Summary
The application of resin composites in dentistry has become increasingly widespread due to the increased aesthetic demands of patients, improvements in the formulation of resin composites, and the ability of these materials to bond to tooth structures, together with concerns about dental amalgam fillings. As resistance to wear is an important factor in determining the clinical success of resin composite restoratives, this review article defines what constitutes wear and describes the major underlying phenomena involved in this process. Insights are further included on both in vivo and in vitro tests used to determine the wear resistance of resin composite and the relationships between these tests. The discussion focuses on factors that contribute to the wear of resin composite. Finally, future perspectives are included on both clinical and laboratory tests and on the development of resin composite restorations.
Keywords: Resin composites, Wear resistance, Wear testing
1. Introduction
The use of resin composites restorations in posterior teeth is increasing worldwide due to improvements in their mechanical properties [1]. An extensive database maintained by the Washington Dental Service in the U.S. reveals that the use of resin composite restorations in posterior teeth only surpassed that of amalgams in 1999 [2]. Among the reason for the switch from amalgam to resin composites might be the desire for restorations that match the color of the tooth and the increasing familiarity and comfort of clinicians with the use of resin composites [3]. In addition, early clinical studies of resin composite restorations in posterior teeth by Wilson et al. [4], [5], [6], [7] in the late 1980s and by Stangel et al. [8], [9] in 1990 reported a probability of success similar to that of amalgam, which might have encouraged the use of these restorations.
The increased use of resin composite restorations in posterior teeth has driven ongoing efforts to improve their clinical performance [10]. Alvanforoush et al. [11] stated that although the overall rates of clinical failure of resin composite restorations in posterior teeth showed little difference between 1995–2005 and 2006–2016, the causes of the failure showed a notable change, with wear becoming more important due to the increasing numbers of resin composite restorations in posterior teeth. Other systematic reviews have shown that the wear of resin composites is one of the reasons for fractures found in restorations in posterior teeth [12], [13]. Resin composite restorations in posterior teeth are subjected to a wide range of mechanical forces and chemical effects, such as food chewing and unconscious bruxism [14], [15], [16]. If the forces applied to the resin composite restorations exceed the mechanical strength of the material wear may occur which is particularly likely to happen in patients who apply greater than average forces during mastication [17]. These occlusal forces may also cause roughening of the surfaces, leading them to lose their shape [18]. Thus, the wear resistance of a resin composite is central to the long-term stability of restorations.
In 1998, Söderholm and Richards [19] published “Wear resistance of composites: a solved problem?” and concluded as follows: “Based on some clinical data, we can conclude that under some conditions, occlusal wear of posterior resin composites restoration remains a clinical problem, although not as bad as it was 10 years ago”. In 2006, Ferracane [20] re-evaluated the same topic and asked “Is the wear of dental composites still a clinical concern? Is there still a need for in vitro wear simulating devices?” He concluded that “While the wear resistance of dental composite restoratives is no longer considered to be a major concern for most restorations, the relatively limited information available suggests that it may still be a concern for very large restorations in direct occlusal contact, or for those patients with bruxing and clenching behavior.”
A PubMed literature search in English for “resin composites” in August 2017 shows that almost 10,000 articles have been published on this topic in the past 10 years (2008–2017). In this period, the number of articles published on “resin composite and wear” was 447 and “resin composite and abrasion” was 365. If the wear of resin composite is no longer of a concern, one must question why so many researchers continue to devote so much time to studying it. Therefore, it is perhaps useful to raise the question again: “Is the wear of resin composite restorations still a clinical concern?”
Based on the amount of resin composite sold, it is estimated that around 800 million resin composite restorations were placed worldwide in 2015 alone; with about 80% placed in the posterior region and 20% in the anterior region [21]. A meta-analysis of resin composite restorations in posterior teeth has shown that at least 5% of them failed due to fracture of the material and about 12% showed noticeable wear over an observation period of 10 years [22]. In other words, almost 77 million resin composite restorations in posterior teeth are likely to show noticeable wear, and about 32 million resin composite restorations placed in posterior teeth in 2015 will need to be repaired or replaced due to fracturing by 2025. This underlines the importance of the wear resistance of resin composite, and the need to understand the factors that influence it. Such understanding can only come from clinical and laboratory measurements and evaluations of wear, and so it is also important to understand the techniques available to do so.
The aim of this report is to review current insights into underlying wear mechanisms, evaluation methods, and the interacting factors that determine the occurrence and magnitude of wear in resin composites.
2. Mechanisms of wear
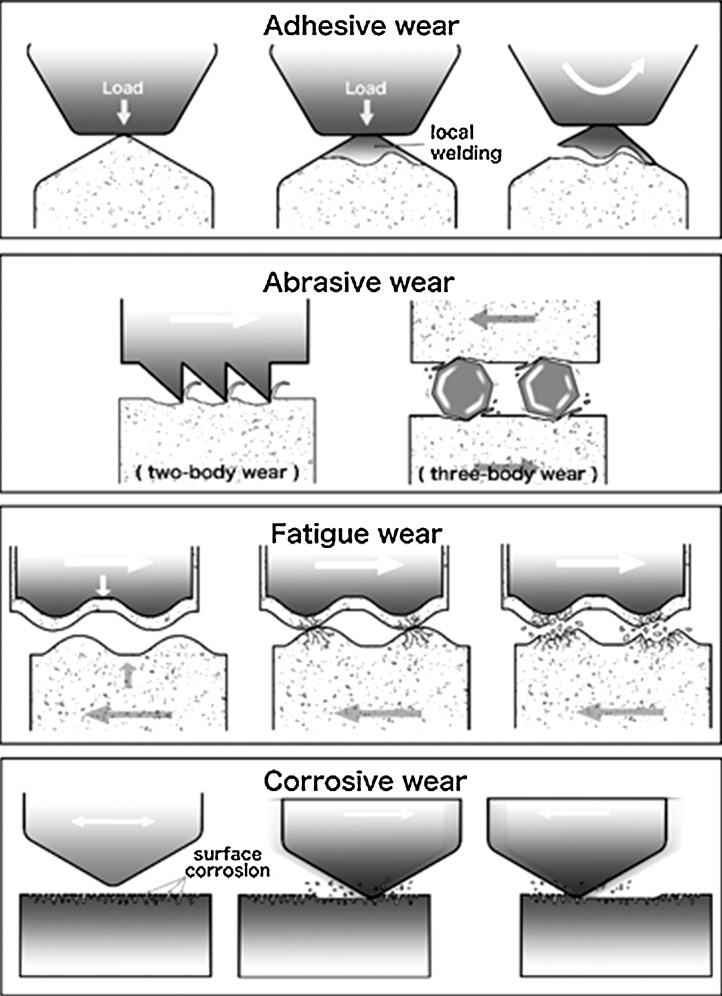
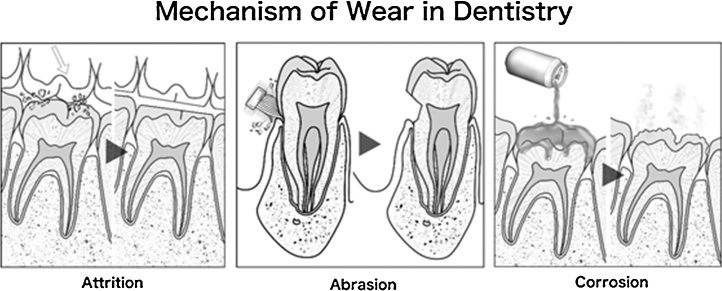
McCabe et al. [23] reports that the Institution of Mechanical Engineers has defined wear as “the progressive loss of substance resulting from mechanical interaction between two contacting surfaces, which are in relative motion”. Wear depends on three broad elements of a system: (1) its structure [24], [25], (2) the interaction conditions [26], [27], and (3) the environment and surface conditions [28], [29], [30]. The structure covers the types of materials and the geometry of their interaction, while the interaction conditions are the forces, stresses, and duration of the interaction. The environment and surface conditions are broad, covering the ambient temperature, surface chemistry, topography and environment. Engineers classify wear by its mechanism; (1) adhesive, (2) abrasive, (3) fatigue and (4) corrosive wear (Fig. 1) [31]. However, in dentistry, wear is typically classified in terms of its clinical manifestations. These include (1) attrition, or wear at contact sites, (2) abrasion, or wear at non-contact sites and (3) corrosion, or wear attributed to chemical effects (Fig. 2) [32]. Attrition and abrasion can arise from any of the four main wear mechanisms, although the meaning of “corrosion” is similar in both engineering and dentistry. In order to understand the problem of wear, it is necessary to first look at the underlying mechanisms.
Figure 1.
Mechanisms of wear in general: adhesive wear; abrasive wear; fatigue wear; corrosive wear.
Figure 2.
Mechanisms of wear in dentistry: attrition; abrasion; corrosion.
2.1. Adhesive wear
When two surfaces are pressed together by a load, local welding can occur at the contact points [33]. These welds are subject to shear forces as the surfaces slide relative to one another and are subject to breakage. When this breakage does not follow the weld interface, some material is transferred from one surface to the other. This transferred material often remains attached, and may even transfer back to the original surface. It is also common for this material to agglomerate and break away as a single entity; the particles thus created can contribute to abrasive wear. Some work has even shown these particles, such as sufficiently small filler particles, may accumulate in tissue [34].
In the oral cavity, as saliva has a lubricating role [35], it is likely that adhesive wear is limited, because the function of lubrication is to reduce and mitigate friction. This type of wear has not been shown to contribute significantly to the wear of resin composites, but it may appear at locations where an opposing cusp or contact point is forced against a resin composite surface [19].
2.2. Abrasive wear
In abrasive wear, material is scraped off a surface either by hard protuberances on the other surface or by hard particles at the interface [36]. The former situation is called two-body abrasion, while the latter is three-body abrasion.
Abrasion is thought to be an important wear mechanism in the oral environment, with the details depending on the location of the restoration. Abrasion from tooth brushing can affect any exposed surfaces, while occlusal wear is limited to contact surfaces [37]. Points of occlusal contact experience two-body abrasion, while other areas experience three-body abrasion from particles present between the teeth during mastication [38]. Most such particles are soft, and not all of them cause wear due to their angle of contact, so occlusal contact areas see much more abrasive wear than non-contact areas. Effects from differences in particles, namely food, on wear are particularly well-studied in the field of dental ecology [39].
The abrasive wear of resin composites is influenced by many factors [40]. First, the shape, size, orientation, and distribution of filler are important, as is the amount of filler [41]. Second, the type of resin matrix and the initiators for polymerization of resin composite influence the hardness of the resin composite surface. Third, bonding between fillers and resin matrix influences the likelihood of plucking of filler particles. All of these factors interact with the details of the loading, making the system extremely complex. Empirical studies have shown that abrasive wear is reduced when the size of or spacing between filler particles is reduced [42], when the degree of conversion of the resin matrix is increased [43], and when bonding between the filler and the resin matrix is improved [24].
The type of abrasive surface and abrasive particle also play an important role. If the resin composite is harder than the abrasive, wear is greatly reduced, and as the abrasive becomes harder, abrasive wear increases [44]. In addition, components of the resin composite that are harder than the abrasive can serve as barriers to abrasion, greatly reducing wear [45]. Shape is important in two-body abrasion [46], with angular protuberances producing more wear than rounded ones, even if the rounded ones are harder. This factor is less important in three-body abrasion, as the loose particles reorient themselves during contact which reduces antagonistic interactions [47].
2.3. Fatigue wear
Fatigue wear is caused by the repeated stressing and unstressing of a material, which can, over time, lead to the formation of microcracks at or below the surface [48]. As these cracks expand, they may join, leading to the detachment of a particle from the surface. An extreme example of this in tooth tissue is abfraction. This particle may go on to contribute to three-body abrasive wear. In the mouth, surface contact fatigue wear is thought to occur during mastication, where opposing teeth repeatedly come into contact [49].
Söderholm and Richards [19] have reported that the sliding of one surface over another can create a compression zone ahead of the motion and a zone of tension behind the motion. This pattern causes cycles of stress that may lead to fatigue wear. Although there has been little direct investigation of this type of wear in dental restorations, mastication seems well-suited to apply the necessary forces, and it is assumed that this process is an important wear mechanism in the oral environment.
2.4. Corrosive wear
Corrosion is due to a chemical reaction between the surface and the environment [36]. In many cases, this reaction is initially rapid, but then forms a cohesive layer of the reaction product, which protects the underlying surface. However, this film may be removed by sliding contact with another surface, exposing the unreacted material and allowing corrosion to continue. This is particularly likely if the corrosion layer is softer than the main material or not tightly bound to it.
Restorations in the oral cavity are exposed to potentially corrosive chemicals in food, drink, the secretions of microbes, and saliva [29], [30]. Plaque acids, food constituents, and enzymes have been shown to soften and roughen resin composites, both factors that are likely to increase vulnerability to abrasive wear [15]. On the other hand, saliva can have a buffering effect, reducing the acidity from foods and bacterial activity [15].
3. Evaluation of wear
The development of new resin composites requires highly accurate investigations of the novel materials with respect to both their wear behavior and their effect on the opposing dentition. It is important to ensure that the resin composites have a similar or, failing that, lesser wear resistance than teeth so as to preserve existing dentition. Hence, the resistance to wear of resin composites has been investigated through both clinical and laboratory methods.
3.1. Clinical wear evaluation methods
The methods used for clinical evaluation of wear can be divided into direct methods, which involve observation of the teeth themselves, and indirect methods, which work from casts [33].
Direct evaluations of resin composite restorations rely on a system of clinical parameters developed by Gunnar Ryge in the 1970s while working in the United States Public Health Service (USPHS) in San Francisco, California, United States [50], [51]. These are known as the USPHS criteria or Ryge Criteria. They involve the observation of restored teeth by a trained assessor and thus can be conducted quickly and accurately. However, the scales lack sensitivity, having only four categories for each aspect, and there are concerns about examiner calibration and comparison of results between studies. As researchers recognized the shortcomings of this scoring system, they modified the criteria according to their needs, which led to many different modified USPHS criteria [53], [54]. In 2007, further developed the USPHS criteria by systematically structuring them based on evidence and objective and subjective guidelines acknowledging, however, that wear can best be quantified by sophisticated equipment.
Indirect methods require impressions to be taken of the restored teeth. It is important that the teeth are cleaned extremely well before the impression is taken with polyvinylsiloxane material. Researchers recommend discarding the first set of impressions, as the impression material removes debris and plaque, making the second impression more accurate [56], [57]. On the other hand, a comparative analysis of the accuracy of clinical wear measurement using replica models revealed no difference between individually fitted and conventional trays [58].
The casts can then be evaluated in a variety of ways. Subjective methods include the Leinfelder scale [59], [60], which has six standards, and the Moffa–Lugassy scale [61], [62], which has 18 standards, and is based on dies with cylindrical defects. The Moffa–Lugassy scale was further developed into the Vivadent scale by Rheinberger, which uses tooth-sized dies with restoration-like defects [63].
A variety of systems can also be used to measure casts and tooth surfaces in order to offer a quantitative assessment of wear (topography, roughness, material loss, fractal dimension, etc.). These fall broadly into mechanical and optical systems. Mechanical systems (such as stylus profilometry and atomic force microscopy) rely on physical contact with the surface where the contours are mapped. Optical systems (such as laser scanning microscopy and white-light optical profilometry) depend on interactions of light with the surface being captured.
Optical systems appear to be superior in general, reaching accuracies of up to 10 μm [58]. The best systems appear to be digital, as Perry et al. [64] reported that three systems achieve a high level of accuracy: a system developed by Clinical Research Associates [65], the Minnesota Dental Research Center for Biomaterials and Biomechanics System (Minnesota System) [66], [67], and a three-dimensional microcomputer controlled mapping system [68]. The greatest advantage of these methods is that they provide a precise and quantitative measure of the extent, location, and morphology of wear, while the subjective systems merely rank wear on a scale. In addition, there are fewer concerns about the consistency of the systems over multiple measurements and experiments. However, they are not without issues.
The main problem is that they are expensive and time-consuming, and may be impractical in large-scale clinical investigations [69]. In addition, inexact casts may lead to high standard deviations, and there are problems with repositioning the samples for multiple measurements, such as before and after wear. An important consideration in the selection of an imaging technique is the lateral distance needed to transverse the entire specimen and the vertical distance needed to accommodate imaging. For example, imaging an entire restored tooth would be impractical with atomic force microscopy. Finally, the resolution of the imaging technique should be assessed. For example, white-light optical profilometry can have a lateral resolution of around 1–5 μm as opposed to 5–10 nm for atomic force microscopy. While wear imaging techniques typically do not achieve lateral resolutions on the nanometer scale, the nanometer scale can affect the surface relationship with bacteria, which may affect mechanical properties [30]. Therefore, techniques could be combined for more robust results for wear: full sample imaging and separate, more detailed surface analysis.
When attempting to use a clinical evaluation of wear to validate a laboratory test, DeLong et al. [70] describe three additional criteria that must be met. First, the clinical study must be of high quality and at least three years’ duration, in order to enable the assessment of fatigue wear. Second, the results from the clinical study must be reported in a format consistent with that used in the simulation. Third, the material used in the clinical study must be available for a simulation test of the same material. While there are clearly many issues facing clinical evaluation of wear, such studies are important if faster and lower cost laboratory wear testing methods are to be validated.
3.2. Laboratory wear evaluation methods
Laboratory wear testing is useful for the rapid assessment of the wear of resin composites, in contrast with clinical studies that may take years to generate results [71]. However, laboratory wear testing is one of the most challenging subjects in dental materials research. Since wear is a very complex process, as described earlier, its analysis is very difficult. Consequently researchers have focused on developing laboratory wear tests in an effort to assess the wear resistance of resin composites [72].
Assessments of the wear resistance of resin composites have been reported frequently in the dental literature since the first in vitro studies were published in 1975 [73]. A variety of laboratory wear testing devices have been developed in an effort to replicate the clinical masticatory process, making the interpretation of performance data more complex. Some laboratory wear tests attempt to re-create and simulate the oral environment as closely as possible, using machines that incorporate most or all of the known mechanisms of wear. Other tests are designed to study one mechanism of wear in isolation. Although there is no internationally recognized standard testing protocol to determine the wear resistance of resin composites, the International Organization for Standardization (ISO) published “Dental materials – Guidance on testing of wear –” in 2001. This report outlined a technical specification providing guidance on eight different methods for evaluating the wear resistance of dental materials in two- and three-body wear machines. These test methods vary with regard to load, the number of cycles and their frequency, abrasive medium, type of force actuator, and sliding versus direct antagonist contact (Table 1). Heintze et al. [75] stated that as the different wear simulator settings measure different wear mechanisms, it seems reasonable to combine at least two different wear settings to assess the wear resistance of a restorative material.
Table 1.
Test methods for wear included in ISO/TS 14569.
| Test method | Antagonist | Medium | Movement | Measurement |
|---|---|---|---|---|
| ACTA | Steel or dental material | Rice, husks of millet spray | Sliding | Profilometry |
| Alabama | Polyacetal | PMMA beads | Impact + sliding | REM |
| Freiburg | Al2O3 | H2O | Sliding | Mass or profilometry |
| DIN | Al2O3 | H2O | Sliding | Mass or profilometry |
| Minnesota | Tooth enamel | H2O | Sliding | Profilometry |
| Newcastle | Steatite or tooth enamel | H2O | Sliding | Profilometry |
| OHSU | Tooth enamel | Poppy seed | Impact + sliding | Profilometry + video-imaging |
| Zurich | Tooth enamel | H2O | Impact + sliding | Profilometry |
3.3. Correlation of laboratory and clinical wear
Ideally, the laboratory wear rates of resin composites should reflect the wear that is clinically measured with the same materials [76]. Research related to three of the methods included in the 2001 ISO publication has tried to establish clinical correlations. A correlation between the Minnesota wear simulation method and clinical wear measurements of resin composites was established, claiming that 300,000 cycles in the wear simulator correspond to about 1 year in vivo [70]. For the Zurich method, a different publication reports that 1,200,000 cycles in the simulator correspond to 5 years in clinic [76]. Barkmeier et al. [67] reported a correlation between the Alabama localized wear simulator and clinical wear measurements, claiming that 100,000 cycles in the simulator correspond to about 3.6 months in clinic for a resin composite. In addition, they also reported that Alabama generalized wear simulation had a good relationship to clinical wear of resin composites [77].
However, a more recent publication of Heintze et al. [76] in 2011 on the correlation of clinical and laboratory wear methods reported that the Oregon Health & Science University (OHSU) abrasion method showed adequate correlation with the clinical wear, but that other methods [Academisch Centrum Tandheelkunde Amsterdam (ACTA), Alabama generalized, Alabama localized, Munich, Ivoclar, and OHSU attrition] were only poorly correlated with the clinical wear. Similarly, in a workshop report on wear, it was stated that laboratory simulation methods are useful for studying fundamental wear characteristics, but they are not able to predict clinical wear [31]. It should, however, be the goal of any laboratory method to determine the relative wear characteristics of restorative materials and provide a predictive assessment of their clinical wear properties prior to use of a material in the oral cavity. Ferracane [69] has identified wear resistance as a property that should be used as a screening tool when selecting a resin composite to replace occlusal surfaces.
Therefore, the wear resistance of resin composites should be evaluated in the laboratory by reliable wear testing methods before they are tested in clinical trials. An effective laboratory wear simulation that reproduced the oral biomechanics would be of widespread value in shortening the time span before the chairside delivery of new dental materials, provided it correlates well with observed clinical outcomes. The need to develop and validate such a method remains pressing.
4. Current insight into the wear of various types of resin composite
Current resin composites cover a wide and complex variety of materials with an increasing range of properties, offering clinicians many choices for the restoration of anterior or posterior teeth [78]. Many studies of the laboratory wear of resin composites, using many different types of wear testing, have been done over the years, demonstrating that, as might be expected, the laboratory wear resistance of resin composites is influenced by the type of resin composite [33].
4.1. Wear of conventional (high viscosity) resin composites
Variations in resin composites arise from differences in manufacturing processes and the ingredients used. Resin composites often include different monomeric resin matrices, silane coupling agents, and filler technologies (filler volume fraction, particle size distribution, and filler density). Many types of monomer, silane coupling agents, and filler technologies have been developed in order to improve the mechanical properties and wear of resin composites [1], [78].
Earlier studies reported that the filler volume fraction played a particularly important role in the wear resistance of conventional resin composites and a higher filler volume fraction was reported to reduce the level of wear. However it should be noted that these studies were performed on older conventional resin composites, which had lower filler volume fractions than current products. In the older resin composites, a marked increase in filler volume fraction would be expected to make a substantial difference to the laboratory wear resistance. Osiewicz et al. [79] concluded that the results of various tests, when taken together, underwrite the use of conventional resin composites with a filler load in excess of 60 vol.%. Finlay et al. [80] performed tests of the laboratory wear resistance of experimental conventional resin composites provided by a dental manufacturer. In these resin composites, the filler volume fraction, diameter, density, and resin monomeric blend were systematically varied to allow the influences of the various factors to be compared. They found that wear of resin composites was primarily influenced by the properties of the filler, although wear was described as a ‘complex process’ and not all resin formulations were reported to behave similarly. The complexity of wear is further underlined by other results [81], [82], in which newly developed conventional resin composites showed variations in wear that could not be explained purely in terms of filler properties, and showed no clear connection to filler volume fraction. This shift may be due to changes in the filler size and volume fraction, as newer resin composites generally have smaller particles occupying a higher volume fraction.
4.2. Wear of nanofilled (high viscosity) resin composites
Considerable improvements have been made in resin composites over the last three decades with the modification of resin matrix formulations and filler characteristics [1]. More recently, improvements in resin composite technology have led to the introduction of nanofilled resin composites for clinical use [83]. Such resin composites have been reported to exhibit good mechanical properties [84], improved surface characteristics [85], better gloss retention [86], and reduced polymerization shrinkage [87]. However, nanofilled resin composites, incorporating a greater amount of nanofiller particles with a more homogeneous distribution in the resin matrix, have a larger interface area between the fillers and resin matrix than conventional resin composites [88]. In clinical situations, the rigid fillers transmit occlusal stress into the more flexible resin matrix during both functional and parafunctional activities [89]. This may lead to stress concentrations at the filler–resin matrix interface, which can result in filler dislodgment and exposure of the resin matrix, leading to wear [90].
Controlled clinical studies [91], [92] on posterior restorations using either nanofillled or conventional resin composites revealed no significant differences in clinical performance. In addition, laboratory studies [89], [90] have reported that some nanofilled resin composites show superior resistance to wear. Nevertheless, Tsujimoto et al. [93] suggested that it is important for clinicians to bear in the mind the risk of wear, even with nanofilled resin composites, and to routinely check restorations to determine whether repair is necessary, especially in restorations in posterior teeth.
In recent years, material development in nanofilled resin composite, such as silorane-based [94], fiber-reinforced [95], [96], [97], and bulk-fill resin composites [98], [99], have led to some changes in the perception of these materials. There is a limited amount of independent research on wear in silorane-based, fiber-reinforced, and bulk-fill resin composites, creating a need for evaluations of their wear properties.
4.3. Wear of flowable (low viscosity) resin composites
Recently, researchers have shown that the physical properties of flowable resin composites can be improved through the development of resin matrix technologies, increasing filler content, and modifying filler size [100]. Such improvements have expanded the clinical application of flowable resin composites to posterior restorations [101]. A recent clinical study [102] reported that the flowable and conventional composites used in that study had similar clinical efficacy after two years of service when placed as Class I occlusal restorations having isthmus widths less than one-half the intercuspal distance. Other studies [103], [104] have examined the wear of flowable resin composites for posterior teeth using various types of wear machines. Sumino et al. [103] compared flowable resin composites for posterior teeth, measuring the localized wear using an Alabama wear testing machine and the flexural properties by following ISO specifications. They concluded that the wear of the tested flowable resin composites was equivalent to that of nanofilled resin composites. In addition, a study conducted by Shinkai et al. [104] did not fully reject the hypothesis that the flowable resin composites would demonstrate differences in wear compared with the nanofilled resin composite. Some flowable resin composites for posterior teeth showed equivalent wear to the nanofilled resin composite. However, Shinkai et al. [104] also reported that the size of the filler might be a more significant factor for the wear of flowable resin composite than the physical properties of the resin matrix, in contrast to conventional resin composites, and that the inclusion of highly dense nano-particle or spherical submicron filler might improve the wear of flowable resin composites.
4.4. Wear of indirect resin composites
Direct resin composite restorations typically require a single appointment and therefore appear to have considerable immediate patient advantages over indirect resin composite restorations. In addition, recent systematic reviews [105], [106] have shown that there is no difference in the longevity of direct and indirect resin composite restorations in posterior teeth. However, the volumetric shrinkage of resin composite is about 2–4% [1], which for indirectly placed resin composite restorations can be partially accommodated by the luting cement. In addition, the stress generated by polymerization shrinkage of direct resin composite restorations is reported to be 13 times that of those placed using an indirect equivalents [107]. Further, indirect resin composite restorations are now prepared using systems that control light, temperature, humidity, pressure, and time, enabling better polymerization and a well-cured restoration [108], minimizing shrinkage stress [109] and improving mechanical properties [110]. Thus, indirect resin composites are widely used, particularly for large restorations in posterior teeth.
Research conducted on indirect resin composites has resulted in many encouraging findings, but the literature is divided. Ferracane et al. [111] reported that post-cure heat treatment at 120 °C for indirect resin composites could significantly improve their mechanical properties. It was suggested that this improvement results from an increased conversion rate of the monomers, which contributes to toughening the resin matrix and possibly improving filler-matrix adhesion, and from the relief of internal stresses through the high temperature treatment. In contrast, other studies [112] have found that indirect resin composites are not vastly superior to direct resin composites. Mandikos et al. [112] compared the wear of four second generation indirect resin composites to one first generation indirect resin composite and one direct resin composite, and concluded that the second generation indirect composites did not exhibit improved wear when compared to the first generation indirect composite, but were as good as direct resin composites. Asmussen et al. [113] investigated the effect of post-curing temperature on selected mechanical properties of indirect resin composites, and concluded that the improvements in physical properties were only moderate, about 9%. Ferracane et al. [114] studied the mechanical properties of four experimental and one commercial resin composite aged in water after heat treatment. The results showed that the improvements in the properties of indirect resin composites produced by heat-treating are of only short-term benefit, and are for the most part lost due to alteration of the resin matrix as the composite reaches equilibrium with water. Leinfelder [115] has reported that resin composites restorations that were exposed to both heat treatment and photo-curing showed very little enhancement of overall clinical performance compared to those that were photo-cured only. Although some situations specifically indicate indirect resin composite restorations, the advantages of using direct resin composite restorations, such as reduced cost and number of clinical sessions, make them an attractive alternative for many clinicians and patients. Nevertheless, it is still important to carefully consider the longevity of indirect resin composite restorations when making treatment decisions. In addition, the stability of indirect resin composite restorations is thought to be increased by using a resin luting cement with high wear resistance [116], [117]. Recently, Takamizawa et al. [118] and Tsujimoto et al. [119], [120] developed laboratory wear testing of resin luting cements using a new gap model, where wear at the marginal closure area is simulated. This kind of wear simulation of resin luting cements should be helpful in ensuring the success of indirect resin composite restorations.
Currently, implant placement with immediate provisionalization is the state of the art in implant dentistry [121]. Although the provisional restoration is usually in place for three months, this period may be prolonged if an extended evaluation period is required [122]. Thus, clinicians are becoming increasingly concerned with the wear of provisional materials [123]. A previous study related to the occlusal wear of provisional implant supported restorations concluded that indirect resin composite was the preferred chairside provisional restorative material when the provisional implant-supported restoration had to be in service for a relatively long period of time [124]. However, as Mandikos et al. [112] noted, limited independent research has been published on indirect materials and the properties specified in advertising literature are largely derived from in-house or contracted testing. Therefore, more research on the wear of indirect resin composites for implant supported provisional restorations would be valuable.
4.5. Wear of CAD/CAM resin composites
Computer-aided design and computer-aided manufacturing (CAD/CAM) systems have come to play a large role in the field of dentistry over the last two decades [125], [126]. CAD/CAM resin composites that include nanoparticle fillers are currently available for clinical use [127]. CAD/CAM resin composites are fabricated by high pressure and high temperature polymerization, resulting in improved physical properties that might make them more suitable as materials for applications ranging from inlays to single crown restorations [128]. In addition, restorations produced from CAD/CAM resin composites can be more easily fabricated and repaired than restorations made from CAD/CAM ceramics [129].
Lawson et al. [130] reported on the laboratory wear of CAD/CAM resin composite. They stated that wear for CAD/CAM resin composites was small, and that infiltrated ceramic and glass ceramics showed greater volume loss than CAD/CAM resin composites. In addition, the resin composites resulted in less opposing enamel wear than infiltrated ceramic or glass ceramics. These results were confirmed by Lauvahutanon et al. [131]. They also reported that the CAD/CAM resin composites investigated displayed low wear compared to that seen in resin composites for direct restorations when using the same wear test. These results suggest that the wear of CAD/CAM resin composites may be lower than that of other types of resin composites. However, Tsujimoto et al. [132] have reported that the laboratory wear of CAD/CAM resin composites differed depending on the material and that care should be taken when selecting one for clinical use. There is, again, a limited amount of independent research on CAD/CAM resin composites, creating a need for additional evaluations of their wear properties.
5. Future perspectives
The prediction of wear for new resin composites based on laboratory testing methods, the refinement of methods for quantifying wear, and the assessment of resin composite restorations from clinical wear studies remain important for future research. There are many questions to consider when performing laboratory wear evaluations, and to date, little has been accomplished in terms of standardizing test methods or data reporting. It is true that wear testing methods provide an indication of the ranking of novel resin composite formulations relative to commercially successful formulations. However, the different wear testing methods used to assess the wear rate of resin composites are based on different concepts, and the results can therefore not be easily compared with each other. Further, most of the laboratory methods used to test the wear of resin composites are not standardized, which makes comparisons among researchers difficult. The low reproducibility and high variability of test results, as well as the low correlation with clinical results, are likely to be the consequences of this lack of standardization and validation.
Mimicking the entire masticatory cycle, with all possible movements of the lower jaw, might be argued to be the best way to test a resin composite, but it is also important that devices be simple, robust, efficient, and only require a low level of simple maintenance. On the other hand, a machine that applies a force in only one direction is inadequate. An effective device would need some level of computer control and multi-directional force actuators in order to approach intraoral conditions, as well as allowing for water exchange in the test chamber. An effective in vitro wear simulation that reproduces oral biomechanics would be of widespread value in shortening the time span to the chairside delivery of resin composites, provided the results correlated with observed clinical outcomes.
There is also a need for more wear-resistant resin composites, so that resin composites can maintain their anatomical shape over longer time periods. Possible directions for future research include better and stronger monomers, better polymerization kinetics and higher conversion rates, intelligent filler technologies with optimal filler distribution and dimensions, as well as stable bonding between filler and matrix, and “self-repairing” resin composites containing microspheres that liberate a polymer that repairs cracks in the material.
6. Conclusion
The management of wear may become more feasible with a greater understanding of the underlying processes of resin composite wear. Further, insight into and criticism of the various methods used to verify the wear resistance of resin composites may be helpful for future in vitro and in vivo studies.
Conflict of interest
The authors of this manuscript certify that they have no proprietary, financial, or other personal interest of any nature or kind in any product, service, and/or company that is presented in this article.
Acknowledgements
This work was supported, in part, by Grant-in-Aid for Scientific Research (C) (Grant numbers: 17K11716 to M.M., 16K11565 to T.T.) and by Grant-in-Aid for Young Scientists (B) (Grant number: 17K17141 to A.T.) from the Japan Society for the Promotion of Science.
Footnotes
Scientific field of dental science: Dental materials.
References
- 1.Ferracane J.L. Resin composite—state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Bogacki R.E., Hunt R.J., del Aguila M., Smith W.R. Survival analysis of posterior restorations using an insurance claims database. Oper Dent. 2002;27:488–492. [Google Scholar]
- 3.Burke F.J.T. Amalgam to tooth-coloured materials — implications for clinical practice and dental education: governmental restrictions and amalgam-usage survey results. J Dent. 2004;32:343–350. doi: 10.1016/j.jdent.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M.A., Wilson N.H., Smith G.A. A clinical trial of a visible light-cured posterior composite resin restorative: two-year results. Quintessence Int. 1986;17:151–155. [PubMed] [Google Scholar]
- 5.Wilson N.H., Smith G.A., Wilson M.A. A clinical trial of a visible light cured posterior composite resin restorative material: three-year results. Quintessence Int. 1986;17:643–652. [PubMed] [Google Scholar]
- 6.Wilson N.H., Wilson M.A., Smith G.A. A clinical trial of a visible light cured posterior composite resin restorative material: four-year results. Quintessence Int. 1988;19:133–139. [PubMed] [Google Scholar]
- 7.Wilson N.H., Wilson M.A., Wastell D.G., Smith G.A. A clinical trial of a visible light cured posterior composite resin restorative material: five-year results. Quintessence Int. 1988;19:675–681. [PubMed] [Google Scholar]
- 8.Stangel I., Barolet R.Y. Clinical evaluation of two posterior composite resins: two-year results. J Oral Rehabil. 1990;17:257–268. doi: 10.1111/j.1365-2842.1990.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Stangel I., Barolet R.Y., Robert D. A five-year evaluation of two posterior composites and amalgam. J Dent Res. 1990;69:308. (Abstract 1600) [Google Scholar]
- 10.Sarrett D.C. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Alvanforoush N., Palamara J., Wong R.H., Burrow M.F. Comparison between published clinical success of direct resin composite restorations in vital posterior teeth in 1995–2005 and 2006–2016 periods. Aust Dent J. 2017;62:132–145. doi: 10.1111/adj.12487. [DOI] [PubMed] [Google Scholar]
- 12.Demarco F.F., Corrêa M.B., Cenci M.S., Moraes R.R., Opdam N.J.M. Longevity of posterior composite restorations: Not only a matter of materials. Dent Mater. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Beck F., Lettner S., Graf A., Bitriol B., Dumitrescu N., Bauer P. Survival of direct resin restorations in posterior teeth within a 19-year period (1996–2015): a meta-analysis of prospective studies. Dent Mater. 2015;31:958–985. doi: 10.1016/j.dental.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 14.de Gee A.J., van Duinen R.M.B., Werner A., Davidson C.L. Early and long-term wear of conventional and resin-modified glass ionomers. J Dent Res. 1996;75:1613–1619. doi: 10.1177/00220345960750081401. [DOI] [PubMed] [Google Scholar]
- 15.de Gee A.J., Wendl S.L., Werner A., Davidson C.L. Influence of enzymes and plaque acids on in vitro wear of dental composites. Biomaterials. 1996;17:1327–1332. doi: 10.1016/0142-9612(96)88679-0. [DOI] [PubMed] [Google Scholar]
- 16.Yip K.H., Smales R.J., Kaidonis J.A. Differential wear of teeth and restorative materials: clinical implications. Int J Prosthodont. 2004;17:350 3–56. [PubMed] [Google Scholar]
- 17.Yap A.U., Teoh S.H., Chew C.L. Effects of cyclic loading on occlusal contact area wear of composite restoratives. Dent Mater. 2002;18:149–158. doi: 10.1016/s0109-5641(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 18.Fujii K., Carrick T.E., Bicker R., McCabe J.F. Effect of the applied load on surface contact fatigue of dental filling materials. Dent Mater. 2004;20:931–938. doi: 10.1016/j.dental.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Söderholm K.J., Richards N.D. Wear resistance of composites: a solved problem. Gen Dent. 1998;46:256–263. [PubMed] [Google Scholar]
- 20.Ferracane J.L. Is the wear of dental composites still a clinical concern? Is there still a need for in vitro wear simulating devices. Dent Mater. 2006;22:689–692. doi: 10.1016/j.dental.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Heintze S.D., Ilie N., Hickel R., Reis A., Loguerucio A., Rousson V. Laboratory mechanical parameters of composite resins and their relation to fractures and wear in clinical trials—a systematic review. Dent Mater. 2017;33:e101–e114. doi: 10.1016/j.dental.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Heintze S.D., Rousson V. Clinical effectiveness of direct class II restorations—a meta-analysis. J Adhes Dent. 2012;14:407–431. doi: 10.3290/j.jad.a28390. [DOI] [PubMed] [Google Scholar]
- 23.McCabe J.F., Molyvda S., Rolland S.L., Rusby S., Carrick T.E. Two- and three-body wear of dental restorative materials. Int Dent J. 2002;52:406–416. [Google Scholar]
- 24.Nihei T., Dabanoglu A., Teranaka T., Kurata S., Ohashi K., Kondo Y. Three-body-wear resistance of the experimental composites containing filler treated with hydrophobic silane coupling agents. Dent Mater. 2008;24:760–764. doi: 10.1016/j.dental.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Lawson N.C., Burgess J.O. Wear of nanofilled dental composites at varying filler concentrations. J Biomed Mater Res B Appl Biomater. 2015;103:424–429. doi: 10.1002/jbm.b.33212. [DOI] [PubMed] [Google Scholar]
- 26.Wonglamsam A., Kakuta K., Ogura H. Effects of occlusal and brushing cycles on wear of composite resins in combined wear test. Dent Mater J. 2008;27:243–250. doi: 10.4012/dmj.27.243. [DOI] [PubMed] [Google Scholar]
- 27.Ghazal M., Kern M. Wear of human enamel and nano-filled composite resin denture teeth under different loading forces. J Oral Rehabil. 2009;36:58–64. doi: 10.1111/j.1365-2842.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- 28.Turssi C.P., Ferracane J.L., Serra M.C. Abrasive wear of resin composites as related to finishing and polishing procedures. Dent Mater. 2005;21:641–648. doi: 10.1016/j.dental.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Mayworm C.D., Camargo S.S., Jr., Bastian F.L. Influence of artificial saliva on abrasive wear and microhardness of dental composites filled with nanoparticles. J Dent. 2008;36:703–710. doi: 10.1016/j.jdent.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.de Paula A.B., Fucio S.B., Ambrosano G.M., Alonso R.C., Sardi J.C., Puppin-Rontani R.M. Biodegradation and abrasive wear of nano restorative materials. Oper Dent. 2011;36:670–677. doi: 10.2341/10-221-L. [DOI] [PubMed] [Google Scholar]
- 31.Mair L.H., Stolarski T.A., Vowles W., Lloyd C.H. Wear: mechanisms, manifestations and measurement. Report of a workshop. J Dent. 1996;24:141–148. doi: 10.1016/0300-5712(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 32.Mair L.H. Wear in dentistry—current terminology. J Dent. 1992;20:140–144. doi: 10.1016/0300-5712(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 33.Turssi C.P., Serra B. Wear of dental resin composites: insights into underlying processes and assessment methods—a review. J Biomed Mater Res B Appl Biomater. 2003;65:280–285. doi: 10.1002/jbm.b.10563. [DOI] [PubMed] [Google Scholar]
- 34.Gatti A.M. Biocompatibility of micro- and nano-particles in the colon. Part II. Biomaterials. 2004;25:385–392. doi: 10.1016/s0142-9612(03)00537-4. [DOI] [PubMed] [Google Scholar]
- 35.Mandel I.D. The functions of saliva. J Dent Res. 1987;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- 36.Ferracane J.L. Lippincott Williams & Wilkins; Philadelphia: 2001. Materials in dentistry: principle and application; pp. 293–311. [Google Scholar]
- 37.Cavalcante L.M., Masouras K., Watts D.C., Pimenta L.A., Silikas N. Effect of nanofillers’ size on surface properties after toothbrush abrasion. Am J Dent. 2009;22:60–64. [PubMed] [Google Scholar]
- 38.Koottathape N., Takahashi H., Iwasaki N., Kanehira M., Finger W.J. Two- and three-body wear of composite resins. Dent Mater. 2012;28:1261–1270. doi: 10.1016/j.dental.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Scott R.S., Ungar P.S., Bergstrom T.S., Brown C.A., Childs B.E., Teaford M.F. Dental microwear texture analysis: technical considerations. J Hum Evol. 2006;51:339–349. doi: 10.1016/j.jhevol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Han J.M., Zhang H., Choe H.S., Lin H., Zheng G., Hong G. Abrasive wear and surface roughness of contemporary dental composite resin. Dent Mater J. 2014;33:725–732. doi: 10.4012/dmj.2013-339. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira G.U., Mondelli R.F., Charantola Rodrigues M., Franco E.B., Ishikiriama S.K., Wang L. Impact of filler size and distribution on roughness and wear of composite resin after simulated toothbrushing. J Appl Oral Sci. 2012;20:510–516. doi: 10.1590/S1678-77572012000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayne S.C., Taylor D.F., Heymann H.O. Protection hypothesis for composite wear. Dent Mater. 1992;8:305–309. doi: 10.1016/0109-5641(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 43.Turssi C.P., Ferracane J.L., Vogel K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials. 2005;26:4932–4937. doi: 10.1016/j.biomaterials.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Manhart J., Kunzelmann K.H., Chen H.Y., Hickel R. Mechanical properties and wear behavior of light-cured packable composite resins. Dent Mater. 2000;16:33–40. doi: 10.1016/s0109-5641(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 45.Sekiya K., Okamoto A., Fukushima M., Iwaku M. In vivo wear pattern of experimental composite resins containing different filler components. Dent Mater J. 1994;13:36–46. doi: 10.4012/dmj.13.36. [DOI] [PubMed] [Google Scholar]
- 46.Krejci I., Albert P., Lutz F. The influence of antagonist standardization on wear. J Dent Res. 1999;78:713–719. doi: 10.1177/00220345990780021201. [DOI] [PubMed] [Google Scholar]
- 47.Lawson N.C., Cakir D., Beck P., Litaker M.S., Burgess J.O. Characterization of third-body media particles and their effect on in vitro composite wear. Dent Mater. 2012;28:e118–26. doi: 10.1016/j.dental.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mair L.H. Subsurface compression fatigue in seven dental composites. Dent Mater. 1994;10:111–115. doi: 10.1016/0109-5641(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 49.McCabe J.F., Wang Y., Braem M.J.A. Surface contact fatigue and flexural fatigue of dental restorative materials. J Biomed Mater Res. 2000;50:375–380. doi: 10.1002/(sici)1097-4636(20000605)50:3<375::aid-jbm11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 50.Cvar J, Ryge G. Criteria for the clinical evaluation of dental restorative materials. US DHEW Document, US Public Health Service 790244, Printing Office, San Francisco: US Government Printing Office; 1971, p. 1–42. Reprinted as Cvar J, Ryge G. Reprint of Criteria for the clinical evaluation of dental restorative materials. Clin Oral Invest 2005; 9: 215–52). [DOI] [PubMed]
- 51.Ryge G., Snyder M. Evaluating the clinical quality of restorations. J Am Dent Assoc. 1973;87:369–377. doi: 10.14219/jada.archive.1973.0421. [DOI] [PubMed] [Google Scholar]
- 53.Vann W.F., Jr., Barkmeier W.W., Mahler D.B. Assessing composite resin wear in primary molars: four-year findings. J Dent Res. 1988;67:876–879. doi: 10.1177/00220345880670051601. [DOI] [PubMed] [Google Scholar]
- 54.Bayne S.C., Schmalz G. Reprinting the classic article on USPHS evaluation methods for measuring the clinical research performance of restorative materials. Clin Oral Investig. 2005;9:209–214. doi: 10.1007/s00784-005-0017-0. [DOI] [PubMed] [Google Scholar]
- 56.Mehl A., Gloger W., Kunzelmann K.H., Hickel R. A new optical 3-D device for the detection of wear. J Dent Res. 1997;76:1799–1807. doi: 10.1177/00220345970760111201. [DOI] [PubMed] [Google Scholar]
- 57.Folwaczny M., Mehl A., Kunzelmann K.H., Hickel R. Determination of changes on tooth-colored cervical restorations in vivo using a three-dimensional laser scanning device. Eur J Oral Sci. 2000;108:233–238. doi: 10.1034/j.1600-0722.2000.108003233.x. [DOI] [PubMed] [Google Scholar]
- 58.Peschke A., Heintze S.D., Roulet J.F. Comparison of two impression methods for clinical wear measurement. J Dent Res. 2005;84 (Abstract 350) [Google Scholar]
- 59.Leinfelder K.F., Taylor D.F., Barkmeier W.W., Goldberg A.J. Quantitative wear measurement of posterior composite resins. Dent Mater. 1986;2:198–201. doi: 10.1016/S0109-5641(86)80013-6. [DOI] [PubMed] [Google Scholar]
- 60.Bayne S.C., Taylor D.F., Rekow E.D., Wilder A.D., Heymann H.O. Confirmation of Leinfelder clinical wear standards. Dent Mater. 1994;10:11–18. doi: 10.1016/0109-5641(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 61.Lugassy A.A., Moffa J.P. Laboratory model for the quantification of clinical occlusal wear. J Dent Res. 1985;64:181. (Abstract 63) [Google Scholar]
- 62.Moffa J.P., Lugassy A.A. Calibration of evaluators utilizing the M–L occlusal loss scale. J Dent Res. 1986;65:302. (Abstract 1197) [Google Scholar]
- 63.Bryant W. Comparison of three standards for quantifying occlusal loss of composite restorations. Dent Mater. 1990;6:60–62. doi: 10.1016/0109-5641(90)90047-i. [DOI] [PubMed] [Google Scholar]
- 64.Perry R., Kugel G., Kunzelmann K.H., Flessa H.P., Estafan D. Composite restoration wear analysis: conventional methods vs. three-dimensional laser digitizer. J Am Dent Assoc. 2000;131:1472–1477. doi: 10.14219/jada.archive.2000.0060. [DOI] [PubMed] [Google Scholar]
- 65.Bangerter V., Christensen R., Christensen G. Method for determination of in vivo wear. J Dent Res. 1987;66:126. (Abstract 258) [Google Scholar]
- 66.Sakaguchi R.L., Douglas W.H., DeLong R., Pintado M.R. The wear of a posterior composite in an artificial mouth. Dent Mater. 1986;2:235–250. doi: 10.1016/s0109-5641(86)80034-3. [DOI] [PubMed] [Google Scholar]
- 67.Barkmeier W.W., Latta M.A., Erickson R.L., Lambrechts P. Comparison of laboratory and clinical wear rates of resin composites. Quintessence Int. 2004;35:269–274. [PubMed] [Google Scholar]
- 68.Hewlett E.R., Orro M.E., Clark G.T. Accuracy testing of three-dimensional digitizing systems. Dent Mater. 1992;8:49–53. doi: 10.1016/0109-5641(92)90053-f. [DOI] [PubMed] [Google Scholar]
- 69.Ferracane J.L. Resin-based composite performance: are there some things we can’t predict. Dent Mater. 2013;29:51–58. doi: 10.1016/j.dental.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeLong R., Pintado M.R., Douglas W.H., Fok A.S., Wilder A.D., Jr., Swift E.J., Jr. Wear of a dental composite in an artificial oral environment: a clinical correlation. J Biomed Mater Res B Appl Biomater. 2012;100:2297–2306. doi: 10.1002/jbm.b.32801. [DOI] [PubMed] [Google Scholar]
- 71.Heintze S.D. How to qualify and validate wear simulation devices and methods. Dent Mater. 2006;22:712–734. doi: 10.1016/j.dental.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Latta M.A., Barkmeier W.W., Wilwerding T.M., Blake S.M. Localized wear of compomer restorative materials. Am J Dent. 2001;14:238–240. [PubMed] [Google Scholar]
- 73.Powell J.M., Phillips R.W., Norman R.D. In vitro wear response of composite resin, amalgam, and enamel. J Dent Res. 1975;54:1183–1195. doi: 10.1177/00220345750540061501. [DOI] [PubMed] [Google Scholar]
- 75.Heintze S.D., Barkmeier W.W., Latta M.A., Rousson V. Round robin test: wear of nine dental restorative materials in six different wear simulators — supplement to the round robin test of 2005. Dent Mater. 2011;27:e1–9. doi: 10.1016/j.dental.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Heintze S.D., Faouzi M.M., Rousson V., Özcan M. Correlation of wear in vivo and six laboratory wear methods. Dent Mater. 2012;28:961–973. doi: 10.1016/j.dental.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Barkmeier W.W., Latta M.A., Erickson R.L., Wilwerding T.M. Wear simulation of resin composites and the relationship to clinical wear. Oper Dent. 2008;33:177–182. doi: 10.2341/07-67. [DOI] [PubMed] [Google Scholar]
- 78.Ilie N., Hickel R. Resin composite restorative materials. Aust Dent J. 2011;56:59–66. doi: 10.1111/j.1834-7819.2010.01296.x. [DOI] [PubMed] [Google Scholar]
- 79.Osiewicz M.A., Werner A., Pytko-Polonczyk J., Roeters F.J., Kleverlaan C.J. Contact- and contact-free wear between various resin composites. Dent Mater. 2015;31:134–140. doi: 10.1016/j.dental.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Finlay N., Hahnel S., Dowling A.H., Fleming G.J.P. The in vitro wear behavior of experimental resin-based composites derived from a commercial formulation. Dent Mater. 2013;29:365–374. doi: 10.1016/j.dental.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Barkmeier W.W., Erickson R.I., Latta M.A., Wilwerding T.M. Wear rates of resin composites. Oper Dent. 2013;38:226–233. doi: 10.2341/12-112-L. [DOI] [PubMed] [Google Scholar]
- 82.Barkmeier W.W., Takamizawa T., Erickson R.L., Tsujimoto A., Latta M., Miyazaki M. Localized and generalized simulated wear of resin composites. Oper Dent. 2015;40:322–335. doi: 10.2341/13-155-L. [DOI] [PubMed] [Google Scholar]
- 83.Mitra S.B., Wu D., Holmes B.N. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 84.Ilie N., Hickel R. Investigations on mechanical behaviour of dental composites. Clin Oral Investig. 2009;13:427–438. doi: 10.1007/s00784-009-0258-4. [DOI] [PubMed] [Google Scholar]
- 85.Ergücü Z., Türkün L.S., Aladag A. Color stability of nanocomposites polished with one-step systems. Oper Dent. 2008;33:413–420. doi: 10.2341/07-107. [DOI] [PubMed] [Google Scholar]
- 86.Egilmez F., Ergun G., Cekic-Nagas I., Vallittu P.K., Lassila L.V. Estimation of the surface gloss of dental nano composites as a function of color measuring geometry. Am J Dent. 2012;25:220–226. [PubMed] [Google Scholar]
- 87.Sideridou I.D., Karabela M.M., Vouvoudi E.Ch. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent Mater. 2011;27:598–607. doi: 10.1016/j.dental.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Ilie N., Rencz A., Hickel R. Investigations towards nano-hybrid resin-based composites. Clin Oral Investig. 2013;17:185–193. doi: 10.1007/s00784-012-0689-1. [DOI] [PubMed] [Google Scholar]
- 89.Hahnel S., Schultz S., Trempler C., Ach B., Handel G., Rosentritt M. Two-body wear of dental restorative materials. J Mech Behav Biomed Mater. 2011;4:237–244. doi: 10.1016/j.jmbbm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Oliveira G.U., Mondelli R.F., Charantola Rodrigues M., Franco E.B., Ishikiriama S.K., Wang L. Impact of filler size and distribution on roughness and wear of composite resin after simulated toothbrushing. J Appl Oral Sci. 2012;20:510–516. doi: 10.1590/S1678-77572012000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palaniappan S., Bharadwaj D., Mattar D.L., Peumans M., Van Meerbeek B., Lambrechts P. Nanofilled and microhybrid composite restorations: five-year clinical wear performances. Dent Mater. 2011;27:692–700. doi: 10.1016/j.dental.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 92.Frankenberger R., Reinelt C., Krämer N. Nanohybrid vs. fine hybrid composite in extended class II cavities: 8-year results. Clin Oral Investig. 2014;18:125–137. doi: 10.1007/s00784-013-0957-8. [DOI] [PubMed] [Google Scholar]
- 93.Tsujimoto A., Barkmeier W.W., Takamizawa T., Latta M.A., Miyazaki M. Influence of thermal stress on simulated localized and generalized wear of nanofilled resin composites. Oper Dent. 2017 doi: 10.2341/16-206-L. in press. [DOI] [PubMed] [Google Scholar]
- 94.Magno M.B., Nascimento G.C., Rocha Y.S., Ribeiro B.D., Loretto S.C., Maia L.C. Silorane-based composite resin restorations are not better than conventional composites — a meta-analysis of clinical studies. J Adhes Dent. 2016;18:375–386. doi: 10.3290/j.jad.a36916. [DOI] [PubMed] [Google Scholar]
- 95.Garoushi S., Säilynoja E., Vallittu P.K., Lassila L. Physical properties and depth of cure of a new short fiber reinforced composite. Dent Mater. 2013;29:835–841. doi: 10.1016/j.dental.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 96.Tsujimoto A., Barkmeier W.W., Takamizawa T., Latta M.A., Miyazaki M. Mechanical properties, volumetric shrinkage and depth of cure of short fiber-reinforced resin composite. Dent Mater J. 2016;35:418–424. doi: 10.4012/dmj.2015-280. [DOI] [PubMed] [Google Scholar]
- 97.Tsujimoto A., Barkmeier W.W., Takamizawa T., Watanabe H., Johnson W.W., Latta M.A. Relationship between mechanical properties and bond durability of short fiber-reinforced resin composite with universal adhesive. Eur J Oral Sci. 2016;124:480–489. doi: 10.1111/eos.12291. [DOI] [PubMed] [Google Scholar]
- 98.Ilie N., Stark K. Curing behavior of high-viscosity bulk-fill composites. J Dent. 2014;42:977–985. doi: 10.1016/j.jdent.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Tsujimoto A., Barkmeier W.W., Takamizawa T., Latta M.A., Miyazaki M. Depth of cure, flexural properties and volumetric shrinkage of low and high viscosity bulk-fill giomers and resin composites. Dent Mater J. 2017;36:205–213. doi: 10.4012/dmj.2016-131. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi H., Finger W.J., Endo T., Kanehira M., Koottathape N., Komatsu M. Comparative evaluation of mechanical characteristics of nanofiller containing resin composites. Am J Dent. 2011;24:264–270. [PubMed] [Google Scholar]
- 101.Seemann R., Pfefferkorn F., Hickel R. Behavior of general dental practitioners in Germany regarding posterior restorations with flowable composites. Int Dent J. 2011;61:252–256. doi: 10.1111/j.1875-595X.2011.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lawson N.C., Radhakrishnan R., Givan D.A., Ramp L.C., Burgess J.O. Two-year randomized, controlled clinical trial of a flowable and conventional composite in Class I restorations. Oper Dent. 2015;40:594–602. doi: 10.2341/15-038-C. [DOI] [PubMed] [Google Scholar]
- 103.Sumino N., Tsubota K., Takamizawa T., Shiratsuchi K., Miyazaki M. Comparison of the wear and flexural characteristics of flowable resin composites for posterior lesions. Acta Odontol Scand. 2013;71:820–827. doi: 10.3109/00016357.2012.734405. [DOI] [PubMed] [Google Scholar]
- 104.Shinkai K., Taira Y., Suzuki S., Suzuki M. In vitro wear of flowable resin composite for posterior restorations. Dent Mater J. 2016;35:37–44. doi: 10.4012/dmj.2015-080. [DOI] [PubMed] [Google Scholar]
- 105.Angeletaki F., Gkogkos A., Papazoglou E., Kloukos D. Direct versus indirect inlay/onlay composite restorations in posterior teeth: A systematic review and meta-analysis. J Dent. 2016;53:12–21. doi: 10.1016/j.jdent.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 106.da Veiga A.M., Cunha A.C., Ferreira D.M., Chianca T.K., Reis T.K., Maia K.R. Longevity of direct and indirect resin composite restorations in permanent posterior teeth: a systematic review and meta-analysis. J Dent. 2016;54:1–12. doi: 10.1016/j.jdent.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Dejak B., Młotkowski A. A comparison of stresses in molar teeth restored with inlays and direct restorations, including polymerization shrinkage of composite resin and tooth loading during mastication. Dent Mater. 2015;31:e77–87. doi: 10.1016/j.dental.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Tanoue N., Murakami M., Koizumi H., Atsuta M., Matsumura H. Depth of cure and hardness of an indirect composite polymerized with three laboratory curing units. J Oral Sci. 2007;49:25–29. doi: 10.2334/josnusd.49.25. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto T., Nakamura Y., Nishide A., Kubota Y., Momoi Y. Contraction stresses in direct and indirect composite restorations compared by crack analysis. J Adhes Dent. 2013;15:47–54. doi: 10.3290/j.jad.a28171. [DOI] [PubMed] [Google Scholar]
- 110.Hirata M., Koizumi H., Tanoue N., Ogino T., Murakami M., Matsumura H. Influence of laboratory light sources on the wear characteristics of indirect composites. Dent Mater J. 2011;30:127–135. doi: 10.4012/dmj.2010-043. [DOI] [PubMed] [Google Scholar]
- 111.Ferracane J.L., Condon J.R. Post-cure heat treatments for composites: properties and fractography. Dent Mater. 1992;8:290–295. doi: 10.1016/0109-5641(92)90102-i. [DOI] [PubMed] [Google Scholar]
- 112.Mandikos M.N., McGivney G.P., Davis E., Bush P.J., Carter J.M. A comparison of the wear resistance and hardness of indirect composite resins. J Prosthet Dent. 2001;85:386–395. doi: 10.1067/mpr.2001.114267. [DOI] [PubMed] [Google Scholar]
- 113.Asmussen E., Peutzfeldt A. Mechanical properties of heat treated restorative resins for use in the inlay/onlay technique. Scand J Dent Res. 1990;98:564–567. doi: 10.1111/j.1600-0722.1990.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 114.Ferracane J.L., Hopkin J.K., Condon J.R. Properties of heat-treated composites after aging in water. Dent Mater. 1995;11:354–358. doi: 10.1016/0109-5641(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 115.Leinfelder K.F. Indirect posterior composite resins. Compend Contin Educ Dent. 2005;26:495–503. [PubMed] [Google Scholar]
- 116.Furuichi T., Takamizawa T., Tsujimoto A., Miyazaki M., Barkmeier W.W., Latta M.A. Mechanical properties and sliding-impact wear resistance of self-adhesive resin cements. Oper Dent. 2016;41:E83–92. doi: 10.2341/15-033-L. [DOI] [PubMed] [Google Scholar]
- 117.Tsujimoto A., Barkmeier W.W., Takamizawa T., Watanabe H., Johnson W.W., Latta M.A. Simulated localized wear of resin luting cement for universal adhesive systems with different curing mode. J Oral Sci. 2017 doi: 10.2334/josnusd.16-0815. in press. [DOI] [PubMed] [Google Scholar]
- 118.Takamizawa T., Barkmeier W.W., Latta M.A., Berry T.P., Tsujimoto A., Miyazaki M. Simulated wear of self-adhesive resin cements. Oper Dent. 2016;41:327–338. doi: 10.2341/14-227-L. [DOI] [PubMed] [Google Scholar]
- 119.Tsujimoto A., Barkmeier W.W., Takamizawa T., Miyazaki M., Latta M.A. Simulated gap wear of resin luting cements. Dent Mater. 2016;32:e42. doi: 10.2341/16-270-L. (Abstract 86) [DOI] [PubMed] [Google Scholar]
- 120.Tsujimoto A., Barkmeier W.W., Takamizawa T., Latta M.A., Miyazaki M. Relationship between simulated gap wear and generalized wear of resin luting cements. Oper Dent. 2017 doi: 10.2341/16-270-L. [DOI] [PubMed] [Google Scholar]
- 121.Lewis S. Anterior single-tooth implant restorations. Int J Periodontics Restorative Dent. 1995;15:30–41. [PubMed] [Google Scholar]
- 122.Tarnow D.P., Eskow R.N. Preservation of implant esthetics: soft tissue and restorative considerations. J Esthet Restor Dent. 1996;8:12–19. doi: 10.1111/j.1708-8240.1996.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 123.Takamizawa T., Barkmeier W.W., Tsujimoto A., Scheidel D., Erickson R.L., Latta M.A. Mechanical properties and simulated wear of provisional resin materials. Oper Dent. 2015;40:603–613. doi: 10.2341/14-132-L.1. [DOI] [PubMed] [Google Scholar]
- 124.Santing H.J., Kleverlaan C.J., Werner A., Feilzer A.J., Raghoebar G.M., Meijer H.J. Occlusal wear of provisional implant-supported restorations. Clin Implant Dent Relat Res. 2015;17:179–185. doi: 10.1111/cid.12072. [DOI] [PubMed] [Google Scholar]
- 125.Miyazaki T., Hotta Y. CAD/CAM systems available for the fabrication of crown and bridge restorations. Aust Dent J. 2011;56:97–106. doi: 10.1111/j.1834-7819.2010.01300.x. [DOI] [PubMed] [Google Scholar]
- 126.Yoshida F., Tsujimoto A., Ishii R., Nojiri K., Takamizawa T., Miyazaki M. Influence of surface treatment of contaminated lithium disilicate and leucite glass ceramics on surface free energy and bond strength of universal adhesives. Dent Mater J. 2015;34:855–862. doi: 10.4012/dmj.2015-123. [DOI] [PubMed] [Google Scholar]
- 127.Ruse N.D., Sadoun M.J. Resin composite block for dental CAD/CAM applications. J Dent Res. 2014;93:1232–1234. doi: 10.1177/0022034514553976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen J.F., Migonney V., Ruse N.D., Sadoun M. Resin composite blocks via high-pressure high-temperature polymerization. Dent Mater. 2012;28:529–534. doi: 10.1016/j.dental.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Rocca G.T., Bonnafous F., Rizcalla N., Krejci I. A technique to improve the esthetic aspects of CAD/CAM composite resin restorations. J Prosthetic Dent. 2010;104:273–275. doi: 10.1016/S0022-3913(10)60138-2. [DOI] [PubMed] [Google Scholar]
- 130.Lawson N.C., Bansal R., Burgess J.O. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent Mater. 2016;32:e275–83. doi: 10.1016/j.dental.2016.08.222. [DOI] [PubMed] [Google Scholar]
- 131.Lauvahutanon S., Takahashi H., Shiozawa M., Iwasaki N., Asakawa Y., Oki M. Mechanical properties of composite resin blocks for CAD/CAM. Dent Mater J. 2014;33:705–710. doi: 10.4012/dmj.2014-208. [DOI] [PubMed] [Google Scholar]
- 132.Tsujimoto A., Barkmeier W.W., Takamizawa T., Latta M.A., Miyazaki M. Influence of thermal cycling on flexural properties and simulated wear of computer-aided design/computer-aided manufacturing resin composites. Oper Dent. 2017;42:101–110. doi: 10.2341/16-046-L. [DOI] [PubMed] [Google Scholar]