Abstract
Backbround
COPD is a common, highly debilitating disease of the airways, primarily caused by smoking. Chronic inflammation and structural remodelling are key pathological features of this disease caused, in part, by the aberrant function of airway smooth muscle (ASM). We have previously demonstrated that hydrogen sulfide (H2S) can inhibit ASM cell proliferation and CXCL8 release, from cells isolated from non-smokers.
Methods
We examined the effect of H2S upon ASM cells from COPD patients. ASM cells were isolated from non-smokers, smokers and patients with COPD (n = 9). Proliferation and cytokine release (IL-6 and CXCL8) of ASM was induced by FCS, and measured by bromodeoxyuridine incorporation and ELISA, respectively.
Results
Exposure of ASM to H2S donors inhibited FCS-induced proliferation and cytokine release, but was less effective upon COPD ASM cells compared to the non-smokers and smokers. The mRNA and protein expression of the enzymes responsible for endogenous H2S production (cystathionine-β-synthase [CBS] and 3-mercaptopyruvate sulphur transferase [MPST]) were inhibited by H2S donors. Finally, we report that exogenous H2S inhibited FCS-stimulated phosphorylation of ERK–1/2 and p38 mitogen activated protein kinases (MAPKs), in the non-smoker and smoker ASM cells, with little effect in COPD cells.
Conclusions
H2S production provides a novel mechanism for the repression of ASM proliferation and cytokine release. The ability of COPD ASM cells to respond to H2S is attenuated in COPD ASM cells despite the presence of the enzymes responsible for H2S production.
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0788-x) contains supplementary material, which is available to authorized users.
Keywords: COPD, Hydrogen sulfide, Proliferation, IL-6, CXCL8, Airway smooth muscle
Background
Hydrogen sulfide (H2S) which was discovered in human tissues over 15 years ago, has emerged as an important gaseous mediator in several biological processes [1]. H2S is now considered the third member of a family of gasotransmitters, together with nitric oxide (NO) and carbon monoxide [2]. The bulk of endogenous H2S synthesis in mammalian tissues appears to be from the pyridoxal-5`-phosphate–dependent enzymes, cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), and also by 3-mercaptopyruvate sulphur transferase (MPST) [3].
Chronic obstructive pulmonary disease (COPD) is a common, highly debilitating disease of the airways, primarily caused by smoking [4]. Serum H2S levels are significantly increased in patients with stable COPD as compared to age matched control subjects or those with acute exacerbation of COPD [5]. Serum H2S levels were negatively correlated with the severity of airway obstruction in patients with stable COPD whereas they were positively correlated with the lung function in all patients with COPD and healthy controls. Patients with acute exacerbations and increased pulmonary artery pressure (PASP) had lower levels of H2S than those with normal PASP, suggesting a negative relation between H2S and PASP in COPD exacerbations. Serum H2S levels are also lower in smokers than non-smokers regardless of their health status (COPD or healthy controls). Furthermore, patients with acute exacerbations, whose serum H2S levels were decreased, demonstrated greater neutrophil numbers but lower lymphocyte numbers in sputum than patients with stable COPD, suggesting a potential role of H2S in regulating inflammatory response at different types or stages of COPD.
We have previously demonstrated that mitogen stimulation increases inflammatory mediator release from both ASM IL-6 and CXCL8 release in COPD patients to a greater degree than those from non-smoker subjects [4]. Furthermore, we have shown that H2S donors inhibit mitogen-induced inflammatory mediator release and proliferation of cells from healthy non-smoking subjects [6]. We therefore set out to determine the effect of H2S in ASM cells isolated from healthy smokers and patients with COPD. We hypothesized that H2S may also mediate ASM proliferation, and cytokine release to varying degrees in these diseased cells. We examined the effect of both exogenous and intracellular sources of H2S in human ASM from 9 donors in each group upon proliferation induced by fetal calf serum (FCS). We used two extracellular H2S donors; the rapidly releasing H2S donor, sodium hydrogen sulfide (NaSH), and modelled endogenous H2S synthesis with the slow H2S-releasing molecule, GYY4137 [6, 7]. To examine the role of endogenously synthesized H2S, we used an inhibitor of H2S synthesis (O-(carboxymethyl)-hydroxylamine hemihydrochloride (CHH)) to inhibit CBS [6]. Finally, we also investigated the role of mitogen-activated protein kinase (MAPK) activation in this process.
Methods
Primary human ASM cell culture
Primary human ASM cells were previously dissected from the lungs of healthy non-smokers, healthy smokers and patients with COPD; disease and smoking status were defined according to guidelines produced by the American Thoracic Society [8]. Healthy smokers had a smoking history of at least 10 pack years. There were significant differences between FEV1 in litres, FEV1 percent predicted, and FEV1/FVC ratio between smokers and patients with COPD compared with non-smokers but matched for age and smoking history (Table 1).
Table 1.
Characteristics of subjects providing airway smooth muscle cells for culture
| Non-smokers | Smokers | COPD | |
|---|---|---|---|
| n | 9 | 9 | 9 |
| Age (y) | 66.4 ± 12.72 | 59.2 ± 7.6 | 65.4 ± 6.6 |
| Sex (♂ - ♀) | 7–2 | 4–5 | 5–5 |
| Pack years smoking | N/A | 29.25 ± 3.3 | 38.32 ± 2.92 |
| FEV1 (L) | 4.02 ± 0.48 | 3.12 ± 0.78 | 1.76 ± 0.45 |
| FEV1 (% predicted) | 104.23 ± 7.28 | 101.5 ± 4.51 | 77 ± 21.97 |
| FEV1:FVC (%) | 78.89 ± 5.98 | 77.57 ± 3.32 | 38.88 ± 15.75 |
Definition of abbreviations: FEV1 forced expiratory volume, FVC forced vital capacity
Data shown as mean ± SEM
ASM cells were cultured and plated as previously described [4, 6, 9–13]. ASM cells were plated onto 96-well plates for the measurement of cytokine release, and six well plates for RNA and protein extraction. Confluent cells were growth-arrested by FCS deprivation for 24 h in Dulbecco’s Modified Eagle’s Medium supplemented with sodium pyruvate (1 mM), L-glutamine (2 mM), nonessential amino acids (1:100), penicillin (100 U/ml)/streptomycin (100 mg/ml), amphotericin B (1.5 mg/ml), and BSA (0.1%) [6]. Cell at passages 3–4 from nine different donors per group were used.
Cells were stimulated in triplicate ±2.5% FCS for 1 h before treatment with an H2S donor (NaSH or GYY4137 [100 μM]). 24 h later CBS, CSE and MPST mRNA and protein expression was measured. At 8 days, cellular proliferation was measured by BrdU assay (Roche Applied Science, West Sussex, UK), cellular viability by MTT assay [14], and IL-6 and CXCL8 levels were determined by DuoSet ELISA (R&D Systems, Abingdon, UK) as previously described [6]. For the inhibitor studies, cells were treated with 1 mM O-(carboxymethyl)-hydroxylamine hemihydrochoride (CHH), 5 μM PD098059 (a MEK-1/2 inhibitor) or 5 μM SB 203580 (a p38 MAP kinase inhibitor) for 30 min before treatment with NaSH (100 μM) for a further 8 days.
RNA isolation and detection of mRNA expression
mRNA was isolated and CBS, CSE and 3-MST mRNA expression levels were measured as previously described [4, 6, 9–11].
Western blotting
Proteins were measured as previously described [4, 6, 9] using mouse anti-CBS (A-2) antibody, a mouse anti-CSE (30.7) antibody, mouse anti-MPST (H-11), rabbit anti-p38 MAPK antibody and rabbit anti–phospho–p38 MAPK (Thr180/Tyr182) antibody (all from Santa Cruz Biotechnology, Middlesex, UK) and, rabbit anti–extracellular signal–regulated kinase (ERK)–1/2 (137F5) and rabbit anti–phospho–ERK-1/2 (Thr202/Tyr204; purchased from Cell Signalling Technology, Ely, Cambridgeshire, UK).
Immunohistochemistry analysis of CSE, CBS and MPST in bronchial biopsies
Cryostat sections from historical biopsies were stained and scored as previously described [15]. Briefly, after blocking non-specific binding sites with horse serum, 1:200 primary antibody was applied in TRIS-buffered saline (0.15 M saline containing 0.05 M TRIS-hydrochloric acid at pH 7.6) and incubated for 1 h at room temperature in a humid chamber. Antibody binding was demonstrated with a secondary horse anti-mouse (Vector, BA 2000) antibody followed by ABC kit HRP Elite, PK6100, Vectastain and diaminobenzidine (DAB) substrate (brown colour). Human tonsil or nasal polyp were used as positive controls. For the negative control, normal mouse, rabbit or goat non-specific immunoglobulins (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used at the same protein concentration as the primary antibody.
Data analysis
Data were analysed using GraphPad Prism, version 5.03 (GraphPad Software, San Diego, CA). Data were not normally distributed (as assessed by the Kolmogorov-Smirnov test), and therefore groups were compared using the Dunn nonparametric test. All data are expressed as means ± SEMs. Significance was defined as a P value of less than 0.05.
Results
The presence of H2S producing enzymes in ASM cells in-situ was determined by immunohistochemical staining of bronchial biopsies from healthy non-smokers, healthy smokers and subjects with COPD. The most intense staining was seen for CSE but no difference in the expression of CSE, CBS or MPST was observed between patient groups (Additional file 1: Figure S1).
Effect of H2S on FCS-induced ASM proliferation and inflammatory mediator release
After 8 days of culture in the absence of FCS, neither NaSH (100 μM) nor GYY4137 (100 μM) had any significant effect upon cell viability in cells from any subject group (Fig. 1a). ASM proliferation increased in the presence of 2.5% FCS (p < 0.001), an effect that was inhibited by both NaSH and GYY4137 (p < 0.05) in all ASM groups. However, there was a significant increase in the level of proliferation between the patient groups, with the highest level seen in the COPD patients compared to non-smokers (p < 0.01). Furthermore, although the H2S donors returned proliferation levels to baseline in the non-smoker ASM cells, this was not the case in the smokers or COPD patients (Fig. 1b). Similar results were observed with regards to FCS-induced IL-6 (Fig. 1c), and CXCL8 release (Fig. 1d) with both being greatest in ASM from COPD subjects and having a lesser response to NaSH and GYY.
Fig. 1.
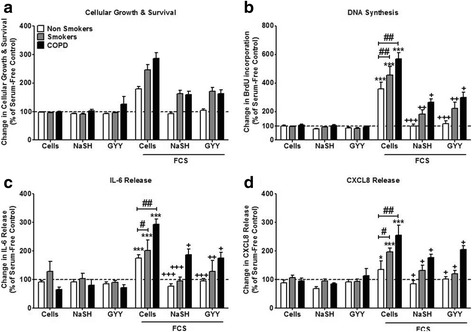
Effect of the hydrogen sulfide (H2S) donors, sodium hydrogen sulfide (NaSH) and GYY4137, on airway smooth muscle (ASM) from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h; NaSH (100 μM) or GYY4137 (100 μM) was added for another 8 days. Cellular growth and survival (a), DNA synthesis (b), IL-6 release (c), and CXCL8 release (d) were subsequently measured by dimethylthiazol-diphenyltetrazolium bromide (MTT) assay, bromodeoxyuridine (BrdU) ELISA, and DuoSet ELISA respectively. Bars represent means (± SEM) of nine ASM donors. */+/#P < 0.05; **/++/##P < 0.01; ***/+++/###P < 0.001
Effect of H2S on CSE, CBS and MPST mRNA expression in non-smokers, smokers and COPD patients
We next examined the effect of exogenous H2S upon basal and FCS-exposed CSE, CBS and MPST mRNA expression. CSE mRNA expression did not alter under any of the experimental parameters studied in any of the patient cohorts (Fig. 2a). NaSH (100 μM) alone had no effect on CBS mRNA levels at 24 h in any of the patient groups. FCS (2.5%) enhanced CBS mRNA expression only in the smokers (p < 0.05) and this was reversed back to baseline by treatment with NaSH (100 μM). In addition, NaSH also suppressed FCS-treated CBS mRNA levels in ASMs from non-smokers (p < 0.05) (Fig. 2b).
Fig. 2.
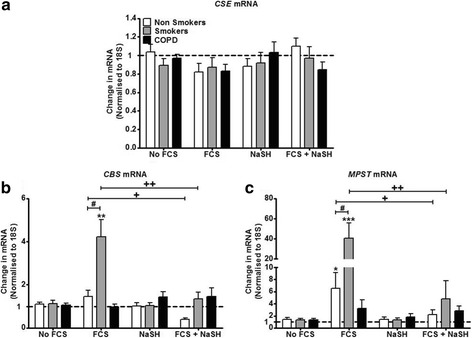
Effect of the H2S donor NaSH on CBS, CSE and MPST mRNA in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h and NaSH (100 μM) was added for another 24 h. Change in CBS (a), CSE (b), and MPST (c) mRNA expression was subsequently measured by TaqMan RT-PCR. Bars represent means (± SEM) of nine ASM donors. */+/#P < 0.05; **/++/##P < 0.01; ***/+++/###P < 0.001
FCS (2.5%) treatment for 24 h significantly up-regulated the expression of MPST mRNA in ASMs from non-smokers and smokers with significantly higher levels in the smokers (p < 0.05) compared with non-smokers (Fig. 2c). There was no effect of FCS on MPST mRNA expression in cells from COPD patients. NaSH (100 μM) alone had no effect on MPST mRNA expression but reduced FCS-stimulated expression back to baseline in cells from non-smokers and smokers (p < 0.05). No effect of NaSH was observed on FCS-treated COPD ASM cells (Fig. 2c).
Effect of H2S on CSE, CBS and MPST protein expression in non-smokers, smokers and COPD patients
Neither FCS nor NaSH had any effect on CSE protein expression in ASM cells from any group studied (data not shown). NaSH (100 μM) treatment alone had no effect on CSE or MPST expression at 24 h (Fig. 3). FCS (2.5%) increased CBS protein in the non-smokers (p < 0.05) and smoker ASM cells (p < 0.01) but no effect on CBS expression in COPD ASM cells. The effect of FCS on CBS expression was significantly greater in cells from smokers than non-smokers (p < 0.05). NaSH (100 μM) suppressed FCS-induced CBS protein production at 24 h in cells from smokers and non-smokers (p < 0.01, p < 0.05 respectively) without affecting expression in COPD cells (Fig. 3a & b).
Fig. 3.
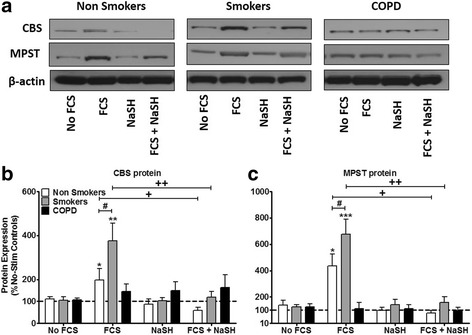
Effect of the H2S donor, NaSH on CBS and MPST protein expression in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h and NaSH (100 μM) was added for another 24 h. CBS, MPST and β-actin were detected by Western blotting (a). Further examples are shown in Additional file 2: Figure S2. Changes in protein expression were quantified by densitometry, normalized against β-actin expression, and then expressed as the percent change versus untreated controls (b & c). Bars represent means (± SEM) of nine ASM donors. */+/#P < 0.05; **/++/##P < 0.01; ***/+++/###P < 0.001
Similarly, FCS (2.5%) enhanced MPST protein expression in ASMs from non-smokers (p < 0.05) and smokers (p < 0.001). Treatment with NaSH (100 μM) completely attenuated the ability of FCS to induce MPST protein at 24 h in cells from smokers and non-smokers (p < 0.01, p < 0.05 respectively) (Fig. 3a & c). Again, no effect on MPST protein expression was observed in COPD ASM cells.
Effect of inhibiting CBS on ASM proliferation induced by FCS in samples isolated from non-smokers, smokers and COPD patients
ASM cells were pre-treated with an inhibitor of CBS (CHH) for 30 min before treatment with 2.5% FCS with or without NaSH (100μM) for 8 days. In ASMs from non-smokers, CHH (1μM) significantly enhanced FCS-stimulated proliferation (p < 0.01) to levels seen with FCS alone in ASM cells from the COPD patients (Fig. 4a). A similar ability of CHH to stimulate FCS-induced proliferation to levels seen in COPD cells was observed with ASM cells from the smokers (p < 0.05). In contrast, CHH had no effect on FCS-induced proliferation in ASMs from patients with COPD (Fig. 4a). The ability of NaSH to suppress FCS-induced proliferation of ASMs from non-smoker and healthy smoker cells was attenuated by the presence of CHH (p < 0.01, p < 0.05 respectively) (Fig. 4a). In contrast, the reduced ability of NaSH to suppress FCS-stimulated proliferation in COPD ASM cells was not affected by CHH.
Fig. 4.
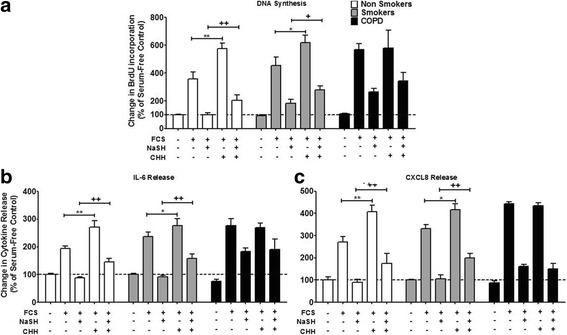
Effect of inhibiting cystathionine-β-synthase (CBS) on ASM from non-smokers, smokers and COPD patients. ASM cells were incubated with 1 mM of an inhibitor of CBS (O-(carboxymethyl)-hydroxylamine hemihydrochloride [CHH]) for 30 min; media with 2.5% FCS was added for a further 8 days with NaSH (100 μM). DNA synthesis (a), IL-6 release (b), and CXCL8 release (c) were subsequently measured by bromodeoxyuridine (BrdU) ELISA and DuoSet ELISA respectively. Bars represent means (± SEM) of nine ASM donors. */+/#P < 0.05; **/++/##P < 0.01; ***/+++/###P < 0.001
A similar profile to that observed for proliferation was seen in relation to FCS-stimulated IL-6 and CXCL8 release (Fig. 4b & c). CHH enhanced FCS-induced IL-6 (Fig. 4b) and CXCL8 (Fig. 4c) release from cells from non-smokers (p < 0.01) and smokers (p < 0.05) to levels with COPD cells. In contrast, CHH had no effect on FCS-induced proliferation in ASMs from patients with COPD (Fig. 4b & c). The ability of NaSH to suppress FCS-induced IL-6 (Fig. 4b) and CXCL8 (Fig. 4c) release from ASMs from non-smoker and healthy smoker cells was attenuated by the presence of CHH (p < 0.01). In contrast, the reduced ability of NaSH to suppress FCS-stimulated IL-6 (Fig. 4b) and CXCL8 (Fig. 4c) from COPD ASM cells was not affected by CHH.
Effect of NaSH on activation of ERK-1/2 and p38 MAPK
We have previously reported that 2.5% FCS significantly phosphorylates both ERK-1/2 and p38 MAPK in ASM cells from non-smokers and that this is prevented by NaSH [6]. We confirmed that FCS stimulates ERK-1/2 activation in non-smokers and demonstrate a similar increase in cells from smokers but a greater activation from COPD cells (Fig. 5a & b). Phosphorylation of ERK-1/2 was significantly reduced by NaSH only in cells from non-smokers (p < 0.05) (Fig. 5a & b). FCS induced a greater level of p38 MAPK phosphorylation in cells from smokers and COPD patients compared to that seen in non-smokers (Fig. 5a & c). This phosphorylation was attenuated by NaSH in cells from non-smokers and smokers but not in cells from COPD patients (Fig. 5a & c).
Fig. 5.
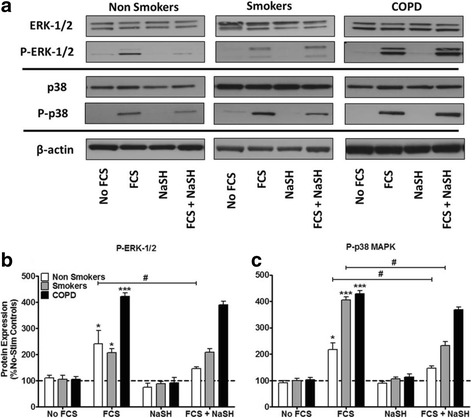
Effect of the H2S donor, NaSH on FCS-induced activation of extracellular signal–regulated kinase (ERK)–1/2 and p38 mitogen-activated protein kinase (MAPK) in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h and NaSH (100 μM) was added for another 24 h. Total and phospho–ERK-1/2, total and phospho-p38 and β-actin were detected by Western blotting (a), and the corresponding densitometry graphs are shown in b & c. Further examples are shown in Additional file 2: Figure S2. Changes in phospho-MAPK expression were quantitated by densitometry, normalized against β-actin expression, and then expressed as the percent change versus non phosphorylated controls. Bars represent means (± SEM) of nine ASM donors. */+/#P < 0.05; **/++/##P < 0.01; ***/+++/###P < 0.001. **P < 0.01. P-ERK, phosphorylated- ERK; P-p38, phosphorylated p38
Finally, we examined the role of the MAPKs, ERK-1/2 and p38, on FCS-induced proliferation (Fig. 6a), IL-6 (Fig. 6b) and CXCL8 (Fig. 6c) release in human ASM cells. The ERK-1/2 inhibitor, PD98059 (5 mM), significantly inhibited FCS-induced proliferation, IL-6 and CXCL8 release (p < 0.05) in all patient groups (Fig. 6a, b & c). However, the magnitude of the effect in COPD cells was less than that observed in smoker and non-smoker cells. The p38 MAPK inhibitor, SB202190 (5 mM), had a reduced effect compared to that seen with PD98059. The combination of PD98059 and SB202190 had a greater suppressive effect on all parameters than the individual inhibitors across all subject groups but again the magnitude of the effect in COPD cells was less.
Fig. 6.
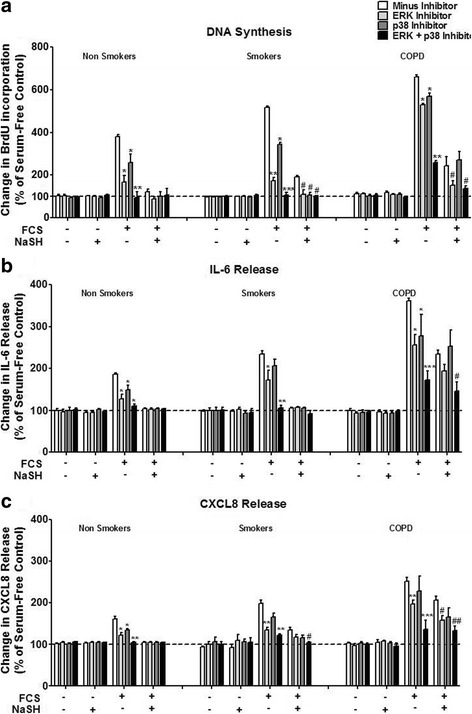
Effect of mitogen-activated protein kinase 1/2 (MEK-1/2) and p38 MAPK inhibition upon FCS-induced proliferation, IL-6 and CXCL8 release in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with an MEK-1/2 (5 mM) inhibitor, a p38 (5 mM) inhibitor, or both for 30 min. The ASM cells were then further incubated with NaSH (100 μM) for another 8 days. DNA synthesis (a), IL-6 release (b), and CXCL8 release (c) were subsequently measured by bromodeoxyuridine (BrdU) ELISA, and DuoSet ELISA respectively. Bars represent means (± SEM) of nine ASM donors. *P < 0.05 versus cells plus 2.5% FCS; **P < 0.01 versus cells plus 2.5% FCS; ***P < 0.001 versus cells plus 2.5% FCS; #P < 0.05 versus cells plus 2.5% FCS plus NaSH; ##P < 0.01 versus cells plus 2.5% FCS plus NaSH
Furthermore, when the ASM cells were further treated with NaSH (100μM) in addition to the MAPK inhibitors, a further decrease in IL-6 and CXCL8 release was observed in the COPD patients (P < 0.05) although this still failed to reach baseline levels as seen in cells from smokers and non-smokers (Fig. 6b & c).
Discussion
For the first time, we demonstrate that both endogenous and exogenous H2S inhibits human ASM cell proliferation and cytokine release induced by FCS, and that this effect was dependent on the patient. Specifically; proliferation and cytokine release from non-smoker ASM cells returned to basal levels (as previously reported [6]) whereas in smokers, both IL-6 and CXCL8 release were reduced to baseline but proliferation although being significantly reduced did not return to basal levels. In contrast, the effect of H2S on proliferation and cytokine release from ASM cells isolated from COPD patients was impaired compared to smokers and non-smoker cells. Furthermore, we have shown that endogenous H2S is produced by the enzymes CBS and MPST, and not by CSE. We found that H2S differentially inhibited phosphorylation of the MAPKs, ERK-1/2 and p38, according to the patient group and propose that this could be a mechanism by which H2S inhibits cellular proliferation and cytokine release [4, 16–19].
ASM proliferation is increased in response to FCS [9, 10, 20] and studies have examined the role of H2S upon cell proliferation. These have concluded that this gas can induce proliferation [21] or, conversely, inhibit it [6, 22, 23] depending upon the cell type examined. Both the fast-release H2S donor, NaSH, and the slow-release donor, GYY4137, have been used previously to affect inflammation in both in-vivo and in-vitro models of inflammation, including a mouse models of vascular inflammation and oxidative stress [24], asthma [25], COPD [26], and a rat model of colitis [27]. Our data extends our previous report demonstrating the inhibitory action of H2S in non-smoker ASM cells [6] and examined its role in smoker and COPD ASM cells. Both NaSH and GYY4137 caused similar inhibitory effects on FCS-induced ASM cell proliferation, IL-6 and CXCL8 release from smokers as well as non-smokers indicating that the rate of release does not modulate the inhibitory effect of H2S in ASM. We also show for the first time, an effect upon primary ASM cells isolated from patients with COPD. However, the effect of H2S donors is reduced compared to that seen in cells from smokers and non-smokers which may explain, in part, the increased inflammatory and proliferative status of COPD cells. Indeed, the H2S enzyme inhibitor CHH had no significant effect on FCS-induced inflammatory protein release from COPD cells in contrast to the effect seen in cells from other subject groups.
We found that, all three H2S producing enzymes are expressed in ASM cells to a similar extent across the subject groups studied. However, our pharmacological studies suggest that endogenous H2S production is these cells is most likely to be through the enzymes, CBS, and MPST. In cultured ASM cells, FCS was able to induce CBS and MPST mRNA and protein in cells from non-smokers and smokers but not in COPD cells suggesting that mitogens may induce cells to produce more H2S. NaSH inhibited both CBS and MPST, likely as a negative-feedback inhibitory mechanism. Currently, CBS appears to be involved in the generation of endogenous H2S in neural pathways, the brain, vascular tissue, and non-smoker ASM cells [6, 28–32]. In contrast, CSE is predominantly involved in endogenous H2S production in rodent smooth muscle and the lung [33–37], and MPST maintains mitochondrial function [extensively reviewed in [38, 39]]. Clearly cell, species and pathology differences should be taken into consideration when investigating the production of endogenous H2S.
A role for the ERK-1/2 and p38 MAPKs in regulating ASM cell proliferation and cytokine release is well documented [4, 16–19] and H2S has been shown to affect the phosphorylation of these kinases [6, 26, 40–43]. Hence, we examined the degree of phosphorylation of these kinases in our COPD ASM cells. We noted that FCS induced both ERK-1/2 and p38 MAPK phosphorylation, which was reduced by NaSH in both the non-smoker and smoker ASM cells, but no effect was seen in the COPD cells. Inhibiting these kinases significantly reduced the ASM proliferation and cytokine release and, when they were used before treatment with NaSH, a further decrease in aberrant phenotype was observed, further supporting the possibility that the mechanism of H2S, at least in part, is via the inhibition of these kinases.
Finally, our data shows that ASM cells of COPD patients indicate an attenuated response to H2S, as compared to the non-smoker and smoker-groups. But the question remains, why? There are numerous reviews discussing both the importance of H2S in chronic respiratory diseases [3, 44] and smooth muscle itself [45], however recent studies demonstrate further actions of this gasotransmitter. For example, Fitzgerald et al. demonstrate that H2S causes the relaxation of human ASM and implicate the role for sarcolemmal KATP channels [46]. In mouse models, Huand et al. indicate that H2S can induce mouse ASM relaxation by activating BKCa [47], and Castro-Piedras et al. indicate that H2S causes ASM relaxation by inhibiting Ca(2+) release through InsP3Rs and consequent reduction of agonist-induced Ca(2+) oscillations [48]. In other rodent models of lung pathology, endogenous H2S has been suggested to have a protective role of anti-inflammation and bronchodilation in chronic cigarette smoke-induced pulmonary injury in rats [49], and H2S provokes tachykinin-mediated neurogenic inflammation that is mediated by stimulation of TRPV1 receptors on the sensory nerve endings in Guinea Pigs [50]. Furthermore, considering the emergence of data suggesting a degree of cross-talk between H2S and epigenetic modifiers such as miRNAs [51, 52], and our own data suggesting broadly different epigenetic profiles between lung pathologies (including COPD) in ASM [4, 9, 10, 12, 13]. Hence, the difference between a COPD ASM cell and a ‘healthy’ ASM cell may incorporate one, or more likely, more of these H2S targets/activators. To address this further we intend to further these and other findings in our murine model of COPD [26].
Conclusion
In conclusion, we have shown for the first time that H2S inhibits both human ASM proliferation and cytokine release induced by FCS, differentially between ASM cells isolated from non-smokers, smokers and patients with COPD. It is likely that exogenous H2S targets the production of endogenous H2S by inhibiting the transcription and subsequent translation of the CBS and MPST enzymes, and proliferation is controlled by H2S through a negative-feedback pathway. H2S inhibits the activity of the ERK-1/2 and p38 MAPKs, in the non-smokers and smokers, but with little effect in the COPD ASM cells. We propose that H2S may provide a novel therapeutic avenue in the stabilization of ASM proliferation but that its effectiveness in COPD may be more limited.
Additional files
Figure S1. Immunohistochemistry staining of CSE, CBS and MPST in bronchial biopsies from non-smokers, smokers and COPD patients. Photomicrographs showing representative photomicrographs of cystathionine-γ-lysase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulphur transferase (MPST) staining in the bronchial mucosa from control non-smokers, control smokers with normal lung function and mild/moderate COPD patients. Immune-stained airway smooth muscle cells are indicated by brown staining. Results are representative of those from 13 non-smokers, 14 smokers with normal lung function, 15 mild/moderate COPD patients. Calibration bar represents 20 μm. Graphical representation of the results are shown in the right hand panels. (JPG 332 kb)
Figure S2. Further examples of the effect of the H2S donor, NaSH on CBS and MPST protein expression, and activation of extracellular signal–regulated kinase (ERK)–1/2 and p38 mitogen-activated protein kinase (MAPK) in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h and NaSH (100 μM) was added for another 24 h. CBS, MPST (A), Total and phospho–ERK-1/2, total and phospho-p38 and β-actin (B) were detected by Western blotting. (JPG 212 kb)
Acknowledgments
Funding
MMP was funded by a Junior Research Fellowship from Imperial College. This project was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. KFC is a Senior Investigator of NIHR, UK. IMA and KFC are supported by the EU- Innovative Medicines Initiative Joint Undertaking project U-BIOPRED (115010). MMP, IMA and KFC are members of Interuniversity Attraction Poles Program-Belgian State-Belgian Science Policy- project P7/30.
This work was supported by grants from Asthma UK (08/041) and The Wellcome Trust (085935) (KFC). This project was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. KFC is a Senior Investigator of NIHR, UK. MMP, IMA and KFC are members of Interuniversity Attraction Poles Program-Belgian State-Belgian Science Policy- project P7/30.
Availability of data and materials
All data is available upon request.
Take Home Message
Hydrogen sulfide production provides a novel mechanism for regulation of airway smooth muscle phenotype in COPD.
Authors’ contributions
MMP designed the study, performed the experiments, analysed the data and wrote the manuscript. BT performed some of the experiments. AP, PC & GC performed the immunohistochemistry. KLR & AJH provided some of the ASM cells. IA helped with writing the manuscript. KFC provided the majority of the ASM and helped with writing the manuscript. MMP conceived the study, performed in-vitro work, analysed the data and wrote the manuscript. BT performed in-vitro work and analysed data. AP, PC and GC performed immunohistochemistry analysis. KD and AH provided healthy smoker and COPD ASM cells. IA helped with writing the manuscript. KFC provided normal, healthy smoker and COPD ASM cells. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Royal Brompton & Harefield NHS Trust Ethics committee and all subjects gave written informed consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1186/s12931-021-01816-7
Change history
8/25/2021
A Correction to this paper has been published: 10.1186/s12931-021-01816-7
Contributor Information
Mark M. Perry, Email: mark.perry@port.ac.uk
Bernadett Tildy, Email: bernadett-tildy@hotmail.co.uk.
Alberto Papi, Email: alberto.papi@unife.it.
Paolo Casolari, Email: paolo.casolari@unife.it.
Gaetano Caramori, Email: gcaramori@unime.it.
Karen Limbert Rempel, Email: klimbertrempel@gmail.com.
Andrew J. Halayko, Email: ahalayk@cc.umanitoba.ca
Ian Adcock, Email: ian.adcock@imperial.ac.uk.
Kian Fan Chung, Email: f.chung@imperial.ac.uk.
References
- 1.Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008;29(2):84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113(1):14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF. Hydrogen sulfide as a potential biomarker of asthma. Expert Review of Respiratory Medicine. 2014;8(1):5. doi: 10.1586/17476348.2014.856267. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary L, Sevinç K, Papazoglou IM, Tildy B, Chung KF, Detillieux K, et al. Airway smooth muscle inflammation is regulated by microRNA-145 in COPD. FEBS Lett. 2016;590(9):1324–1334. doi: 10.1002/1873-3468.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y-H, Yao W-Z, Geng B, Ding Y-L, Lu M, Zhao M-W, et al. Clinical investigations: COPD: endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128:3205–3211. doi: 10.1378/chest.128.5.3205. [DOI] [PubMed] [Google Scholar]
- 6.Perry MM, Hui CK, Whiteman M, Wood ME, Adcock I, Kirkham P, et al. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2011;45(4):746. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 8.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121. [PubMed]
- 9.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50(1):7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry MM, Tsitsiou E, Austin PJ, Lindsay MA, Gibeon DS, Adcock IM, et al. Role of non-coding RNAs in maintaining primary airway smooth muscle cells. Respir Res. 2014;15:58. doi: 10.1186/1465-9921-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry MM, Durham AL, Austin PJ, Adcock IM, Kian FC. BET Bromodomains regulate transforming growth factor-β-induced proliferation and cytokine release in asthmatic airway smooth muscle. J Biol Chem. 2015;290(14):9111–9121. doi: 10.1074/jbc.M114.612671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry MM, Lavender P, Scott Kuo CH, Galea F, Michaeloudes C, Flanagan JM, et al. DNA methylation modules in airway smooth muscle are associated with asthma severity. Eur Respir J. 2018; 10.1183/13993003.01068-2017. [DOI] [PMC free article] [PubMed]
- 13.Austin PJ, Tsitsiou E, Boardman C, Jones SW, Lindsay MA, Adcock IM, et al. Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J Allergy Clin Immunol. 2017;139(3):780–789. doi: 10.1016/j.jaci.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Contoli M, Papi A, Cappello F, Adcock I, Durham A, Di Stefano A, et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax. 2014;69(6):516–524. doi: 10.1136/thoraxjnl-2012-203062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L245–LL53. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Liang Q, Gorska M, Alam R, Balzar S, Wenzel S. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J Allergy Clin Immunol. 2008;121(4):893–902. doi: 10.1016/j.jaci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Nath P, Leung S-Y, Williams A, Noble A, Chakravarty SDS, Luedtke GR, et al. Importance of p38 mitogen-activated protein kinase pathway in allergic airway remodelling and bronchial hyperresponsiveness. Eur J Pharmacol. 2006;544(1–3):160–167. doi: 10.1016/j.ejphar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Larner-Svensson HM, Williams AE, Tsitsiou E, Perry MM, Jiang X, Chung KF, et al. Pharmacological studies of the mechanism and function of interleukin-1beta-induced miRNA-146a expression in primary human airway smooth muscle. Respir Res. 2010;11:68. doi: 10.1186/1465-9921-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiels IA, Taylor SM, Bowler SD. Airway smooth muscle proliferation in asthma: the potential of vascular leakage to contribute to pathogenesis. Med Hypotheses. 1995;45(1):37–40. doi: 10.1016/0306-9877(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 21.Christl SU, Eisner HD, Dusel G, Kasper H, Scheppach W. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: a potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci. 1996;41(12):2477–2481. doi: 10.1007/BF02100146. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, et al. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessel. 2004;19(2):75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- 23.Yang G. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21(Cip/WAK-1) J Biol Chem. 2004;279(47):49199–49205. doi: 10.1074/jbc.M408997200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Lu H, Meng G, Li X, Xie L, Chen Q, et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Br J Pharmacol. 2013;169(8):1795–1809. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Wang P, Yang G, Cao Q, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol. 2013;182(4):1188–1195. doi: 10.1016/j.ajpath.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Zhang P, Zhang M, Sun X, Bao A, Zhou X, et al. Hydrogen sulfide prevents and partially reverses ozone-induced features of lung inflammation and emphysema in mice. Am J Respir Cell Mol Biol. 2016;55(1):72–81. doi: 10.1165/rcmb.2015-0014OC. [DOI] [PubMed] [Google Scholar]
- 27.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolution of Colitis in Rats. Gastroenterology. 2009;137(2):569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Jhee K-H, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279(50):52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 30.Eto K, Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. United States: WAVERLY PRESS INC; 2002. p. 42680. [DOI] [PubMed] [Google Scholar]
- 31.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26(1):13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 32.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya N, Mikami Y, Kimura Y, Kimura H, Nagahara N. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146(5):623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Wu L, Bryan S, Mani S, Wang R, Khaper N. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res. 2010;86(3):487–495. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Wang R, Wu L. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20(3):553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y-H, Wu R, Geng B, Qi Y-F, Wang P-P, Yao W-Z, et al. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–123. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Chassé JF, Paly E, Paris D, Paul V, Sinet PM, Kamoun P, et al. Genomic organization of the human cystathionine beta-synthase gene: evidence for various cDNAs. Biochem Biophys Res Commun. 1995;211(3):826–832. doi: 10.1006/bbrc.1995.1886. [DOI] [PubMed] [Google Scholar]
- 38.Szabo C, Módis K, Coletta C, Olah G, Yanagi K, Ransy C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171(8):2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Módis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171(8):2123–2146. doi: 10.1111/bph.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papapetropoulos A, Pyriochou A, Marazioti A, Altaany Z, Yang G, Wang R, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuhlmeier KM, Bröll J, Iliev B. NF-KappaB independent activation of a series of proinflammatory genes by hydrogen sulfide. Exp Biol Med. 2009;234(11):1327–1338. doi: 10.3181/0904-RM-137. [DOI] [PubMed] [Google Scholar]
- 42.Hu LF, Lu M, Wu ZY, Wong PTH, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75(1):27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Zhang CY, Yan H, Jin HF, Du JB, Zhang LK, et al. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res. 2008;31(8):1619–1630. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Wang R. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol. 2012;184(2):130–138. doi: 10.1016/j.resp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Dunn WR, Alexander SP, Ralevic V, Roberts RE. Effects of hydrogen sulphide in smooth muscle. Pharmacol Ther. 2016;158:101–113. doi: 10.1016/j.pharmthera.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald R, DeSantiago B, Lee DY, Yang G, Kim JY, Foster DB, et al. H2S relaxes isolated human airway smooth muscle cells via the sarcolemmal K(ATP) channel. Biochem Biophys Res Commun. 2014;446(1):393–398. doi: 10.1016/j.bbrc.2014.02.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Luo YL, Hao Y, Zhang YL, Chen PX, Xu JW, et al. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: role of BKCa. Eur J Pharmacol. 2014;741:55–63. doi: 10.1016/j.ejphar.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Castro-Piedras I, Perez-Zoghbi JF. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J Physiol. 2013;591(23):5999–6015. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YH, Wang PP, Wang XM, He YJ, Yao WZ, Qi YF, et al. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine. 2011;53(3):334–341. doi: 10.1016/j.cyto.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, et al. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145(8):1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hackfort BT, Mishra PK. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2016;310(7):H802–H812. doi: 10.1152/ajpheart.00660.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhai Y, Tyagi SC, Tyagi N. Cross-talk of MicroRNA and hydrogen sulfide: A novel therapeutic approach for bone diseases. Biomed Pharmacother. 2017;92:1073–1084. doi: 10.1016/j.biopha.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Immunohistochemistry staining of CSE, CBS and MPST in bronchial biopsies from non-smokers, smokers and COPD patients. Photomicrographs showing representative photomicrographs of cystathionine-γ-lysase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulphur transferase (MPST) staining in the bronchial mucosa from control non-smokers, control smokers with normal lung function and mild/moderate COPD patients. Immune-stained airway smooth muscle cells are indicated by brown staining. Results are representative of those from 13 non-smokers, 14 smokers with normal lung function, 15 mild/moderate COPD patients. Calibration bar represents 20 μm. Graphical representation of the results are shown in the right hand panels. (JPG 332 kb)
Figure S2. Further examples of the effect of the H2S donor, NaSH on CBS and MPST protein expression, and activation of extracellular signal–regulated kinase (ERK)–1/2 and p38 mitogen-activated protein kinase (MAPK) in human ASM cells from non-smokers, smokers and COPD patients. ASM cells were incubated with FCS (2.5%) for 1 h and NaSH (100 μM) was added for another 24 h. CBS, MPST (A), Total and phospho–ERK-1/2, total and phospho-p38 and β-actin (B) were detected by Western blotting. (JPG 212 kb)
Data Availability Statement
All data is available upon request.


