Introduction
Key Teaching Points.
-
•
Holt-Oram syndrome should be considered as a possible cause of conduction disease in patients who present with nonspecific cardiac symptoms and skeletal abnormalities.
-
•
Left ventricular noncompaction presents with a broad range of additional cardiac and extracardiac features, which should be independently considered.
-
•
Genetic testing is a useful tool for facilitating correct diagnoses and can provide a basis for additional, comprehensive clinical review, so-called “reverse phenotyping.”
Left ventricular noncompaction (LVNC) in adults can be found in isolation or in association with a range of structural and arrhythmogenic cardiac phenotypes. LVNC is characterized by noncompaction of the left ventricle with prominent trabeculations and deep intertrabecular recesses. Unlike in severe congenital LVNC, the causes of LVNC in adults remain largely unknown, and treatment and prognosis depend primarily on coexisting pathologies. Recent studies found that up to 43% of healthy individuals fulfill LVNC diagnostic criteria on cardiac magnetic resonance imaging (cMRI),1 raising the question whether LVNC in adults represents a pathologic process or a normal anatomic variant.2 Given this significant uncertainty, the relevance and management of LVNC in adults can be challenging for clinicians.
In addition to cardiac phenotypes, LVNC can occur in patients with various neuromuscular and skeletal disorders, including Holt-Oram syndrome (HOS), a rare developmental disorder resulting in hand-heart disease.3 HOS is characterized by heart and upper limb abnormalities and is primarily caused by autosomal dominant mutations in the T-box protein 5 gene (TBX5), important in heart development.4, 5 Typical cardiac manifestations of HOS include septal defects and cardiac conduction disease, particularly in middle age. It remains unclear whether LVNC in patients with HOS is an incidental finding or an associated cardiac defect.
In the setting of complex phenotypes such as LVNC, which commonly present with coexisting cardiac and extracardiac features, genetic testing can facilitate a correct diagnosis and appropriate management of patients and family members. Here we show how whole exome sequencing and thorough clinical phenotyping facilitated a diagnosis of HOS in 2 families presenting with LVNC and cardiac conduction disease.
Methods
Clinical assessment
A multidisciplinary team including cardiologists, cardiac genetic counselors, rheumatologists, and clinical geneticists performed clinical assessments, including medical and family history, physical examination, electrocardiogram (ECG), echocardiography, 24-hour Holter monitoring, exercise testing, and cMRI. HOS was diagnosed on finding upper limb anomalies with a personal or family history of cardiac conduction disease or congenital heart disease.6
Genetic analysis
Informed consent was obtained from probands and family members. Whole exome sequencing was performed on genomic DNA isolated from whole blood of 2 unrelated probands, as previously reported.7 Rare variants (minor allele frequency < 0.01%) located in protein coding regions or splice signal sequences of 174 cardiac-associated genes were classified according to American College of Medical Genetics and Genomics (ACMG) criteria.8 Variants of interest were Sanger validated and genotyped in affected family members.
Case report
Family 1
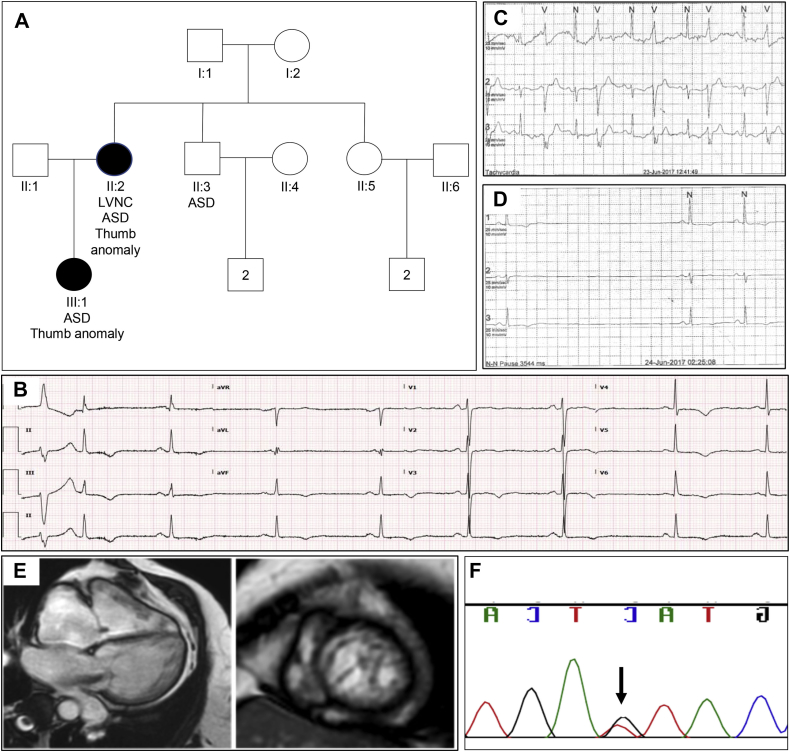
The female proband presented for cardiac review at age 50 years with light-headedness, fatigue, and exertional dyspnea. Her medical history included an atrial septal defect (ASD) repair at age 34 years, hip replacement, hypertension, hypercholesterolemia, and depression. Review of the family revealed ASDs in her daughter and brother (Figure 1A). Her resting ECG showed sinus bradycardia and anterolateral T-wave inversion (Figure 1B). Holter monitoring revealed symptomatic sinus node disease with diurnal pauses, isolated ventricular ectopy, and predisposition to atrial fibrillation (Figure 1C and D). Echocardiography revealed possible LVNC affecting the mid to apical segments of the left ventricle, which was confirmed with cMRI according to Petersen criteria (NC:C ratio > 2.3 at end-diastole) (Figure 1E). Additional cMRI findings included mild right ventricle dilation with normal systolic function, biatrial dilation, severe enlargement of main and branch pulmonary arteries, and normal left ventricular systolic function. The patient received a dual-chamber pacemaker.
Figure 1.
Family 1. A: Family pedigree revealing atrial septal defects (ASD) and thumb anomalies in multiple family members. LVNC = left ventricular noncompaction. B: Electrocardiogram demonstrating sinus bradycardia with widespread T-wave inversion and ventricular ectopic beat in proband. C: Ventricular bigeminy on Holter monitor in proband. D: Sinus pause on Holter monitor in proband. E: Short- and long-axis cardiac magnetic resonance imaging from proband. F: Sanger sequencing validation of TBX5 predicted splice variant c.510+5G>T in the proband.
Genetic analysis
Whole exome sequencing of the proband revealed 1 variant: a c.510+5G>T substitution in intron 5 of the predominant TBX5 transcript (NM_000192.3). The variant is absent from general population databases of more than 120,000 people and alters an evolutionarily conserved nucleotide (GERP score = 4.35), 3 bases from the donor splice site of exon 5. In silico tools predict that the variant disrupts the canonical splice sequence. The variant was validated with Sanger sequencing (Figure 1F) and subsequently confirmed to segregate to the daughter; DNA was not available from the proband's brother, who has an ASD. We classified the c.510+5G>T substitution as a variant of unknown significance. Functional studies showing that the variant alters splicing of TBX5, or additional segregation data, would be required to reclassify this variant as likely pathogenic to facilitate clinical use.
Outcome
The combination of a predicted splice site variant in TBX5, a family history of ASDs, and conduction disease in the proband raised a suspicion of HOS, prompting a more comprehensive review of the family's medical records. The daughter had a neonatal diagnosis of HOS, with radiographs revealing radial hypoplasia and triphalangeal thumbs; echocardiography did not show LVNC. Moreover, the daughter's medical notes mentioned prior diagnosis of HOS in the proband, with radiographs showing dysplasia of all carpal bones and an extra carpal bone.
Family 2
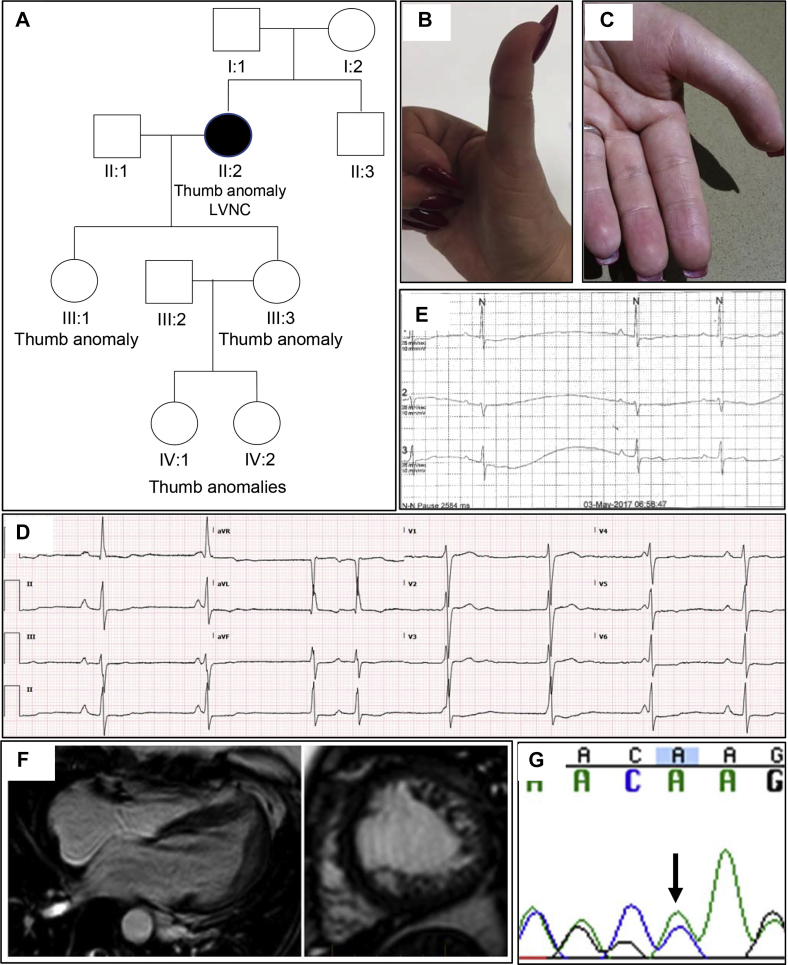
The female proband presented at age 49 years with general fatigue, chest tightness, palpitations, and presyncope. Her medical history was significant for a recent diagnosis of ankylosing spondylitis, anxiety, and depression. Her family history revealed triphalangeal thumbs across 3 generations and ankylosing spondylitis in her brother (Figure 2A–C). Clinical examination of the proband revealed brachydactyly and a pectus deformity. Her ECG and Holter monitoring showed sinus node dysfunction, intermittent sinus pauses, and evidence of isorhythmic dissociation (Figure 2D and E). Holter monitoring also revealed sinus bradycardia with frequent episodes of junctional rhythm and possible periods of wandering atrial pacemaker owing to variable P-wave morphologies. Echocardiography revealed LVNC, which fulfilled Petersen criteria on cMRI (Figure 2F) in the mid to apical regions, particularly involving the anterolateral, inferolateral, and apical walls. There was also mild left ventricular dilation with normal systolic function and prominent right ventricular trabeculation. The patient does not have a pacemaker, but one may be considered in the future.
Figure 2.
Family 2. A: Family pedigree revealing thumb anomalies in multiple family members. B: Thumb anomaly in proband. C: Thumb anomaly in proband's daughter. D: Electrocardiogram revealing sinus bradycardia and isorhythmic junctional rhythm, and nonspecific ST-T wave abnormality in proband. E: Sinus pause on Holter monitor in proband. F: Long- and short-axis cardiac magnetic resonance imaging from proband. G: Sanger sequencing validation of TBX5 frameshift variant p.Ser36ThrFs*25 in the proband.
Genetic analysis
Whole exome sequencing of the proband revealed 1 variant: a novel frameshift p.Ser36Thrfs*25 in exon 2 of the TBX5 predominant transcript (NM_000192.3). The variant is located at an evolutionarily conserved nucleotide (GERP score = 4.84). The variant was validated with Sanger sequencing (Figure 2G). A different frameshift variant at the same amino acid position segregates with 3 affected members of a family with HOS and has been classified as pathogenic (ClinVar ID 369677).
Outcome
We classified the p.Ser36Thrfs*25 variant as pathogenic according to ACMG criteria8 as a result of the following: (1) it is a null variant in a gene with an established loss-of-function mechanism, (2) the variant is absent from over 120,000 population controls, and (3) the phenotype is specific for a disease with a single genetic etiology in TBX5. Identification of a pathogenic TBX5 variant in this family confirmed a suspected diagnosis of HOS.
Discussion
This report describes the diagnosis of HOS in 2 families with a cardiac presentation including conduction disease and an initial diagnosis of LVNC. A broad genetic testing approach revealed 2 variants in TBX5, which causes HOS, prompting more extensive clinical investigation. Both probands were found to have skeletal involvement, and family 1 had a history of congenital ASDs. These families highlight the utility of genetic testing in facilitating the correct diagnosis of patients with conduction disease, LVNC, and skeletal manifestations.
The identification of HOS resulted in familial screening and appropriate clinical management. An overview of the clinical features of HOS, a rare disorder with a prevalence of 1 in 100,000 individuals, is provided in Table 1. HOS is transmitted as an autosomal dominant trait with frequent de novo mutations.4 Diagnosis most commonly occurs in the perinatal or neonatal period and requires comprehensive assessment of upper limb size, shape, and range of movement.9 Clinical features are highly variable even within families; patients present with a variety of cardiac and skeletal phenotypes, which range from subtle to severe.5 No correlation between severity of cardiac and skeletal defects exists.10
Table 1.
Clinical features of Holt-Oram syndrome
| Feature | Manifestation |
|---|---|
| Inheritance | Autosomal dominant |
| Genetic causes | TBX5 mutations |
| Gene penetrance | Complete |
| Prevalence | 1 in 100,000 individuals |
| Age at symptom onset | Typically identified in perinatal (severe skeletal defects) or neonatal period; conduction disease frequently develops at middle age |
| Clinical features | Upper limb and cardiac abnormalities, ocular (refractive error, squint, and Duane anomaly) and skin defects, sudden cardiac death |
| Skeletal abnormalities | Radial-ray defects (most prevalent), triphalangeal thumbs, absent thumbs, elongated thumbs, hypoplasia of thenar eminence, reduced finger length, clinodactyly, limited supination of the forearm, radial hypoplasia, rhyzomelic shortening of the upper arm, narrow sloping shoulders with reduced movement, pectus deformity, vertebral fusion and/or stenosis |
| Structural cardiac abnormalities | Atrial and ventricular septal defects, tetralogy of Fallot, mitral valve prolapse, aortic stenosis, patent ductus arteriosus, sinus venosus atrial septal defect, dextrocardia, pulmonary stenosis, right isomerism |
| Electrophysiological cardiac abnormalities | Sinus bradycardia, atrioventricular block, complete heart block, arrhythmias, first-degree heart block with a long PR interval > 0.2 s (most common electrocardiogram abnormality), right and left bundle branch block, left or right axis deviation, P mitrale, sudden cardiac death |
| Prognosis | Based on the severity of cardiac disease |
All HOS patients have upper limb anomalies, which are bilateral and often asymmetrical.10 Patients frequently present with triphalangeal thumbs, and occasionally present with phocomelia (underdeveloped or absent limbs) or vertebral anomalies, including vertebral stenosis and fusion.9 Ankylosing spondylitis, as identified in family 2, is a feature of HOS; however, further investigation is required to better understand this association.
Cardiac pathologies are present in up to 95% of HOS and most commonly include septal defects and cardiac conduction disease featuring bradycardia, bundle branch block, and atrial fibrillation. Although no specific cardiac presentation is required for a diagnosis of HOS, the majority of families have at least 1 family member with a septal defect.10 Management of HOS involves treatment of associated cardiac and skeletal manifestations. Cardiac screening, including yearly ECG and Holter monitoring for individuals with conduction disease and echocardiography every 1 to 5 years, is recommended depending on the degree of cardiac involvement.
A common feature of the 2 families is the existence of significant conduction disease. This mainly involved bradycardia and sinus pauses requiring a permanent pacemaker. Rhythm abnormalities occur frequently in HOS patients and include sinus bradycardia, first-degree heart block, right and left bundle branch block, atrioventricular block, sinus node dysfunction, Wolff-Parkinson-White syndrome, and sudden cardiac death (Table 1).5, 10 Though the majority of patients with rhythm abnormalities have additional structural defects, up to 40% of patients experience isolated conduction disease.10
Genetic testing reveals a TBX5 variant in up to 74% of HOS.11 TBX5, a T-box family transcription factor, plays a critical role in early cardiac and upper limb development, with key interactions with developmental transcription factors including NKX2-5 and GATA4.12 Consistent with the variants identified in this report, the majority of disease-causing variants in TBX5 are highly penetrant loss-of-function variants that result in haploinsufficiency. However, a small number of missense variants have also been identified.12 Genetic testing in HOS facilitates cascade genetic testing in the family and provides the opportunity for affected relatives to consider preimplantation genetic diagnosis. Furthermore, a genetic test result can distinguish HOS from other syndromes that have overlapping features.
Recent studies have raised uncertainty surrounding LVNC in adults as a unique pathologic entity. LVNC did not segregate with disease in family 1, raising the possibility that LVNC was an incidental finding. Further, LVNC was not identified in the proband of family 1 during her earlier ASD repair, suggesting that this was an acquired change rather than a defect of cardiac development. It is currently unclear whether the identification of LVNC in the presence of other cardiac or neuromuscular phenotypes signifies a worse prognosis.13, 14
Conclusion
Patients who present with conduction disease and LVNC should be examined for subtle skeletal and syndromic anomalies that can be suggestive of additional diagnoses such as HOS. Given the broad spectrum of cardiac and noncardiac pathologies associated with LVNC, a diagnosis in adult patients can be distracting and needs to be considered in the clinical context of the patient, and of other associated cardiac abnormalities. Genetic testing is a useful tool for informing correct diagnoses in patients and guiding management of families. As the uptake of genetic testing increases, it is likely that more patients with less severe forms of rare diseases such as HOS will be identified. Clinical approaches that utilize specialized multidisciplinary teams are required to facilitate appropriate diagnosis and management in these patients and their families.
Acknowledgments
The authors thank the patients and their families for their interest and involvement in the study. The authors thank Dr Ghassan Charbel, Dr Caroline Medi, Dr Belinda Gray, and Ms Charlotte Burns for assistance with patient management; Ms Natalie Nowak for genetic analysis; and Dr Raj Puranik for imaging.
Footnotes
Dr Semsarian is the recipient of a National Health and Medical Research Council, Australia (NHMRC) Practitioner Fellowship (#1059156). This research is supported by a grant from HeartKids Australia.
References
- 1.Kawel N., Nacif M., Arai A.E., Gomes A.S., Hundley W.G., Johnson W.C., Prince M.R., Stacey R.B., Lima J.A., Bluemke D.A. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5:357–366. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aung N., Zemrak F., Mohiddin S.A., Petersen S.E. LV Noncompaction cardiomyopathy or just a lot of trabeculations? JACC Cardiovasc Imaging. 2017;10:704–707. doi: 10.1016/j.jcmg.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Kapadia R., Choudhary P., Collins N., Celermajer D., Puranik R. Left ventricular non-compaction in Holt-Oram syndrome. Heart Lung Circ. 2016;25:626–630. doi: 10.1016/j.hlc.2015.12.098. [DOI] [PubMed] [Google Scholar]
- 4.Basson C.T., Bachinsky D.R., Lin R.C. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 5.Basson C.T., Cowley G.S., Solomon S.D., Weissman B., Poznanski A.K., Traill T.A., Seidman J.G., Seidman C.E. The clinical and genetic spectrum of the Holt-Oram syndrome (heart-hand syndrome) N Engl J Med. 1994;330:885–891. doi: 10.1056/NEJM199403313301302. [DOI] [PubMed] [Google Scholar]
- 6.Hurst J.A., Hall C.M., Baraitser M. The Holt-Oram syndrome. J Med Genet. 1991;28:406–410. doi: 10.1136/jmg.28.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnall R.D., Weintraub R.G., Ingles J. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687. [DOI] [PubMed] [Google Scholar]
- 8.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barisic I., Boban L., Greenlees R. Holt Oram syndrome: a registry-based study in Europe. Orphanet J Rare Dis. 2014;9:156. doi: 10.1186/s13023-014-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newbury-Ecob R.A., Leanage R., Raeburn J.A., Young I.D. Holt-Oram syndrome: a clinical genetic study. J Med Genet. 1996;33:300–307. doi: 10.1136/jmg.33.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott D.A., Bressan M.C., He J. TBX5 genetic testing validates strict clinical criteria for Holt-Oram syndrome. Pediatr Res. 2005;58:981–986. doi: 10.1203/01.PDR.0000182593.95441.64. [DOI] [PubMed] [Google Scholar]
- 12.Boogerd C.J., Dooijes D., Ilgun A., Mathijssen I.B., Hordijk R., van de Laar I.M., Rump P., Veenstra-Knol H.E., Moorman A.F., Barnett P., Postma A.V. Functional analysis of novel TBX5 T-box mutations associated with Holt-Oram syndrome. Cardiovasc Res. 2010;88:130–139. doi: 10.1093/cvr/cvq178. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K., Takenaka K., Ebihara A. Prognostic impact of left ventricular noncompaction in patients with Duchenne/Becker muscular dystrophy–prospective multicenter cohort study. Int J Cardiol. 2013;168:1900–1904. doi: 10.1016/j.ijcard.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov A., Dabiesingh D.S., Bhumireddy G.P., Mohamed A., Asfour A., Briggs W.M., Ho J., Khan S.A., Grossman A., Klem I., Sacchi T.J., Heitner J.F. Prevalence and prognostic significance of left ventricular noncompaction in patients referred for cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006174. [DOI] [PubMed] [Google Scholar]