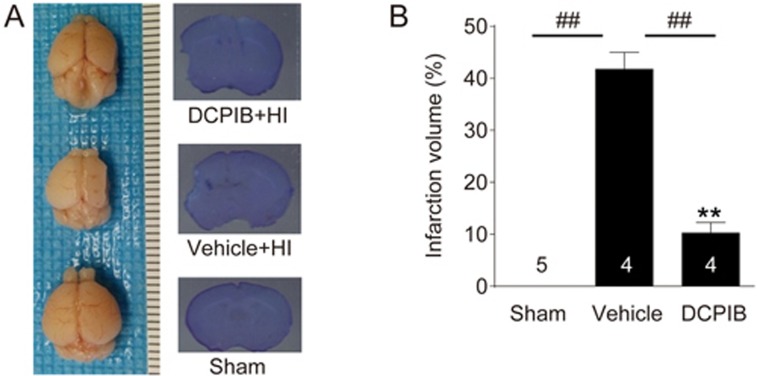
Seven days after the HI, the brains of mouse pups were extracted to examine the extent of morphological and histological damage. As shown in Figure 4, integrity of brain morphology in the control HI group is severely compromised seven days after HI brain injury. However, for animals in the DCPIB-treated HI group, the morphology of these brains is qualitatively more comparable to the brains in the sham group. Consistent with this observation, Nissl staining to assess histological damage also shows a similar trend. That is, the DCPIB-treated HI group showed significantly reduced infarction volume from 41.79%±3.23% to 10.21%±2.08% (P<0.05; n=4/group; Figure 4). This is the first evidence to suggest the lasting neuroprotective effect of DCPIB against HI brain injury, as even only one administration of DCPIB ∼20 min before CCAO elicited an improvement one week after the HI.
Figure 4.
DCPIB-treated animals showed less morphological and histological damage following HI. (A) Left panel shows representative whole brain images of P14 mouse pups in either the sham group (bottom), control HI group (middle), or DCPIB-treated HI group (top). Note that morphological integrity is severely compromised for the control group brain, when compared to the treatment and sham group brains. Right panel displays the corresponding representative images of Nissl staining. (B) Summary column chart showing that intraperitoneal (ip) treatment of DCPIB (10 mg/kg in 0.2% DMSO) 20 min before induction of HI significantly reduced infarct volume measured seven days following the HI episode (**P<0.01 vs Sham. ##P<0.01 vs vehicle, respectively; n=4/group; One-way ANOVA with Bonferonni multiple comparison test), thus suggesting the lasting neuroprotective effectiveness of DCPIB.
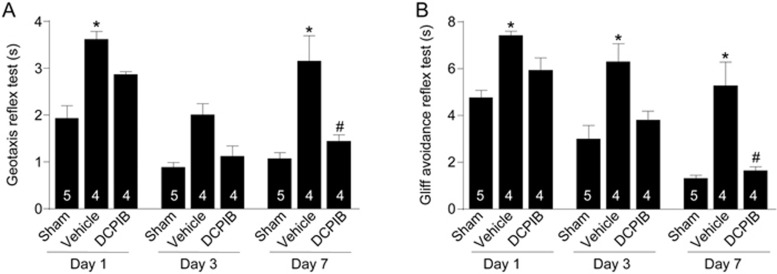
We further quantitatively assessed recovery outcomes of the HI by employing short term neurobehavioural tests (ie geotaxis reflex, and cliff aversion reflex). These reflex tests were chosen because they are representative of the earliest development stages in mice, and thus are good indicators of sensorimotor function20. Specifically, the geotaxis reflex assesses vestibular and proprioceptive functions, whereas the cliff aversion reflex measures the maladaptive impulse behaviour20. These neurobehavioral tests determine the functional recovery of animals after the insult. A longer time indicates slower reflex, which is considered a negative score (ie, lower time is better). Each test was conducted one, three, and seven days after the HI. Animals in all groups (ie, sham, control HI, and DCPIB-treated HI) were subjected to these tests (Figure 5). On Day 1, pups in the control HI group had the slowest reflex for both geotaxis and cliff avoidance (at 3.61±0.17 and 7.42±0.18 s, respectively; P<0.05 for both). Treatment with DCPIB improved reflex speed of animals in the DCPIB-treated HI group (to 2.86±0.06 and 5.94±0.51 s, respectively), which is not significant compared to animals in the sham group (1.93±0.27 and 4.76±0.31 s, respectively). On Day 3, control HI group animals still had the slowest cliff avoidance reflex, at 6.30±0.93 s (P<0.05), which was improved with DCPIB treatment (3.80±0.38 s; note that this score was not significantly different compared with the animals in the sham group at 2.99±0.58 s). Although pups in the control HI group did not significantly have a slower geotaxis reflex on Day 3, it was clearly gravitating towards this trend. One possible explanation is that at Day 3, mouse pups become P10, which is when their eyes begin to open. The sudden acquisition of vision can potentially confer drastic improvement to vestibular and proprioceptive performance despite suffering HI. This remains to be tested. Nevertheless, by Day 7, geotaxis reflex of the control HI group had markedly worsened. This was ameliorated with inhibition of the swelling-induced chloride current ICl,swell by DCPIB (from 3.15±0.54 s in the control HI group to 1.44±0.14 s in the DCPIB-treated HI group; P<0.05). However, note that at this time point, DCPIB-treated HI group animals still performed worse than sham group animals (at 1.07±0.13 s; P<0.05); nevertheless, the difference was minimal and the trend of improvement was clearly present. Similarly, animals in the DCPIB-treated group also demonstrated improvement in cliff avoidance reflex at Day 7; reflex time was reduced from 5.27±1.02 s (control HI group) to 1.64±0.17 s (DCPIB-treated HI group; P<0.05). However, although evidently trending towards the same reflex levels as animals in the sham group (at 1.31±0.14 s), pups in the DCPIB-treated HI group remained significantly slower (P<0.05). Nevertheless, we provide the first evidence that a single injection of DCPIB ∼20 min prior to CCAO can elicit a lasting neuroprotective effect that ultimately improves neurobehavioural performance.
Figure 5.
Neurobehavioural performance after HI is improved with DCPIB treatment. (A) Summary of geotaxis reflex test on Day 1, Day 3, and Day 7 (when mouse pups were P8, P10, and P14, respectively) across animals from all groups: sham (n=5), control HI (n=4), and DCPIB-treated HI (n=4). Animals in the control HI group consistently had a slower reflex, either significant (*P<0.05) or clearly trending towards significance. Note that comparisons were made between groups on each respective day (compared to the sham group). The general trend is that DCPIB-treatment improves geotaxis reflex towards that of sham animals. (B) Summary of cliff avoidance reflex test on Day 1, Day 3, and Day 7 (when mouse pups were P8, P10, and P14, respectively) across animals from all groups: sham (n=5), control HI (n=4), and DCPIB-treated HI (n=4). Animals in the control HI group consistently had a significant slower reflex (*P<0.05). Note that comparisons were made between groups on each respective day (compared to the sham group). The general trend is that DCPIB-treatment improves cliff avoidance reflex towards that of sham animals. (One-way ANOVA with Bonferonni multiple comparison test).
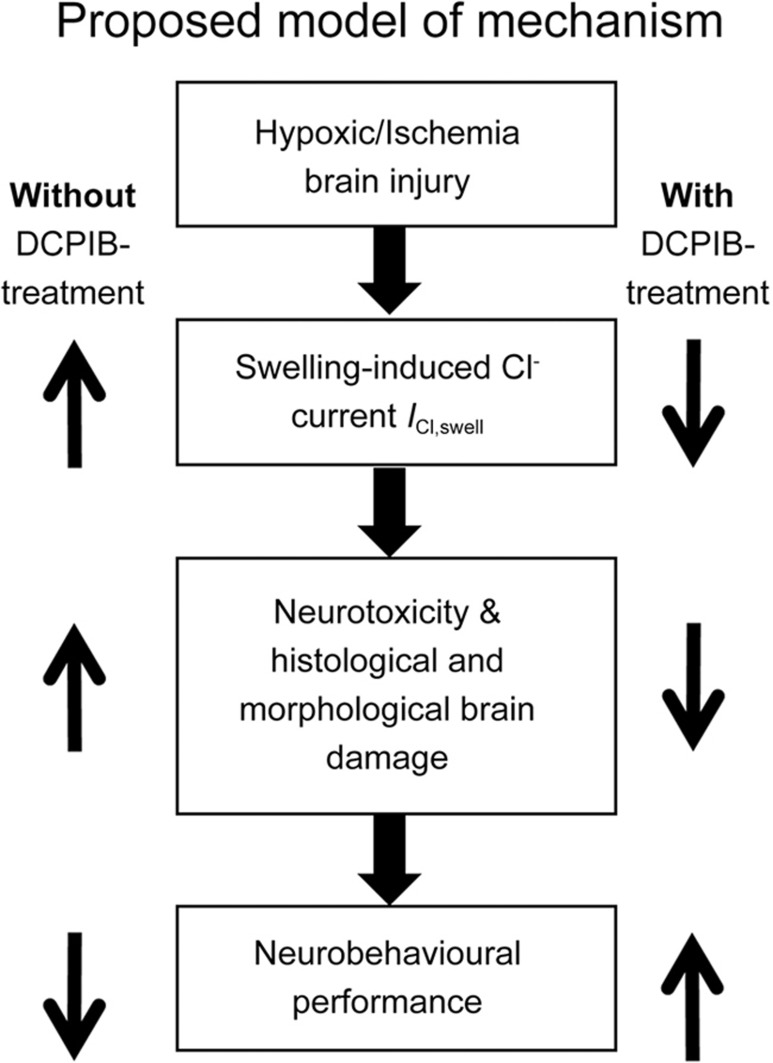
In our previous study, we focused on the in vitro effects of DCPIB by demonstrating the effects of DCPIB on OGD and intracellular chloride concentration17. Specifically, we showed that the resting [Cl−]i significantly increased following OGD in the PC12 cell line, and that DCPIB (10 μmol/L) reduced this influx. Furthermore, we demonstrated that treating PC12 cells with DCPIB significantly reduced cell death induced by OGD, and ultimately increased cell survival. In addition, DCPIB alone did not show any toxicity on cultured cells, which was suggestive of safety17. This in vitro work gave evidence that inhibition of the swelling-induced Cl− current ICl,swell can ameliorate OGD-induced injury. Recently, we showed that DCPIB inhibited the swelling-induced Cl− current ICl,swell, and consequently suppressed the malignant cellular functions (ie, proliferation, migration, and invasion) of glioblastoma cell lines (ie, U87, U251)18. This suggests that DCPIB is an effective swelling-induced Cl− current ICl,swell antagonist across multiple cell types. In our present study, we examined the in vivo effects of DCPIB using a neonatal mouse HI model. Specifically, we assessed the behavioural, histological, and morphological aspects of the HI insult with or without DCPIB treatment. This is unknown in the literature and represents a knowledge gap, because inhibition of the swelling-induced Cl− current ICl,swell can potentially be exploited as an effective therapeutic strategy for neonatal HI brain injury. Moreover, DCPIB has been reported to be effective in reducing the infarct volumes of adult MCAO model16, which further articulates the broad implications and therapeutic feasibility of DCPIB in ischemia. However, in adult brain ischemia, DCPIB only demonstrated neuroprotection when given intracisternally, but not intravenously. This was in part due to an impedance of drug delivery by the developed blood brain barrier (BBB)16. In our previous17 and current studies, however, we showed evidence that intraperitoneal administration of DCPIB was able to cross the immature BBB of the perinatal or neonatal brain. That is, DCPIB treatment exerted neuroprotection during HI insults by significantly reducing brain damage and improving neurobehavioural outcomes. The BBB in neonates may be immature and undergoing developmental changes, thus favouring drug penetration into the neonatal brain, especially during ischemic conditions which can disrupt the BBB integrity27. Here, we showed critical in vivo evidence for the pathophysiological role of the swelling-induced Cl− current ICl,swell in the HI brain injury. Moreover, we provided important findings for potential drug development of swelling-induced Cl− current ICl,swell antagonists such as DCPIB as a readily administrable drug for prevention and treatment of the HI brain injury. In conclusion, we established that the effects mediated by the swelling-induced Cl− current ICl,swell represent a non-glutamate mechanism of the neonatal HI brain injury (Figure 6). Thus, the swelling-induced Cl− current ICl,swell is exploitable as a drug target (by using selective potent antagonists; eg, DCPIB) in order to prevent and treat human neonatal HI brain injury in addition to its consequential neurological disorders such as hypoxic-ischemic encephalopathy and cerebral palsy.
Figure 6.
Schematic of proposed model of mechanism underlying the swelling-induced Cl− current ICl,swell in HI brain injury. Following hypoxic and ischemic insult, there is aberrant activity of the swelling-induced Cl− current ICl,swell. This can lead to neuronal death and result in brain damage. Ultimately, neurobehavioural performance may be negatively affected. However, this outcome can be reversed with treatment using the specific swelling-induced Cl− current ICl,swell antagonist DCPIB. Aberrant swelling-induced Cl− current ICl,swell activity is reduced. Consequently, there is less neuronal death and brain damage, which would contribute to improved neurobehavioural performance.
Author contribution
Hong-shuo SUN and Zhong-ping FENG developed the concepts and designed the study. Raymond WONG, Ahmed ABUSSAUD, Joseph WH LEUNG, Bao-feng XU, Sammen HUANG, and Fei-ya LI performed experiments and analyzed the data; Raymond WONG and Hong-shuo SUN wrote the manuscript; all authors discussed the results and commented on the manuscript.
Acknowledgments
This work was supported by the following grants: Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants to Hong-shuo SUN (RGPIN-2016-04574); a Canadian Institutes of Health Research (CIHR) China-Canada Joint Health Research Initiative (CIHR, FRN #132571) to Hong-shuo SUN; Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants to Zhong-ping FENG (RGPIN-2014-06471) and to Hong-shuo SUN (RGPIN-2016-04574); the NSFC-CIHR Joint Health Research Initiative Proposal (No 81361128011) to Guan-lei WANG; a NSERC Postgraduate Scholarship-Doctoral to Raymond WANG.
References
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol 2004; 207: 3149–54. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ. Cerebral ischemia and the developing brain: introduction. Stroke 2007; 38: 723. [Google Scholar]
- Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol 2000; 17: 113–20. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol 2004; 3: 150–8. [DOI] [PubMed] [Google Scholar]
- Nelson KB. Perinatal ischemic stroke. Stroke 2007; 38: 742–5. [DOI] [PubMed] [Google Scholar]
- Little WJ. On the influence of abnormal parturition, difficult labours, premature birth, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Clin Orthop Relat Res 1966; 46: 7–22. [PubMed] [Google Scholar]
- Kruse M, Michelsen SI, Flachs EM, Bronnum-Hansen H, Madsen M, Uldall P. Lifetime costs of cerebral palsy. Dev Med Child Neurol 2009; 51: 622–8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment. MMWR Morb Mortal Wkly Rep 2004; 53: 57–9. [PubMed] [Google Scholar]
- de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2010; 95: F220–F224. [DOI] [PubMed] [Google Scholar]
- Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 2008; 29: 268–75. [DOI] [PubMed] [Google Scholar]
- Guan YY, Wang GL, Zhou JG. The ClC-3 Cl− channel in cell volume regulation, proliferation and apoptosis in vascular smooth muscle cells. Trends Pharmacol Sci 2006; 27: 290–6. [DOI] [PubMed] [Google Scholar]
- Kim SY, Shin DH, Kim SJ, Koo BS, Bae CD, Park J, et al. Chloride channel conductance is required for NGF-induced neurite outgrowth in PC12 cells. Neurochem Int 2010; 56: 663–9. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature 1997; 390: 417–21. [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of ICl,swell and prevents swelling induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol 2001; 134: 1467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Okada Y. Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J Neurosci 2007; 27: 1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAO and the release of glutamate in the ischemic cortical penumbra. Exp Neurol 2008; 210: 514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibrahim A, Zhao LY, Bae CY, Barszczyk A, Sun CLF, Wang GL, et al. Neuroprotective effects of volume-regulated anion channel blocker DCPIB on neonatal hypoxic-ischemic injury. Acta Pharmacol Sin 2013; 34: 113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Chen W, Zhong X, Rutka JT, Feng ZP, Sun HS. Swelling-induced chloride current in glioblastoma proliferation, migration and invasion. J Cell Physiol 2017; 233: 363–70. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 2009; 12: 1300–7. [DOI] [PubMed] [Google Scholar]
- Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res 2005; 157: 157–65. [DOI] [PubMed] [Google Scholar]
- Chen W, Xu B, Xiao A, Liu L, Fang X, Liu R, et al. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol Brain 2015; 8: doi:10.1186/s13041-015-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao AJ, Chen W, Xu B, Liu R, Turlova E, Barszczyk A, et al. Marine compound xyloketal B reduces neonatal hypoxic-ischemic brain injury. Mar Drugs 2014; 13: 29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Xiao AJ, Chen W, Turlova E, Liu R, Barszczyk A, et al. Neuroprotective effects of a PSD-95 inhibitor in neonatal hypoxic-ischemic brain injury. Mol Neurobiol 2016; 53: 5962–70. [DOI] [PubMed] [Google Scholar]
- Huang S, Turlova E, Li F, Bao MH, Szeto V, Wong R, et al. Transient receptor potential melastatin 2 channels (TRPM2) mediate neonatal hypoxic-ischemic brain injury in mice. Exp Neurol 2017; 296: 32–40. [DOI] [PubMed] [Google Scholar]
- Huang S, Wang H, Turlova E, Abussaud A, Ji X, Britto LR, et al. GSK-3β inhibitor TDZD-8 reduces neonatal hypoxic-ischemic brain injury in mice. CNS Neurosci Ther 2017; 23: 405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang S, Yan K, Fang X, Abussaud A, Martinez A, et al. Tideglusib, a chemical inhibitor of GSK3β, attenuates hypoxic-ischemic brain injury in neonatal mice. Biochim Biophys Acta 2016; 1860: 2076–85. [DOI] [PubMed] [Google Scholar]
- Sun HS, Xu B, Chen W, Xiao A, Turlova E, Alibraham A, et al. Neuronal KATP channels mediate hypoxic preconditioning and reduce subsequent neonatal hypoxic-ischemic brain injury. Exp Neurol 2015; 263: 161–71. [DOI] [PubMed] [Google Scholar]
- Turlova E, Bae CY, Deurloo M, Chen W, Barszczyk A, Horgen FD, et al. TRPM7 regulates axonal outgrowth and maturation of primary hippocampal neurons. Mol Neurobiol 2014; 53: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Chen W, Zhong X, Rutka JT, Feng ZP, Sun HS. Swelling-induced chloride current in glioblastoma proliferation, migration and invasion. J Cell Physiol 2017; 233: 363–70. [DOI] [PubMed] [Google Scholar]
- Chen WL, Turlova E, Sun CL, Kim JS, Huang S, Zhong X, et al. Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways. Mar Drugs 2015; 13: 2505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WL, Barszczyk A, Turlova E, Deurloo M, Liu B, Yang BB, et al. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015; 6: 16321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]