Summary
The continuity of epithelial tissue is collapsed by tooth eruption. The junctional epithelium (JE) is attached to the tooth surface by hemidesmosomes, which constitutes the front-line defense against periodontal bacterial infection. JE constitutively expresses intercellular adhesion molecule-1 (ICAM-1), and neutrophils and lymphocytes penetrate into JE via interaction between ICAM-1 and LFA-1 expressed on the surface of these migrating cells. JE also expresses cytokines and chemokines. These functions of JE are maintained even in germ-free condition. Therefore, the constitutive expression of adhesion molecules, cytokines, and chemokines might be used not only for anti-pathogenic defense but also for maintaining the physiological homeostasis of JE. In this review, we have mainly focused on the structural and functional features of JE, and discussed the function of intraepithelial lymphocytes in JE as a front-line anti-microbial defense barrier and regulator of JE hemostasis.
Keywords: The junctional epithelium, Intraepithelial lymphocytes, ICAM-1, Cytokines, Chemokines, Enamel organ
1. Introduction
Epithelial tissues form junctional complexes to maintain a continuous demarcation against the external environment and prevent the invasion of foreign substances inside the living body. Tooth eruption collapses the continuity of epithelial tissue in the oral cavity. Erupted teeth are surrounded by the gingival epithelium, which is one of the most specialized tissues of the body because the discontinuity of the gingival epithelial sheath renders it susceptible to bacterial infection from dental plaques and periodontal disease [1], [2], [3].
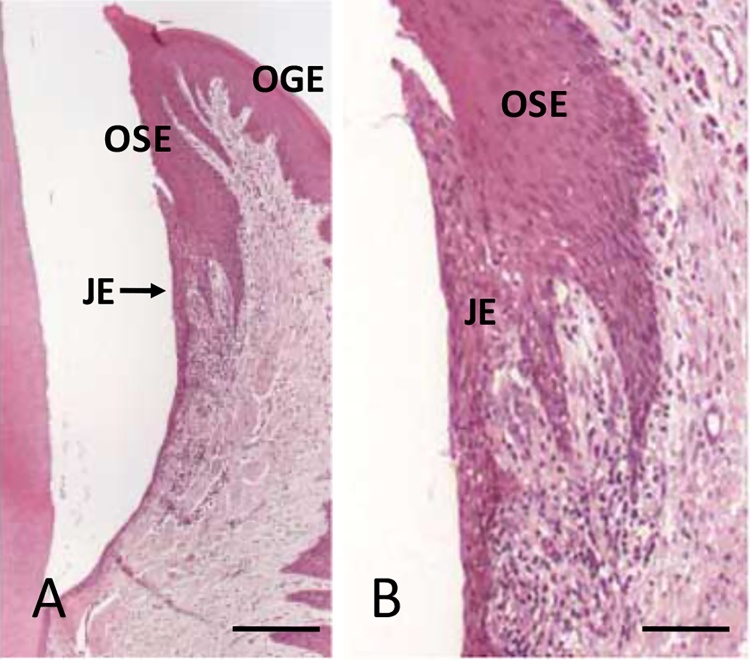
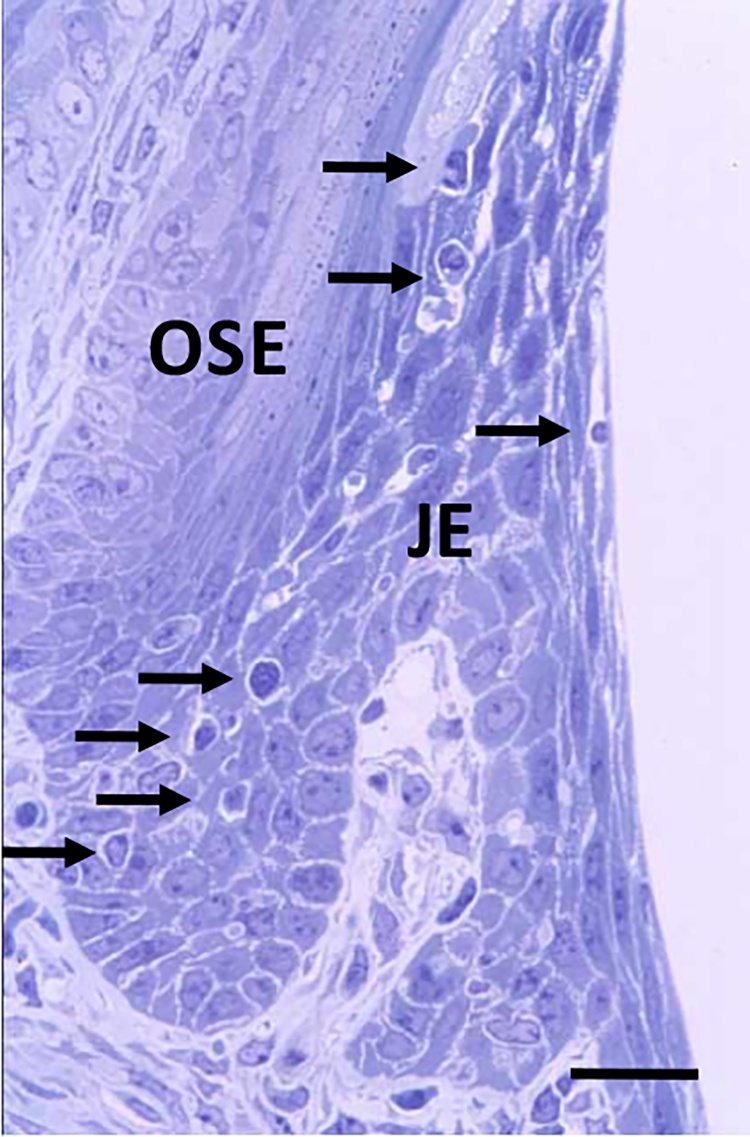
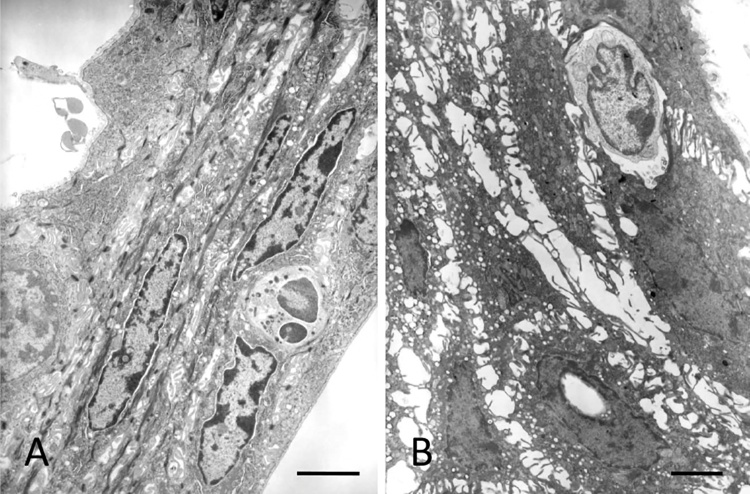
The gingival epithelium consists of the oral gingival epithelium (OGE), oral sulcular epithelium (OSE), and junctional epithelium (JE) (Fig. 1A, B). Among them, the JE attaches to the tooth surface via hemidesmosomes, which forms the front-line of defense against periodontal bacterial infection [4], [5]. In rodents, the JE is clearly distinguished from OSE (Fig. 2). The JE originates from the enamel organ and constructs the gingival epithelium along with OGE and OSE, which are derived from the oral epithelium [6], [7]. The OGE and OSE are keratinized squamous epithelial cells with narrow intercellular space [8], [9]. On the contrary, the JE is non-keratinized epithelium and has wide intercellular space (Fig. 3A, B). In addition, many migrating cells such as polymorphonuclear cells (PMNs) and lymphocytes are located within JE [10], [11], [12], [13], [14], [15] (Fig. 3A, B). The JE shows rapid turnover, which might contribute to its defense against dental plaque [16], [17]. The structure of the gingival epithelium is maintained by the coordination of epithelial tissues of two different origins. Therefore, the structure, origin, and function of the JE at the dento-gingival junction have been the subject of interest and controversy.
Figure 1.
Human gingiva. A: Inner gingival epithelium consists of OSE and JE. Bar = 250 μm. B: Higher magnification of the JE. Bar = 100 μm.
Figure 2.
Mouse gingiva. The JE is clearly distinguished from OSE. Many migrating cells (arrows) are detected in the JE. Bar = 50 μm.
Figure 3.
Ultrastructure of the JE showing wide intercellular space. Neutrophil (A) and lymphocyte are migrating within the JE. Bars = 10 μm.
2. Structure of the JE
JE is classified as non-keratinized stratified squamous epithelium. Intercellular junctions formed in JE are relatively loose and contain only few desmosomes, adherens junctions, and gap junctions, which allow tissue exudate and inflammatory cells to penetrate toward the gingival sulcus [1], [4], [5], [18], [19], [20].
JE has a true basement membrane towards the connective tissue of gingiva (called the external basal lamina, EBL) and a simple ECM (called the internal basal lamina, IBL) against the enamel [21] (Fig. 3A, B). The EBL contains structures that are identical to those of a typical basement membrane, with the lamina lucida against the basal keratinocytes and the lamina densa towards the underlying connective tissue. The protein composition of the IBL differs significantly from that of a typical basement membrane. The IBL does not contain any typical basement membrane-forming proteins such as laminin 111, laminin 511, type IV and VII collagens, and perlecan [22]. The main cell adhesion protein identified so far in the IBL is laminin 332 [22], [23], [24]. Furthermore, reports show the presence of type VIII collagen and versican at the JE-tooth interface [25], [26]. These proteins were not detected in other typical basement membranes. These findings indicate the uniqueness of JE and provide important information regarding the biological relationship between dental implants and the gingival epithelium to establish the effective interface.
In general, epithelial cells form basement membranes for survival in cell culture systems [27], [28], [29], [30]. However, detailed studies regarding the protein composition of basement membrane are lacking. Therefore, an extensive study of the protein composition of epithelial cell basement membranes in culture might clarify the structure and function of the JE basement membrane.
3. Origin of the JE
The structure and the function of the JE are influenced by the underlying connective tissue and by contact with a solid substratum, such as enamel, dentin or cementum, the exact mechanisms that lead to the formation and regeneration of the JE remain unclear. Common notion posits that during tooth development, the primary JE is formed by the fusion of the reduced enamel organ, which is replaced in time by a secondary JE derived solely from cells of the OE [31], [32], [33], [34], [35]. The reduced enamel epithelium is composed of reduced ameloblasts and papillary cells located at the side of the connective tissue. The histological analysis clearly distinguishes OSE from the JE. The function of JE is also entirely different from OSE. Therefore, is might be reasonable that the JE is derived from the reduced enamel epithelium.
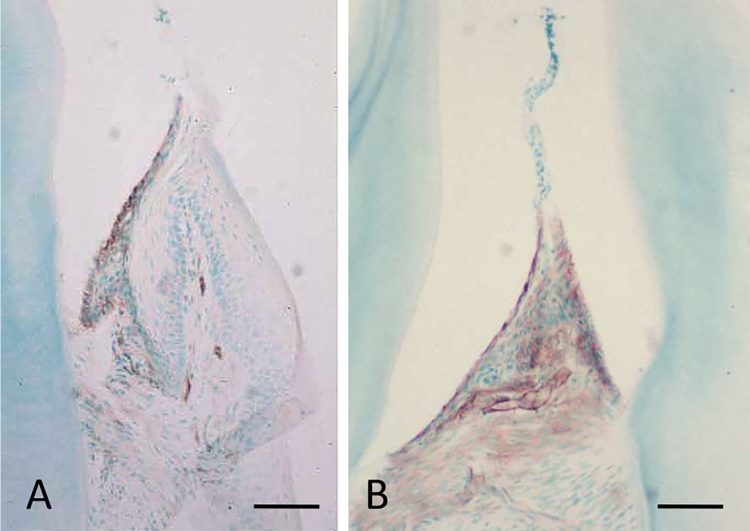
We previously demonstrated that the constitutive expression of ICAM-1 in the gingival epithelium is restricted in the JE of erupted molar teeth of mouse [36] (Fig. 4A, B). The expression of ICAM-1 in the JE was also reported in human and experimental animal gingiva [37], [38], [39]. The expression of ICAM-1 is constitutively maintained even in the JE of germ-free mouse [36].
Figure 4.
Immunohistichemical localization of ICAM-1 in the JE of labial side (A) and intertooth area (B) of a germ-free-mouse. Intensive immunoreactions are detected at the JE. B: The JE between the two molars. Endothelial cells beneath the JE also expressed ICAM-1. Bars = 80 μm.
Studies demonstrated that keratinocytic ICAM-1 expression in dermatological disease is localized precisely in the lymphocyte infiltration site of the epithelium. Keratinocytes do not express ICAM-1 after recovery from disease [40], [41], [42]. These results indicate that the expression of ICAM-1 is regulated by pro-inflammatory cytokines such as interluekin-1 (IL-1) and tumor necrosis factor-α (TNF-α) [43], [44], [45], [46]. Therefore, regulation of ICAM-1 expression and differences in ICAM-1 levels in JE might be regulated not only by bacteria-derived LPS, which stimulates macrophages to produce cytokines [46], but also by intrinsic factors. In fact, JE constitutively express IL-1β, which regulates the expression of ICAM-1 in JE [36], [47].
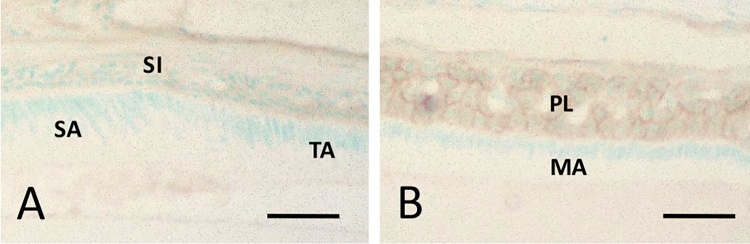
During tooth development, the inner enamel epithelium differentiates into the secretary, transitional, mature, and reduced stages of ameloblasts, and the stratum intermedium, adjacent to the secretary stage of ameloblasts, differentiates into the papillary layer [48], [49]. ICAM-1 expression is initiated during the formation of the papillary layer (Fig. 5A, B). However, all stages of ameloblasts do not express ICAM-1. The papillary layer adheres to the oral epithelial tissue during tooth eruption. These results indicate that the JE might have originated from papillary layer cells and not the reduced ameloblasts. ICAM-1 expression may be regulated by certain autocrine or paracrine factors regulating cell death of ameloblasts at the transition stage. Although IL-1β might be the key molecule for ICAM-1 expression [43], [44], [45], [46], further studies are required to clarify the mechanism of ICAM-1 expression during tooth development.
Figure 5.
Immunohistochemical localization of ICAM-1 during the tooth development. A: ICAM-1 is not detected at the stratum intermedium adjacent to the secretoru stage of ameloblasts and expresses form the transition stage of ameloblasts at the papillary layer. B: The papillary layer cells highly express ICAM-1 at the maturation and the reduced stages of ameloblasts. SI: stratum intemedium, SA: secretory stage of ameloblast, TA: transition stage of ameloblast, PL: papillary layer, MA: maturation stage of ameloblast. Bars = 30 μm.
4. Functional specificity of the JE
The JE has several specific functions that differ from those of other oral epithelium. JE expresses defensive factors such as β-defensins, secretory leukocyte protease inhibitor (SLPI), and S100A8 against inflammation [50], [51], [52]. β-Defensins are small cationic peptides that contribute to innate host defense against bacterial challenge [50], [52]. SLPI protects the intestinal epithelium from proteases secreted as part of the inflammatory response and is associated with the maintenance of tissue integrity [53]. S100A8 and S100A9 form a heterodimeric complex and constitute calprotectin, an antimicrobial peptide [54].
The JE constitutively produces chemokines and cytokines, such as keratinocyte-derived chemokine (KC), macrophage inflammatory protein-2 (MIP-2), and IL-1β in conventional and germ-free mice [47]. KC and MIP-2 are potent chemoattractants for neutrophils. The expression of these chemokines is up-regulated by bacterial stimulation.
The JE also constitutively express follicular dendritic cell-secreted protein (FDC-SP) and odontogenic ameloblast-associated protein (ODAM) [55], [56], [57]. The genes encoding FDC-SP and odontogenic ameloblast-associated protein appose at the gene cluster for secretory calcium-binding phosphoproteins [58], [59]. Secretory calcium-binding phosphoproteins interact with several bioactive molecules to regulate cell adhesion, migration, proliferation, and tumorigenesis [60], [61]. Furthermore, the gene encoding FDC-SP lies adjacent to the cluster of genes encoding C–X–C chemokines [58]. These results suggest a close association between the expression of pro-inflammatory cytokines and chemokines and the expression of FDC-SP and odontogenic ameloblast-associated protein.
Histological and immunohistological investigation indicates massive infiltration of lymphocyte function-associated antigen-1 (LFA-1)-positive PMNs and lymphocytes in the JE [36], [37]. The interaction of LFA-1 and ICAM-1 plays a key role in the migration of these cells in JE. Endothelial cells and fibroblasts underlying the JE also express ICAM-1, which might provide a pathway for granulocytic and lymphocytic migration into JE.
5. Role of commensal microflora
All the factors mentioned above are up-regulated in conventional conditions [47]. The commensal microflora plays a critical role in the postnatal development of the system, as the physiological inflammatory response in the gut during early postnatal ontogenesis is essential for the development of the immune system and its appropriate functioning. An interaction between commensal bacteria and the innate defense system of the periodontal tissue has also been reported [62]. Hayashi et al. observed the increased expression of SLPI in the JE compared to that in oral gingival epithelium [50]. The expression of SLPI is regulated by a cross-talk between epithelial cells and neutrophils. Thus, the constitutive expression of these factors and the migration of neutrophils in the JE might be induced by intrinsic genetic regulation rather than by bacterial infection for the maintenance of the functional specificity of the JE.
Histological examination indicated an increase in the JE area in conventional mice [47]. Immunohistochemistry also indicated an increase in the number of PCNA-positive epithelial cells within the JE upon bacterial infection [47]. Furthermore, the number of neutrophils and the expression of chemokines (KC and MIP-2) in the junctional epithelium are significantly higher in conventionalized mice than in germ-free mice [47]. Tsukamoto et al. evaluated the relationship between epithelial cell proliferation and inflammation in clinically healthy and inflamed human gingival tissue and found that epithelial cell proliferation and epithelial thickness were associated with gingival inflammation [63]. Similarly, chemokines were induced by bacterial lipopolysaccharide [64], [65]. Thus, commensal bacteria might be important for up-regulating the physiological barrier function of the JE.
6. Intraepithelial cells in the JE
Intraepithelial cells (IELs) are lymphocytes located within the epithelial layer of mucosal tissues such as the gastrointestinal and reproductive tract. IELs were localized in the middle layer of the JE as indicated by mucosa associated lymphoid tissue (MALT) [66]. IELs are characterized by the presence of higher numbers of TCRγδ+ lymphocytes than those in the systemic immune organs such as the spleen and lymph nodes [67].
The vast majority of TCRγδ+ IELs expresses CD8αα, but a small proportion lacks CD8α and CD8β, which is obviously different from the TCRγδ+ T cells located in lymphoid tissues, which show no CD8 expression [68], [69]. The TCR repertoires of IELs in each mucosal tissue were restricted by the in situ microbial colonizations in the tissue [70]. In humans, the γδ TCRs expressed by IELs predominantly use the Vγ1 gene and Vδ1, whereas in mice, Vγ5 T cells are enriched in the intraepithelial tissues of the gut [71], [72].
Non-classical molecules such as the thymus leukemia antigen or the major histocompatibility complex (MHC) class I-like molecules are candidate ligands for TCRγδ. TCRγδ+ T cells, including IELs, may be involved in a totally distinct type of host defense, whereas TCRαβ-positive T cells, a major population in peripheral lymphoid organs, elicit the MHC class II-restricted or MHC class I-restricted immune responses [73], [74].
The TCRγδ+ lymphocytes play an important role in the defense mechanism as a front-line competent cell population. IELs are frequently exposed to a number of microbial antigens and might be cytotoxic against infected epithelial cells [73], [74]. IELs have also been suggested to be involved in graft-versus-host diseases and abrogation of oral tolerance [75], [76]. In contrast, IELs produce growth factors to regulate the epithelial cell proliferation [77], [78], [79].
IELs in the JE express TCRγδ and CD3 in conventional and germ-free mice [36], [47]. The existence of TCRγδ T cells in the JE was also reported in humans [80]. Furthermore, TCRαβ-expressing T cells and B cells could not be detected in the JE, whereas in other mucosal epithelial tissues, TCRαβ-positive T cells constituted the main population of IELs [73], [81]. These results suggest that the JE might be a specialized mucosal tissue in the body. The identification of TCR repertoires might be the next step for clarifying the exact function and IEL antigens in JE.
7. The expected function of IELs in the JE
Previous studies from our and other laboratories indicated that IELs in the duodenum and jejunum induced enterocyte apoptosis within 30 min after activation by anti-CD3ε antibody treatment [82], [83], [84]. Cytotoxic activity of IELs is inhibited by immunosuppressive agents such as cyclosporine A (CsA) and FK506, which induce the elongation of intestinal villi and decrease enterocyte activity [82], [84].
As mentioned above, the JE plays an active role in the synthesis of a variety of molecules that are involved in anti-bacterial defense. Therefore, the balance between cell death and mitotic activity is critical for maintaining the number of JE cells, which affects the defensive properties of the JE, and eventually, disease progression.
The JE and OSE form the inner gingival epithelium. The origins of these two epithelial tissues are different. The JE and OSE have a high rate of cell turnover. The JE shows higher proliferation than the OSE [16], [85]. To maintain the structure of the inner gingival epithelium over time, either the cell cycle of the two epithelial tissues must be identical or cells of the epithelium showing high proliferative activity should be eliminated.
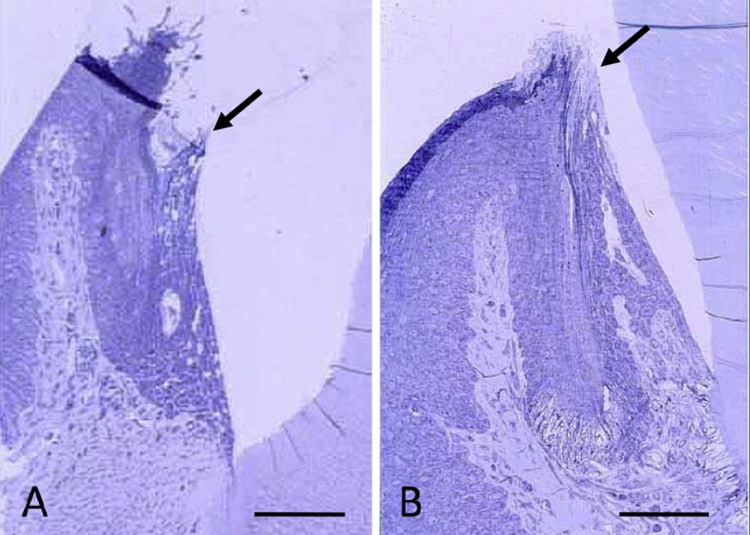
Three weeks treatment with CsA (25 mg/kg) induced the elongation of JE. In normal conditions, the tips of the JE are always located under the OSE, while the tips of the JE are guided to a position higher than the OSE by CsA treatment (Fig. 6A, B). which indicate that the cytotoxic activity of IELs expressing TCRγδ might be inhibited by CsA. These results suggest that IELs in the JE regulate epithelial cell survival for maintaining barrier function against bacterial stimulation similar to that in the gastrointestinal tract. The inhibition of rapid turnover of JE might decrease the survival activity and the barrier function of JE, and permit the easy penetration of bacterial LPS into the gingival connective tissue.
Figure 6.
The effect of cyclosporine A on the JE. Mice are intraperioneally injected with cyclosporine A (25 mg/kg) for 21 days. A: Normal JE. The tip of the JE (arrow) is lower than the OSE. B: By cyclosporine A treatment, the JE elongated and the tip of the JE (arrow) reaches almost to the same level with the OSE. Bars = 50 μm.
8. Conclusion
The JE is the only discontinuous epithelial tissue in the body. The JE functions as a front-line barrier against foreign antigens with structural and functional features such as adhesion to tooth surface via formation of basal lamina, large intercellular space, expression of several cytokines and chemokines, and the penetration of neutrophils and lymphocytes to play as the front-line innate immune system and to maintain the JE homeostasis. Further understanding of the structure and function of the JE would provide better understanding of systemic host defense system and specificity of the oral mucosal tissue.
Conflicts of interest
The author declare that no competing financial interests exist.
Acknowledgment
This study was partially supported by the Grants-in Aid for Scientific Research (24592777, 15K11022) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Schroeder H.E., Listgarten M.A. The gingival tissues: the architecture of periodontal protection. Periodontology. 2000;1997(13):91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder H.E., Listgarten M.A. The junctional epithelium: from strength to defense. J Dent Res. 2003;82:158–161. doi: 10.1177/154405910308200302. [DOI] [PubMed] [Google Scholar]
- 3.Pöllänen M.T., Salonen J.I., Uitto V.J. Structure and function of the tooth-epithelial interface in health and disease. Periodontology. 2000;2003(31):12–31. doi: 10.1034/j.1600-0757.2003.03102.x. [DOI] [PubMed] [Google Scholar]
- 4.Luke D. The structure and functions of the dentogingival junction and periodontal ligament. Br Dent J. 1992;172:187–190. doi: 10.1038/sj.bdj.4807818. [DOI] [PubMed] [Google Scholar]
- 5.Ten Cate A.R. The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Dis. 1996;2:55–62. doi: 10.1111/j.1601-0825.1996.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanci A., Bosshardt D.D. Structure of periodontal tissues in health and disease. Periodontology. 2000;2006(40):11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 7.Bampton J.L., Shirlaw P.J., Topley S., Weller P., Wilton J.M. Human junctional epithelium: demonstration of a new marker, its growth in vitro and characterization by lectin reactivity and keratin expression. J Invest Dermatol. 1991;96:708–717. doi: 10.1111/1523-1747.ep12470948. [DOI] [PubMed] [Google Scholar]
- 8.Squier C.A. Keratinization of the sulcular epithelium—a pointless pursuit? J Periodontol. 1981;52:426–429. doi: 10.1902/jop.1981.52.8.426. [DOI] [PubMed] [Google Scholar]
- 9.Matsson L., Theilade J., Attström R. Electron microscopic study of junctional and oral gingival epithelia in the juvenile and adult beagle dog. J Clin Periodontol. 1979;6:425–436. [Google Scholar]
- 10.Maruyama S., Itagaki M., Ida-Yonemochi H., Kubota T., Yamazaki M., Abé T. Perlecan-enriched intercellular space of junctional epithelium provides primary infrastructure for leukocyte migration through squamous epithelial cells. Histochem Cell Biol. 2014;142:297–305. doi: 10.1007/s00418-014-1198-x. [DOI] [PubMed] [Google Scholar]
- 11.Kido M.A., Yamaza T., Goto T., Tanaka T. Immunocytochemical localization of substance P neurokinin-1 receptors in rat gingival tissue. Cell Tissue Res. 1999;297:213–222. doi: 10.1007/s004410051349. [DOI] [PubMed] [Google Scholar]
- 12.Scott D.A., Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura H., Nakakura-Ohshima K., Maeda T., Ohshima H. Different distribution of immunocompetent cells in the dentogingival junction during root formation in rat molars. J Periodontal Res. 2003;38:10–19. doi: 10.1034/j.1600-0765.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- 14.Tonetti M.S., Straub A.M., Lang N.P. Expression of the cutaneous lymphocyte antigen and the alpha IEL beta 7 integrin by intraepithelial lymphocytes in healthy and diseased human gingiva. Arch Oral Biol. 1995;40:1125–1132. doi: 10.1016/0003-9969(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Séguier S., Godeau G., Brousse N. Immunohistological and morphometric analysis of intra-epithelial lymphocytes and Langerhans cells in healthy and diseased human gingival tissues. Arch Oral Biol. 2000;45:441–452. doi: 10.1016/s0003-9969(00)00018-2. [DOI] [PubMed] [Google Scholar]
- 16.Shimono M., Ishikawa T., Enokiya Y., Muramatsu T., Matsuzaka K., Inoue T. Biological characteristics of the junctional epithelium. J Electron Microsc. 2003;52:627–639. doi: 10.1093/jmicro/52.6.627. [DOI] [PubMed] [Google Scholar]
- 17.Salonen J., Kallajoki M. Immunohistochemical localization of transferrin receptors in junctional and sulcular epithelium of human gingiva. Arch Oral Biol. 1986;31:345–349. doi: 10.1016/0003-9969(86)90155-x. [DOI] [PubMed] [Google Scholar]
- 18.Skougaard M.R. Cell renewal, with special reference to the gingival epithelium. Adv Oral Biol. 1970;4:261–288. doi: 10.1016/b978-0-12-030504-9.50015-9. [DOI] [PubMed] [Google Scholar]
- 19.Stern I.B. Current concepts of the dentogingival junction: the epithelial and connective tissue attachments to the tooth. J Periofontol. 1981;52:465–476. doi: 10.1902/jop.1981.52.9.465. [DOI] [PubMed] [Google Scholar]
- 20.Bosshardt D.D., Lang N.P. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 21.Larjava H., Koivisto L., Häkkinen L., Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res. 2011;90:1367–1376. doi: 10.1177/0022034511402207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hormia M., Sahlberg C., Thesleff I., Airenne T. The epithelium-tooth interface—a basal lamina rich in laminin-5 and lacking other known laminin isoforms. J Dent Res. 1998;77:1479–1485. doi: 10.1177/00220345980770070201. [DOI] [PubMed] [Google Scholar]
- 23.Hormia M., Owaribe K., Virtanen I. The dento-epithelial junction: cell adhesion by type I hemidesmosomes in the absence of a true basal lamina. J Periodontol. 2001;72:788–797. doi: 10.1902/jop.2001.72.6.788. [DOI] [PubMed] [Google Scholar]
- 24.Oksonen J., Sorokin L.M., Virtanen Hormia M. The junctional epithelium around murine teeth differs from gingival epithelium in its basement membrane composition. J Dent Res. 2001;80:2093–2097. doi: 10.1177/00220345010800121401. [DOI] [PubMed] [Google Scholar]
- 25.Abiko Y., Nishimura M., Rahemtulla F., Mizoguchi I., Kaku T. Immunohistochemical localization of large chondroitin sulphate proteoglycan in porcine gingival epithelia. Eur J Morphol. 2001;39:99–104. doi: 10.1076/ejom.39.2.99.7372. [DOI] [PubMed] [Google Scholar]
- 26.Salonen J., Oda D., Funk S.E., Sage H. Synthesis of type VIII collagen by epithelial cells of human gingiva. J Periodontal Res. 1991;26:355–360. doi: 10.1111/j.1600-0765.1991.tb02074.x. [DOI] [PubMed] [Google Scholar]
- 27.Hahn U., Schuppan D., Hahn E.G., Merker H.J., Riecken E.O. Intestinal cells produce basement membrane proteins in vitro. Gut. 1987;28(Supple):143–151. doi: 10.1136/gut.28.suppl.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecay T.W., Valentich J.D. Basal lamina formation by epithelial cell lines correlates with laminin A chain synthesis and secretion. Exp Cell Res. 1992;203:32–38. doi: 10.1016/0014-4827(92)90036-8. [DOI] [PubMed] [Google Scholar]
- 29.Patrone L.M., Cook J.R., Crute B.E., Van Buskirk R.G. Differentiation of epithelial cells on microporous membranes. J Tissue Culture Methods. 1992;14:225–234. [Google Scholar]
- 30.Nguyen N.M., Miner J.H., Pierce R.A., Senior R.M. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol. 2002;246:231–244. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- 31.Glavind L., Zander H.A. Dynamics of dental epithelium during tooth eruption. J Dent Res. 1970;49:549–555. doi: 10.1177/00220345700490031401. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder H.E., Münzel-Pedrazzoli S. Morphometric analysis comparing junctional and oral epithelium of normal human gingiva. Helv Odontol Acta. 1970;14(2):53–66. [PubMed] [Google Scholar]
- 33.Massoth D.L., Dale B.A. Immunohistochemical study of structural proteins in developing junctional epithelium. J Periodontol. 1986;57:756–763. doi: 10.1902/jop.1986.57.12.756. [DOI] [PubMed] [Google Scholar]
- 34.Heymann R., Wroblewski J., Terling C., Midtvedt T., Obrink B. The characteristic cellular organization and CEACAM1 expression in the junctional epithelium of rats and mice are genetically programmed and not influenced by the bacterial microflora. J Periodontol. 2001;72:454–460. doi: 10.1902/jop.2001.72.4.454. [DOI] [PubMed] [Google Scholar]
- 35.Larjava H., Koivisto L., Häkkinen L., Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res. 2011;90(12):1367–1376. doi: 10.1177/0022034511402207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujioka M., Sasa R., Inoue M., Nakamura M. Immunological characterization of junctional epithelium: an immunohistochemical study. Dent Med Res. 2009;29:253–258. [Google Scholar]
- 37.Crawford J.M., Hopp B. Junctional epithelium expresses the intercellular adhesion molecule ICAM-1. J Periodontal Res. 1990;25:254–256. doi: 10.1111/j.1600-0765.1990.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 38.Crawford J.M. Distribution of ICAM-1, LFA-3 and HLA-DR in healthy and diseased gingival tissues. J Periodontal Res. 1992;27:291–298. doi: 10.1111/j.1600-0765.1992.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 39.Moughal N.A., Adonogianaki E., Thornhill M.H., Kinane D.F. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J Periodontal Res. 1992;27:623–630. doi: 10.1111/j.1600-0765.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 40.Dustin M.L., Singer K.H., Tuck D.T., Springer T.A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon and is mediated by intercellular adhesion molecule 1 (ICAM-1) J Exp Med. 1988;167:1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caughman S.W., Li L.J., Degitz K. Human intercellular adhesion molecule-1 gene and its expression in the skin. J Invest Dermatol. 1992;98(6 Suppl):61S–65S. doi: 10.1111/1523-1747.ep12462226. [DOI] [PubMed] [Google Scholar]
- 42.Krutmann J., Grewe M. Involvement of cytokines, DNA damage, and reactive oxygen intermediates in ultraviolet radiation-induced modulation of intercellular adhesion molecule-1 expression. J Invest Dermatol. 1995;105(1 Suppl):67S–70S. doi: 10.1111/1523-1747.ep12316095. [DOI] [PubMed] [Google Scholar]
- 43.Norris D.A. Cytokine modulation of adhesion molecules in the regulation of immunologic cytotoxicity of epidermal targets. J Invest Dermatol. 1990;95(6 Suppl):111S–120S. doi: 10.1111/1523-1747.ep12874977. [DOI] [PubMed] [Google Scholar]
- 44.Van der Meide P.H., Schellekens H. Cytokines and the immune response. Biotherapy. 1996;8:243–249. doi: 10.1007/BF01877210. [DOI] [PubMed] [Google Scholar]
- 45.Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39:37–55. doi: 10.1002/biof.1041. [DOI] [PubMed] [Google Scholar]
- 46.Boyle J.J. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–68. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 47.Tsukamoto Y., Usui M., Yamamoto G., Takagi Y., Tachikawa T., Yamamoto M. Role of the junctional epithelium in periodontal innate defense and homeostasis. J Periodontal Res. 2012;47:750–757. doi: 10.1111/j.1600-0765.2012.01490.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith C.E., Nanci A. Secretory activity as a function of the development and maturation of ameloblasts. Connect Tissue Res. 1989;22:147–156. [PubMed] [Google Scholar]
- 49.Smith C.E., Nanci A. Overview of morphological changes in enamel organ cells associated with major events in amelogenesis. Int J Dev Biol. 1995;39:153–161. [PubMed] [Google Scholar]
- 50.Hayashi Y., Matsunaga T., Yamamoto G., Nishii K., Usui M., Yamamoto M. Comprehensive analysis of gene expression in the junctional epithelium by laser microdissection and microarray analysis. J Periodontal Res. 2010;45:618–625. doi: 10.1111/j.1600-0765.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- 51.Nishii K., Usui M., Yamamoto G., Yajima S., Tsukamoto Y., Tanaka J. The distribution and expression of S100A8 and S100A9 in gingival epithelium of mice. J Periodontal Res. 2013;48:235–242. doi: 10.1111/jre.12000. [DOI] [PubMed] [Google Scholar]
- 52.Dinulos J.G., Mentele L., Fredericks L.P., Dale B.A., Darmstadt G.L. Keratinocyte expression of human beta defensin 2 following bacterial infection: role in cutaneous host defense. Clin Diagn Lab Immunol. 2003;10:161–166. doi: 10.1128/CDLI.10.1.161-166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Si-Tahar M., Merlin D., Sitaraman S., Madara J.L. Constitutive and regulated secretion of secretory leukocyte proteinase inhibitor by human intestinal epithelial cells. Gastroenterology. 2000;118:1061–1071. doi: 10.1016/s0016-5085(00)70359-3. [DOI] [PubMed] [Google Scholar]
- 54.Goyette J., Geczy C.L. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 55.Shinomura T., Nakamura S., Ito K., Shirasawa S., Höök M., Kimura J.H. Adsorption of follicular dendritic cell-secreted protein (FDC-SP) onto mineral deposits. Application of a new stable gene expression system. J Biol Chem. 2008;283:33658–33664. doi: 10.1074/jbc.M800719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishio C., Wazen R., Kuroda S., Moffatt P., Nanci A. Expression pattern of odontogenic ameloblast-associated and amelotin during formation and regeneration of the junctional epithelium. Eur Cell Mater. 2010;20:393–402. doi: 10.22203/ecm.v020a32. [DOI] [PubMed] [Google Scholar]
- 57.Dos Santos Neves J., Wazen R.M., Kuroda S., Francis Zalzal S., Moffatt P., Nanci A. Odontogenic ameloblast-associated and amelotin are novel basal lamina components. Histochem Cell Biol. 2012;137:329–338. doi: 10.1007/s00418-011-0901-4. [DOI] [PubMed] [Google Scholar]
- 58.Marshall A.J., Du Q., Draves K.E., Shikishima Y., HayGlass K.T., Clark E.A. FDC-SP, a novel secreted protein expressed by follicular dendritic cells. J Immunol. 2002;169:2381–2389. doi: 10.4049/jimmunol.169.5.2381. [DOI] [PubMed] [Google Scholar]
- 59.Kawasaki K., Lafont A.G., Sire J.Y. The evolution of milk casein genes from tooth genes before the origin of mammals. Mol Biol Evol. 2011;28:2053–2061. doi: 10.1093/molbev/msr020. [DOI] [PubMed] [Google Scholar]
- 60.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiodoni C., Colombo M.P., Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metasitasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 62.Darveau R.P. The oral microbial consortium’s interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Celenligil-Nazliel H., Palali A., Ayhan A., Ruacan S. Analysis of in situ proliferative activity in oral gingival epithelium in patients with xerostomia. J Periodontol. 2003;74:247–254. doi: 10.1902/jop.2003.74.2.247. [DOI] [PubMed] [Google Scholar]
- 64.Miyauchi M., Sato S., Kitagawa S., Hiraoka M., Kudo Y., Ogawa I. Cytokine expression in rat gingival periodontal tissues after topical application of lipopolysaccharide. Histochem Cell Biol. 2001;116:57–62. doi: 10.1007/s004180100298. [DOI] [PubMed] [Google Scholar]
- 65.Miyauchi M., Kitagawa S., Hiraoka M., Saito A., Sato S., Kudo Y., Ogawa I., Takata T. Immunolocalization of CXC chemokine and recruitment of polymorphonuclear leukocytes in the rat molar periodontal tissue after topical application of lipopolysaccharide. Histochem Cell Biol. 2004;121:291–297. doi: 10.1007/s00418-004-0636-6. [DOI] [PubMed] [Google Scholar]
- 66.Azzali G. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol Rev. 2003;195:178–189. doi: 10.1034/j.1600-065x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 67.Sheridan B.S., Lefrançois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep. 2010;12:513–521. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodman T., Lefrancois L. Expression of the gdT-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 69.Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Helgeland L., Dissen E., Dai K.Z., Midtvedt T., Brandtzaeg P., Vaage J.T. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur J Immunol. 2004;34:3389–3400. doi: 10.1002/eji.200425122. [DOI] [PubMed] [Google Scholar]
- 71.Pang D.J., Neves J.F., Sumaria N., Pennington D.J. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136:283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edelblum K.L., Shen L., Weber C.R., Marchiando A.M., Clay B.S., Wang Y. Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109:7097–7102. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayday A., Theodoridis E., Ramsburg E., Shires J. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 74.Cheroutre H., Lambolez F., Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ke Y., Pearce K., Lake J.P., Ziegler H.K., Kapp J.A. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618. [PubMed] [Google Scholar]
- 76.Wang X., Sherman A., Liao G., Leong K.W., Daniell H., Terhorst C. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev. 2013;65:759–773. doi: 10.1016/j.addr.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang H., Antony P.A., Wildhaber B.E., Teitelbaum D.H. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172(April (7)):4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 78.Cai Y.J., Wang W.S., Liang H.Y., Sun L.H., Teitelbaum D.H., Yang H. Keratinocyte growth factor up-regulates Interleukin-7 expression following intestinal ischemia/reperfusion in vitro and in vivo. Int J Clin Exp Pathol. 2012;5:569–580. [PMC free article] [PubMed] [Google Scholar]
- 79.Toulon A., Breton L., Taylor K.R., Tenenhaus M., Bhavsar D., Lanigan C. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;13(206):743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lundqvist C., Hammarstrom S., Hammarstrom M.L. Intraepithlial lymphocytes expressing TCRγδ in human gingival tissues. Adv Exp Med Biol. 1995;371B:1097–1102. [PubMed] [Google Scholar]
- 81.Tonetti M.S., Imboden M., Gerber L., Lang N.P. Compartmentalization of inflammatory cell phenotypes in normal gingiva and peri-implant keratinized mucosa. J Clin Periodontol. 1995;22:735–742. doi: 10.1111/j.1600-051x.1995.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 82.Hidaka M., Nakamura M., Ohmichi Y., Itoh J., Fukuzawa K., Masuko T. Involvement of intestinal intraepithelial lymphocytes in turnover of intestinal epithelial cells: morphological and functional alterations due to daily administration of FK506. Cell Immunol. 2012;279:124–133. doi: 10.1016/j.cellimm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Tamura A., Soga H., Yaguchi K., Yamagishi M., Toyota T., Sato J. Distribution of two types of lymphocytes (intraepithelial and lamina-propria-associated) in the murine small intestine. Cell Tissue Res. 2003;313:47–53. doi: 10.1007/s00441-003-0706-4. [DOI] [PubMed] [Google Scholar]
- 84.Yaguchi K., Kayaba S., Soga H., Yamagishi M., Tamura A., Kasahara S. DNA fragmentation and detachment of enterocytes induced by anti-CD3 mAb-activated intraepithelial lymphocytes. Cell Tissue Res. 2004;315:71–84. doi: 10.1007/s00441-003-0795-0. [DOI] [PubMed] [Google Scholar]
- 85.Jue S.S., Kim J.Y., Na S.H., Jeon K.D., Bang H.J., Park J.H. Localization of ODAM, PCNA, and CK14 in regenerating junctional epithelium during orthodontictooth movement in rats. Angle Orthod. 2014;84:534–540. doi: 10.2319/051613-378.1. [DOI] [PMC free article] [PubMed] [Google Scholar]