Abstract
Transient receptor potential melastatin 2 (TRPM2) is a calcium (Ca2+)-permeable non-selective cation channel belonging to the TRP ion channel family. Oxidative stress-induced TRPM2 activation provokes aberrant intracellular Ca2+ accumulation and cell death in a variety of cell types, including neurons. Aberrant TRPM2 function has been implicated in several neurological disorders including ischemia/stroke, Alzheimer's disease, neuropathic pain, Parkinson's disease and bipolar disorder. In addition to research identifying a role for TRPM2 in disease, progress has been made in the identification of physiological functions of TRPM2 in the brain, including recent evidence that TRPM2 is necessary for the induction of N-methyl-D-aspartate (NMDA) receptor-dependent long-term depression, an important form of synaptic plasticity at glutamate synapses. Here, we summarize recent evidence on the role of TRPM2 in the central nervous system (CNS) in health and disease and discuss the potential therapeutic implications of targeting TRPM2. Collectively, these studies suggest that TRPM2 represents a prospective novel therapeutic target for neurological disorders.
Keywords: transient receptor potential melastatin 2 (TRPM2), synaptic plasticity, neuropathic pain, Alzheimer's disease, Parkinson's disease, bipolar disorder, neurodegeneration
Introduction
Transient receptor potential melastatin 2 (TRPM2) is a calcium (Ca2+)-permeable non-selective cation channel first cloned and described in 19981. The last 20 years have led to the identification of mechanisms that regulate TRPM2 and a better understanding of factors that augment or diminish the function of the channel. However, the mechanisms by which these factors dynamically regulate TRPM2 function in health and disease remains poorly understood. In the central nervous system (CNS), TRPM2 mRNA is most abundant among transient receptor potential (TRP) channels2. An increasing body of evidence has revealed a number of important contributions of TRPM2 channels to CNS physiology and pathophysiology. This review will summarize this evidence, with a focus on synaptic plasticity and neurodegeneration.
TRPM2 channel structure
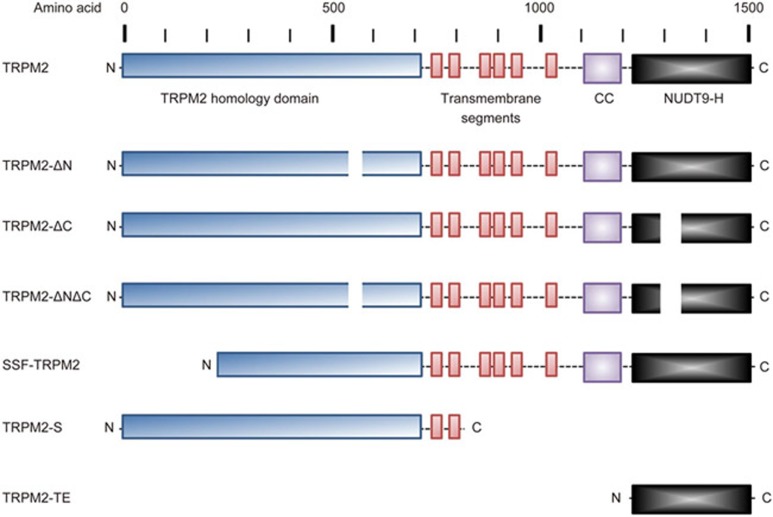
The first TRP gene was originally identified in Drosophila, where it participates in phototransduction3. Mammalian TRP channels are divided into six subfamilies including the canonical (C), vanilloid (V), polycystin (P), mucolipin (ML), ankyrin (A), and melastatin (M) subfamilies4,5. The gene encoding human TRPM2, previously known as TRPC7 and LTRPC2, is located on chromosome 21q22.31. The gene is 90 kb in length and contains 33 exons, producing a 5876-bp full-length transcript. This transcript codes for the full-length 1503-amino acid protein in humans, with a predicted molecular mass of ∼170 kDa. In addition to the full-length transcript, there are also several splice variants of TRPM2 (Figure 1). These variants include TRPM2-ΔN, with a deletion of amino acids 538–557; TRPM2-ΔC, with a deletion of amino acids 1292–1325; TRPM2-ΔNΔC, missing both amino acids 538-557 and 1292-1325; striatum short form TRPM2 (SSF-TRPM2), a variant identified in the striatum in which amino acids 1–214 are deleted; TRPM2-S, a variant that is truncated after the second transmembrane domain, producing an 845-residue splice variant; and TRPM2-TE, a splice variant identified using computational analysis that is up-regulated in melanoma and other tumor types6,7,8,9,10,11.
Figure 1.
TRPM2 Isoforms. Schematic representation of full-length TRPM2 and TRPM2 isoforms, demonstrating the approximate locations of domains and deletions. The N-terminus of TRPM2 contains the TRPM homology domain and the IQ-like motif. This is followed by six transmembrane domains, with a pore-forming re-entry loop between domains 5 and 6. The C-terminus contains the coiled-coiled (CC) motif and the NUDT9-H motif. TRPM2 isoforms include TRPM2-ΔN (deletion of amino acids (aa) 538-557); TRPM2-ΔC (deletion of aa 1292-1325); TRPM2-ΔNΔC (deletion of aa538-557 and aa1292-1325); striatum short form TRPM2 (SSF-TRPM2, deletion of aa1-214); TRPM2-S, a variant that is truncated after the second transmembrane domain, producing an 845-residue splice variant; and TRPM2-TE, a splice variant identified with computational analysis demonstrating up-regulated expression in melanoma and other tumor types6,7,8,9,10,11.
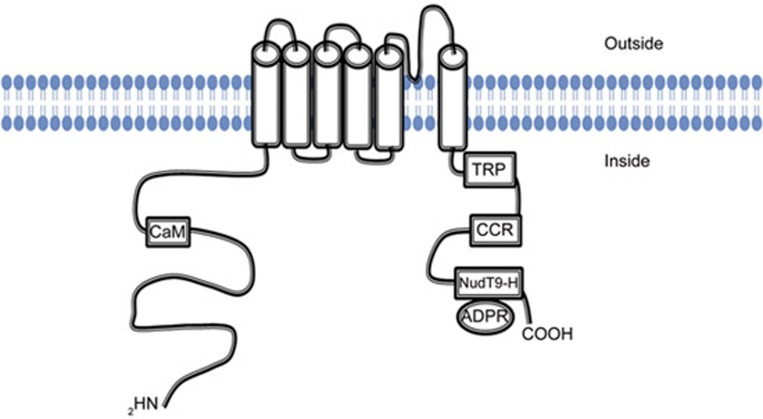
The TRPM2 protein structure includes 6 transmembrane domains, with a pore-forming re-entry loop located between domains 5 and 6. Regulatory features of the channel are contained within the cytoplasmic N- and C-terminal regions of the channel (Figure 2). The N-terminus contains the TRPM homology domain, a region of approximately 700 amino acids that is largely conserved across all TRPM subfamily members12. Additionally, there is an IQ-like motif that is involved in Ca2+-calmodulin binding to the channel and participates in TRPM2 activation13,14. In the C-terminus of TRPM2, the coiled-coiled motif participates in subunit interaction and assembly of the channel into its functional tetrameric form15. The C-terminus of TRPM2 also includes a nucleoside diphosphate-linked moiety X-type homology motif (NUDT9-H). This motif is required for gating of the channel by adenosine diphosphate ribose (ADPR), an intracellular ligand for TRPM2. Although the sequence in this region is highly homologous to NUDT9 (50% sequence identity), a mitochondrial ADPR hydrolase that converts ADPR to adenosine monophosphate (AMP) and ribose-5-phosphate, this region demonstrates less than 1% of the enzymatic activity16,17. More recently, NUDT9-H has been suggested to not possess enzymatic activity. Rather, the previously reported apparent activity is proposed to be due to the instability and spontaneous degradation of ADPR18. The impaired enzyme activity of NUDT9-H, when compared to the enzyme activity of NUDT9, is attributed to amino acid substitutions within the NUDT9-H motif at E1405I/F1406L19. Consistent with this suggestion, the introduction of the reverse substitution within NUDT9-H (ie, I1405E/L1406F) restores the enzyme activity to that of NUDT9 and abolishes TRPM2 activation by ADPR14,20,21. This observation suggests that NUDT9-H has adapted during evolution to function as an intracellular ligand-binding domain.
Figure 2.
TRPM2 Structure. TRPM2 is comprised of intracellular amino and carboxy-terminal regions and six transmembrane segments with a re-entry loop that forms a pore between segments 5 and 6. The amino terminus contains the TRPM2 homology domain, which is largely conserved across all TRPM family members. The amino terminus also contains a Ca2+-calmodulin (CaM) binding motif that participates in channel activation. The carboxy terminus contains a highly conserved TRP box (TRP), a coiled-coiled region (CCR) that may participate in tetrameric assembly, and the nucleoside diphosphate-linked moiety X-type homology motif (NUDT9-H), which binds adenosine diphosphate ribose (ADPR), the intracellular agonist for TRPM2.
Gating mechanisms and channel properties
The TRPM2 channel has a single-channel conductance of approximately 60–80 pS, with a linear current-voltage relationship and a reversal potential of 0 mV16,22,23. These characteristics demonstrate that TRPM2 is a non-selective cation channel without voltage-dependent behavior. The permeability ratio of Ca2+ to Na+ (PCa:PNa) is approximately 0.7, indicating that the inward current is predominantly carried by Na+23,24. Despite this, substantial Ca2+ is able to flux into the cell due to the channel's long open time of several hundred milliseconds16,23,24. The permeability of the channel to divalent cations including Ca2+ and Mg2+ is regulated by glutamic acid, aspartate, and glutamine residues located between the pore helix and the selectivity filter in the TRPM2 channel pore25.
TRPM2 represents a particularly unique channel in that it is activated in response to reactive oxygen and nitrogen species (ROS/RNS)26. Exposure to ROS/RNS is generally accepted to promote channel activation through the generation of ADPR, an agonist for TRPM216. The precise interaction between the ligand ADPR and the NUDT9-H domain continues to be investigated, with recent evidence further characterizing the precise residues critical for binding and activation27,28. Oxidative stress may generate ADPR through two pathways. In the first pathway, ROS/RNS leads to DNA damage and activation of poly-ADPR polymerase (PARP) and poly-ADPR glycohydrolase (PARG). PARP and PARG then act in concert to convert nicotinamide adenine dinucleotide (NAD+) into polymers of ADPR, which are subsequently degraded into ADPR monomers. Monomeric ADPR then acts as an intracellular ligand to activate TRPM2 channels29,30. The second proposed mechanism involves the breakdown of NAD+ into ADPR in the mitochondria by NUDT9 ADPRase. To demonstrate this mechanism, Perraud and colleagues used an elegant approach in which they targeted NUDT9, which is able to degrade ADPR, to the mitochondria. When mitochondria-targeted NUDT9 was expressed in human embryonic kidney-293 (HEK-293) cells stably expressing human TRPM2 (HEK293-TRPM2), they demonstrated that TRPM2 channel activation in response to H2O2 exposure was largely abrogated21. These findings suggest that the mitochondrial production of ADPR is critical for the activation of TRPM2 channels in response to oxidative stress.
In addition to ADPR, several studies have suggested that NAD+ is capable of activating TRPM2 channels23,31,32; however, this finding has not been consistently reproduced in the literature8,16. In comparison to the latency of TRPM2 activation by ADPR, the latency to TRPM2 activation after NAD+ application is significantly longer, raising the possibility that NAD+ activates TRPM2 indirectly following its conversion to ADPR23. TRPM2 has also been shown to be activated by structural analogues of ADPR, including cyclic ADPR (cADPR), nicotinic acid adenine dinucleotide phosphate (NAADP) and 2'-O-acetyl-ADP-ribose (OAADPr), a product of the sirtuin family of protein deacetylases33. Whether cADPR and NAADP act as direct agonists, contributing to TRPM2 activation through their conversion to ADPR, or modify the concentration-response curve of ADPR required for channel activation is still unclear34,35,36,37,38. Intriguingly, a recent study seeking to identify the structural requirements for TRPM2 channel activation assessed the agonist activity of a series of ADPR analogues harboring modifications to the purine base, the pyrophosphate group or the terminal ribose39. This investigation led to the identification of 2'-deoxy-ADPR, a TRPM2 superagonist capable of eliciting whole-cell currents >10-fold larger than those elicited by ADPR. Using HPLC and mass spectrometry, 2'-deoxy-ADPR was identified as an endogenous metabolite generated through the concerted activity of cytosolic nicotinamide mononucleotide adenylyltransferase 2 (NMNAT-2) and the nicotinamide adenine dinucleotide glycohydrolase CD38. Progress in understanding the relative influence of these candidate intracellular ligands in regulating TRPM2 activity will require knowledge of the signaling context and concentrations achieved under various physiological and pathological conditions.
In addition to NUDT9-H agonist binding, TRPM2 activation has a strict intracellular Ca2+ requirement16,22,40,41,42. Ca2+ dependence is imparted via the Ca2+-sensitive association of calmodulin (CaM) to an IQ-like domain localized within the intracellular N-terminus of TRPM2 (amino acids 406–416)13,14. Mutation of the IQ-like CaM binding motif abrogates channel activation by hydrogen peroxide13 and ADPR14. Similarly, ADPR-evoked TRPM2 currents are inhibited by calmidazolium, an inhibitor of CaM41. Interestingly, TRPM2 splice isoforms (TRPM2-ΔN, TRPM2-ΔC, TRPM2-ΔNΔC, and SSF-TRPM2) and mutants that do not respond to ADPR can be activated by Ca2+, albeit at very high concentrations (EC50=∼17 μmol/L in the absence of exogenously supplied ADPR)14. This Ca2+-dependent activation suggests that elevated Ca2+ alone may be sufficient to activate TRPM2. In this regard, the rise in the intracellular Ca2+ concentration within micro- and nano-domains surrounding open Ca2+-permeable channels is estimated to reach as high as 1–100 μmol/L43. Whether Ca2+ reaches a concentration sufficient to directly promote Ca2+/CaM-dependent TRPM2 channel openings, for example, in response to Ca2+ influx via voltage- or ligand-operated channels localized in close proximity to TRPM2, remains to be determined. More realistically, intracellular Ca2+ and ADPR likely act co-operatively to effect TRPM2 channel activation. Such co-operativity between ADPR and Ca2+ is likely to have important functional implications. In neutrophil granulocytes, endogenous levels of ADPR, estimated at ∼5 μmol/L, are sufficient to allow TRPM2 to respond to varying intracellular levels of Ca2+, for example, in response to the release of Ca2+ from intracellular stores22. Analogously, in hippocampal pyramidal neurons, intracellular application of ADPR alone is insufficient to initiate robust TRPM2 activation. However, supplemental Ca2+ entry via voltage- or NMDA receptor-gated Ca2+-permeable channels is necessary42. Collectively, these results suggest that TRPM2 integrates intracellular signaling events that provoke changes in the intracellular concentrations of ADPR (and possibly other endogenous agonists) and Ca2+.
Additional gating modifiers
In addition to ADPR and Ca2+, several other factors may influence TRPM2 gating. For example, extracellular pH has been shown to inhibit TRPM2 channels with a half-maximal inhibitory concentration (IC50) ranging from pH 4.7 to 6.5, depending on the concentration of Ca2+ used in the solution. Acidic pH is proposed to reduce TRPM2 channel conductance by binding to external residues and/or through permeation of H+ and subsequent competition for intracellular Ca2+ binding sites on the channel44,45,46. Similarly, an intracellular acidic environment also inhibits TRPM2 channels. This inhibition appears to involve Asp933, since substitution of this residue changes the IC50 from pH 6.7 to pH 5.5. Furthermore, increasing the concentration of intracellular calcium shifts the IC50 from pH 6.7 to pH 6.3, suggesting that intracellular protons may compete with calcium for a binding site44. In addition to pH, glutathione (GSH), a naturally occurring antioxidant, can act as an inhibitor of TRPM2 channels47. GSH has been shown to inhibit TRPM2-mediated cell death induced by H2O2 and TNF-α in insulinoma and monocyte cell lines31. Conversely, chemical depletion of intracellular GSH induces an increase in intracellular Ca2+ through TRPM2 channels expressed in glia or dorsal root ganglion sensory neurons48,49. Our own results have demonstrated that TRPM2 activity is enhanced in cultured hippocampal neurons over time in vitro due to the loss of tonic inhibition by GSH47. Although the precise mechanism has yet to be defined, we have demonstrated that GSH causes a rightward shift in the ADPR concentration-response curve, increasing the EC50 for channel activation by ADPR from 77 μmol/L to 269 μmol/L. This observation suggest that GSH may compete for the ADPR binding site47.
Physiological roles of TRPM2
TRPM2 mRNA expression assessed by quantitative real-time polymerase chain reaction (qRT-PCR) is nearly ubiquitous in all tissues examined, except for bone and cartilage. As such, TRPM2 has been implicated in physiological processes in a host of tissues and organ systems. First, TRPM2 participates in insulin secretion from pancreatic β-cells32,50,51. A role for TRPM2 in inflammation has also been established whereby TRPM2 activation has been shown to promote inflammation and immune responses through the production of cytokines CXCL8 (previously known as interleukin-8 [IL-8]), IL-6, IL-10, and TNF-α52,53. More recently, TRPM2 has been implicated in phagosome maturation, which has been shown to increase bacterial clearance and reduce mortality in a mouse model of E coli sepsis54. Interestingly, TRPM2 dampens the inflammatory response through cellular depolarization and subsequent reduction of ROS production in phagocytes, thereby minimizing excess inflammation55. Additionally, the channel has been implicated in lung inflammation and associated diseases56. Lastly, oxidative stress-induced TRPM2 activation mediates Ca2+ entry into endothelial cells, leading to vascular barrier dysfunction via opening of interendothelial junctions, although the precise mechanism(s) involved requires further investigation57,58.
TRPM2 in the CNS
TRPM2 mRNA is most abundant in the brain2,24,59. Within the CNS, TRPM2 expression has been demonstrated in microglia, astrocytes, and neuronal populations in the hippocampus, substantia nigra, striatum, and cortex, as well as dorsal root ganglion (DRG) sensory neurons in the spinal cord2,24,42,59,60,61,62,63. Within the hippocampus, TRPM2 mRNA is localized to the pyramidal cell layer, and preliminary evidence has demonstrated a predominantly extrasynaptic distribution in cultured hippocampal neurons42. TRPM2 currents in these cells can be activated by ROS/RNS exposure or by intracellularly applied ADPR contingent on supplemental Ca2+ influx through voltage-gated calcium channels (VGCCs) or N-methyl-D-aspartate (NMDA) receptors42.
The requirement for Ca2+ entry via VGCCs and/or NMDA receptors (NMDARs) is in keeping with the recognized Ca2+/CaM co-dependence of TRPM2 activation by NUDT9-H ligands. The strict requirement for co-stimulation of VGCCs or NMDARs to activate TRPM2 in hippocampal neurons is intriguing given the importance of each of these channels in neuronal function. In light of the essential contribution of NMDARs to the induction of multiple forms of synaptic plasticity64,65, the functional link between NMDARs and TRPM2 is of particular interest to us. In a recent study, we demonstrated that TRPM2 channels are necessary for the establishment of long-term depression (LTD), a specific form of NMDAR-dependent synaptic plasticity66. Impaired NMDAR-dependent LTD in TRPM2 knock-out (KO) hippocampal slices was associated with a reduced expression of postsynaptic density protein 95 (PSD-95) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPARs). Moreover, the loss of NMDAR-dependent LTD was recapitulated with the TRPM2 antagonist clotrimazole, demonstrating that the observed changes in synaptic plasticity were not due to developmental changes induced by genetic deletion of TRPM2. Mechanistically, the resulting changes could be attributed to inactivation of glycogen synthase kinase 3β (GSK-3β) based on two key findings: 1) loss of TRPM2 was associated with increased inactivation of GSK-3β through its phosphorylation at Ser9, and 2) LTD deficits were rescued by stimulating GSK-3β activity downstream of dopamine D2 receptors 66. The signaling pathway through which TRPM2 regulates GSK-3β activity is likely to involve Ca2+. Calcineurin (also known as PP2B), a phosphatase regulated by Ca2+, dephosphorylates GSK-3β at Ser9 and may mediate the change in GSK-3β activation downstream of TRPM2. Evidence supporting such a mechanism was recently provided67.
Importantly, we have demonstrated that the loss of TRPM2 expression does not affect the expression of NMDAR subunits GluN1, GluN2A and GluN2B 66, the predominant NMDAR subunits expressed in the hippocampus. In contrast, a subsequent study reported that knockout of TRPM2 impacted the expression of both GluN2A and GluN2B but not GluN168. In this study, the reported changes in expression were evident by western blot analysis of whole hippocampal lysates but not when examined by immunofluorescence68. The reason for these discordant western blot findings is not clear. Importantly, the lack of altered expression was confirmed in our study by the assessment of NMDAR function in hippocampal pyramidal neurons. In this way, we demonstrated that NMDA-evoked current amplitude, desensitization (steady state/peak) and sensitivity to Ro25-6981, a highly specific GluN2B antagonist, are identical in hippocampal neurons derived from TRPM2 WT and KO mice66. Similarly, the amplitude of synaptically evoked NMDAR-mediated responses was not impacted by genetic deletion of TRPM266. These findings strongly suggest that genetic deletion of TRPM2 does not impact NMDAR expression, at least among the complement of functioning receptors expressed at the cell surface.
In addition to regulating the induction of NMDAR-dependent synaptic plasticity, TRPM2 has been shown to have a physiological role in temperature sensation and thermoregulation. These roles are consistent with previous evidence demonstrating that TRPM2 can be activated by exposure to warm temperatures50,69. Indeed, TRPM2 was recently shown to be expressed in a subpopulation of neurons in the preoptic area (POA) of the hypothalamus where it is involved in the sensing of hyperthermia and plays an important role in temperature homeostasis69. In this study, the authors demonstrated that TRPM2, expressed within POA neurons, becomes active under conditions of elevated core body temperatures (ie, >37 °C) and drives a compensatory hypothermic response, suggested to limit the upper fever range during heat stress. Interestingly, TRPM2 has also been shown to play a role in heat sensation by somatosensory and autonomic neurons70. In this report, the authors exposed mice to temperatures ranging from 23 °C to 38 °C and demonstrated that TRPM2 knockout mice showed a preference for non-noxious warmer temperatures, whereas wild-type littermates preferred cooler temperatures. Both groups tended to avoid the noxious temperature of 43 °C70. Collectively, these studies suggest that TRPM2 may guide both an autonomic and behavioral response that seeks to maintain thermal homeostasis.
Contribution of TRPM2 to CNS pathology
The influx of Ca2+ resulting from TRPM2 channel activation is key to its contribution to physiological as well as pathological processes. The intracellular concentration of Ca2+ is normally buffered to very low resting levels by numerous homeostatic mechanisms. Calcium signals, generated when the concentration of this divalent cation rises above this resting level, are sensed by a number of Ca2+-dependent signaling molecules and serve a critical role in regulating cellular functions. Paradoxically, prolonged or excessive alterations in Ca2+ concentrations can lead to cytotoxicity71,72,73. Cell death as a result of cytotoxic Ca2+ entry via TRPM2 activated in response to oxidative stress is among the best and earliest characterized role for TRPM2. Initial evidence in HEK cells expressing TRPM2 heterologously, as well as in insulinoma and monocyte cell lines expressing TRPM2 endogenously, revealed that TRPM2 conferred susceptibility to cell death in response to H2O2 treatment31. A role for TRPM2 in cell death has since been confirmed in a variety of cell types including neuronal cell populations in response to H2O2, TNF-α, and Aβ peptide74,75,76,77. Aberrant TRPM2 function has been implicated in various neurological disorders, which is not surprising when one considers that TRPM2 expression levels are highest in the CNS, channel activation occurs in response to oxidative and nitrosative stress, and channel-mediated Ca2+ entry has been linked to cell death. In the sections to follow, we summarize recent progress in the understanding of the cause and consequence of pathological TRPM2 activation in the CNS.
Aging
Aging represents the leading risk factor for neurodegenerative diseases including Alzheimer's and Parkinson disease78. A major theory of aging as a risk factor for CNS disease suggests that neuronal Ca2+ dysregulation contributed by reduced antioxidant defense, increased oxidative stress and perturbed energy metabolism is a likely cause of aging-associated CNS disease79. TRPM2 is thought to participate in the normal aging processes within the brain. For example, normal aging is associated with a decreased concentration of glutathione (GSH) in vitro and in vivo80,81,82,83,84. The depletion of GSH with age not only increases the oxidative stress status of the cell but may also be associated with increased intracellular calcium and subsequent toxicity85,86,87,88,89. This process may involve TRPM2, as recent work has demonstrated that TRPM2 activity is enhanced in cultured hippocampal neurons over time in vitro due to the loss of tonic inhibition by glutathione47. Relief of constitutive TRPM2 inhibition due to reduced intracellular GSH levels with aging could contribute to elevations in intracellular Ca2+, consequently disrupting synaptic plasticity and reducing cell viability. Interestingly, although NMDAR-dependent long-term potentiation (LTP) is impaired with age, the total magnitude of LTP is largely preserved during normal aging, potentially through a compensatory upregulation in voltage gated calcium channel dependent LTP90. Given that TRPM2 is necessary for the induction of NMDAR-dependent LTD66, we might expect that increased TRPM2 activity, in part due to reduced GSH levels with age, is associated with increased LTD. In fact, LTD has been shown to be enhanced with normal aging91. Combined, these changes in synaptic plasticity produce an age-dependent decrease in synaptic strength that may underlie the memory impairment associated with normal aging. Additional studies will be required to assess whether aberrant TRPM2 activity contributes to age-related alterations in plasticity and whether moderating TRPM2 activity in the aged brain could serve as a novel therapeutic intervention.
Ischemic stroke
Excitotoxicity by glutamate acting upon NMDARs is long established as the dominant conceptual model underlying neuronal cell death associated with ischemic stroke. However, numerous studies have demonstrated an important additional contribution of Ca2+-permeable non-selective cation channels including large-pore pannexin channels92,93 and TRPM7, which have been suggested to be recruited by conditions of elevated oxidative stress associated with ischemia94. Similarly, a number of studies have linked TRPM2 activation to conditions of elevated oxidative stress and dysregulated Ca2+ associated with ischemic neuronal death. Among the earliest suggestive evidence in this regard came the demonstration that cell death of cultured neurons in response to H2O2 treatment (50 μmol/L for 6 h or 1 mmol/L for 20 min) was greatly attenuated by knockdown of TRPM276. More definitive evidence that TRPM2 contributes to ischemia-induced neuronal cell death was provided with the demonstration that pharmacological inhibitors and RNA interference using shRNA targeting TRPM2 reduced infarct volume in vivo and decreased neuronal cell death in vitro following oxygen glucose deprivation (OGD)75. Interestingly, this protection appeared to be specific to males, suggesting a potential sex difference in the contribution of TRPM2 to ischemic cell death95,96. Other groups have confirmed the ability of TRPM2 knockout to protect against ischemic cell death (notably, only male mice were used in these studies)68,97,98. In addition to its direct contribution to neuronal death in response to ischemia, TRPM2 may contribute to the sequelae of stroke by modulating stress-induced activation of microglia, as suggested in a previous study. Here, in the transient middle cerebral artery occlusion (tMCAO) rat model of ischemia, expression of TRPM2 mRNA was demonstrated to be elevated from 1–4 weeks following stroke induction. This increase in TRPM2 expression was attributed to transcriptional upregulation of TRPM2 in microglia in response to oxidative stress and the cytokine IL-1β59. Lastly, TRPM2 may also participate in neonatal ischemic brain injury. One recent study looked at the role for TRPM2 in hypoxic-ischemic injury in postnatal day 7 mouse pups by ligating the right common carotid artery and subsequently exposing animals to a hypoxic environment for 2 h. This group demonstrated that TRPM2 knockout was neuroprotective, with reduced brain infarct size, improved sensorimotor function, and reduced expression of inflammatory markers99.
Alzheimer's disease
In addition to a potential role in stroke and ischemic brain injury, TRPM2 has also been implicated in Alzheimer's disease (AD). Primary striatal cultures exposed to 20 μmol/L monomeric β-amyloid demonstrated an increase in intracellular calcium and cell death, which was partially blocked when cultures were transfected with a dominant-negative splice variant of TRPM274. More definitive evidence that TRPM2 contributes to the pathology and cognitive decline was recently provided in an AD mouse model77. Elimination of TRPM2 was shown to reduce expression of endoplasmic reticulum stress response markers and microglia activation in the APP/PS1 Alzheimer's disease mouse model77. Importantly, this study also demonstrated that deletion of TRPM2 rescued the spatial memory deficits in aged APP/PS1 mice. As TRPM2 is broadly expressed in a variety of cell types in the brain, and TRPM2 was deleted globally in this study, the extent to which the beneficial effects noted were due to loss of TRPM2 function in neurons, microglia or other cell types remains to be determined. Along these lines, subsequent research has demonstrated that TRPM2 participates in Aβ-induced neuroinflammation through microglia activation and generation of TNF-α in a pathway involving ROS activation of PARP-1100. Moreover, Aβ has been shown to provoke TRPM2 activation in vascular endothelial cells, where TRPM2 has been proposed to contribute to cerebrovascular dysfunction in AD101.
Neuropathic pain
Recent evidence also suggests that TRPM2 may play a role in spinal cord injury and neuropathic pain. In a rat model of spinal cord injury, intraperitoneal (IP) injection of clotrimazole conjugated to polyethylene glycol significantly reduced lipid peroxidation, an indicator of oxidative stress, when administered 5 min after spinal cord compression102. Whether inhibition or knockdown of TRPM2 results in higher functional performance compared to untreated controls in a spinal cord injury model is unclear. Additionally, a recent study demonstrated that TRPM2 mRNA is elevated following sciatic nerve injury. When compared with WT littermate controls, TRPM2 knockout mice show a reduced immune response and attenuation of the heightened mechanical and thermal pain responses elicited by sciatic nerve injury, suggesting that TRPM2 plays a role in neuropathic pain103.
Bipolar disorder and other disorders associated with TRPM2 mutations
Genetic studies have associated single nucleotide polymorphisms (SNPs) in TRPM2 to an increased susceptibility for bipolar disorder104,105,106,107. Interestingly, a recent study demonstrated that a TRPM2 mutation identified in patients with bipolar disorder (D543E) is associated with a loss of TRPM2 function66. This finding led the authors to posit that the loss of TRPM2 function may be associated with the behavioral manifestations of bipolar disorder, including mood disorders and impaired social interaction. Consistent with this assumption, TRPM2 knockout mice were found to exhibit increased anxiety and impaired social cognition. In agreement with previous reports demonstrating an important role of TRPM2 in regulating GSK-3 activity66,68, the authors provided further evidence suggesting that bipolar disorder-related behavior associated with loss of TRPM2 function may be due to dysregulated GSK-3 activity.
A single SNP (P1018L) in TRPM2 has also been identified in tissue from Guamanian amyotrophic lateral sclerosis (ALS) and Parkinson's disease subjects108. In addition to the potential genetic link with Parkinson's disease, a recent study also demonstrated that TRPM2 channels are necessary for NMDA-induced burst firing in substantia nigra pars reticulate GABAergic neurons. These authors noted that previous studies have demonstrated an increase in burst firing observed in Parkinson's disease, which may implicate TRPM2 in Parkinson's disease pathology62. Whether other neurological disorders are associated with TRPM2 mutations requires further investigation.
Pharmacology and therapeutic potential of drugs targeting TRPM2
Initial research into TRPM2 and its role in normal physiology and disease involved a combination of non-selective pharmacological agents and genetic knockout models. The channel is inhibited by agents such as N-(p-amycinnamoyl) anthranilic acid (N-ACA), econazole, clotrimazole, and flufenamic acid42,109. Notably, many previously identified TRPM2 inhibitors are non-selective and thus have additional effects unrelated to TRPM2. For example, N-ACA inhibits calcium-activated chloride channels110 as well as several TRP family members, including TRPC6 and TRPM8111. Clotrimazole, an antifungal agent, inhibits Ca2+-activated potassium channels (KCa3.1)112, cytochrome P-450113 and NMDARs114. Lastly, flufenamic acid, a non-steroidal anti-inflammatory, inhibits chloride channels, calcium-activated chloride channels, and voltage-gated Ca2+ channels115. Experimentally, the limited specificity of existing inhibitors can be addressed in part through the utilization of a panel of inhibitors that have the ability to block TRPM2 in common but differ with respect to their additional off-target effects (eg, N-ACA and clotrimazole). Additionally, potential confounds related to the use of non-specific antagonists can be circumvented through judicious use of established TRPM2 knockout mice53. Therapeutically, the lack of specificity of existing TRPM2 inhibitors is a major limitation. In this regard, some recent developments have provided hope that TRPM2 is, in fact, a druggable target.
Research in the last 5 years has led to the identification of several novel inhibitors of TRPM2. These inhibitors certainly have a role in experimental application, and some may also show promise as compounds that may be developed into novel therapeutics. The use of a modified ADPR analogue, 8Br-ADPR, was initially shown to inhibit ADPR-activated calcium influx in mouse neutrophils and dendritic cells116. Following this paper, several other modified compounds were designed, and their ability to antagonize TRPM2 was evaluated, including the antagonist 8-Ph-2′-deoxy-ADPR, which displays an IC50 of 3 μmol/L117. Luo and colleagues also recently synthesized novel ADPR analogues capable of selectively inhibiting TRPM2 channel currents in vitro at low concentrations without affecting TRPM7, TRPM8, TRPV1, or TRPV3118. Additionally, a TRPM2 peptide inhibitor tat-M2NX, a cell-permeable peptide, was recently designed to correspond to the C-terminal NUDT9-H domain. This peptide has been shown to decrease calcium influx in vitroand decrease infarct volume following transient middle cerebral artery occlusion when provided either prior to the infarct or 3 hours following the insult119.
Summary
As highlighted in this review, numerous studies have now established the important contribution of TRPM2 to a wide variety of CNS diseases, including ischemia/stroke, Alzheimer's disease, neuropathic pain, bipolar disorder, and Parkinson's disease. Collectively, these studies suggest that therapeutic interventions able to moderate aberrant TRPM2 activation may be effective in treating these debilitating neurological disorders. This therapeutic potential is counterbalanced by reports demonstrating that TRPM2 also contributes to many physiological processes. The involvement of TRPM2 in these numerous physiological processes raises some concerns with regards to the potential side effects of drugs able to block TRPM2 function. These concerns predominantly relate to the roles of the channel in immune function, insulin release, and temperature sensation and regulation. Of note, genetic deletion of TRPM2 is well tolerated and does not appear to alter behavior, including locomotor activity assessed on the open field test, anxiety behaviors assessed by the elevated plus maze, or spatial memory deficits assessed by the Barnes maze77. Ultimately, assessment of the risk-benefit profile of TRPM2 as a therapeutic target will require the development of specific TRPM2 inhibitors with favorable pharmacokinetic and pharmacodynamic properties. Further research will be needed to identify which specific patient populations would derive the most benefit and to assess whether side effects of TRPM2 inhibition are clinically significant and thus preclude the consideration of drugs targeting this channel.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research CIHR (grants MOP-125901 and MOP-97771 to Michael Frederick JACKSON).
References
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 1998; 54: 124–31. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 2006; 26: 159–78. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 1989; 2: 1313–23. [DOI] [PubMed] [Google Scholar]
- Clapham DE. SnapShot: mammalian TRP channels. Cell 2007; 129: 220. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 2011; 12: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hoffman NE, Shanmughapriya S, Bao L, Keefer K, Conrad K, et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem 2014; 289: 36284–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N. Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 2005; 328: 1232–43. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 2002; 277: 23150–6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 2003; 278: 16222–9. [DOI] [PubMed] [Google Scholar]
- Lavorgna G, Triunfo R, Santoni F, Orfanelli U, Noci S, Bulfone A, et al. AntiHunter 2.0: increased speed and sensitivity in searching BLAST output for EST antisense transcripts. Nucleic Acids Res 2005; 33: W665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanelli U, Wenke AK, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res 2008; 18: 1128–40. [DOI] [PubMed] [Google Scholar]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch 2005; 451: 204–11. [DOI] [PubMed] [Google Scholar]
- Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, et al. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem 2006; 281: 9076–85. [DOI] [PubMed] [Google Scholar]
- Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci U S A 2009; 106: 7239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH. Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem Soc Trans 2007; 35: 86–8. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001; 411: 595–9. [DOI] [PubMed] [Google Scholar]
- Shen BW, Perraud AL, Scharenberg A, Stoddard BL. The crystal structure and mutational analysis of human NUDT9. J Mol Biol 2003; 332: 385–98. [DOI] [PubMed] [Google Scholar]
- Iordanov I, Mihályi C, Tóth B, Csanády L. The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity. eLife 2016; 5. pii: e17600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Shen B, Dunn CA, Rippe K, Smith MK, Bessman MJ, et al. NUDT9, a member of the Nudix hydrolase family, is an evolutionarily conserved mitochondrial ADP-ribose pyrophosphatase. J Biol Chem 2003; 278: 1794–801. [DOI] [PubMed] [Google Scholar]
- Kuhn FJ, Luckhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J Biol Chem 2004; 279: 46431–7. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 2005; 280: 6138–48. [DOI] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jüngling E, Lückhoff A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J 2006; 398: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001; 293: 1327–30. [DOI] [PubMed] [Google Scholar]
- Kraft R, Grimm C, Grosse K, Hoffmann A, Sauerbruch S, Kettenmann H, et al. Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am J Physiol Cell Physiol 2004; 286: C129–37. [DOI] [PubMed] [Google Scholar]
- Xia R, Mei ZZ, Mao HJ, Yang W, Dong L, Bradley H, et al. Identification of pore residues engaged in determining divalent cationic permeation in transient receptor potential melastatin subtype channel 2. J Biol Chem 2008; 283: 27426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium 2011; 50: 279–87. [DOI] [PubMed] [Google Scholar]
- Fliegert R, Watt JM, Schöbel A, Rozewitz MD, Moreau C, Kirchberger T, et al. Ligand-induced activation of human TRPM2 requires the terminal ribose of ADPR and involves Arg1433 and Tyr1349. Biochem J 2017; 474: 2159–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Xue X, Zhang J, Hu X, Wu Y, Jiang LH, et al. Identification of the ADPR binding pocket in the NUDT9 homology domain of TRPM2. J Gen Physiol 2017; 149: 219–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenn C, Wyrsch P, Bader J, Bollhalder M, Althaus FR. Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death. Cell Mol Life Sci CMLS 2011; 68: 1455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall ICB, Benham CD, Boyfield I, Brown JD, Hill K, et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 2004; 143: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 2002; 9: 163–73. [DOI] [PubMed] [Google Scholar]
- Inamura K, Sano Y, Mochizuki S, Yokoi H, Miyake A, Nozawa K, et al. Response to ADP-ribose by activation of TRPM2 in the CRI-G1 insulinoma cell line. J Membr Biol 2003; 191: 201–7. [DOI] [PubMed] [Google Scholar]
- Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL, et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem 2006; 281: 14057–65. [DOI] [PubMed] [Google Scholar]
- Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J 2006; 20: 962–4. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 2005; 18: 61–9. [DOI] [PubMed] [Google Scholar]
- Lange I, Penner R, Fleig A, Beck A. Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium 2008; 44: 604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Sun HY, Lau CP, Tse HF, Lee HC, Li GR. Cyclic ADP ribose is a novel regulator of intracellular Ca2+ oscillations in human bone marrow mesenchymal stem cells. J Cell Mol Med 2011; 15: 2684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B, Csanády L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem 2010; 285: 30091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegert R, Bauche A, Wolf Pérez AM, Watt JM, Rozewitz MD, Winzer R, et al. 2′-Deoxyadenosine 5′-diphosphoribose is an endogenous TRPM2 superagonist. Nat Chem Biol 2017; 13: 1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 2003; 278: 11002–6. [DOI] [PubMed] [Google Scholar]
- Starkus J, Beck A, Fleig A, Penner R. Regulation of TRPM2 by extra- and intracellular calcium. J Gen Physiol 2007; 130: 427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Jackson MF, Li H, Perez Y, Sun HS, Kiyonaka S, et al. Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J Physiol 2009; 587: 965–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron 2008; 59: 873–81. [DOI] [PubMed] [Google Scholar]
- Du J, Xie J, Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol 2009; 134: 471–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Fleig A, Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol 2010; 588: 1227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Zou J, Xia R, Vaal ML, Seymour VA, Luo J, et al. State-dependent inhibition of TRPM2 channel by acidic pH. J Biol Chem 2010; 285: 30411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belrose JC, Xie YF, Gierszewski LJ, MacDonald JF, Jackson MF. Loss of glutathione homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol Brain 2012; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J 2010; 24: 2533–45. [DOI] [PubMed] [Google Scholar]
- Nazıroğlu M, Özgül C, Çiğ B, Doğan S, Uğuz AC. Glutathione modulates Ca2+ influx and oxidative toxicity through TRPM2 channel in rat dorsal root ganglion neurons. J Membr Biol 2011; 242: 109–18. [DOI] [PubMed] [Google Scholar]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 2006; 25: 1804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 2011; 60: 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol 2010; 184: 2386–93. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008; 14: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cui P, Zhang K, Chen Q, Fang X. Transient receptor potential melastatin 2 regulates phagosome maturation and is required for bacterial clearance in Escherichia coli sepsis. Anesthesiology 2017; 126: 128–39. [DOI] [PubMed] [Google Scholar]
- Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 2011; 13: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Steinritz D, Gudermann T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 2017; 67: 123–37. [DOI] [PubMed] [Google Scholar]
- Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 2008; 102: 347–55. [DOI] [PubMed] [Google Scholar]
- Mittal M, Nepal S, Tsukasaki Y, Hecquet CM, Soni D, Rehman J, et al. Neutrophil activation of endothelial cell-expressed TRPM2 mediates transendothelial neutrophil migration and vascular injury. Circ Res 2017; 121: 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Mattei C, Hill K, Brown JT, Randall A, Benham CD, et al. TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J Recept Signal Transduct Res 2006; 26: 179–98. [DOI] [PubMed] [Google Scholar]
- Hill K, Tigue NJ, Kelsell RE, Benham CD, McNulty S, Schaefer M, et al. Characterisation of recombinant rat TRPM2 and a TRPM2-like conductance in cultured rat striatal neurones. Neuropharmacology 2006; 50: 89–97. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Kawakami S, Hara Y, Wakamori M, Itoh E, Minami T, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci 2006; 101: 66–76. [DOI] [PubMed] [Google Scholar]
- Lee CR, Machold RP, Witkovsky P, Rice ME. TRPM2 channels are required for NMDA-induced burst firing and contribute to H2O2-dependent modulation in substantia nigra pars reticulata GABAergic neurons. J Neurosci 2013; 33: 1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazıroğlu M, Özgül C, Çelik Ö, Çiğ B, Sözbir E. Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J Membr Biol 2011; 241: 69–75. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004; 304: 1021–4. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol 2006; 18: 71–84. [DOI] [PubMed] [Google Scholar]
- Xie YF, Belrose JC, Lei G, Tymianski M, Mori Y, Macdonald JF, et al. Dependence of NMDA/GSK-3β mediated metaplasticity on TRPM2 channels at hippocampal CA3-CA1 synapses. Mol Brain 2011; 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y, Lee SH, Lee B, Jung S, Khalid A, Uchida K, et al. TRPM2, a susceptibility gene for bipolar disorder, regulates glycogen synthase kinase-3 activity in the brain. J Neurosci 2015; 35: 11811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim I, Teves L, Li R, Mori Y, Tymianski M. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J Neurosci 2013; 33: 17264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Wang H, Kamm GB, Pohle J, Reis F de C, Heppenstall P, et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016; 353: 1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C-H, McNaughton PA. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016; 536: 460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 1985; 58: 293–7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22: 391–7. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–65. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Marshall ICB, Boyfield I, Skaper SD, Hughes JP, Owen DE, et al. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 2005; 95: 715–23. [DOI] [PubMed] [Google Scholar]
- Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, et al. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab 2011; 31: 2160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Kawakami S, Hara Y, Wakamori M, Itoh E, Minami T, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci 2006; 101: 66–76. [DOI] [PubMed] [Google Scholar]
- Ostapchenko VG, Chen M, Guzman MS, Xie YF, Lavine N, Fan J, et al. The transient receptor potential melastatin 2 (TRPM2) channel contributes to β-amyloid oligomer-related neurotoxicity and memory impairment. J Neurosci 2015; 35: 15157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol Mech Dis 2008; 3: 41–66. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell 2007; 6: 337–50. [DOI] [PubMed] [Google Scholar]
- Chen TS, Richie JP, Lang CA. The effect of aging on glutathione and cysteine levels in different regions of the mouse brain. Proc Soc Exp Biol Med 1989; 190: 399–402. [DOI] [PubMed] [Google Scholar]
- Liu RM. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res 2002; 68: 344–51. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res 2008; 86: 2339–52. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res 2007; 1127: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Senda M, Kim S, Kojima S, Kubodera A. Age-related changes of glutathione content, glucose transport and metabolism, and mitochondrial electron transfer function in mouse brain. Nucl Med Biol 2001; 28: 25–31. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 2006; 9: 119–26. [DOI] [PubMed] [Google Scholar]
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev 1997; 25: 335–58. [DOI] [PubMed] [Google Scholar]
- Gabby M, Tauber M, Porat S, Simantov R. Selective role of glutathione in protecting human neuronal cells from dopamine-induced apoptosis. Neuropharmacology 1996; 35: 571–8. [DOI] [PubMed] [Google Scholar]
- Jurma OP, Hom DG, Andersen JK. Decreased glutathione results in calcium-mediated cell death in PC12. Free Radic Biol Med 1997; 23: 1055–66. [DOI] [PubMed] [Google Scholar]
- Thanislass J, Raveendran M, Devaraj H. Buthionine sulfoximine-induced glutathione depletion. Its effect on antioxidants, lipid peroxidation and calcium homeostasis in the lung. Biochem Pharmacol 1995; 50: 229–34. [DOI] [PubMed] [Google Scholar]
- Robillard JM, Gordon GR, Choi HB, Christie BR, MacVicar BA. Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS One 2011; 6: e20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci 1996; 16: 5382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger NL, Lohman AW, Rakai BD, Ma EMM, Bialecki J, Maslieieva V, et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 2016; 19: 432–42. [DOI] [PubMed] [Google Scholar]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A 2011; 108: 20772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003; 115: 863–77. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Grewal H, Klawitter J, Herson PS. Sex steroids do not modulate TRPM2-mediated injury in females following middle cerebral artery occlusion. eNeuro 2014; 1. pii: ENEURO.0022-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Quillinan N, Orfila JE, Herson PS. Sirtuin-2 mediates male specific neuronal injury following experimental cardiac arrest through activation of TRPM2 ion channels. Exp Neurol 2016; 275: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Melzer N, Schattling B, Göb E, Hicking G, Arunachalam P, et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke 2014; 45: 3395–402. [DOI] [PubMed] [Google Scholar]
- Ye M, Yang W, Ainscough JF, Hu XP, Li X, Sedo A, et al. TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 2014; 5: e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Turlova E, Li F, Bao MH, Szeto V, Wong R, et al. Transient receptor potential melastatin 2 channels (TRPM2) mediate neonatal hypoxic-ischemic brain injury in mice. Exp Neurol 2017; 296: 32–40. [DOI] [PubMed] [Google Scholar]
- Alawieyah Syed Mortadza S, Sim JA, Neubrand VE, Jiang LH. A critical role of TRPM2 channel in Aβ42 -induced microglial activation and generation of tumor necrosis factor-α. Glia 2018; 66: 562–75. [DOI] [PubMed] [Google Scholar]
- Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, et al. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat Commun 2014; 5: 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usul H, Cakir E, Arslan E, Peksoylu B, Alver A, Sayin OC, et al. Effects of clotrimazole on experimental spinal cord injury. Arch Med Res 2006; 37: 571–5. [DOI] [PubMed] [Google Scholar]
- Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, et al. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci 2012; 32: 3931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyrko A, Hauser J, Rybakowski JK, Trzeciak WH. Screening of chromosomal region 21q22.3 for mutations in genes associated with neuronal Ca2+ signalling in bipolar affective disorder. Acta Biochim Pol 2006; 53: 317–20. [PubMed] [Google Scholar]
- McQuillin A, Bass NJ, Kalsi G, Lawrence J, Puri V, Choudhury K, et al. Fine mapping of a susceptibility locus for bipolar and genetically related unipolar affective disorders, to a region containing the C21ORF29 and TRPM2 genes on chromosome 21q22.3. Mol Psychiatry 2006; 11: 134–42. [DOI] [PubMed] [Google Scholar]
- Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet Part B Neuropsychiatr Genet 2006; 141B: 36–43. [DOI] [PubMed] [Google Scholar]
- Xu C, Li PP, Cooke RG, Parikh SV, Wang K, Kennedy JL, et al. TRPM2 variants and bipolar disorder risk: confirmation in a family-based association study. Bipolar Disord 2009; 11: 1–10. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Cui AM, Go RCV, Davenport B, Shetler CM, Heizer JW, et al. Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci U S A 2008; 105: 18029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld J, Lückhoff A. TRPM2. Handb Exp Pharmacol 2007; (179): 237–52. [DOI] [PubMed] [Google Scholar]
- Gwanyanya A, Macianskiene R, Bito V, Sipido KR, Vereecke J, Mubagwa K. Inhibition of the calcium-activated chloride current in cardiac ventricular myocytes by N-(p-amylcinnamoyl)anthranilic acid (ACA). Biochem Biophys Res Commun 2010; 402: 531–6. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Frenzel H, Kraft R. N-(p-amylcinnamoyl)anthranilic acid (ACA): a phospholipase A2 inhibitor and TRP channel blocker. Cardiovasc Rev 2007; 25: 61–75. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A 2000; 97: 8151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, Sellers EM. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab Dispos Biol Fate Chem 2002; 30: 314–8. [DOI] [PubMed] [Google Scholar]
- Isaev NK, Stelmashook EV, Dirnagl U, Andreeva NA, Manuhova L, Vorobjev VS, et al. Neuroprotective effects of the antifungal drug clotrimazole. Neuroscience 2002; 113: 47–53. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol Ther 2013; 138: 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol 2007; 179: 7827–39. [DOI] [PubMed] [Google Scholar]
- Moreau C, Kirchberger T, Swarbrick JM, Bartlett SJ, Fliegert R, Yorgan T, et al. Structure-activity relationship of adenosine 5'-diphosphoribose at the transient receptor potential melastatin 2 (TRPM2) channel: rational design of antagonists. J Med Chem 2013; 56: 10079–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Li M, Zhan K, Yang W, Zhang L, Wang K, et al. Selective inhibition of TRPM2 channel by two novel synthesized ADPR analogues. Chem Biol Drug Des 2017; [DOI] [PMC free article] [PubMed]
- Shimizu T, Dietz RM, Cruz-Torres I, Strnad F, Garske AK, Moreno M, et al. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Exp Neurol 2016; 283: 151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]