Abstract
Arctigenin (AR) and its glycoside, arctiin, are two major active ingredients of Arctium lappa L (A lappa), a popular medicinal herb and health supplement frequently used in Asia. In the past several decades, bioactive components from A lappa have attracted the attention of researchers due to their promising therapeutic effects. In the current article, we aimed to provide an overview of the pharmacology of AR and arctiin, focusing on their anti-inflammatory effects, pharmacokinetics properties and clinical efficacies. Compared to acrtiin, AR was reported as the most potent bioactive component of A lappa in the majority of studies. AR exhibits potent anti-inflammatory activities by inhibiting inducible nitric oxide synthase (iNOS) via modulation of several cytokines. Due to its potent anti-inflammatory effects, AR may serve as a potential therapeutic compound against both acute inflammation and various chronic diseases. However, pharmacokinetic studies demonstrated the extensive glucuronidation and hydrolysis of AR in liver, intestine and plasma, which might hinder its in vivo and clinical efficacy after oral administration. Based on the reviewed pharmacological and pharmacokinetic characteristics of AR, further pharmacokinetic and pharmacodynamic studies of AR via alternative administration routes are suggested to promote its ability to serve as a therapeutic agent as well as an ideal bioactive marker for A lappa.
Keywords: arctigenin, arctiin, Arctium lappa L, Fructus Arctii, anti-inflammatory agents, pharmacokinetics, clinical efficacy
Introduction
Arctigenin (AR) (Figure 1), a phenylpropanoid dizbenzylbutyrolactone lignan, was first identified in Arctium lappa L (A lappa), a popular medicinal herb and health supplement frequently used for anti-influenza treatment in Asia, especially China, Korea and Japan. AR and its glycoside, arctiin, are listed as both the chemical marker compounds and major active ingredients of Fructus Arctii in Chinese Pharmacopeia1. In the past several decades, bioactive components from A lappa, especially AR, have attracted the attention of researchers due to their promising therapeutic effects on inflammation2,3,4, infection5,6,7, metabolic disorders8,9,10, and central nervous system dysfunctions11,12,13.
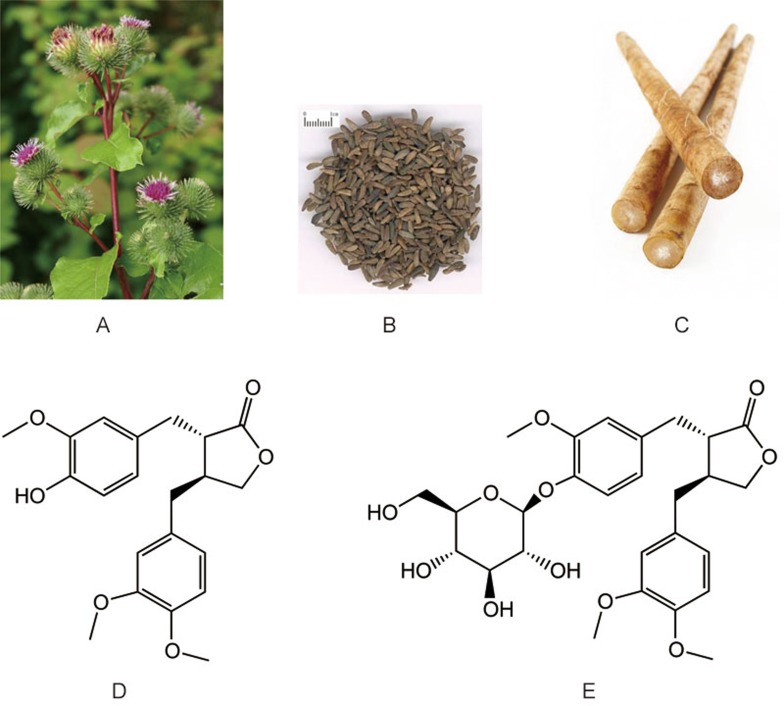
Figure 1.
Arctium lappa L: plant (A), fruit (Fructus Arctii) (B), root (C), and two major bioactive compounds, AR (D) and arctiin (E).
AR and arctiin have been extensively studied for their anti-inflammatory effects in both in vitro and in vivo models. Inflammation is a series of protective responses of the body against exogenous pathogens and to repair tissue damage resulting from infection or trauma. Acute inflammation is characterized by vasodilatation, fluid exudation and neutrophil infiltration14. Severe inflammation can cause organ injury, shock and even death, presenting major management problems14. Furthermore, when the inflammatory response does not eradicate the primary stimulus, a chronic form of inflammation ensues and contributes to further tissue damage. A number of chronic diseases, including atherosclerosis, cancer, type II diabetes, and Alzheimer's disease, have a pathophysiologically important inflammatory component15. Therefore, developing novel compounds targeting the inflammatory response can be beneficial for the treatment of acute inflammation and infection, as well as many widespread chronic diseases. Studies on the pharmacology of AR and arctiin as natural compounds with significant anti-inflammatory effects may contribute to the development of novel anti-inflammatory therapeutics.
Pharmacokinetic profiles, including the absorption, distribution, metabolism and excretion properties, determine the efficacy and safety of a potential therapeutic. Extensive in vitro and in vivo studies have been conducted on AR and arctiin to elucidate their absorption, metabolite profiles, and plasma concentration profiles, as well as the mechanisms involved. The results of these studies provide comprehensive data on the pharmacokinetic properties of AR and arctiin, leading to further optimization strategies for the use of these natural compounds as potential anti-inflammatory therapeutics.
Although research interest in AR, arctiin and A lappa has been growing rapidly, there are few published review articles on their pharmacological characteristics. In the current article, we aim to provide an overview of the pharmacology of AR and arctiin, especially their anti-inflammatory effects, pharmacokinetics properties and clinical efficacy.
Distribution of AR and arctiin in plants
AR and arctiin belong to the family of lignans, which is a class of phytoestrogens characterized by their dibenzylbutane skeleton. Lignans were first identified in plants and are believed to play a role in the construction of plant cell wall as the precursor of lignin. The contents of AR and arctiin, as well as the total lignans, were found to be the highest in the fruit of A lappa among all the plant parts16. AR and arctiin account for approximately 0.5%–2% (w/w) and 2%–10% (w/w) of the dry weight of the fruit, respectively, depending on place of origin, processing methods, and other factors17,18. AR is regarded as marker compound in dozens of other medicinal herbs, probably due to its promising therapeutic activities19,20. AR and arctiin have been identified in not only A lappa but also more than 38 other plant species, among which 71% belong to the Asteraceae family. Table 1 summarizes the distribution of AR and arctiin in different plant species from eight families, including Aspleniaceae, Asteraceae, Convolvulaceae, Linaceae, Oleaceae, Styracaceae, Taxacea, and Thymelaeaceae. In the family Asteraceae, Centaurea is a genus that includes many AR- and arctiin-containing plants, although the AR and arctiin contents are lower than that in A lappa. Fruits and seeds had high levels of AR and arctiin2,21, while the other parts, such as flower, leaves, stem, and roots, had low levels (Table 1). As shown in Table 1, many of these AR- and arctiin-containing plants are recorded as medical plants in their growing areas and are well-recognized for the treatment of diseases, such as rheumatic arthritis, inflammatory diseases, infection, and others.
Table 1. Plants species containing AR or arctiin and their medical usages.
| Family | Species | Parts | Compounds (content, w/w) | Medical usages | Ref |
|---|---|---|---|---|---|
| Aspleniaceae | Asplenium trichomanes | Frond | AR | The frond is used as expectorant, anti-cough remedy, laxative, and emmenagogue in the Italian folk medicine, as well as abortifacient in North America. | 89 |
| Asteraceae | Arctium lappa | Fruit Root Stem Flower | Arctiin (2%–10%), AR (0.5%–2%) Arctiin (0.04%) Arctiin (0.06%) Arctiin (0.05%), AR (0.05%) | The roots are traditionally used to treat diseases such as sore throat and infections such as rashes, boils and various skin problems. The seed and fruits are used for skin conditions as well as in cold/flu formulae of traditional Chinese medicine. | 2,21,90,91,92,93 |
| Arctium tomentosum | Root Seed | Arctiin (0.68%) Arctiin (10.3%), AR (0.2%) | The root and leaf are used for therapy of rheumatism and paraesthesia of skin in Xinjiang, China. | 94 | |
| Carduus micropterus | Aerial parts | Arctiin, AR | No medical use has been reported for this species. | 95 | |
| Centaurea affinis | Aerial parts | AR (0.007%) | No use has been reported for specific species. | 96 | |
| Centaurea americana | Seed | Arctiin (0.2%), AR (0.015%) | Many species of the genus Centaurea have long been used in traditional medicine to cure diabetes, diarrhoea, rheumatism, malaria, hypertension etc. | 95 96 97 | |
| Centaurea arenaria | Whole plant | Arctiin, AR | 98 | ||
| Centaurea cuneifolia | whole plant | AR | 97,99 | ||
| Centaurea dealbata | Fruit | Arctiin, AR | 100 | ||
| Centaurea macrocephala | Seed | Arctiin (0.3%) | 101 | ||
| Centaurea nigra | Seed | Arctiin (0.3%), AR (0.06%) | 102 | ||
| Centaurea phrygia | Flower | AR | 103 | ||
| Centaurea ptosimopappa | Aerial parts | AR | 104 | ||
| Centaurea scabiosa | Fruit | AR | 100 | ||
| Centaurea sclerolepis | Fruit | Arctiin | 97,100 | ||
| Centaurea scoparia | Aerial parts | AR | 105 | ||
| Centaurea schischkinii | Seed | Arctiin, AR | 19,96 | ||
| Centaurea scoparia | Aerial parts | AR (0.004%) | 106 | ||
| Centaurea tweediei | Aerial parts | AR | 97,107 | ||
| Cirsium oleraceum | Fruit | Arctiin (3.9%), AR (0.1%) | No medical use has been reported for this species. | 108 | |
| Cirsium palustre | Fruit | Arctiin (2.8%), AR (0.07%) | No medical use has been reported for this species. | 100 | |
| Saussurea conica | Whole plant | AR | No medical use has been reported for this species. | 109 | |
| Saussurea medusa | Aerial part | Arctiin, AR (0.7%) | The aerial part has been used for the treatment of rheumatoid diseases in Northwest China and Nepal. | 55 | |
| Saussurea salicifolia | Aerial part | Arctiin, AR | The aerial part has been used to treat gynaecological diseases, hepatitis, and gallbladder disorder in Mongolian traditional medicine. | 110,111 | |
| Saussurea involucrata | Seed | Arctiin (0.24%) | No medical use has been reported for this species. | 112 | |
| Onopordum cynarocephalum | Aerial part | AR | A decoction or a tea of the whole plant is used in folk medicine for digestion, cough sedating, and in biliary diseases in Lebanon. The decoction or infusion of flowering tops is used for the alleged treatment of malarial fever and for washing exanthematic skin. | 113 | |
| Onopordon laconicum | Aerial part | AR | No medical use has been reported for this species. | 114 | |
| Onopordon sibthorpianum | Aerial part | AR | No medical use has been reported for this species. | 114 | |
| Convolvulaceae | Ipomoea cairica | Whole plant | AR | No medical use has been reported for this species. | 42 |
| Linaceae | Linum usitatissimum | Seed | AR | Traditionally been used for the management of diarrhea and gastrointestinal infections. | 115 |
| Oleaceae | Forsythia intermedia | Flower, leave | Arctiin | No medical use has been reported for this species. | 116 |
| Forsythia koreana | Fruit | Arctiin, AR | Forsythia fruits from the three species are known in China, Korea, and Japan as an anti-inflammatory, diuretics, antidote, and anti-bacteria medicine in traditional herbal medicine. | 25,33 | |
| Forsythia suspense | Fruit | AR (0.36%) | 25 | ||
| Forsythia viridissima | Fruit, flower | Arctiin, AR | 8,116,117 | ||
| Styracaceae | Styrax japonica | Stem bark | Arctiin | The stem bark has been used to treat inflammatory diseases. | 118 |
| Taxaceae | Torreya nucifera | Bark | Arctiin, AR | The fruits are widely used in folk medicine for the treatment of tapeworm infestation in Korea. | 119 |
| Torreya jackii | Leaves | AR | No medical use has been reported for this species. | 120 | |
| Thymelaeaceae | Wikstroemia indica | Whole plant | AR (0.06%) | Has long been employed as an antipyretic, detoxicant, expectorant, vermifuge, and abortifacient agent in clinical practice in China. | 121 |
Effect of AR and arctiin against inflammatory diseases
Effect of AR and arctiin on acute inflammation and its mechanism
Multiple studies have found that A lappa exhibits anti-inflammatory activities, which were attributed to AR in most research focusing on the traditional Chinese herb22,23,24. The anti-inflammatory effect and the reported mechanism of AR are summarized in Table 2.
Table 2. Summary of the studies on anti-inflammatory effects and related mechanisms of AR.
| Model | Cell line/species | Dose | Effect | Mechanism | Ref |
|---|---|---|---|---|---|
| LPS-induced inflammation | RAW264.7 | 0.01 to 1 μmol/L | Suppression of NO production | Inhibition of iNOS protein expression; suppression of IκBα phosphorylation and p65 nuclear translocation | 30 |
| 0.01 to 1 μmol/L | NR | Decrease of TNF-α production and mRNA level; inhibition of binding between AP-1 and its consensus oligonucleotide; inhibition of phosphorylation and activation of MAPKs | 36 | ||
| 0.1 to 10 μmol/L | Inhibition of NO and PGE2 production | Reduction of iNOS and COX-2 expression; inhibition of NF-κB expression and binding; Suppression of phosphorylation of IκB, IKK and activation of MAPKs | 33 | ||
| 0.3 to 32 μmol/L | Inhibition of NO production | Inhibition of TNF-α production | 22 | ||
| 10 to 50 μmol/L | Inhibition of iNOS activity; slight inhibition on COX-2 activity | Suppression of iNOS expression, IL-1β and IL-6 gene expression; reduction of phosphorylation and nucleus translocation of JAK, STAT1 and STAT3 | 4 | ||
| 50 μmol/L | Inhibition of iNOS activity | Suppression of iNOS expression; promotion of ubiqitination and degradation of iNOS by CHIP-associated proteasomes | 32 | ||
| 3 to 100 μmol/L | Inhibition of iNOS activity; no inhibition of COX-2 activity | Suppression of iNOS expression; inhibition of TNF-α and IL-6 production | 31 | ||
| U937 | 1 to 16 μmol/L | NR | Inhibition of TNF-α production | 22 | |
| Mice peritoneal macrophage | 10 to 20 μmol/L | NR | Decrease of IL-1β, IL-6, and TNF-α level with increased IL-10 and CD204; inhibition of NF-κB activation and p65 nuclear translocation; suppression of PI3K and AKT phosphorylation | 2 | |
| Silica-induced inflammation | RAW264.7 | 0.1 to 10 μmol/L | Inhibition of ROS production | NR | 25 |
| Concanavalin A and LPS induced proliferation | Mice primary splenocyte | 0.5 to 16 μmol/L | Inhibition of T cell and B cell proliferation | NR | 22 |
| Anti-CD3/CD28 Ab induced proliferation | Primary human T lymphocyte | 8.25 to 25 μmol/L | Inhibition of lymphocytes proliferation | Suppression of IL-2 and IFN-γ production and gene expression; decrease of NF-AT-mediated reporter gene expression | 3 |
| TGF-β1-induced EMT-like changes in renal tubular epithelial cells | Human proximal tubular cell line HK-2 | 0.5 to 1 μmol/L | Protection against TGF-β1-induced MCP-1 upregulation and the resulting EMT-like phenotypic changes | Inactivation of the ROS/ERK1/2 MAPK/NF-κB pathway | 122 |
| TNF-α induced inflammation | BEAS-2B cells | 50 μmol/L | NR | Inhibition of PI3K/AKT and Ras/MAPK pathways; inhibition of NF-κB activation | 35 |
| Acetic acid-induced inflammation | Rats | 12.5, 25, 100 mg/kg, po, single dose | Decrease of writhing response and capillary permeability accentuation | NR | 25 |
| Arachidonic acid-induced ear edema | Rats | 0.1–1 mg/ear painting, single dose | Decrease of edema volume, tissue MPO and EPO activities | NR | 25 |
| Carrageenan-induced paw edema | Rats | 10, 30, 100 mg/kg, po, single dose | Decrease of paw edema volumn | NR | 25 |
| LPS-Induced acute lung injury | Rats | 30, 100 mg/kg, iv, single dose | Reduced histological damage, myeloperoxidase activity, and wet-to-dry weight ratio of lung tissues | Decrease of TNF-α, IL-1β, and IL-6 levels; down-regulation of NF-κB and p65 expression; activation of AMPKa | 37 |
| Mice | 50 mg/kg, ip, single dose | Decreased infiltration of inflammatory cells into BALF; production of pro-inflammatory cytokines; reduced the malondialdehyde level; increased superoxide dismutase and catalase activities and glutathione peroxidase/glutathione disulfide ratio in the lung | Significantly reduction of NO production and iNOS expression; enhancement of heme oxygenase-1 expression, and decrease of MAPKs phosphorylation | 27 | |
| LPS-induced colitis | Mice | 5 mg/kg, ip, single dose | NR | Suppression of blood IL-1β and TNF-α level TNBS-induced colitis | 2 |
| Mice | 30, 60 mg/kg, po, qd, 3 days | Reduced loss of body weight, colon shortening, macroscopic scores and MPO activity | Inhibition of IL-1β, TNF-α and IL-6 expression and increase of IL-10 and CD204 expression; inhibition of NF-κB activation, as well as PI3K, AKT and IKKβ phosphorylation | 2 | |
| Dextran sulphate sodium-induced colitis | Mice | 25, 50 mg/kg, po, qd, 10 days | Reduced loss of body weight, disease index and histological damage; recovered intestinal epithelial cells; decreased infiltration of neutrophils and macrophages | Down-regulation of cytokines expressions, including TNF-α and IL-6 at protein and mRNA levels; suppression of MAPKs phosphorylation and NF-κB activation; blockage of Th1 and Th17 responses; inhibition of mTORC1 associated with down-regulation of Th1/ Th17 responses | 26,123 |
| Convection enhanced delivery induced brain injury | Mice | 20, 40, 80 mg/kg, po, qd, 14 days | Reduced brain water content and hematoma; accelerated wound closure; reduced number of allograft inflammatory factor and MPO-positive cells | Decrease of number of allograft inflammatory factor and MPO-positive cells, TNF-α and IL-6 level; Elevation of IL-10 level | 28 |
Abbreviation: iv: intravenous administration; po: oral administration; ip: intraperitoneal; qd: once daily. NR: not reported.
The anti-inflammatory effects of AR were demonstrated in various disease models, including local edema, colitis, acute lung injury, and brain trauma. AR was effective in relieving symptoms such as writhing response, capillary permeability accentuation, and edema volume in local tissue inflammation of rats induced by various stimulators25. Protective effects of AR against LPS-induced acute lung injury through suppression of MAPK, HO-1, and iNOS signaling was observed26,27. Furthermore, AR reduced the infiltration of leukocytes into local tissues, a typical hallmark of acute inflammation. This was observed in various colitis mouse models by the decreased activity of myeloperoxidase (MPO), eosinophil peroxidase (EPO), and cluster of differentiation 68 (CD68), indicators of neutrophils, eosinophils and macrophages, respectively2,26,28. In addition, AR reduced brain water content and hematoma and accelerated wound closure in convection-enhanced delivery induced brain injury in mice through regulation of various inflammatory factors and numbers of MPO-positive cells28.
The anti-inflammatory effect of AR was first shown to be mediated through the suppression of NO production via inhibition of inducible nitric oxide synthase (iNOS) at both the expression and activity levels. These findings have been confirmed in multiple in vitro studies that were primarily conducted on a lipopolysaccharide (LPS)-induced inflammatory model of RAW264.7 cells22,29,30,31,32, an immortalized murine macrophage cell line, and on U937 cells22, a human pro-macrophage cell line. The modulatory effects of AR on cyclooxygenase-2 (Cox-2)31,33 have also been reported, but there is controversy regarding the effect of AR on Cox-2. Although both studies were carried out on the same LPS-induced RAW 267.4 cells, Zhao et al reported that AR did not affect Cox-2 expression or enzyme activity at 3–100 μmol/L31, whereas Lee et al found that 0.1 μmol/L of AR could decrease COX-2 expression and PGE2 production by 26.70%±4.61% and 32.84%±6.51%, respectively33. Other in vitro anti-inflammatory effects of AR include inhibition of LPS-induced primary murine splenocyte proliferation22, inhibition of anti-CD3/CD28 antibody-induced primary human T lymphocyte proliferation3, and polarization of M1 macrophages to M2-like macrophages2. The anti-inflammatory effect was also confirmed on silica-induced and peptidoglycan-induced inflammatory cell models25. In addition, AR was reported to have immunomodulatory effects towards type I–IV allergic inflammation34, as well as inhibiting mast cell-mediated allergic responses35.
Molecular mechanisms accounting for the anti-inflammatory effect of AR have been widely investigated in the past decade. Generally, upon sensing infection or tissue damage, transcription factors such as nuclear factor κB (NF-κB) are activated to induce the expression of genes participating in the inflammatory response (eg, iNOS and COX-2). Cytokine-mediated feed-forward loops can amplify and coordinate this inflammatory response15. The anti-inflammatory effect of AR has been attributed to its potent in vitro and in vivo modulating effects on several important cytokines, such as tumor necrosis factor-α (TNF-α)2,22,26,28,31,36,37, interleukin-6 (IL-6)2,26,28,29,31,37, interleukin-1β (IL-1β)2,29,37, and interleukin-10 (IL-10)2,28. Inhibitory effects of AR on the expression levels of other cytokines, such as interleukin-2 and interferon-γ (IFN-γ), were also found in vitro3. Multiple upstream mechanisms for the modulating effect of AR on cytokines were proposed. Both in vivo and in vitro studies showed that AR inactivated NF-kB by inhibiting p65 nuclear translocation, suppressing I-κ phosphorylation2,26,30,37, suppressing phosphorylation of mitogen-activated protein kinases (MAPKs)26,36, and inhibiting phosphorylation of phosphatidylinositide 3-kinases (PI3K) and protein kinase B (AKT)2,26. Other proposed mechanisms include suppression of the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) pathway4,29, promotion of degradation of iNOS synthase through the carboxyl terminus of Hsc70-interacting protein (CHIP)-associated proteasome32, and activation of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPKα)37. However, there are few studies on the anti-inflammatory effects of arctiin. In three studies, arctiin was reported to have similar anti-inflammatory effects to those of AR in vitro and in vivo33,38,39.
Effect of AR and arctiin against exogenous pathogens
AR, arctiin, and A lappa also demonstrated inhibitory effects on microorganism (Table 3), including viruses and bacteria, common exogenous stimuli for inflammatory responses. Studies attributed the anti-viral effects of A lappa to the major component AR. AR was reported to have strong anti-viral activities against influenza A in both in vitro and in vivo settings, and the mechanism of the anti-influenza effect of AR was related to the direct inhibitory effect on viral replication5,6,40. Furthermore, protective effects of AR against more lethal pathogens, such as human immunodeficiency virus and Japanese encephalitis virus, were also reported with in vitro and in vivo models7,41,42,43. AR demonstrated inhibitory effects on the bacteria Helicobacter pylori, but this effect was not sufficient to attenuate the gastric carcinogenesis in Mongolian gerbils44. Other anti-bacterial activities of A lappa against pathogens such as Escherichia coli and Pseudomonas aeruginosa were all demonstrated using the extract of the herb on in vitro disk diffusion models45,46,47,48. In addition, inhibitory effects of A lappa on other microorganisms, such as fungi, were also demonstrated46. However, whether these effects are attributed to AR and arctiin is still unclear.
Table 3. Summary of the studies on pharmacological effect of AR and arctiin against exogenous pathogens.
| Pathogen | Compound | Model | Dose | Findings | Ref |
|---|---|---|---|---|---|
| Japanese encephalitis virus (JEV) | AR | BALB/c mice | 10 mg/kg, ip | AR treatment provided complete protection from JEV infection, reduced virus titres and demonstrated neuron rescue and gliosis reducing effects. | 41 |
| AR | Mouse Neuro2a cells | 0.1% | AR decreased viral titre. | 41 | |
| Human immunodeficiency virus (HIV) | AR | HTLV-III B/H9-Jurkat cell system | 0.5 μmol/L | The expression and reverse transcriptase activity of HIV-1 proteins p17 and p24, was inhibited by AR. | 42 |
| AR | 3′-processing and integration assays | 100 μmol/L | AR suppressed the integration of proviral DNA into the cellular DNA genome but was inactive in the cleavage (3′-processing) and integration (strand transfer) assays. | 43 | |
| AR | CHME5 cells | 5–20 μmol/L | AR regulated the upstream PI3K enzyme to abolish the cytoprotective phenotype of HIV virus type 1 Tat-expressing CHME5 cells. | 7 | |
| Influenza A | Arctiin | BALB/c mice | 5 mg/d, po | Lethal infection was decreased, virus production was reduced, antibody response was elevated by treatment of arctiin. | 5 |
| AR | NIH mice | 10, 100 μg/kg, po | Lung consolidation due to viral infection was significantly inhibited by AR. Survival time of infected mice was prolonged by AR treatment. | 6 | |
| AR | MDCK cells | 5, 25, 50 μmol/L | IC50 of AR were 3.8 μmol/L and 2.9 μmol/L in plaque yield reduction assay. Synergistic effect of AR with oseltamivir was found. | 5 | |
| AR | Hemagglutination titer | 6.7–53.6 μmol/L | Hemagglutination titer was inhibited, indicating direct inhibitory effect against influenza virus replication of AR. | 40 | |
| Helicobacter pylori | AR | Mongolian gerbil | in diet10 μmol/L, 0.1% | AR showed inhibitory effect of H. pylori colonies at 10 μmol/L, but failed to attenuate neoplasia in vivo. | 44 |
| porcine circovirus type 2 | AR | Mice | 200 μg/kg, ip | Significant inhibition of PCV2 proliferation in the lungs, spleens and inguinal lymph nodes. | 124 |
Abbreviation: ip: intraperitoneal injection; po: oral administration.
Anti-inflammatory activities of AR and arctiin on chronic diseases
AR and arctiin have also been associated with beneficial effects on some chronic diseases, such as metabolic disorders and central nervous system dysfunctions, partially due to their anti-inflammatory activity. AR and arctiin demonstrated their effects on ameliorating metabolic disorders in various cell lines49,50,51, ob/ob mice, and streptozotoxin (SZT)-induced diabetic rats8,9,10. Neuroprotective effects of AR were demonstrated on cultured neuron cells, cerebral ischemia rats, memory deficit mice, experimental autoimmune encephalomyelitis in mice, Aβ-induced AD mice, and transgenic Alzheimer's disease mice11,12,13,52,53. Multiple mechanisms for these neuroprotective effects were proposed, including scavenging free radicals, down-regulating pro-inflammatory cytokines, regulating AMPK and PPAR-γ/ROR-γt signaling, reducing Tau hyperphosphorylation and inhibiting Aβ production11,12,13,52,53,54. In addition, although AR and A lappa demonstrated their potential anti-cancer activities on various cancer cell lines, there is still a lack of sufficient evidence for their anti-cancer activities on in vivo models21,55,56,57,58,59.
In summary, AR was reported as the most potent bioactive component of A lappa in the majority of studies, while the bioactivities of arctiin were lower than those of AR in most reports evaluating both compounds. AR demonstrated potent effects on inflammatory responses. The anti-inflammatory effect of AR may function synergistically with its anti-viral effect to manage some infectious conditions. However, inflammatory responses also have a role in the progression of several chronic diseases, and AR may serve as an auxiliary treatment for these chronic diseases, including metabolic disorders and central nervous system dysfunctions.
Pharmacokinetic properties of AR and arctiin
Despite the research attention AR has received due to its promising therapeutic potential, biopharmaceutic and pharmacokinetic investigations of AR and arctiin are rare. In this section, we will discuss the pharmacokinetic properties of arctiin and AR, including the absorption, distribution, metabolism and excretion characteristics, focusing on the biotransformation of arctiin and AR. Furthermore, comparison will be made among the pharmacokinetic profiles of AR after various routes of administration.
Pharmacokinetic properties of arctiin
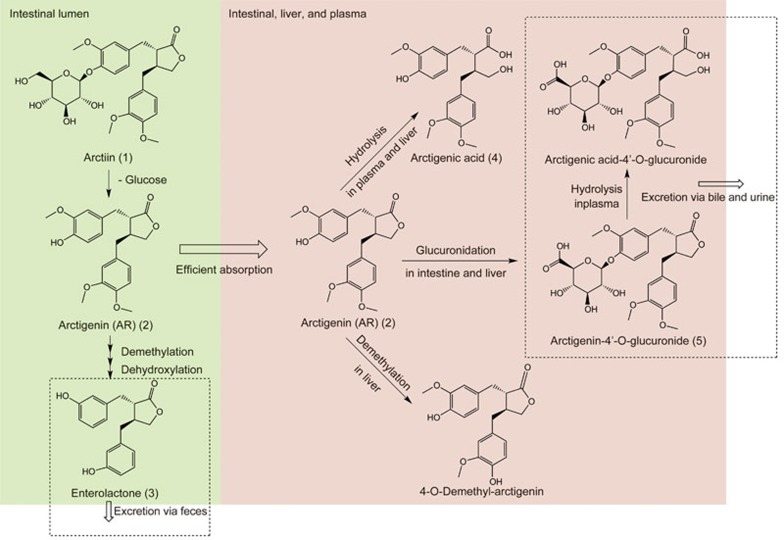
AR was regarded as the only metabolite in most in vivo pharmacokinetic studies of arctiin, due to its much higher concentration in plasma compared with that of arctiin5,60. However, in vitro incubation studies of arctiin or AR with intestinal content or feces revealed that intestinal microbiota mediated the biotransformation of arctiin to AR in the intestine61,62, followed by demethylation and a series of other biotransformation processes leading to the formation of enterolactone (3)63,64,65. Wang et al reported three metabolites in rat urine and feces after oral administration of 30 mg/kg arctiin (1), including AR (2), enterolactone (3) and (2R,3R)-2-(3′-hydroxybenzyl)-3-(3″,4″-dimethoxybenzyl)-butyrolactone, an intermediate metabolite66 (Figure 2).
Figure 2.
Summary of major in vivo metabolic pathways of AR and arctiin.
Pharmacokinetic properties of AR
The pharmacokinetic properties of AR are summarized in Table 4. After oral ingestion, efficient absorption of AR was demonstrated in a Caco-2 cell monolayer transport study and a rat in situ intestinal perfusion model67,68. The duodenum was found to be the best absorption segment of AR among all the intestinal segments67. Although the Caco-2 cell monolayer model showed that no significantly active efflux was involved during the absorption of AR, with an efflux ratio of 1.1768, an in situ intestinal perfusion model showed that absorption of AR in the duodenum was significantly improved by co-treatment with the P-glycoprotein (P-gp) inhibitor verapamil67, suggesting AR is a potential P-gp substrate.
Table 4. Summary of absorption, distribution, metabolism and elimination of AR.
| Model | Dose | Findings | Ref |
|---|---|---|---|
| Absorption | |||
| Caco-2 cell monolayer | 50 μg/mL | AR efficiently passed through the cell monolayer with Papp of (1.76±0.48)×10−5 (apical to basolateral) and (1.50±0.61)×10−5 (basolateral to apical). | 68 |
| In situ SD rat intestinal perfusion model | 25 μg/mL | Efficient absorption of AR was observed with extensive intestinal first-pass metabolism demonstrated. | 68 |
| In situ intestinal perfusion on normal and diabetic SD rats | 5, 10, 20 μg/mL | AR belongs to easily absorbed agents. Duodenum was the best absorption segment of AR. The Peff and Ka of AR were increased by 60% and 52% in duodenum with co-treatment of verapamil. The absorption of AR was promoted in diabetic rats. | 67 |
| Distribution | |||
| In vitro plasma incubation | 0.0672, 0.269, 1.075 μmol/L | R exhibited a strong binding capacity (99.8%–100%) with plasma, including human, beagle dog, and rat. | 69 |
| Wistar rat | 0.806 μmol/kg, ih | AR concentration in the intestine was the highest, followed by heart, liver, pancreas, and kidney. No accumulation of AR in tissues after 6 h. | 69 |
| SD rat | 30, 50, 70 mg/kg, po | AR was rapidly distributed into organs, and Cmax in tissues was observed at 30 min. The content of AR in spleen was the highest. | 70 |
| Metabolism and elimination | |||
| Human fecal inoculum incubation | 0.2 to 0.35 mmol/L | Three metabolites of AR were identified under anaerobic condition: enterolactone (3), 3′-demethyl-4′-dehydroxyarctigenin and 3′-demethylarctigenin. | 65 |
| Eubacterium ARC-2 incubation | 0.6 mmol/L | After 24-h incubation, AR was transformed to 4′, 4′-dihydroxylenterolatone through 3 types of demethylation products under anaerobic condition. | 61,71 |
| Rat intestinal content solution | After 4-h incubation, AR was stable in rat small and large intestinal content solution, while arctigenic acid was converted back to arctigenin in rat large intestinal content. All three glucuronides, were hydrolysed back to corresponding parent compounds. | 72 | |
| In situ SD rat intestinal perfusion model | 25 μg/mL | Extensive intestinal first-pass metabolism of AR to arctigenic acid (4) and arctigenin-4′-O-glucuronide (5) was identified. | 68 |
| Human recombinant paraoxonase 1 | 0.27 to 134.4 μmol/L | Paraoxonase 1 was confirmed to be the enzyme responsible for AR hydrolysis. | 73 |
| V79 Chinese hamster cells with rat Cyp2b1 | 1 mmol/L | AR was converted to 3′-demethyl-arctigenin in cells expressing rat Cyp2b1. | 125 |
| Rat liver/intestine microsome | 0.269 to 67.2 μmol/L | Extensive glucuronidation of AR was observed in both liver and intestine microsome. No further glucuronidation or demethylation of arctigenic acid (4) in liver and intestine microsome. | 72 |
| Rat liver cytosol incubation | 10 nmol/L | 3′-Demethyl-arctigenin was converted back to AR. | 126 |
| Human liver/intestine microsomes | 100 μmol/L | AR was metabolized to 4'-O-glucuronide (5) in human liver and intestinal microsome mainly via UGT1A9, UGT2B7 and UGT2B17. | 74 |
| Human, monkey, dog, and rat liver microsome | 100 μmol/L | Around 62%, 3.7%, 25.9% and 15.7% of AR remained after incubated in human, monkey, dog, and rat liver microsome for 90 min. | 69 |
| SD rats | 3 mg/kg, po | Arctigenic acid (4) and arctigenin-4′-O-glucuronide (5) was identified as major metabolites in rat plasma after oral administration of AR. 4-O-demethylarctigenin was also identified in vivo. | 73 |
| SD rats | 0.48 to 2.4 mg/kg, iv; 2.4 to 12 mg/kg, po | Rapid formation of arctigenic acid (4) and arctigenin-4′-O-glucuronide (5) with quick elimination of both parent and metabolites were observed after both intravenous and oral administrations. No quantifiable AR was identified after oral administration due to extensive first-pass metabolism | 72 |
| SD rats | 0.96 mg/kg, iv | Arctigenin-4′-O-glucuronide (5), arctigenic acid-4′-O-glucuronide, 4-O-demethyl-arctigenin -4,4′-O-di-glucuronide, and trace amount of arctigenic acid (4) were found in bile at 0–15 min. | 72 |
| Wistar rat | 0.806 μmol/kg ih | Within 72 h after drug-delivery, the urine accumulative excretion ratio was 1.93% and the excretory amount of AR in faeces and bile were 0.248% and 0.182%, respectively. | 69 |
Abbreviation: iv: intravenous administration; ih: hypodermic injection; po: oral administration.
After entering the systemic circulation, AR exhibited a strong binding capacity (99.8%–100%) to plasma. The high plasma binding was found in various species, including human, beagle dog, and rat69. The tissue distribution of AR was only investigated after hypodermic or oral administration to rats. After hypodermic injection of 0.806 μmol/kg AR to rats, the AR concentrations were reduced at 6 h to approximately 1/10 of their peak values at 0.25 h in most organs, indicating no accumulation in tissues, and the peak concentration of AR was in the intestine, followed by the heart, liver, pancreas, and kidney69. After oral administration of 70 mg/kg AR to rats, the tissue concentration of AR peaked at 30 min and was quickly eliminated within 4 h, and the concentration of AR was highest in the spleen, followed by the liver and the other organs70.
AR was eliminated via extensive metabolism to various metabolites. The dominant metabolic pathways of AR are summarized in Figure 2. In vitro incubation of AR with the intestinal microbiota demonstrated that similar to acrtiin, AR can be biotransformed into a series of demethylation and dehydroxylation products, such as 3'-demethylarctigenin, 3'-demethyl-4'-dehydroxyarctigenin, and eventually to the enterolactone (3) anaerobically within 24 h61,65,71. In rats, extensive first-pass metabolism of AR occurred in both the intestine and liver, with the formation of two major in vivo metabolites, namely, arctigenic acid (4) and arctigenin-4'-O-glucuronide (5)68,72,73,74. Further in vitro and clinical studies confirmed that similar biotransformation also occurred in humans. The hydrolysis of AR was mediated by human paraoxonase 1 in plasma73, and glucuronidation of AR was mediated by UGT1A9, UGT2B7 and UGT2B17 in the liver and intestine74. A phase I clinical trial of the herbal product GBS-01 on pancreatic cancer patients demonstrated that after oral administration of GBS-01 at a dose of 12 g AR per person, the area under the plasma concentration versus time curve (AUC) of arctigenin-glucuronide was almost 1000 times higher than that of AR75. Notably, the extent of metabolism of AR might be different between species. As reported by Li et al, approximately 62%, 3.7%, 25.9% and 15.7% of the AR remained after incubation in human, monkey, dog, and rat liver microsomes for 90 min69. Other minor in vivo metabolites found in SD rats include 4-O-demethylarctigenin, arctigenin 4-O'-sulfate, arctigenic acid-4'-O-glucuronide, and 4-O-demethyl-arctigenin-4,4'-O-di-glucuronide72,73. Following rapid formation, fast elimination of the two major metabolites was observed after both intravenous and oral administration of AR to rats. Several glucuronidation products of AR, including arctigenin-4'-O-glucuronide (5), were excreted via bile, with potential enterohepatic circulation suggested72. These complex metabolic pathways of AR were described and verified by an integrated semi-mechanistic pharmacokinetic model of rats72 and warrant further verification in human trials.
Due to the extensive first-pass metabolism of AR, it is likely that most AR, either as single compound or as active component in herbal preparations, would be quickly metabolized after oral administration. As shown in Table 5, after oral administration, the plasma concentrations of AR were very low and even undetectable in various animal models, suggesting poor oral bioavailability. The pharmacokinetic profile of AR in humans after oral administration was investigated in a phase I clinical trial of the herbal product GBS-01. After oral administration of GBS-01 at a dose of 12 g AR per person, the peak concentration of AR in the plasma of the pancreatic cancer patients was 66.56±26.81 ng/mL, and the AUC was 487.97±368.86 ng*h/mL75. Given the low molecular weight of AR and its high permeability demonstrated in the absorption models, the poor oral bioavailability of AR should be mainly due to its extensive first-pass metabolism rather than limitations of membrane permeability. Thus, delivering AR through alternative administration routes might be plausible to bypass the first-pass metabolism and improve its bioactivities. Alternative routes for administration of AR, including hypodermic injection and sublingual administration, were tested on experimental animals. The results demonstrated substantially improved AUC and bioavailability of AR after hypodermic or sublingual administration compared with that from the oral administration (Table 5)69. These results suggested that optimization of the administration routes for AR may potentially improve its therapeutic efficacy by increasing the systemic and target organ exposure. Further pharmacokinetic/pharmacodynamic studies of AR after different routes of administration are warranted.
Table 5. Pharmacokinetic parameters of AR after different routes of administration in rat, dog, and human.
| Species | Route | Dose (μmol/kg) | Pharmacokinetic parameters |
Ref | ||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μmol/L) | Tmax (min) | T1/2 (min) | AUC (min* μmol/L) | Bioavailability | ||||
| Beagle dogs | iv | 0.403 | N/A | N/A | 96±24.84 | 15.64±2.08a | N/A | 69 |
| sl | 0.20 (2.687 μmol/dog) | 0.04±0.01 | 60±16.44 | 61.8±19.26 | 6.56±1.16a | N/A | ||
| 0.403 (5.374 μmol/dog) | 0.07±0.24 | 112±53.64 | 70.8±24.36 | 12.01±2.26a | 72.5% | |||
| 0.81 (10.748 μmol/dog) | 0.10±0.02 | 130.2±70.20 | 84±34.98 | 23.22±3.06a | N/A | |||
| ih | 0.134 | 0.03±0.01 | 60±0 | 94.8±31.74 | 4.76±0.75a | N/A | ||
| 0.403 | 0.11±0.03 | 70.2±15.48 | 74.4±12.18 | 16.93±5.42a | 108% | |||
| 1.209 | 0.25±0.04 | 75±16.44 | 117±25.44 | 35.63±4.21a | N/A | |||
| Wistar rats | iv | 2.687 | N/A | N/A | 217.8±74.04 | 66.26±18.7a | N/A | 69 |
| ih | 2.687 | 1.26±0.30 | 15±0 | 116.4±21.72 | 77.87±24.67a | 116% | ||
| po | 2.687 | Concentrations were lower than the lowest limit of quantitation at most time points. | ||||||
| Wistar rats | iv | 0.8 | 0.87±0.18 | N/A | 40.8±10.0 | 13.1±3.6b | N/A | 127 |
| SD rats | iv | 0.32 | N/A | N/A | 9.35±2.15 | 9.32±2.80a | N/A | 72 |
| 0.64 | N/A | N/A | 13.4±2.2 | 19.4±2.9a | N/A | |||
| 1.61 | N/A | N/A | 13.0±1.8 | 54.1±8.1a | N/A | |||
| po | 1.61, 3.22, 8.16 | No quantifiable AR was determined. | ||||||
| SD rats | iv | 27 | N/A | N/A | 627.6±214.8 | 199.8±49.51a | N/A | 67 |
| po | 538 | 0.34±0.04 | 4.32±5.46 | 414.6±107.4 | 116.18±38.69a | N/A | ||
| Diabetic rats | iv | 27 | N/A | N/A | 469.2±227.4 | 151.05±46.84a | N/A | 67 |
| po | 538 | 1.22±0.45 | 3.42±0.9 | 322.8±106.2 | 197.99±48.28a | N/A | ||
| Human with pancreatic cancer | po | 134 (3 g/person) 269 (6 g/person) 538 (12 g/person) | 0.05±0.01 0.07±0.02 0.18±0.07 | 60±30 30±0 52.2±37.2 | 430.8 183.6 ±176.4 340.8 ±380.4 | 40.1±33.3b 23.0±9.2b 78.7±59.5b | N/A N/A N/A | 75 |
Abbreviation: iv: intravenous administration; sl: sublingual administration; ih: hypodermic injection; po: oral administration. N/A: not applicable.
a: AUC0-∞;
b: AUC0-t.
Clinical usages
As described previously, AR and arctiin served as marker compounds in the quality control of numerous proprietary Chinese medicines. Most of these products are for treatment of common cold, flu and related symptoms, such as various dosage forms of Yinqiaojiedu decoction76,77,78, Lingyang ganmao decoction79,80 and Fengreganmao granules81,82. Despite its popularity, Fructus Arctii is not commonly used alone. Therefore, reports on the clinical trials of AR, arctiin or A lappa alone are rather limited. As summarized in Table 6, only four clinical trials were identified for evaluation of the therapeutic effects of AR, arctiin or A lappa, with diverse indications. Despite their high Jadad scores (2 out of 3 received full score of 5)83, three randomized controlled trials demonstrating the efficacy of arctiin (0.5–1 g, t.i.d.) or Fructus Arctii (20 g, t.i.d.) against diabetic nephropathy were actually reported by the same group, with a similar study design and dose regiments84,85,86. A recent phase I clinical study co-sponsored by the Japanese National Institute for Cancer Research and Kracie Pharmaceutical Co, Ltd confirmed the safety of an oral product containing a high content of AR (GBS-01) (dose equal to 3 to 12 g daily)75. Moreover, a study protocol for evaluating A lappa-containing moisturizing cream for dry skin and itch relief in a randomized, double-blind, placebo-controlled trial was published87. Similarly, A lappa was also demonstrated to effectively treat acne vulgaris in a recent uncontrolled observational interventional study in India88. The anti-inflammatory effects of AR, arctiin or A lappa have not yet been confirmed in the clinic. Further randomized controlled trials are needed to evaluate the therapeutic efficacy of AR and arctiin.
Table 6. Summary of clinical trials on AR- or arctiin-containing products.
| Disease | Drug | Study design | Jadad score* | Patient numbers | Oral dose | Duration | Clinical efficacy | Ref |
|---|---|---|---|---|---|---|---|---|
| Diabetic nephropathy | Fructus Arctii powder | Randomized controlled | 2 | Placebo: 61; Treatment: 60 | 20 g, t.i.d. | 8 weeks | Urinary albumin excretion rate (UAER) and 24 h quantitative examination of urinary protein (UPQ) were improved by the treatment, which was significantly better than placebo (P<0.001). | 86 |
| Tangjiang-shenkang Granule (125 mg arctiin/g) | Randomized double blind controlled | 5 | Placebo: 60; Low dose (L): 64; High dose (H): 62 | L: 4 g, t.i.d.; H: 8 g, b.i.d. | 8 weeks | The treatment efficacy of L and H dose was better than those of placebo (P<0.01). UAER and UPQ were improved by the treatment (P<0.05). | 85 | |
| Arctiin Granule | Randomized double blind controlled | 5 | Placebo: 102; Treatment: 307 | 500 mg/dose, t.i.d. | 8 weeks | The treatment efficacy of arctiin granule was better than those of placebo (P<0.01). UAER and UPQ were improved by the treatment (P<0.05). | 84 | |
| Advanced pancreatic cancer refractory to gemcitabine | GBS-01 | Uncontrolled Phase I clinical trial | 0 | 15 | 3–12 g AR qd | 4 weeks | No dose limited toxicities observed. Response was found in 1/15 patient while another 4 showed stable disease. | 75 |
Abbreviation: b.i.d.: twice a day; t.i.d.: three times a day; qd: once daily.
Conclusions
Inflammatory responses are an important part of various acute and chronic disease conditions. AR, as the most potent bioactive component of A lappa with anti-inflammatory activities, is a promising therapeutic compound against acute inflammation as well as several chronic diseases. However, pharmacokinetic investigations suggested that the extensive first-pass metabolism of AR would hinder its in vivo and clinical efficacy after oral administration. To optimize the in vivo and clinical efficacy of AR, alternative administration routes other than oral administration are suggested. AR could be delivered through sublingual or buccal routes that allow rapid onset of the treatment of acute inflammation and influenza; transdermal routes for the treatment of skin conditions; and the intranasal route for targeting central nervous system dysfunctions. In addition, considering the extensive first-pass metabolism of AR and the higher plasma concentrations of metabolites compared with parent compound observed, the potential pharmacological effects of the metabolites of AR should be studied. Further reports with simultaneous monitoring of pharmacokinetics and pharmacological properties are essential for a better understanding of the effects of AR.
Acknowledgments
This work is supported by Direct Grant 3800005 from the Chinese University of Hong Kong and research fund 7010213 from the Health Authority of Hong Kong, China.
References
- China Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, Part 1. People's Medical Publishing House 2010. Beijing, China.
- Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, et al. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol 2013; 708: 21–9. [DOI] [PubMed] [Google Scholar]
- Tsai WJ, Chang CT, Wang GJ, Lee TH, Chang SF, Lu SC, et al. Arctigenin from Arctium lappa inhibits interleukin-2 and interferon gene expression in primary human T lymphocytes. Chin Med 2011; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou XJ, Qi SM, Dai WX, Luo L, Yin ZM. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int Immunopharmacol 2011; 11: 1095–102. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Narutaki K, Nagaoka Y, Hayashi T, Uesato S. Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol Pharm Bull 2010; 33: 1199–205. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu N, Huang B, Wang Y, Hu Y, Zhu Y. Effect of anti-influenza virus of arctigenin in vivo. Zhongyaocai 2005; 28: 1012–4. [PubMed] [Google Scholar]
- Kim Y, Hollenbaugh JA, Kim DH, Kim B. Novel PI3K/Akt inhibitors screened by the cytoprotective function of humanimmunodeficiency virus type 1 Tat. PLoS One 2011; 6: e21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Rhee SJ, Choi SW, Choi Y. Effects of forsythia fruit extracts and lignan on lipid metabolism. Biofactors 2004; 22: 161–3. [DOI] [PubMed] [Google Scholar]
- Li X, Liu C, Wei J. The effect of amioguanidine and Arctium lappa on renal tissue nonenzymatic glycation and apoptosis in diabetic rats. Linchuang Heli Yongyao Zazhi 2011; 4: 19–20. [Google Scholar]
- Lu LC, Zhou W, Li ZH, Yu CP, Li CW, Luo MH, et al. Effects of arctiin on streptozotocin-induced diabetic retinopathy in Sprague-Dawley rats. Planta Med 2012; 78: 1317–23. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both beta-amyloid production and clearance. J Neurosci 2013; 33: 13138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Jiang WL, Zhu J, Feng Zhang Y. Arctigenin protects focal cerebral ischemia-reperfusion rats through inhibiting neuroinflammation. Biol Pharm Bull 2012; 35: 2004–9. [DOI] [PubMed] [Google Scholar]
- Jang YP, Kim SR, Choi YH, Kim J, Kim SG, Markelonis GJ, et al. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J Neurosci Res 2002; 68: 233–40. [DOI] [PubMed] [Google Scholar]
- Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 2004; 18: 385–405. [DOI] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013; 339: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Guo M, Yu X, Zhao L, Duan W. Determination of total lignanoids and arctinin in different parts of Arctium lappa L. Zhongguo Xiandai Yingyong Yaoxue 2012; 29: 506–8. [Google Scholar]
- Shao J, Ni JN, Zhao L, Yu XH, Duan WD. Comparative study of content of arctiin in burdock fruit of seven different place. Gansu Zhongyi Xueyuan Xuebao 2009; 26: 41–3. [Google Scholar]
- Yuan Y, Dou D, Kang T. Evaluating the quality of Arctiin Lappa L with different origins. Shijie Kexue Jishu-Zhongyiyao Xiandaihua 2008; 10: 75–7. [Google Scholar]
- Shoeb M, Celik S, Jaspars M, Kumarasamy Y, Macmanus SM, Nahar L, et al. Isolation, structure elucidation and bioactivity of schischkiniin, a unique indole alkaloid from the seeds of Centaurea schischkinii. Tetrahedron 2005; 61: 9001–6. [Google Scholar]
- Paska C, Innocenti G, Kunvari M, Laszlo M, Szilagyi A. Lignan production by Ipomoea cairica callus cultures. Phytochemistry 1999; 52: 879–83. [Google Scholar]
- Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, et al. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 2006; 66: 1751–7. [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim AR, Yoo ES, Baik KU, Park MH. Immunomodulatory effect of arctigenin, a lignan compound, on tumour necrosis factor-alpha and nitric oxide production, and lymphocyte proliferation. J Pharm Pharmacol 1999; 51: 1267–73. [DOI] [PubMed] [Google Scholar]
- Sohn EH, Jang SA, Joo H, Park S, Kang SC, Lee CH, et al. Anti-allergic and anti-inflammatory effects of butanol extract from Arctium Lappa L. Clin Mol Allergy 2011; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Li G, Chai O, Song C. Inhibitory effect of Arctium lappa Linne on compound 48/80-induced mast cell activation and vascular permeability. Korean J Phys Anthropol 2004; 17: 55–66. [Google Scholar]
- Kang HS, Lee JY, Kim CJ. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol 2008; 116: 305–12. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang Y, Dou Y, Ye J, Bian D, Wei Z, et al. Arctigenin but not arctiin acts as the major effective constituent of Arctium lappa L fruit for attenuating colonic inflammatory response induced by dextran sulfate sodium in mice. Int Immunopharmacol 2014; 23: 505–15. [DOI] [PubMed] [Google Scholar]
- Zhang WZ, Jiang ZK, He BX, Liu XB. Arctigenin protects against Lipopolysaccharide-induced pulmonary oxidative stress and inflammation in a mouse model via suppression of MAPK, HO-1, and iNOS signaling. Inflammation 2015; 38: 1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Li N, Xia Y, Gao Z, Zou SF, Kong L, et al. Arctigenin treatment protects against brain damage through an anti-inflammatory and anti-apoptotic mechanism after needle insertion. Front Pharmacol 2016; 7: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Zhu F, Zhao Z, Liu C, Luo L, Yin Z. Arctigenin enhances chemosensitivity of cancer cells to cisplatin through inhibition of the STAT3 signaling pathway. J Cell Biochem 2011; 112: 2837–49. [DOI] [PubMed] [Google Scholar]
- Cho MK, Park JW, Jang YP, Kim YC, Kim SG. Potent inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by dibenzylbutyrolactone lignans through inhibition of I-kappaBalpha phosphorylation and of p65 nuclear translocation in macrophages. Int Immunopharmacol 2002; 2: 105–16. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang L, Liu K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L, through inhibition on iNOS pathway. J Ethnopharmacol 2009; 122: 457–62. [DOI] [PubMed] [Google Scholar]
- Yao X, Li G, Lü C, Xu H, Yin Z. Arctigenin promotes degradation of inducible nitric oxide synthase through CHIP-associated proteasome pathway and suppresses its enzyme activity. Int Immunopharmacol 2012; 14: 138–44. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cho BJ, Park TW, Park BE, Kim SJ, Sim SS, et al. Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-b and mitogen-activated protein kinase in RAW264.7 cells. Biol Pharm Bull 2010; 33: 1847–53. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim CJ. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I-IV allergic inflammation and pro-inflammatory enzymes. Arch Pharm Res 2010; 33: 947–57. [DOI] [PubMed] [Google Scholar]
- Hou Y, Nie Y, Cheng B, Tao J, Ma X, Jiang M, et al. Qingfei Xiaoyan Wan, a traditional Chinese medicine formula, ameliorates Pseudomonas aeruginosa-induced acute lung inflammation by regulation of PI3K/AKT and Ras/MAPK pathways. Acta Pharm Sin B 2016; 6: 212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-alpha inhibition. Int Immunopharmacol 2004; 4: 1419–29. [DOI] [PubMed] [Google Scholar]
- Shi X, Sun H, Zhou D, Xi H, Shan L. Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammation 2015; 38: 623–31. [DOI] [PubMed] [Google Scholar]
- Park SY, Hong SS, Han XH, Hwang JS, Lee D, Ro JS, et al. Lignans from Arctium lappa and their inhibition of LPS-induced nitric oxide production. Chem Pharm Bull 2007; 55: 150–2. [DOI] [PubMed] [Google Scholar]
- Wu JG, Wu JZ, Sun LN, Han T, Du J, Ye Q, et al. Ameliorative effects of arctiin from Arctium lappa on experimental glomerulonephritis in rats. Phytomedicine 2009; 16: 1033–41. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dong X, Kang T, Zhao C, Huang Z, Zhang X. Activity of in vitro anti-influenza virus of arctigenin. Zhongcaoyao 2002; 33: 724–6. [Google Scholar]
- Swarup V, Ghosh J, Mishra MK, Basu A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J Antimicrob Chemother 2008; 61: 679–88. [DOI] [PubMed] [Google Scholar]
- Schroder HC, Merz H, Steffen R, Muller WE, Sarin PS, Trumm S, et al. Differential in vitro anti-HIV activity of natural lignans. Z Naturforsch C 1990; 45: 1215–21. [DOI] [PubMed] [Google Scholar]
- Eich E, Pertz H, Kaloga M, Schulz J, Fesen MR, Mazumder A, et al. (-)-Arctigenin as a lead structure for inhibitors of human immunodeficiency virus type-1 integrase. J Med Chem 1996; 39: 86–95. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Tsukamoto T, Mizoshita T, Nishibe S, Deyama T, Takenaka Y, et al. Inhibitory effect of nordihydroguaiaretic acid, a plant lignan, on Helicobacter pylori-associated gastric carcinogenesis in Mongolian gerbils. Cancer Sci 2007; 98: 1689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JV, Bergamo DCB, Pereira JO. Franca SdC, Pietro RCLR, Silva-Sousa YTC. Antimicrobial activity of Arctium lappa constituents against microorganisms commonly found in endodontic infections. Braz Dentl J 2005; 16: 192–6. [DOI] [PubMed] [Google Scholar]
- Elsayed A. Aboutabla, El-Tantawyb ME, Shamsb MM. Chemical composition and antimicrobial activity of volatile constituents from the roots, leaves, and seeds of Arctium lappa L (Asteraceae) grown in Egypt. Egypt Pharm J 2013; 12: 173–6. [Google Scholar]
- Gentil M, Pereira JV, Sousa YTCS, Pietro R, Neto MDS, Vansan LP, et al. In vitro evaluation of the antibacterial activity of Arctium lappa as a phytotherapeutic agent used in intracanal dressings. Phytother Res 2006; 20: 184–6. [DOI] [PubMed] [Google Scholar]
- He J, Zhao Y, Sun X, Wu Q, Pan Y. Antibacterial effects of burdock (Arctium lappa L) concentrate on Vibrio parahemolyticus. Nat Prod Res Dev 2012; 24: 381–4. [Google Scholar]
- Gu Y, Sun XX, Ye JM, He L, Yan SS, Zhang HH, et al. Arctigenin alleviates ER stress via activating AMPK. Acta Pharmacol Sin 2012; 33: 941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XL, Li Q, Pang LW, Huang GQ, Huang JC, Shi M, et al. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-gamma/LXR-alpha signaling pathway. Biochem Bioph Res Co 2013; 441: 321–6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, et al. Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay. PLoS One 2013; 8: e63354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Z, Zhang K, Xue Z, Li Y, Zhang Z, et al. Arctigenin suppress Th17 cells and ameliorates experimental autoimmune encephalomyelitis through AMPK and PPAR-gamma/ROR-gammat signaling. Mol Neurobiol 2016; 53: 5356–66. [DOI] [PubMed] [Google Scholar]
- Qi Y, Dou DQ, Jiang H, Zhang BB, Qin WY, Kang K, et al. Arctigenin attenuates learning and memory deficits through PI3k/Akt/GSK-3beta pathway reducing Tau hyperphosphorylation in abeta-induced AD mice. Planta Med 2017; 83: 51–6. [DOI] [PubMed] [Google Scholar]
- Song J, Li N, Xia Y, Gao Z, Zou SF, Yan YH, et al. Arctigenin confers neuroprotection against mechanical trauma injury in human neuroblastoma SH-SY5Y cells by regulating miRNA-16 and miRNA-199a expression to alleviate inflammation. J Mol Neurosci 2016; 60: 115–29. [DOI] [PubMed] [Google Scholar]
- Takasaki M, Konoshima T, Komatsu K, Tokuda H, Nishino H. Anti-tumor-promoting activity of lignans from the aerial part of Saussurea medusa. Cancer Lett 2000; 158: 53–9. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Yokohira M, Takeuchi H, Saoo K, Yamakawa K, Matsuda Y, et al. Lack of significant modifying effect of arctiin on prostate carcinogenesis in probasin/SV40 T antigen transgenic rats. Cancer Lett 2005; 222: 145–51. [DOI] [PubMed] [Google Scholar]
- Hasumura M, Ueda M. Onose J-i, Imai T, Hirose M. Lack of a significant effect of arctiin on development of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in ovariectomized Sprague-Dawley rats. Nutr Cancer 2007; 57: 201–8. [DOI] [PubMed] [Google Scholar]
- Kato T, Hirose M, Takahashi S, Hasegawa R, Kohno T, Nishibe S, et al. Effects of the lignan, arctiin, on 17-beta ethinyl estradiol promotion of preneoplastic liver cell foci development in rats. Anticancer Res 1998; 18: 1053–7. [PubMed] [Google Scholar]
- Hirose M, Yamaguchi T, Lin C, Kimoto N, Futakuchi M, Kono T, et al. Effects of arctiin on PhIP-induced mammary, colon and pancreatic carcinogenesis in female Sprague-Dawley rats and MeIQx-induced hepatocarcinogenesis in male F344 rats. Cancer Lett 2000; 155: 79–88. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cai S, Xu X, Fu S. Dyuamical studies on metabolic chemistry of lignans from seeds of Arctium lappa. Zhongguo Zhongyao Zazhi 2005; 30: 1287–9. [PubMed] [Google Scholar]
- Zhao YF, Song FR, Zhao LP, Liu SY. Studies on the biotransformation of arctigenin using electrospray ionization mass spectrometry. Acta Chim Sinica 2009; 67: 1123–6. [Google Scholar]
- Jin JS, Hattori M. Human intestinal bacterium, strain END-2 is responsible for demethylation as well as lactonization during plant lignan metabolism. Biol Pharm Bull 2010; 33: 1443–7. [DOI] [PubMed] [Google Scholar]
- Xie L, Ahn E, Akao T, Abdel-Hafez AA, Nakamura N, Hattori M. Transformation of arctiin to estrogenic and antiestrogenic substances by human intestinal bacteria. Chem Pharm Bull 2003; 51: 378–84. [DOI] [PubMed] [Google Scholar]
- Nose M, Fujimoto T, Takeda T, Nishibe S, Ogihara Y. Structural transformation of lignan compounds in rat gastrointestinal-tract. Planta Med 1992; 58: 520–3. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wahala K, Deyama T, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem 2001; 49: 3178–86. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan Q, Han XY, Wang J, Tan RQ, He F, et al. Simultaneous determination of arctiin and its metabolites in rat urine and feces by HPLC. Fitoterapia 2013; 86: 6–12. [DOI] [PubMed] [Google Scholar]
- Zeng XY, Dong S, He NN, Jiang CJ, Dai Y, Xia YF. Comparative pharmacokinetics of arctigenin in normal and type 2 diabetic rats after oral and intravenous administration. Fitoterapia 2015; 105: 119–26. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhang Y, Wo S, Zuo Z. Extensive intestinal first-pass metabolism of arctigenin: evidenced by simultaneous monitoring of both parent drug and its major metabolites. J Pharm Biomed Anal 2014; 91: 60–7. [DOI] [PubMed] [Google Scholar]
- Li J, Li X, Ren YS, Lv YY, Zhang JS, Xu XL, et al. Elucidation of arctigenin pharmacokinetics and tissue distribution after intravenous, oral, hypodermic and sublingual administration in rats and beagle dogs: integration of in vitro and in vivo findings. Front Pharmacol 2017; 8: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Dou DQ, Hou Q, Sun Y, Kang TG. Pharmacokinetic study of arctigenin in rat plasma and organ tissue by RP-HPLC method. Nat Prod Res 2013; 27: 903–6. [DOI] [PubMed] [Google Scholar]
- Jin J, Zhao Y, Nakamura N, Akao T, Kakiuchi N, Hattori M. Isolation and characterization of a human intestinal bacterium, Eubacterium sp. ARC-2, capable of demethylating arctigenin, in the essential metabolic process to enterolactone. Biol Pharm Bull 2007; 30: 904–11. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhang Y, Wo S, Zuo Z. Elucidation of arctigenin pharmacokinetics after intravenous and oral administrations in rats: integration of in vitro and in vivo findings via semi-mechanistic pharmacokinetic modeling. AAPS J 2014; 16: 1321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhang Y, Wo S, Zuo Z. Hydrolysis is the dominating in vivo metabolism pathway for arctigenin: identification of novel metabolites of arctigenin by LC/MS/MS after oral administration in rats. Planta Med 2013; 79: 471–9. [DOI] [PubMed] [Google Scholar]
- Xin H, Xia YL, Hou J, Wang P, He W, Yang L, et al. Identification and characterization of human UDP-glucuronosyltransferases responsible for the in-vitro glucuronidation of arctigenin. J Pharm Pharmacol 2015; 67: 1673–81. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sato A, Mochizuki N, Toyosaki K, Miyoshi C, Fujioka R, et al. Phase I trial of GBS-01 for advanced pancreatic cancer refractory to gemcitabine. Cancer Sci 2016; 107: 1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang N. Deterimination of the content of arctiin in yinqiaojiedu granules by HPLC. Shanxi Zhongyi Zazhi 2012; 33: 229–30. [Google Scholar]
- Sun G, Yanling S, Li Y, Liang J. Quantitative determination of Yinqiao Jiedu pill reference fingerprint criteria by 3 wavelength quantified fingerprints coupled with 4 compounds analysis. Zhongnan Yaoxue 2013; 11: 366–71. [Google Scholar]
- Ouyang J, Kang L, Hu W. Determination of forsythin and arctiin in vitamin C Yinqiao tablets by HPLC. Zhongguo Yaoshi 2008; 22: 692–4. [Google Scholar]
- Yang Q, Zhang J, Li J. Determination of forsythin and arctiin in lingyangganmao tablets by HPLC. Zhongchengyao 2007; 29: 383–5. [Google Scholar]
- Li D. Simultaneous detection of forsythin and arctiin in lingyangganmao tables by HPLC. Shoudu Yiyao 2008; 1: 51–2. [Google Scholar]
- Zhou X, Jin F, Li T, Sha M, Meng X, Cao A. Determination of arctiin in fengre ganmao granule s by HPLC. Yaoxue Jinbu 2004; 28: 327–9. [Google Scholar]
- Shi C, Tu L, Cao Y. Determination of arctiin in fengreganmao granules by HPLC. Yunnan Daxue Xuebao 2009; 31: 456–8. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Ma S, Liu D, Niu R, Liu R, Ji Q, Zhan J, et al. Double-blind, randomized, placebo-controlled multi-center phase III clinical trial of Arctiin granule in the treatment of diabetic nephropathy. Zhongguo Linchuang Yaolixue Zazhi 2011; 27: 15–8. [Google Scholar]
- Ma S, Liu D, Niu R, Liu R, Ji Q, Zhan J, et al. Tangjiangshenkang granule in treatment of diabetic nephropathy:a doubleblind, randomized, placebo-controlled multicentre clinical trial. Zhongguo Xinyao yu Linchuang Zazhi 2011; 30: 16–9. [Google Scholar]
- Zhang L, Li P, Zhang X, Wu J, Bao M. Clinical observation on treatment of diabetic nephropathy with fructus arctii powders. Sichuan Yixue 2011; 32: 656–8. [Google Scholar]
- Lee DH, Seo ES, Hong JT, Lee GT, You YK, Lee KK, et al. The efficacy and safety of a proposed herbal moisturising cream for dry skin and itch relief: a randomised, double-blind, placebo-controlled trial-study protocol. BMC Complement Altern Med 2013; 13: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglani A, Manchanda R. Observational study of Arctium lappa in the treatment of acne vulgaris. Homeopathy 2014; 103: 203–7. [DOI] [PubMed] [Google Scholar]
- Dall'acqua S, Tome F, Vitalini S, Agradi E, Innocenti G. In vitro estrogenic activity of Asplenium trichomanes L extracts and isolated compounds. J Ethnopharmacol 2009; 122: 424–9. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen K, Schliemann W, Strack D. Isolation and identification of arctiin and arctigenin in leaves of burdock (Arctium lappa L) by polyamide column chromatography in combination with HPLC-ESI/MS. Phytochem Anal 2005; 16: 86–9. [DOI] [PubMed] [Google Scholar]
- Predes FS, Ruiz ALTG, Carvalho JE, Foglio MA, Dolder H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement Altern Med 2011; 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B, Greger H, Marian B. Naturally occurring lignans efficiently induce apoptosis in colorectal tumor cells. J Cancer Res Clin Oncol 2003; 129: 569–76. [DOI] [PubMed] [Google Scholar]
- Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SMY, et al. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011; 19: 245–54. [DOI] [PubMed] [Google Scholar]
- Ge L, Zhang H, Tian S. Content determination of arctiin and arctigen from different parts of Arctium tomentosum by RP-HPLC. Zhongguo Shiyan Fangjixue Zazhi 2010; 16: 41–3. [Google Scholar]
- Tundis R, Statti G, Menichini F, Delle Monache F. Arctiin and onopordopicrin from Carduus micropterus ssp perspinosus. Fitoterapia 2000; 71: 600–1. [DOI] [PubMed] [Google Scholar]
- Janackovic P, Tesevic V, Milosavljevic S, Vajs V, Marin PD. Sesquiterpene lactones, lignans and flavones of Centaurea affinis. Biochem Syst Ecol 2004; 32: 355–7. [Google Scholar]
- Shoeb M, Macmanus SM, Kumarasamy Y, Jaspars M, Nahar L, Thoo-Lin PK, et al. Americanin, a bioactive dibenzylbutyrolactone lignan, from the seeds of Centaurea americana. Phytochemistry 2006; 67: 2370–5. [DOI] [PubMed] [Google Scholar]
- Csapi B, Hajdu Z, Zupko I, Berenyi A, Forgo P, Szabo P, et al. Bioactivity-guided isolation of antiproliferative compounds from Centaurea arenaria. Phytother Res 2010; 24: 1664–9. [DOI] [PubMed] [Google Scholar]
- Aslan Ü, Öksüz S. Chemical constituents of Centaurea cuneifolia. Turk J Chem 1999; 23: 15–20. [Google Scholar]
- Szokol-Borsodi L, Solyomvary A, Molnar-Perl I, Boldizsar I. Optimum yields of dibenzylbutyrolactone-type lignans from Cynareae fruits, during their ripening, germination and enzymatic hydrolysis processes, determined by on-line chromatographic methods. Phytochem Anal 2012; 23: 598–603. [DOI] [PubMed] [Google Scholar]
- Shoeb M, Rahman MM, Nahar L, Delazar A, Jaspars M, Macmanus SM. Bioactive lignans from the seeds of Centaurea macrocephala. DARU J Pharm Sci 2004; 12: 87–93. [Google Scholar]
- Middleton M, Cox PJ, Jaspars M, Kumarasamy Y, Nahar L, Reid R, et al. Dibenzylbutyrolactone lignans and indole alkaloids from the seeds of Centaurea nigra (Asteraceae). Biochem Syst Ecol 2003; 31: 653–6. [Google Scholar]
- Christensen LP. Flavones and other constituents from Centaurea species. Phytochemistry 1991; 30: 2663–5. [Google Scholar]
- Çelik S, Rosselli S, Maggio AM, Raccuglia RA, Uysal I, Kisiel W, et al. Guaianolides and lignans from the aerial parts of Centaurea ptosimopappa. Biochem Syst Ecol 2006; 34: 349–52. [Google Scholar]
- Youssef D, Frahm AW. Constituents of the Egyptian Centaurea scoparia; III. Phenolic constituents of the aerial parts. Planta Med 1995; 61: 570–3. [DOI] [PubMed] [Google Scholar]
- Bruno M, Diaz JG, Herz W. Guaianolides and lignans from Centaurea solstitialis subs schouwii. Phytochemistry 1991; 30: 4165–6. [Google Scholar]
- Fortuna AM, De Riscala EC, Catalan CA, Gedris TE, Herz W. Sesquiterpene lactones from Centaurea tweediei. Biochem Syst Ecol 2001; 29: 967–71. [DOI] [PubMed] [Google Scholar]
- Boldizsar I, Kraszni M, Toth F, Noszal B, Molnar-Perl I. Complementary fragmentation pattern analysis by gas chromatography-mass spectrometry and liquid chromatography tandem mass spectrometry confirmed the precious lignan content of Cirsium weeds. J Chromatogr A 2010; 1217: 6281–9. [DOI] [PubMed] [Google Scholar]
- Fan CQ, Zhu XZ, Zhan ZJ, Ji XQ, Li H, Yue JM. Lignans from Saussurea conica and their NO production suppressing activity. Planta Med 2006; 72: 590–5. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Lee HJ, Kang K, Jho EH, Kim CY, Baturen D, et al. Lignans inhibit cell growth via regulation of Wnt/beta-catenin signaling. Food Chem Toxicol 2010; 48: 2247–52. [DOI] [PubMed] [Google Scholar]
- Kang K, Lee HJ, Kim CY, Lee SB, Tunsag J, Batsuren D, et al. The chemopreventive effects of Saussurea salicifolia through induction of apoptosis and phase II detoxification enzyme. Biol Pharm Bull 2007; 30: 2352–9. [DOI] [PubMed] [Google Scholar]
- Lin HR. Sesquiterpene lactones from Tithonia diversifolia act as peroxisome proliferator-activated receptor agonists. Bioorg Med Chem Lett 2012; 22: 2954–8. [DOI] [PubMed] [Google Scholar]
- Formisano C, Rigano D, Russo A, Cardile V, Caggia S, Apostolides Arnold N, et al. Phytochemical profile and apoptotic activity of Onopordum cynarocephalum. Planta Med 2012; 78: 1651–60. [DOI] [PubMed] [Google Scholar]
- Lazari D, Garcia B, Skaltsa H, Pedro JR, Harvala C. Sesquiterpene lactones from Onopordon laconicum and O-sibthorpianum. Phytochemistry 1998; 47: 415–22. [Google Scholar]
- Attoumbre J, Bienaime C, Dubois F, Fliniaux MA, Chabbert B, Baltora-Rosset S. Development of antibodies against secoisolariciresinol-application to the immunolocalization of lignans in Linum usitatissimum seeds. Phytochemistry 2010; 71: 1979–87. [DOI] [PubMed] [Google Scholar]
- Tokar M, Klimek B. The content of lignan glycosides in Forsythia flowers and leaves. Acta Pol Pharm 2004; 61: 273–8. [PubMed] [Google Scholar]
- Lee JH, Lee JY, Kim TD, Kim CJ. Antiasthmatic action of dibenzylbutyrolactone lignans from fruits of Forsythia viridissima on asthmatic responses to ovalbumin challenge in conscious guinea-pigs. Phytother Res 2011; 25: 387–95. [DOI] [PubMed] [Google Scholar]
- Kim M, Moon HT, Lee DG, Woo E. A new lignan glycoside from the stem bark of Styrax japonica S et Z. Arch Pharm Res 2007; 30: 425–30. [DOI] [PubMed] [Google Scholar]
- Jang YP, Kim SR, Kim YC. Neuroprotective dibenzylbutyrolactone lignans of Torreya nucifera. Planta Med 2001; 67: 470–2. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Nookandeh A, Schneider B, Sun XF, Schmitt B, Stockigt J. Lignans from Torreya jackii identified by stopped-flow high-performance liquid chromatography nuclear magnetic resonance spectroscopy. J Chromatogr A 1999; 837: 83–91. [Google Scholar]
- Sun L, Liu L, Yang W, Zhao T, Tong L. Simultaneous determination of matairesinol and arctigenin in the Wikstroemia indica (Linn) C A Mey by HPLC. Shenyang Yaoke Daxue Xuebao 2010; 27: 893–7. [Google Scholar]
- Li A, Wang J, Zhu D, Zhang X, Pan R, Wang R. Arctigenin suppresses transforming growth factor-beta1-induced expression of monocyte chemoattractant protein-1 and the subsequent epithelial-mesenchymal transition through reactive oxygen species-dependent ERK/NF-kappaB signaling pathway in renal tubular epithelial cells. Free Radic Res 2015; 49: 1095–113. [DOI] [PubMed] [Google Scholar]
- Wu X, Dou Y, Yang Y, Bian D, Luo J, Tong B, et al. Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway. Biochem Pharmacol 2015; 96: 323–36. [DOI] [PubMed] [Google Scholar]
- Chen J, Li W, Jin E, He Q, Yan W, Yang H, et al. The antiviral activity of arctigenin in traditional Chinese medicine on porcine circovirus type 2. Res Vet Sci 2016; 106: 159–64. [DOI] [PubMed] [Google Scholar]
- Kasper R, Gansser D, Doehmer J. Biotransformation of the naturally-occurring lignan (-)-arctigenin in mammalian-cell lines genetically-engineered for expression of single cytochrome-P450 isoforms. Planta Med 1994; 60: 441–4. [DOI] [PubMed] [Google Scholar]
- Nose M, Fujimoto T, Nishibe S, Ogihara Y. Structural transformation of lignan compounds in rat gastrointestinal-tract; II. Serum concentration of lignans and their metabolites. Planta Med 1993; 59: 131–4. [DOI] [PubMed] [Google Scholar]
- Zou Q, Gu Y, Lu R, Zhang T, Zhao GR, Liu C, et al. Development of an LC/MS/MS method in order to determine arctigenin in rat plasma: its application to a pharmacokinetic study. Biomed Chromatogr 2013; 27: 1123–8. [DOI] [PubMed] [Google Scholar]