Abstract
Objective
To evaluate the efficacy and tolerability of single pulse transcranial magnetic stimulation (sTMS) for the preventive treatment of migraine.
Background
sTMS was originally developed for the acute treatment of migraine with aura. Open label experience has suggested a preventive benefit. The objective of this trial was to evaluate the efficacy and tolerability of sTMS for migraine prevention.
Methods
The eNeura SpringTMS Post-Market Observational U.S. Study of Migraine (ESPOUSE) Study was a multicenter, prospective, open label, observational study. From December 2014 to March 2016, patients with migraine (n = 263) were consented to complete a 1-month baseline headache diary followed by 3 months of treatment. The treatment protocol consisted of preventive (four pulses twice daily) and acute (three pulses repeated up to three times for each attack) treatment. Patients reported daily headache status, medication use, and device use with a monthly headache diary. The primary endpoint, mean reduction of headache days compared to baseline, was measured over the 28-day period during weeks 9 to 12. The primary endpoint was compared to a statistically-derived placebo estimate (performance goal). Secondary endpoints included: 50% responder rate, acute headache medication consumption, HIT-6, and mean reduction in total headache days from baseline of any intensity.
Results
Of a total of 263 consented subjects, 229 completed a baseline diary, and 220 were found to be eligible based on the number of headache days. The device was assigned to 217 subjects (Safety Data Set) and 132 were included in the intention to treat Full Analysis Set. For the primary endpoint, there was a −2.75 ± 0.40 mean reduction of headache days from baseline (9.06 days) compared to the performance goal (−0.63 days) (p < 0.0001). The 50% responder rate of 46% (95% CI 37%, 56%) was also significantly higher (p < 0.0001) than the performance goal (20%). There was a reduction of −2.93 (5.24) days of acute medication use, headache impact measured by HIT-6, −3.1 (6.4) (p < 0.0001), and total headache days of any intensity −3.16 days (5.21) compared to the performance goal (−0.63 days) (p < 0.0001). The most common adverse events were lightheadedness (3.7%), tingling (3.2%), and tinnitus (3.2%). There were no serious adverse events.
Conclusions
This open label study suggests that sTMS may be an effective, well-tolerated treatment option for migraine prevention.
Trial registration number
Keywords: Migraine, transcranial magnetic stimulation, preventive treatment, single-pulse
Abbreviations
- sTMS
single pulse Transcranial Magnetic Stimulation
- rTMS
repetitive Transcranial Magnetic Stimulation
- CSD
cortical spreading depression
- DLPFC
dorsolateral prefrontal cortex
- TMS
Transcranial Magnetic Stimulation
Introduction
Migraine is a highly prevalent neurologic disease affecting over 38 million people in the United States (1). It is the leading cause of neurological disability and the seventh most disabling medical illness in the world (2). Currently available acute and preventive medications are often associated with suboptimal efficacy, tolerability and adherence (3). In those with chronic migraine, less than 20% of patients are able to adhere to preventive medications over a period of one year (3).
Transcranial magnetic stimulation employs the principles of electromagnetic induction to deliver an electrical current across resistive layers of the scalp, skull, meninges, cerebrospinal fluid, and into the superficial layers of the cortex where it modulates the electrical environment of neurons. Single pulse transcranial magnetic stimulation (sTMS) is considered a noninvasive, safe, and well-tolerated diagnostic and treatment modality (4). In preclinical animal models, sTMS stimulation raises the threshold for cortical spreading depression (CSD) and modulates the activity of nociceptive thalamic trigeminovascular neurons (5). sTMS is FDA-approved and has been shown to be effective in the acute treatment of migraine with aura (6).
It has been hypothesized that sTMS may be effective for the acute treatment of migraine through CSD inhibition, and repetitive transcranial magnetic stimulation (rTMS) may be effective for the preventive treatment of migraine through changes in cortical excitability (7,8,9). However, the exact changes in cortical excitability in rTMS seem to be highly dependent on stimulation characteristics. For example, rTMS at high frequency (20 Hz) has demonstrated an increase in cortical excitability and rTMS at low frequency (1 Hz) has demonstrated a decrease in cortical excitability (10,11). This type of variability is reflected in the clinical studies involving rTMS in the treatment of migraine. Studies involving rTMS in the prevention of migraine have had conflicting results, likely due to differences in the location, frequency, and strength of stimulation. High frequency tabletop clinic-based rTMS of the primary motor cortex in a cohort of migraine patients demonstrated a reduction of headache frequency (−15.6 days) compared to placebo (−8.1 days) (12). Conversely, low frequency rTMS was ineffective for the preventive treatment of migraine (13). In addition to frequency, location of stimulation is likely a confounding variable in rTMS studies. Conforto and colleagues found that stimulation of the dorsolateral prefrontal cortex (DLPFC) using a tabletop clinic-based rTMS did not decrease headache days in migraine (14). However, other studies demonstrate efficacy with high frequency rTMS of the DLPFC (15). There are ongoing studies with rTMS for the prevention of migraine in efforts to develop a more consistently effective treatment protocol.
Interestingly, patient experience in the United Kingdom with sTMS suggested that sTMS may have not only an acute, but also a preventive benefit for migraine with and without aura (16). Both episodic and chronic migraine patients using sTMS reported a reduction in headache days and acute medication use over time. The results of this study led to a CE Mark for migraine prevention. Given that CSD inhibition is a shared mechanism in many effective migraine preventive therapies (17), the ability to raise the threshold for CSD and inhibit dural specific thalamic sensory neurons may be a plausible underlying mechanism resulting in acute and preventive benefits in patients with migraine with or without aura. The ESPOUSE Study was a multicenter, prospective, single-arm, open label, post-market observational study to evaluate sTMS for the preventive treatment of migraine with or without aura.
Methods
The eNeura ESPOUSE study was a prospective, non-randomized, single arm study intended to support a labeling extension to migraine prevention from its FDA approved indication of acute pain relief in migraine with aura (NCT02357381). Subjects were recruited from December 2014 to March 2016 from headache subspecialty clinics. Using Pass 2008, the sample size required to detect a difference between an observed reduction of −1.8 days against a performance goal of −0.63 required 92 completed patients in the study. Assuming a lost-to-follow-up (LTF) rate of approximately 15%, the enrolled sample size needed to be109 subjects. The protocol and consent forms were approved by site-specific institutional review boards. All subjects provided signed informed consent prior to enrollment.
In this study, a performance goal, a statistically derived placebo response, was used as the comparator. To calculate the estimated placebo response, the inverse variance weighted estimate by the method of Fleiss (18) was used, combining two placebo-controlled clinical trials evaluating topiramate for the preventive treatment of episodic migraine (19,20) and one device study (21) in similar patient populations: Predominantly episodic migraine patients with consistent age and sex distribution.
Subjects were recruited from selected headache centers and were provided use of the device for the duration of the study. After informed consent, subjects in the ESPOUSE study completed a 28-day baseline paper headache diary to establish the baseline headache days and determine eligibility based on the inclusion and exclusion criteria (Table 1). Subjects 18 to 65 years of age, who had migraine with or without aura based on the International Classification of Headache Disorders (ICHD), 3rd edition, beta version (22), with 4–25 headache days per month confirmed, were included. A headache day was defined as a calendar day with ≥4 hours of headache that at any point resulted in at least moderate pain intensity. Subjects with a history of epilepsy or seizures, metal-containing implants, concurrent use of other neurostimulation or neuromodulation devices within the past month, use of onabotulinumtoxin A injections for chronic migraine within the past 4 months, or extracranial nerve blocks within the past 3 months, were excluded. Subjects were allowed to continue preventive medications and instructed not to change dosage for the duration of the study. Details regarding additional inclusion and exclusion criteria can be found in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria 1. Patients 18–65 years of age 2. Patients able to understand and communicate in English 3. Migraine with or without aura 4. 4–25 headache days per month (confirmed by 1-month baseline diary, minimum of five completely headache-free days/month) 5. Understand and willing to provide diary and survey data | Exclusion criteria 1. Severe co-existing disease having a life expectancy of less than 1 year 2. Involved in any other clinical trials that have not completed their primary endpoint or that may interfere with the SpringTMS study results 3. Mental impairment or other conditions which may not allow the subject to understand the nature, significance and scope of the study and to cooperate with the follow-up requirements 4. Known drug and/or alcohol addiction 5. Patients with epilepsy or history of seizure 6. Severe active major depression or major psychiatric illness 7. Use of other neurostimulation or neuromodulation devices within past month 8. Use of onabotulinum toxin A within the past 4 months 9. Extracranial nerve block within past 3 months 10. Patients with implants containing metal |
Subjects who met the inclusion and exclusion criteria underwent the 3-month (12 ± 1 weeks) treatment protocol (Figure 1), consisting of both preventive and acute treatment. To treat, the patient placed the portable sTMS device on the occiput and pressed the button to deliver the sTMS pulse. Time to deliver each pulse is less than 1 minute. The device weighs 1.5 Kg with dimensions H: 81 mm; W: 220 mm; D: 134 mm. A brief sound is heard as the pulse is delivered. At treatment, a single magnetic field pulse is delivered of nominally 0.9 T, measured 1 cm from the device surface, with a rise time of 180 μ sec and a total pulse length of less than 1 ms.
Figure 1.
ESPOUSE treatment protocol.
The preventive daily treatment was four pulses twice per day. The time needed to deliver four pulses is about 2 minutes. The acute treatment dose was three consecutive pulses. If there was no relief after 15 minutes, the dose could be repeated an additional two times. Subjects were allowed to rescue with acute medication 30 minutes after the first three pulses were delivered. Patients reported daily headache status, medication use, and device use to the clinical centers with a monthly paper headache diary.
Pre-specified endpoints were measured over weeks 9 to 12. The primary endpoint was the mean reduction from baseline in headache days compared to the performance goal. Secondary endpoints included the percentage of subjects achieving a ≥50% reduction in headache days, reduction from baseline in days acute headache medication was taken, the HIT-6 score, and total headache days of any pain intensity.
Efficacy analysis was performed in the subjects who completed the baseline assessment and had at least 1 month of data from a diary that indicated device use. Subjects who did not complete the 3-month diary had their 3-month headache days imputed 20 times by an unbiased random selection of headache days from a patient who had completed a diary (consistent with the recommendation of the National Research Council Committee on Missing Data (2010)), unless their withdrawal from the study was due to an adverse event or other confirmed reason that indicated lack of effectiveness. These patients were assigned the worst outcome from among similar patients because their data were missing as a result of the device or its performance. After confirmation that data from the study sites was not heterogeneous, data from 20 imputed data sets were combined into a single t-statistic inference by the method of Rubin (23), and sensitivity analyses were done to demonstrate the robustness of the unbiased imputation. Within imputation testing was done by a one-sample t-statistic. As is customary for imputation analysis submitted to FDA, based on the 2010 recommendations from the National Research Council on Missing Data, secondary endpoint analyses were done in the completed cases population without imputation using a one-sample binomial test for percentages (percentage of patients with ≥50% reduction in headache days from baseline) or a one-sample t-test for quantitative observations (reduction in acute medication days, HIT-6, and reduction to total headache days of any pain intensity). Confirmatory analyses of secondary endpoints were done in the per protocol population.
The alpha inflation due to multiple hypothesis tests was controlled by the gatekeeper method between the primary and secondary endpoints, and among the four secondary endpoints by the modified Bonferroni method of Hochberg (24).
Results
Sample overview
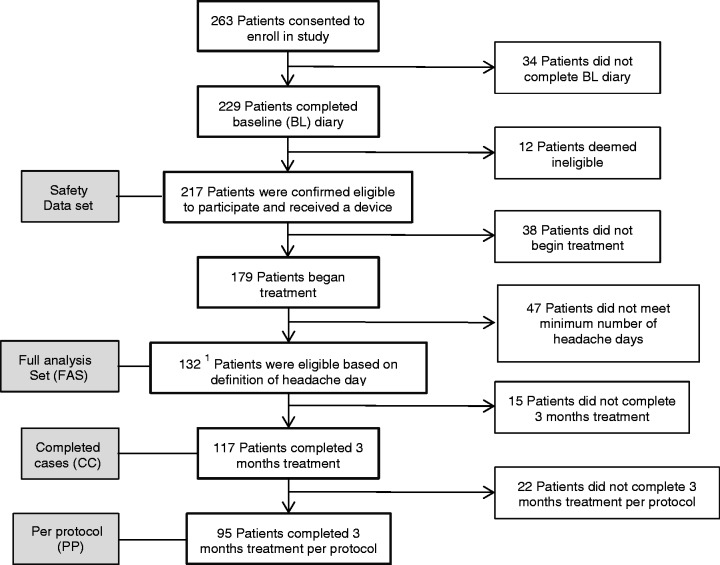
There were 263 subjects consented for enrollment in the study between December 2014 and March 2016. As demonstrated in the Subject Accountability Flow Chart (Figure 2), 229 subjects completed the baseline headache diary, 217 subjects were assigned and received a device and therefore included in the safety analysis set. Of the 217 subjects, 179 subjects were confirmed to have used the device at least once. Additionally, it was confirmed after site monitoring that 47 of these 179 subjects did not meet the inclusion criteria minimum of four headache days per month, which was defined as a calendar day with ≥ 4 hours of headache that at any point resulted in at least moderate pain intensity, and thus these 47 subjects were excluded from the Full Analysis Set (FAS). The FAS was the intention-to-treat population. There were 132 subjects in the FAS, of whom 15 subjects withdrew for reasons not associated with the primary endpoint. Of the 15 withdrawn subjects, two subjects were missing for cause and were not considered missing at random. These subjects were assigned the worst outcome from the groups of subjects with a similar baseline number of migraine headache days. The remaining 13 subjects were assumed to be missing at random and were assigned a 3-month outcome based on a random selection with replacement from subjects within the same imputation group based on the baseline migraine headache days. There were 117 subjects in the Completed Cases (CC) set who met eligibility requirements and completed the 3-month diary. The Per Protocol (PP) set was comprised of the 95 patients that met eligibility and used the device as directed for the entire 3-month treatment period. Treatment adherence was confirmed by patient diary.
Figure 2.
Subject accountability flow chart.
Quantitative and qualitative baseline demographics of the FAS, CC, and PP subjects are listed in Supplementary Table 1 and Supplementary Table 2. There were 119/132 (90%) subjects with episodic migraine and 13/132 (10%) with chronic migraine. There were 44/132 (33%) subjects with aura and 88/132 (67%) without aura. There were 9.06 (3.83) headache days at baseline in the FAS. Although subjects were allowed to continue preventive medications during the study, only six (2.3%) patients reported usage of preventive medications at baseline and no patients added preventive medications during the treatment period. Preventive medications used included topiramate and propranolol. Medication overuse was not analyzed in this study.
Effectiveness
There were 9.06 (3.83) headache days at baseline in the FAS. For the primary endpoint there was a −2.75 ± 0.40 (SE) days reduction compared to the performance goal of −0.63 (p < 0.0001) (Figure 3). Statistical significance persisted in sensitivity analyses with a modified placebo estimate using only the topiramate clinical trials of −1.1 days.
Figure 3.
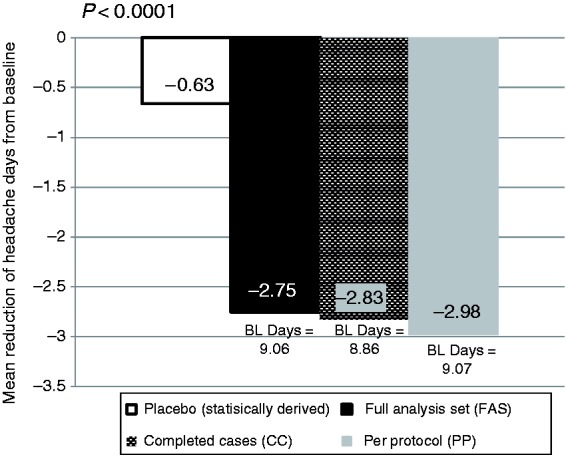
Primary effectiveness endpoint: Mean reduction in headache days.
The first secondary endpoint analysis was the percentage of subjects who had at least a 50% reduction in headache days. This hypothesis was tested in the Completed Cases population against a performance goal responder rate of 20%. Of the 117 subjects in the Completed Cases group, 46% (54/117) had a ≥ 50% reduction in headache days (Figure 4), (95% CI 37–56%). Since the lower limit of the 95% confidence interval is greater than 20%, the null hypothesis is rejected and this secondary endpoint was met.
Figure 4.
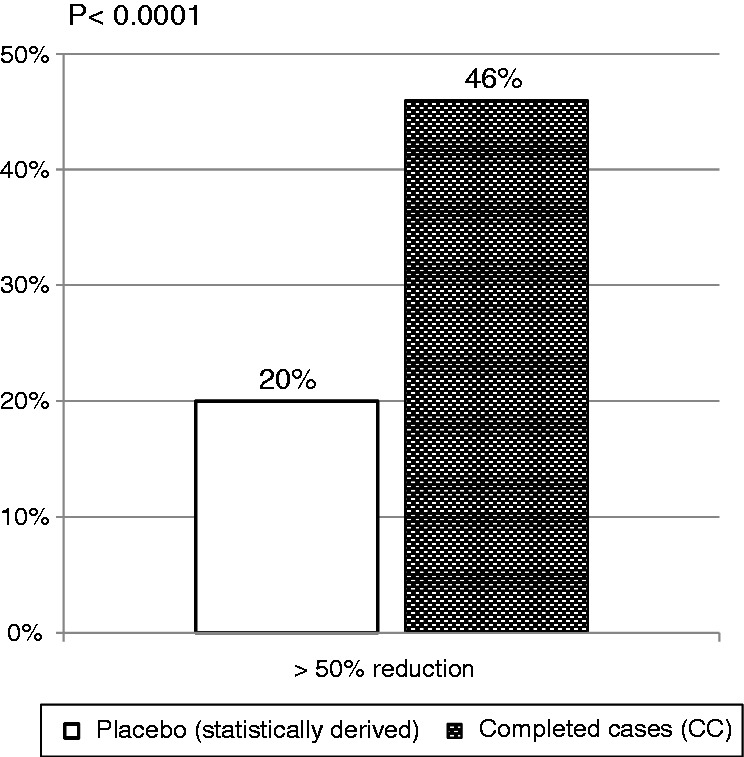
≥ 50% responder rate.
The second secondary endpoint was the reduction from baseline in the days of acute headache medication use. The test was to determine if the amount of reduction is greater than zero. There was a −2.93 (5.24) reduction in acute medication days (Table 2). The results demonstrate that the mean change in days of acute medication use was significantly greater than zero. The null hypothesis was rejected, and this secondary endpoint was met.
Table 2.
Reduction in acute medication days in CC and PP populations.
| Endpoint | Baseline Mean, (SD) N Med (min, max) | Change Mean, (SD) N Med (min, max) | 95% confidence interval | t-statistic | p-value |
|---|---|---|---|---|---|
| Acute medication days (CC) | 9.95 (5.63) 117 10.0 (0, 28) | −2.93 (5.24) 117 −2.0 (−23, 10) | (−3.89, −1.97) | −6.05 | <0.0001 |
| Acute medication days (PP) | 10.38 (5.76) 95 10 (0, 29) | −3.18 (5.45) 95 −3 (−23, 9) | (−4.29, −2.07) | −5.69 | <0.0001 |
The third secondary endpoint is the reduction from baseline of HIT-6 impact questionnaire. This hypothesis was tested in the Completed Cases cohort. The test was to determine if the amount of reduction was greater than zero. The results (Table 3) demonstrate that the mean change in the HIT-6 is significantly greater than zero, thus the null hypothesis is rejected and this secondary endpoint was met.
Table 3.
Reduction in HIT-6 score in CC and PP populations.
| Endpoint | Baseline Mean, (SD) N Med (min, max) | Change Mean, (SD) N Med (min, max) | 95% confidence interval | t-statistic | p-value |
|---|---|---|---|---|---|
| HIT6 (CC) | 63.85 (4.56) 117 64.0 (50, 76) | −3.10 (6.42) 114a −2.0 (−25, 11) | (−4.29, −1.90) | −5.15 | <0.0001 |
| HIT6 (PP) | 64.04 (4.56) 95 64 (52, 76) | −3.63 (6.79) 94b −2 (−25, 11) | (−5.02, −2.24) | −5.18 | <0.0001 |
aThree subjects in the CC group did not have a HIT6 score at 12 weeks. bOne subject in the PP group did not have a HIT6 score at 12 weeks.
The fourth secondary endpoint was the difference from baseline in total headache days of any pain intensity (mild, moderate, or severe). This hypothesis was tested in both Completed Cases and Per Protocol populations. The results (Table 4) demonstrate that the mean change in total headache days of any pain intensity lasting for 4 or more hours is significantly less than the performance goal of −0.63, thus the null hypothesis is rejected and this secondary endpoint was met.
Table 4.
Reduction in total headache days in CC and PP populations.
| Endpoint | Baseline Mean, (SD) N Med (min, max) | Change Mean, (SD) N Med (min, max) | 95% confidence interval | t-statistic | p-value |
|---|---|---|---|---|---|
| Headache daysa (CC) | 10.58 (4.33) 117 10.0 (4, 24) | −3.16 (5.21) 117 −4.0 (−22, 9) | (−4.12, −2.21) | −5.25 | <0.0001 |
| Headache daysa (PP) | 10.79 (4.32) 95 10 (4, 24) | −3.28 (5.16) 95 −4 (−22, 9) | (−4.34, −2.23) | −5.01 | <0.0001 |
A headache day is defined as a day with a headache lasting ≥4 or more hours with pain of any intensity.
Tolerability and safety
Adverse events were recorded from the Safety Data Set of 217 subjects who received the device. Overall, there were no serious adverse events reported. Nine patients withdrew from the study due to adverse events. In total, there were 62 adverse events. Sixteen were determined by the investigator to be unrelated to the treatment or device, 30 were thought to be possibly treatment related and 12 were thought to be probably or definitely related to the device. The most commonly reported adverse events are listed in Table 5.
Table 5.
Most common adverse events possibly or probably device related.
| Adverse event | n | % |
|---|---|---|
| Light headedness | 8/217 | 4% |
| Tingling | 7/217 | 3% |
| Ringing in ears (Tinnitus) | 7/217 | 3% |
| Dizziness | 6/217 | 3% |
| Headache | 5/217 | 2% |
| Scalp discomfort | 5/217 | 2% |
| Discomfort from noise | 5/217 | 2% |
| Any reported adverse events | 62/217 | 29% |
Discussion
This open label study met its primary endpoint and pre-specified, multiplicity-protected secondary endpoints, suggesting that sTMS may be an effective preventive treatment for migraine with or without aura. Overall, 46% of the subjects had a greater than 50% reduction in the number of headache days, and there was a significant reduction in disability and in the days of acute medication use. In addition, the treatment was safe and well tolerated. There were no serious adverse events and only 4% (9/217) withdrew due to adverse events.
Based on epidemiological studies, episodic and chronic migraine are undertreated (25,26). Only 26.3% of patients with episodic migraine and 4.5% of patients with chronic migraine have overcome the barriers for minimally appropriate care (25,26). Even for those who ultimately received preventive care, adherence and compliance is poor (27–30). In the study by Hepp et al., regardless of the class of preventive medication, there was a sharp decline or discontinuation of the preventive treatment of choice within 30 days, with 50% of patients stopping the medication within 60 days. Persistence with treatment at 12 months occurred in only 15% of patients (30). Studies in patient preference in migraine have demonstrated a desire for a treatment option that is simple, effective, rapid in onset of action, and with minimal side effects (31,32). In clinical practice, patients would like a variety of options including vitamins, supplements, non-pharmacologic and non-oral preventive treatment options (32). Treatment that is aligned with patient preference and meets the needs of the vast majority of migraine patients is still lacking, which is likely one of the major reasons why adherence remains poor. Poor adherence is most commonly a result of poor outcomes, such as inefficacy or side effects (33,34). In addition, as the complexity of a treatment regimen increases, adherence declines (28). The simplicity, efficacy and tolerability of sTMS, combined with its dual utility as both an acute and preventive treatment option, may possibly increase adherence and compliance and improve patient outcomes.
sTMS technology has been safely used for decades in tens of thousands of subjects and patients for diagnosis, monitoring, and treatment of a variety of neurologic and psychiatric disorders without serious adverse events (35). The results of this study support previous studies in migraine in demonstrating good tolerability and no serious device-related adverse events (6,16). In addition, it is a non-oral, non-pharmacologic treatment option that modulates targets and pathophysiological events considered to be important in the pathophysiology of migraine. The relatively few contraindications to treatment with sTMS also render it an appealing choice in patients with episodic and chronic migraine, who often have a high number of medical and psychiatric comorbidities that may preclude the use of many other therapies (36).
The UK post-market pilot study broadened the population of those potentially responsive to sTMS to include both migraine with and without aura, and expanded the indication to include preventive treatment of episodic and chronic migraine (16). In addition, similar to clinical practice, the treatment protocol was tailored to the patient response. In subjects with frequent or even daily headache, the treatment protocol that was eventually recommended was daily pulses (16). The mean baseline frequency of migraine days per month was 15 at baseline, 11 at 6 weeks, and 8 at 12 weeks (16). The positive results from the UK pilot study demonstrated that sTMS was possibly effective in both migraine with and without aura. In addition, daily use of sTMS treatments in subjects with frequent migraine attacks demonstrated a reduction in mean monthly migraine days. However, a limitation of the UK pilot program was that it did not have a consistent treatment protocol designed to investigate a preventive benefit. The ESPOUSE Study was therefore designed to determine the preventive benefit of daily sTMS treatment with a consistent daily protocol of four pulses twice a day in both migraine with and without aura. The current data demonstrate a preventive benefit of sTMS in patients with episodic migraine and chronic migraine.
Additional analyses compared ESPOUSE results to post market observational data collected in the UK study for preventive treatment of chronic migraine (16). Sixty-two chronic migraine patients were prescribed and used sTMS preventatively for a minimum of 3 months (16). The baseline headache days for these patients was 24.9 (SD = 5.61) (16). The average reduction in headache days was −8.58 days (SD = 12.82) with 95% confidence limits of (−11. 8 days, −5.3 days) and the 50% responder rate was 47% with 95% confidence limits (33.98%, 59.88%) (16). Average number of pulses used per daily treatment was 2.9 (SD = 0.86) for an average of 29 days (SD = 2.3) (16). Acute medication days were reduced by a mean of −7 days (SD = 9.3) in the 41 patients who provided information on acute medication use (16). The similarity of the results between two independent studies provides support for the consistency of effect experienced by patients treated with the sTMS device for reduction in migraine days in different populations (UK versus US) with different investigators.
In addition, recent basic science research has demonstrated a plausible mechanism of action for sTMS for the treatment of migraine (5). Migraine pathophysiology involves first order neurons from the trigeminal nerve that synapse on second order neurons in the trigeminocervical complex that synapse on third order neurons in the ventroposteromedial (VPM) thalamus via the trigeminothalamic tract (37). STMS not only inhibited CSD in the rodent animal model, but also VPM thalamic spontaneous neuronal activity and C-fiber-mediated activity for greater than 90 minutes (5). The thalamus may be a potential target for the acute and preventive treatment of migraine, as the thalamus is an important player in not only migraine attacks (38), but also in the development of central sensitization (39,40).
It may be important to note that sTMS failed to inhibit CSD in the gyrencephalic cat cortex (5), which may be a result of the study design and stimulation protocol as it was optimized for the rodent. However, it must also be considered that human physiologic responses may be more similar to the cat, and thus this is still an area of further investigation, although previous CSD studies have demonstrated that treatment targets with known positive clinical outcomes in humans have been less consistently effective in the cat compared to the rodent (41).
Limitations and future research
Study limitations include the open-label post-marketing study design, involving a relatively heterogeneous migraine population, with the lack of a control or sham device group. The gold standard for clinical trials is a well-conducted randomized controlled trial (RCT) with confirmed blinding throughout the study period, minimal loss to follow-up and balance among withdrawn patients. Migraine preventive RCTs can have significant drop out rates that often differ between treatment groups. In the test arm, patients may withdraw due to adverse effects or lack of tolerance to the drug, but in the placebo arm, patients may withdraw due to lack of efficacy. In addition, the challenge of developing a true sham comparator for device studies is well known. Blinding is difficult for neuromodulation studies because patients can detect the difference between active and placebo (sham) devices. Developing a true placebo or sham device that emits no energy but looks and feels like an active device is often not possible. In the previous sTMS study of acute treatment for migraine with aura, a true (no energy) sham was developed and successful blinding was confirmed. However, because headache prevention studies require more frequent (daily) treatment over an extended period of time, creating a sham that would support blinding throughout the study was a significant limitation. Transcutaneous supraorbital neurostimulation (tSNS) and noninvasive vagal nerve stimulation (nVNS) studies have used lower energy sham devices (21,42). It is unclear if blinding was sustained, because a low energy comparator device feels and sounds much weaker than the active device. Additionally, the assumption that the low energy (sham) is delivering a sub-therapeutic dose is not always supported. In one nVNS study, the low energy sham device showed a statistically significant reduction of headache days when compared to the active device (42). This was the inverse of the expected results. For the current study, a differential loss to follow-up and difficulty in blinding a preventive device study led to the decision to conduct a single arm study.
In these cases, the FDA proposes the use of a Performance Goal, a statistically derived estimate of the placebo effect based on historical controls, but only in extensively studied populations where the expected placebo rates are well characterized. The historical controls chosen for the performance goal estimate in this study were three contemporary randomized placebo-controlled trials (RCTs) with documented efficacy in migraine prevention. These were trials in episodic migraine, given that the majority of the study subjects had episodic migraine. One of the trials used was the only self-administered, daily administration, patient-administered device study in migraine prevention that was available at the time of study design (21). The other two trials utilized in the generation of a performance goal were contemporary studies of topiramate – the most commonly used FDA-approved migraine preventive treatment in the US. Each of these studies used comparable patient populations and patient-administered treatments. Further, Macedo et al. completed a meta-analysis of the placebo response in 22 RCTs for all FDA-approved preventive treatment options in migraine, demonstrating a 23% placebo responder rate that is comparable to the statistically-derived comparator in this study of 20% obtained from the three studies used to calculate the performance goal in this study (Table 6) (43).
Table 6.
Data derived from a meta analysis of the prophylaxis of migraine vs. ESPOUSE Study.
| Macedo et al., meta analysis prophylaxis of migraine | eNeura ESPOUSE Study | |
|---|---|---|
| Active responder rate (≥50% reduction) | 41% | 46% |
| Placebo responder rate | 23% | 20%* |
| Active mean attack reduction | 36% | 34% |
| Placebo mean attack reduction | 18% | 23%* |
| Proportion with adverse events in active arm | 39% | 30% |
| Proportion with adverse events in placebo arm | 30% | NA |
Statistically derived placebo rate (performance goal).
NA: not applicable.
Other limitations include missing data. Randomized controlled studies of daily use medication for migraine prevention exhibit approximately 50% missing data in both arms (19,20). In 2010, The National Research Council (NRC) recommended imputation of missing data combined with a sensitivity analysis to demonstrate the robustness of the imputation. Following the NRC recommendation, the FDA is now requesting the more rigorous imputation analysis in place of last data point carried forward. The ESPOUSE statistical analysis plan specified imputation analysis. In this study, 15/132 (11%) subjects included in the Full Analysis Set did not complete the 3-month treatment diary. In this intention to treat analysis, a multiple imputation technique and multiple sensitivity analyses were performed to confirm the strength of the analyses. In the imputation sensitivity analysis, the worst-case analysis assigned missing data with the worst observed response in patients completing the study.
While an acute treatment paradigm was utilized, acute treatment efficacy was not assessed in the present study. Future studies specifically in migraine without aura are needed to assess acute treatment response. Although treatment adherence was documented by the paper headache diary, in the future, more objective device monitoring would be helpful to directly evaluate treatment adherence. Additionally, studies that evaluate clinical factors that predict treatment response to sTMS would be useful. Medication overuse was not analyzed in the present study. Although the majority of subjects had episodic migraine, it was a heterogeneous patient population with episodic and chronic migraine, migraine with and without aura. Due to the relatively small size of the subject population in specific subsets, subset analyses were not performed in this study. The heterogeneity of the patient population in a study of this size is a limitation. Finally, the study sample consisted mainly of Caucasian women, which may limit generalizability to other populations.
In conclusion, this study adds to the current evidence for acute migraine treatment that sTMS is a safe and possibly effective preventive treatment for migraine. Prior studies have demonstrated acute treatment benefits in those who have migraine with aura. Prior open label patient experience suggested a preventive benefit, and this current open label study provides additional evidence for the preventive benefit in patients with migraine when used daily. All studies support the safety and tolerability of this treatment method. Based on a combination of the ESPOUSE study, a previous randomized controlled trial (6) and the UK program results (16), the FDA has approved the sTMS device for the acute and preventive treatment of migraine. In addition, sTMS has received the CE Mark for acute and preventive treatment of migraine.
Clinical implications
sTMS is possibly effective for the preventive treatment of migraine.
This study adds to the current evidence that sTMS is a safe, well-tolerated treatment for migraine.
Supplemental Material
Supplemental material, Supplementary Table 1 for A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study) by Amaal J Starling, Stewart J Tepper, Michael J Marmura, Ejaz A Shamim, Matthew S Robbins, Nada Hindiyeh, Andrew C Charles, Peter J Goadsby, Richard B Lipton, Stephen D Silberstein, Amy A Gelfand, Richard P Chiacchierini and David W Dodick in Cephalalgia
Supplemental Material
Supplemental material, Supplementary Table 2 for A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study) by Amaal J Starling, Stewart J Tepper, Michael J Marmura, Ejaz A Shamim, Matthew S Robbins, Nada Hindiyeh, Andrew C Charles, Peter J Goadsby, Richard B Lipton, Stephen D Silberstein, Amy A Gelfand, Richard P Chiacchierini and David W Dodick in Cephalalgia
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Amaal J Starling is on the Advisory Board for eNeura, she has also attended advisory board meetings for Alder, Amgen, and Eli Lilly and Company. She has consulted for Amgen and Eli Lilly and Company. Stewart J Tepper has received personal compensation for activities with Acorda, Alder, Allergan, Amgen, ATI, Avanir, Dr Reddy's, eNeura, Pfizer, Scion Neurostim, Teva, Zosana, Depomed, Impax, Pernix, Teva, Amgen, and Merck. He has received personal compensation in an editorial capacity for Headache Currents, holds stock and/or stock options in ATI, and received research support from Alder, Allergan, Amgen, ATI, Scion Neurostim, Teva, and Zosano. Michael J Marmura has received personal compensation for activities with Teva and Supernus as a consultant ad has received research support for Teva and eNeura. Ejaz A Shamim has nothing to disclose. Matthew S Robbins has received personal compensation in an editorial capacity for Current Pain and Headache Reports as well as book royalties from Wiley. He has received research support from eNeura. Nada Hindiyeh has nothing to disclose. Andrew C Charles has received personal compensation for activities with Amgen as a consultant and with eNeura and Eli Lilly for serving on the Scientific Advisory Board, and has received research support from Takeda. Peter J Goadsby reports grants and personal fees from Allergan, Amgen, and Eli Lilly and Company; and personal fees from Akita Biomedical, Alder Biopharmaceuticals, Avanir Pharma, Cipla, Dr Reddy's Laboratories, eNeura, Electrocore, Novartis, Pfizer, Quest Diagnostics, Scion, Teva Pharmaceuticals, Trigemina, and Scion; and personal fees from Medico Legal work, Journal Watch, Up-to-Date, Massachusetts Medical Society, and Oxford University Press; and in addition, he has a patent Magnetic stimulation for headache assigned to eNeura without fees. Richard B Lipton has received personal compensation from Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol Myers Squibb, Cognimed, Colucid, Eli Lilly and Company, eNeura, Merck, Novartis, Pfizer, TEVA, and Vedanta. Stephen D Silberstein has received consulting fees and/or honoraria from Allergan, Amgen, Avanir Pharmaceuticals, eNeura, ElectroCore Medical LLC, Medscape, LLC, Medtronic, Mitsubishi Tanabe Pharma America, NINDS, Pfizer, Supernus Pharmaceuticals and Teva Pharmaceuticals. Amy A Gelfand consults for Eli Lilly, Zosano, eNeura and Biohaven. She receives honoraria from UpToDate. Her spouse receives compensation for medical legal consulting, consulting compensation from Genentech, and research support from Genentech, Quest Diagnostics and MedDay. Richard P Chiacchierini has nothing to disclose. David W Dodick discloses the following: Consulting: Acorda, Allergan, Amgen, Alder, Merck, Dr Reddy’s, Promius, eNeura, Eli Lilly and Company, Insys Therapeutics, Autonomic Technologies, Teva, Xenon, Tonix, Trigemina, Boston Scientific, GBS, Colucid, Zosano, Laydenburg Thalmann, Biocentric, Biohaven, Magellan, Pfizer (Japan), Charleston Laboratories. Royalties: Oxford University Press and Cambridge University Press (book royalty). Uptodate – editorial/honoraria. CME companies honoraria/publishing honoraria/royalites: Chameleon Communications, Medscape, WebMD, Academy for Continued Healthcare Learning, Haymarket Medical Education, Global Scientific Communications, HealthLogix, Academy for Continued Healthcare Learning, Meeting LogiX, Health LogiX, Wiley Blackwell, Oxford University Press, Cambridge University Press. Stock/options: GBS/Nocira, Epien, and Mobile Health. Consulting use agreement: NAS. Board position: King-Devick.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ESPOUSE Study was supported by eNeura Inc.
References
- 1.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia 2017; 37: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker AT, Shields K. Transcranial magnetic stimulation: Basic principles and clinical applications in migraine. Headache 2017; 57: 517–524. [DOI] [PubMed] [Google Scholar]
- 5.Andreou AP, Holland PR, Akerman S, et al. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain 2016; 139: 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 2010; 9: 373–380. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003; 2: 145–156. [DOI] [PubMed] [Google Scholar]
- 8.Maeda F, Keenan JP, Tormos JM, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 2000; 111: 800–805. [DOI] [PubMed] [Google Scholar]
- 9.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clin Neurophysiol 2001; 112: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 10.Gangitano M, Valero-Cabre A, Tormos JM, et al. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol 2002; 113: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 11.Romero JR, Anschel D, Sparing R, et al. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol 2002; 113: 101–107. [DOI] [PubMed] [Google Scholar]
- 12.Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: A randomized, placebo-controlled study. J Neurol 2013; 260: 2793–2801. [DOI] [PubMed] [Google Scholar]
- 13.Teepker M, Hotzel J, Timmesfeld N, et al. Low-frequency rTMS of the vertex in the prophylactic treatment of migraine. Cephalalgia 2010; 30: 137–144. [DOI] [PubMed] [Google Scholar]
- 14.Conforto AB, Amaro E, Jr, Goncalves AL, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia 2014; 34: 464–472. [DOI] [PubMed] [Google Scholar]
- 15.Brighina F, Piazza A, Vitello G, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: A pilot study. J Neurol Sci 2004; 227: 67–71. [DOI] [PubMed] [Google Scholar]
- 16.Bhola R, Kinsella E, Giffin N, et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: Evaluation of outcome data for the UK post market pilot program. J Headache Pain 2015; 16: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 18.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993; 2: 121–145. [DOI] [PubMed] [Google Scholar]
- 19.Silberstein SD, Neto W, Schmitt J, et al. Topiramate in migraine prevention: Results of a large controlled trial. Arch Neurol 2004; 61: 490–495. [DOI] [PubMed] [Google Scholar]
- 20.Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: A randomized controlled trial. JAMA 2004; 291: 965–973. [DOI] [PubMed] [Google Scholar]
- 21.Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013; 80: 697–704. [DOI] [PubMed] [Google Scholar]
- 22.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 23.Rubin D. Multiple imputation for nonresponse in surveys, New York: John Wiley and Sons, 1987. [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple testing of significance. Biometrika 1988; 75: 800–802. [Google Scholar]
- 25.Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013; 53: 81–92. [DOI] [PubMed] [Google Scholar]
- 26.Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache 2016; 56: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78: 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rains JC, Penzien DB, Lipchik GL. Behavioral facilitation of medical treatment of headache: Implications of noncompliance and strategies for improving adherence. Headache 2006; 46: S142–S143. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: A systematic review. Headache 2014; 54: 795–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia 2017; 37: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment?. Headache 2002; 42: S3–S9. [DOI] [PubMed] [Google Scholar]
- 32.Peres MF, Silberstein S, Moreira F, et al. Patients' preference for migraine preventive therapy. Headache 2007; 47: 540–545. [DOI] [PubMed] [Google Scholar]
- 33.Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015; 35: 478–488. [DOI] [PubMed] [Google Scholar]
- 34.Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: An observational study. J Headache Pain 2011; 12: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodick DW, Schembri CT, Helmuth M, et al. Transcranial magnetic stimulation for migraine: A safety review. Headache 2010; 50: 1153–1163. [DOI] [PubMed] [Google Scholar]
- 36.Buse DC, Manack A, Serrano D, et al. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010; 81: 428–432. [DOI] [PubMed] [Google Scholar]
- 37.Goadsby PJ, Charbit AR, Andreou AP, et al. Neurobiology of migraine. Neuroscience 2009; 161: 327–341. [DOI] [PubMed] [Google Scholar]
- 38.Afridi SK, Goadsby PJ. Neuroimaging of migraine. Curr Pain Headache Rep 2006; 10: 221–224. [DOI] [PubMed] [Google Scholar]
- 39.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010; 13: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010; 68: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland PR, Akerman S, Goadsby PJ. Cortical spreading depression-associated cerebral blood flow changes induced by mechanical stimulation are modulated by AMPA and GABA receptors. Cephalalgia 2010; 30: 519–527. [DOI] [PubMed] [Google Scholar]
- 42.Straube A, Ellrich J, Eren O, et al. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): A randomized, monocentric clinical trial. J Headache Pain 2015; 16: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macedo A, Banos JE, Farre M. Placebo response in the prophylaxis of migraine: A meta-analysis. Eur J Pain 2008; 12: 68–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Table 1 for A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study) by Amaal J Starling, Stewart J Tepper, Michael J Marmura, Ejaz A Shamim, Matthew S Robbins, Nada Hindiyeh, Andrew C Charles, Peter J Goadsby, Richard B Lipton, Stephen D Silberstein, Amy A Gelfand, Richard P Chiacchierini and David W Dodick in Cephalalgia
Supplemental material, Supplementary Table 2 for A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study) by Amaal J Starling, Stewart J Tepper, Michael J Marmura, Ejaz A Shamim, Matthew S Robbins, Nada Hindiyeh, Andrew C Charles, Peter J Goadsby, Richard B Lipton, Stephen D Silberstein, Amy A Gelfand, Richard P Chiacchierini and David W Dodick in Cephalalgia