Summary
Human cancer tissues are heterogeneous in nature and become differentiated during expansion of cancer stem cells (CSCs). CSCs initiate tumorigenesis, and are involved in tumor recurrence and metastasis. Furthermore, data show that CSCs are highly resistant to anticancer drugs. Cetuximab, a specific anti-epidermal growth factor receptor (EGFR) monoclonal antibody, is used in cancer treatment. Although development of resistance to cetuximab is well recognized, the underlying mechanisms remain unclear. Lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR)/ErbB2, has antiproliferative effects and is used to treat patients with ErbB2-positive metastatic breast cancer. In this review, cetuximab and lapatinib-resistant oral squamous cell carcinoma (OSCC) cells proliferation and migration signal transduction passway is discussed by introducing our research.
Keywords: Cancer stem cells, Cetuximab, Lapatinib, Epidermal growth factor receptor, Oral squamous cell carcinoma, Molecularly-targeted therapy, Sphere formation
1. Introduction
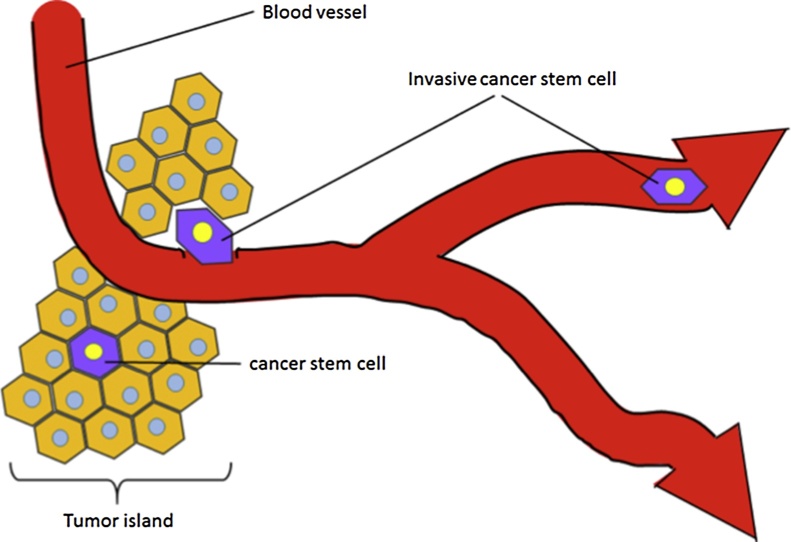
Cancer tissue is a complex “organ”. The tumor tissue microenvironment is composed of a variety of cells, including tumor cells, cancer stem cells, inflammatory cells, and cancerassociated fibroblasts, along with blood vessels (Fig. 1). It is possible that cancer stem cells participate in the processes that lead to resistance to therapy and the establishment of distant metastases.
Figure 1.
Cancer tissue is a complex “organ”. The tumor tissue microenvironment is composed of a variety of cells, including tumor cells, cancer stem cells along with blood vessels. The cancer stem cells are rare cells found primarily in the invasive edge of tumors close to blood vessels.
The epidermal growth factor receptor [(EGFR)/ErbB1/HER1] is a member of the ErbB tyrosine kinase family. All receptors of the ErbB family activate and regulate diverse cellular processes, including proliferation, survival, adhesion, migration and differentiation [1]. Ligand binding potentiates receptor interaction with either a homologous molecule (homodimerization), a different ErbB-family receptor [2], [3], [4], [5]. Upregulation of EGFR expression in many human epithelial cancers is associated with advanced tumor stage and an unfavorable prognosis [6], [7]. Thus, EGFR is considered to be not only a useful prognostic biomarker but also a promising therapeutic target, have been developed and used in cancer treatment.
Molecularly-targeted therapies, which include monoclonal antibodies and small molecule inhibitors, such as EGFR, have significantly changed the treatment of cancer over the past 10 years. These drugs are now a component of therapy for many common malignancies, including breast, colorectal, lung, and pancreatic cancers, as well as oral cancer. The mechanisms of action and toxicities of targeted therapies differ from those of traditional cytotoxic chemotherapy. Targeted therapies are generally better tolerated than traditional chemotherapy. Targeted therapy has raised new questions about the tailoring of cancer treatment to an individual patient’s tumor, the assessment of drug effectiveness and toxicity, the economics of cancer care, and resistance following treatments.
Cetuximab is a chimeric IgG1 monoclonal antibody that binds with high affinity to the extracellular domain of EGFR [8]. The antibody blocks EGFR activation by preventing tyrosine kinase-mediated phosphorylation of the protein [9]. Cetuximab has been prescribed for patients with metastatic colorectal cancer (mCRC) [10], [11], [12], [13], [14] and head and neck squamous cell carcinoma (HNSCC) [15], [16], [17], [18], [19]. For clinical setting of metastatic or recurrent oral cavity cancers, cetuximab 400 mg/m2 IV loading dose on day 1, followed 250 mg/m2 IV weekly until disease progression.
The EGFR/ErbB2 dual inhibitor lapatinib is used to treat ErbB2-positive breast cancer. Despite intensive efforts investigating a large number of ligands identified for EGFR, ErbB3 and ErbB4, no direct ligand for ErbB2 binding has been identified. However, ErbB2 dimerizes with other ErbB receptors and acts as a co-receptor [20], and overexpression of ErbB2 can induce transformation of cells without the ligand [21]. In addition, since heterodimeric formation of ErbB2 with other ErbBs can enhance ligand binding, receptor tyrosine phosphorylation, and cell proliferation compared with EGFR homodimers, lapatinib has better efficacy than those of single inhibitors of EGFR signal transduction for preventing tumor growth and survival [22]. For clinical use, oral lapatinib 1500 mg daily or oral lapatinib 1000 mg daily in combination with intravenous trastuzumab 2 mg/kg weekly (after the initial 4 mg/kg loading dose).
However, use of EGFR inhibitors containing cetuximab or lapatinib is resistance following treatments. Thus, it is important to understand not only how cetuximab or lapatinib acts but also the mechanisms of resistance. In this review, cetuximab and lapatinib-resistant oral squamous cell carcinoma (OSCC) cells proliferation and migration signal transduction passway is discussed by introducing our research.
2. Proliferation of OSCC cell lines in monolayer culture
2.1. Cetuximab inhibits proliferation of HSC3 and HSC4 cells, but not SAS cells
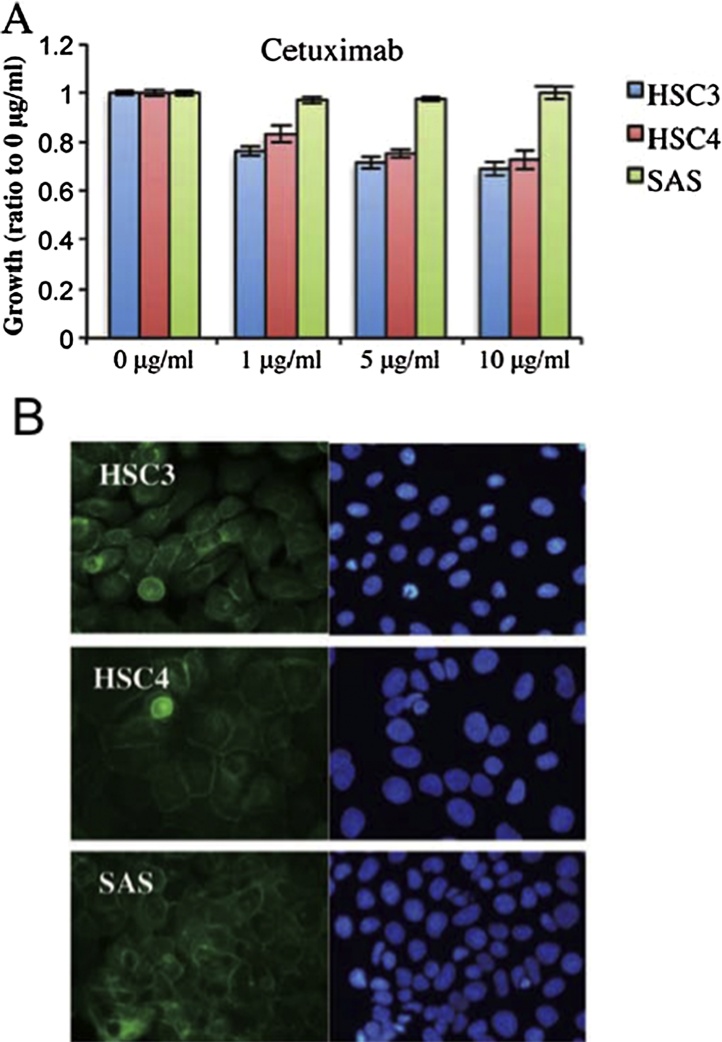
Although Cetuximab inhibits the growth of squamous cell carcinoma, it may not be effective for some cancers, or may acquire resistant. In the results of our research, cetuximab reduce the proliferation of HSC3 and HSC4 cells, but SAS cells proliferate (Fig. 2A). Thus, HSC3 and HSC4 cells were cetuximab-sensitive and SAS cells were cetuximab-resistant. Accordingly, HSC3 and HSC4 proliferation was regulated principally by EGFR, whereas SAS proliferation was not controlled by EGFR. Fathermore, immunofluorescence from anti-EGFR antibody was detected in the cell-cell contact regions of all cell lines, indicating that EGFR was located in the cell membrane (Fig. 2B). These data are consistent with those of a previous report that EGFR biomarker analysis in non-small cell lung carcinoma patients showed that those with higher EGFR expression levels obtained more therapeutic benefit from cetuximab than did patients with lower EGFR levels [23]. However, we found that SAS proliferation was not affected by cetuximab, although SAS cells expressed EGFR, which was localized in the cell membrane, as did HSC3 and HSC4 cells. Chung et al. showed that colorectal cancer patients with EGFR-negative tumors could nonetheless respond to cetuximab-based therapies [10]. Collectively, EGFR status does not seem to have predictive value when used to gauge the efficacy of cetuximab treatment in oral cancer patients.
Figure 2.
EGFR expression by cell lines and sensitivity of the cells to various inhibitors.
(A) Three human OSCC cell lines, HSC3, HSC4 and SAS provided by the RIKEN BioResource Center (Ibaraki, Japan), were used in the present study. Human OSCC cells (2 × 103/well) were plated in 96-well plates. After 24 h of growth, cetuximab were added at the indicated concentrations. All experiments were performed in triplicate. Cell proliferation was assessed using the CellTiter 96® Non-Radioactive Cell Proliferation Assay (Promega, Tokyo, Japan).The effects of cetuximab on growth of HSC3, HSC4 and SAS cells, measured using the MTT assay. The values are means ± SD of data from triplicate samples from one representative experiment. (B) Immunofluorescence analysis. Cultured cells and cell aggregates were fixed in 3.5% (w/v) formaldehyde, permeabilized in 0.2% (v/v) Triton X-100, and blocked in 2% (w/v) BSA. The primary antibodies were rabbit anti-EGFR. Alexa Fluor 488-conjugated IgG (Life Technologies) was used as the secondary antibody. After incubation with the antibodies, SlowFade Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) was added. The specimens were observed using fluorescence microscopy. HSC3, HSC4 and SAS cells were stained with an anti-EGFR antibody (left panels) or DAPI (right panels).
2.2. Cetuximab-resistant OSCC cell lines proliferate autonomously in monolayer culture and form spheres in floating culture, and growth of SAS aggregates is inhibited by cetuximab
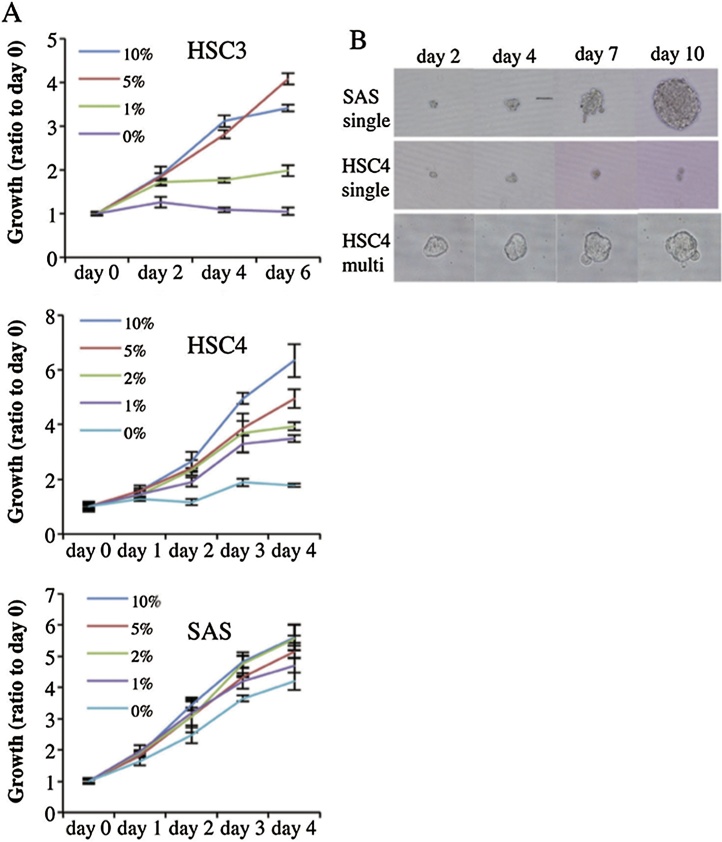
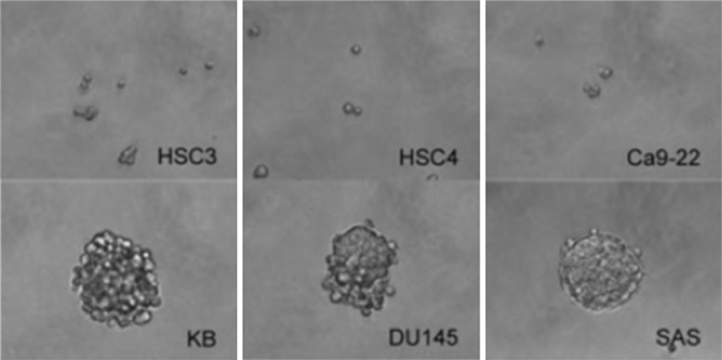
Fig. 3A shows that proliferation in monolayer culture was promoted by serum in a concentration-dependent manner. HSC3 and HSC4 cells proliferated only slightly over 6 and 4 days, respectively of serum-free culture. Notably, however, SAS cells proliferated strongly in serum-free medium. Analyzing the sphere formation capacity of single cells growing in serum-free sphere formation medium supplemented with bFGF and EGF [24], SAS cells form spheres from single cells (Fig. 3B), and sphere diameter increase as culture time rose. In contrast, HSC4 cells did not form spheres from either single (Fig. 3B) or multiple cells (Fig. 3B). Thus, cetuximab-resistant SAS cells exhibite cancer stem cell-like characteristics but cetuximab-sensitive cells were incapable of forming growing aggregates. Sphere formation revealed the stem cell-like properties of SAS cells. HSC3 and HSC4 cells essentially lacked such properties, being unable to form spheres from single cells. This suggests that expression of stem cell-like features is associated with cetuximab resistance under anchorage-dependent growth conditions.
Figure 3.
Serum-dependence of OSCC cell growth and sphere formation activity.
(A) Serum dependence of OSCC cells. HSC3, HSC4 and SAS cells were cultured in DMEM with various concentrations of serum (0, 1, 2, 5 or 10%; all v/v) for 4–6 days, and cells were enumerated using the MTT assay. The values are means ± SD of data from triplicate samples from one representative experiment. (B) When aggregation culture was performed, 1 × 103 cells were seeded into each well of low adhesive 96-well plates (Sumitomo, Tokyo, Japan) and cultured in DMEM supplemented with 10% (v/v) FCS at 37 °C under 5% (v/v) CO2. To allow sphere formation, 1:1000 dilution of a suspension of 1 × 103 cells was added to the well of low-adhesive 96-well U-shaped plates in ‘sphere medium’, which was DMEM/F12 supplemented with 2 mM glutamine, 2% (v/v) B27, 20 ng/ml EGF, 20 ng/ml bFGF, penicillin, and streptomycin. Phase-contrast micrographs of spheres cultured from single SAS cells and single or multiple HSC4 cells growing in low-adhesive 96-well culture plates.
3. Proliferation of OSCC cell lines in floating culture
3.1. Cetuximab sensitivity of SAS aggregate growth is EGFR-PI3K-AKT pathway-dependent
Signaling through the EGFR is transmitted to the nucleus through various routes. We analyzed the MAPK/ERK and the PI3K-AKT pathways downstream of EGFR activation in SAS aggregates [25]. The EGFR-PI3K-AKT pathway plays a crucial role in SAS growth under anchorage-independent conditions. Anchorage-mediating structures on the extracellular matrix of epithelial cells serve a mechanical function and provide important survival signals to the cell. Detachment from the substrate, loss of cell anchorage, and concomitant loss of such survival signals leads to the induction of apoptosis, which is termed anoikis, in the majority of adherent cells [26], [27], [28]. Acquisition of anoikis resistance of cancer cells constitutes an essential prerequisite for tumor progression and metastases in most cancers of epithelial origin [29], [30]. Herein, we demonstrated that activation of the ligand-dependent EGFR/PI3K/Akt pathway, other than anoikis resistance, is necessary for anchorage-independent growth during metastasis, consistent with a recent report [31].
3.2. EGFR is stimulated in lipid rafts in SAS aggregates
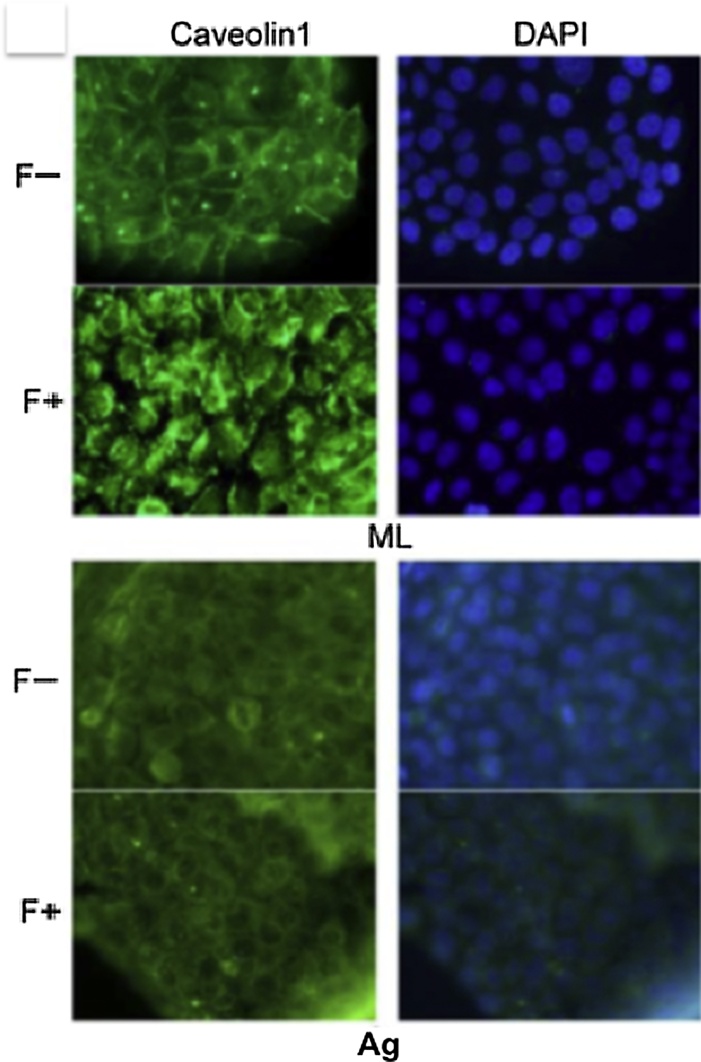
The phosphorylation of EGFR and AKT was upregulated by aggregation in floating cultures of SAS cells indicates that distinct EGFR signaling pathways are used in different culture conditions. EGFR is localized mainly at the plasma membrane and is activated by signals from the environment. The plasma membrane contains discrete heterogeneous microdomains [32], including lipid rafts that act as platforms for cellular signaling [33]. EGFR has been reported to be localized in the lipid rafts [34]. To explore whether lipid rafts may also provide such a platform, we use the Filipin III, which preferentially removes cholesterol from plasma membranes, to perturb the lipid rafts [35], [36], [37]. Filipin III treatment inhibited the phosphorylation of EGFR and AKT in SAS aggregates, but not in monolayer cultures [25], indicating that lipid rafts are involved in EGFR transactivation in SAS aggregates. A previous report showed that caveolin-1 (cav1) phosphorylation is required for EGFR and AKT activation in a specific environment [38]. Examining the cav1 expression and its phosphorylation in SAS aggregates, Cav1 was detected in equal amounts in both SAS cell aggregates and in monolayers. However, its phosphorylation levels were only downregulated in aggregates [25]. Immunocytological staining demonstrated that in SAS monolayer cultures, cav1 was localized on the cell surface and translated into the cytoplasm in response to filipin III treatment (Fig. 4, ML). However, cav1 was localized in the cytoplasm in SAS aggregates and its localization was not affected by filipin III treatment (Fig. 4, Ag). Accordingly, in SAS aggregates, non-phosphorylated cav1 was localized in the cytoplasm and not involved with lipid rafts. Thus, we speculate that lipid rafts serve as a platform in which EGFR and PI3K co-localize in the plasma membrane of SAS aggregates, thereby transmitting growth signals to the PI3K-Akt pathway through EGFR activation by ligand binding.
Figure 4.
Monolayer cultures (upper panel) and aggregates (lower panel) of SAS cells with (F+) or without (F−) filipin III were immunostained for cav1.
For immunofluorescence staining, cultured cells were fixed in 3.5% (w/v) formaldehyde, permeabilized in 0.2% (v/v) Triton x-100, and blocked in 2% (w/v) BSA. The primary antibodies were incubated at 4 °C overnight. Alexa fluor 488-conjugated IgG (Life Technologies) was used as the secondary antibody. After incubation with the antibodies, SlowFade Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (dAPI; Invitrogen/Life Technologies) was added. The specimens were observed using fluorescence microscopy. Nuclei were stained with dAPI.
3.3. Lapatinib reduces proliferation of OSCC cell lines lacking sphere formation capability
Lapatinib inhibite the proliferation of HSC3, HSC4 and Ca9-22 cells, but lapatinib did not inhibit the proliferation of KB, SAS and DU145 cells [39]. Thus, HSC3, HSC4 and Ca9-22 cells were lapatinib-sensitive, and KB, SAS and DU145 cells were lapatinib-resistant. Previous studies reported that lapatinib reduced the formation of atmospheres and proliferation of the progenitor/stem-enriched ductal carcinoma in situ population regardless of the ErbB2 status [40]. Analyzing the sphere formation capacities of these cell lines. HSC3, HSC4 and Ca9-22 cells remained single cells in floating cultures, while KB, DU145 and SAS cells formed spheres (Fig. 5). Therefore, lapatinib-sensitive cells lack stem cell properties, while lapatinib-resistant cells exhibit cancer stem cell-like characteristics (Fig. 5B). Lapatinib inhibited the proliferation of HSC3, HSC4 and Ca9-22 OSCC cell lines, in monolayer cultures. After lapatinib treatment, levels of AKT S473 phosphorylation and cyclin D1 protein expression, as well as phosphorylation of EGFR, ErbB2 and ErbB3, decreased in these cell lines, consistent with previous studies of breast cancer cells [41], [42]. However, growth of OSCC cell lines KB and SAS, and the prostate cancer cell line DU145 were resistant to lapatinib in monolayer cultures. In breast cancer, the causes of lapatinib resistance involve both ErbB2-dependent and ErbB2-independent mechanisms. In the former case, expression and structural changes of ErbB2 induce lapatinib resistance [43], [44]. In the latter case, activation of a pathway other than ErbB2 overcomes the inhibitory effects of lapatinib [45].
Figure 5.
Sphere formation cultures.
Cells (1 × 103) were seeded into each well of ultra-low attachment 6-well plates (Corning, NY, USA) and cultured in DMEM supplemented with 10% (v/v) FBS at 37 °C under 5% CO2. Phase-contrast photomicrographs of cell masses derived from single cells; 1 × 103 HSC3, HSC4, Ca9-22, KB, DU145 and SAS cells were grown on ultra-low attachment 6-well plates.
3.4. Sphere formation of SAS cells via activation of the ErbB/AKT/cyclin D2 signalling pathway is inhibited by lapatinib
Anchorage-dependent growth of SAS cells is resistant to EGFR inhibitors, cetuximab and AG1478, but anchorage-independent growth becomes sensitive to these inhibitors [25]. Examining the effects of lapatinib on sphere formation in KB, SAS and DU145 cancer cell lines possessing sphere formation capabilities,the levels of EGFR phosphorylation in KB and SAS cells increase during sphere formation process, but remaine during sphere formation in DU145 cells. ErbB2 phosphorylation levels increase during sphere formation in all the cell lines. AKT phosphorylation increase during sphere formation of SAS cells but remaine at low levels during sphere formation of KB and DU145 cells. Cyclin D1 levels were moderately altered during sphere formation in all cells. Cyclin D2 levels were increased along with sphere formation of SAS cells but were not detected in KB or DU145 spheres [39]. Analyzing the formation of lapatinib-resistant cell lines develops sensitivity to lapatinib, sphere formation was inhibite by lapatinib in SAS cells, but not in KB or DU145 cells [39]. Phosphorylation of EGFR and ErbB2 was reduced by lapatinib in these cell lines. Furthermore, lapatinib treatment reduced AKT phosphorylation and cyclin D2 protein expression in SAS spheres. AKT phosphorylation and cyclin D2 protein expression were not detected in untreated KB and DU145 spheres [39]. Thus, ErbB/AKT/cyclin D2 pathway plays an important role in sphere formation of SAS cells (Figure 6, Figure 7).
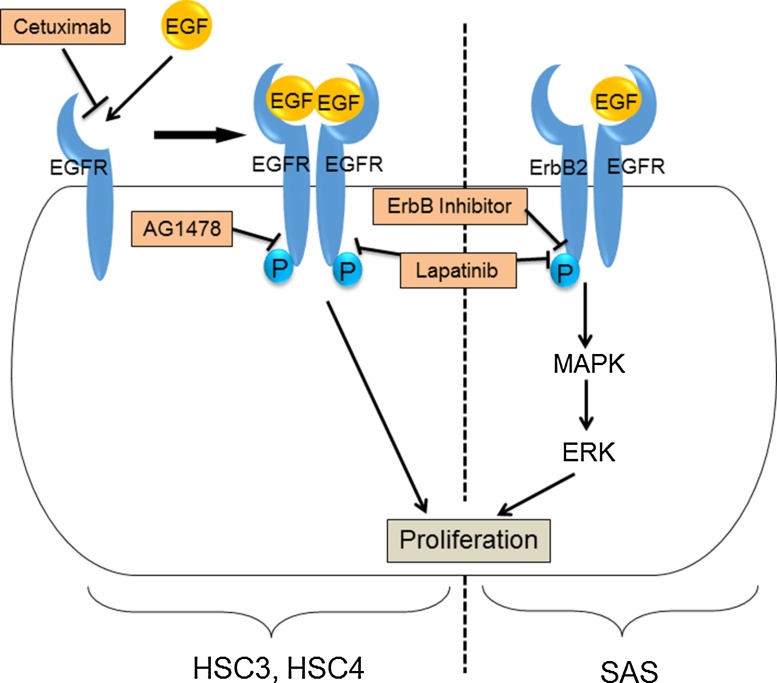
Figure 6.
Proliferation of OSCC cell lines in monolayer culture. (Left side) EGFR signaling has an important role for the proliferation of HSC3 and HSC4. Addition of EGF induces the promotion of cell proliferation. Moreover, addition of EGFR inhibitor, such as cetuximab, AG1478, and lapatinib, inhibit the proliferation of these cell lines. (Right side) SAS also express EGFR on the plasma membrane. The cell proliferation is not inhibited by the EGFR inhibitors (cetuximab and AG1478). On the other hand, the addition of lapatinib, a dual inhibitor of EGFR and ErbB2 tyrosine kinase activity, inhibit the cell proliferation. Moreover, the addition of ErbB inhibitor II significantly inhibit the cell proliferation. These results suggest that proliferation of SAS needs the signaling of ErbB other than EGFR. In addition, the ErbB signaling phosphorylates and activates MAPK, which in turn phosphorylates and activates ERK. This signaling cascade promotes the SAS cell proliferation, consequently.
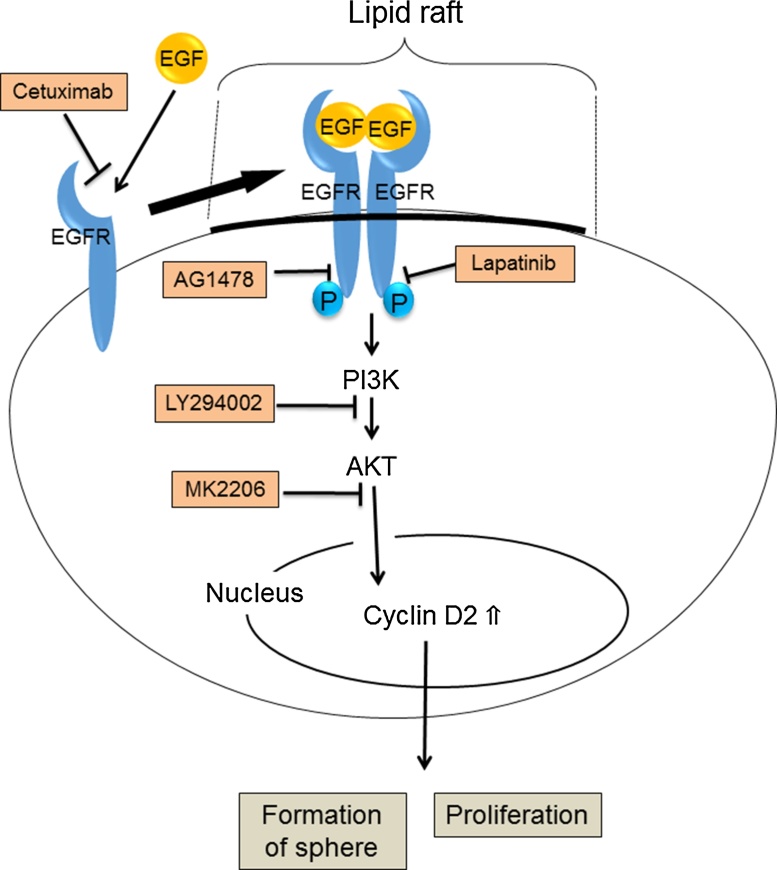
Figure 7.
Proliferation of SAS in floating culture. SAS can proliferate in the anchorage-independent growth condition and form sphere. In this condition, activated receptors, including EGFR, assemble at lipid raft, which is a kind of platform of the plasma membrane. Not only lapatinib but also cetuximab and AG1478, inhibit the proliferation of SAS sphere. Moreover, LY294002, which is a PI3K inhibitor, and MK2206, which is an AKT inhibitor, suppress the amount of cyclin D2. Cyclin D2 is one of a regulator of cell cycle and contributes to the proliferation of SAS sphere.
4. Migration of OSCC cell lines
4.1. Cetuximab treatment markedly inhibited the migratory activity of SAS cells
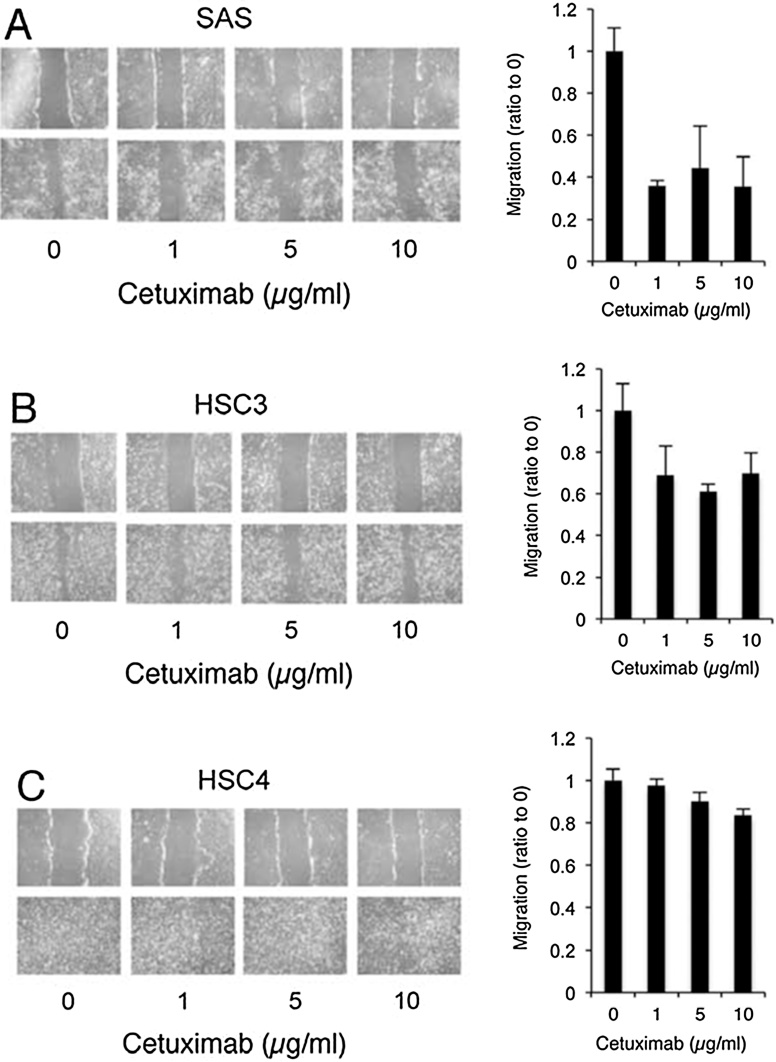
In addition to cell proliferation, EGFR signaling impacts other important physiological properties, including migration, differentiation, and apoptosis [46], [47]. Although cetuximab inhibits the growth of OSCC, it may not be effective for some cancers on migration. By the wound healing assay in the presence of cetuximab, cetuximab treatment markedly inhibited the migratory activity of SAS cells (Fig. 8A). Inhibitory effects of cetuximab were moderate in HSC3 cells, and almost absent in HSC4 cells (Fig. 8B and C). So, alterations in EGFR signaling induced by cetuximab play an important role in SAS cell migration [25], [48]. Cetuximab binds to the EGFR with a high affinity comparable to that of its ligands [26], and prevents ligand binding and receptor activation. Thus, the effects of cetuximab are restricted to cellular physiology regulated by ligand-dependent EGFR activation. Growth of SAS cells in monolayer cultures persists in serum-free medium and was not inhibited by cetuximab treatment. Wound repair by SAS cells was inhibited by cetuximab treatment (Fig. 8). These data show that dual systems of EGFR activation, including ligand-independent and ligand-dependent EGFR activation, play distinct roles in SAS monolayer cultures, in cell growth and in wound repair, respectively.
Figure 8.
Cetuximab inhibits SAS cell migration.
Cell migration was determined by a scratch wound healing assay as described [59], with slight modifications. Briefly, cells at a semi-confluence in 12-well plates were treated with 10 μg/ml of mitomycin C for 4 h to block proliferation and subsequently wounded with a sterile 200-μl pipette tip to generate a cell-free gap ∼1 mm in width. Cells were then washed with PBS and photographed to record the wound width at 0 h. Next, one group of cells was cultured in dMEM with 10% FBS for 24 h as a control. Other groups were treated with various concentrations of cetuximab. Twenty-four hours later, photographs were taken to evaluate migration. Scratch wound healing assays were performed to compare the migration capability of SAS (A), HSC3 (B), and HSC4 (C) cells in the presence or absence of cetuximab treatment. The width of the scratches were measured at 0 h and after 24 h of culture using ImageJ software. The relative distance was calculated as the mean width of the cell scratch. The effect of cetuximab treatment on cell migration was investigated by comparing the width of treated and non-treated cells; the non-treated width was set at 1.0.
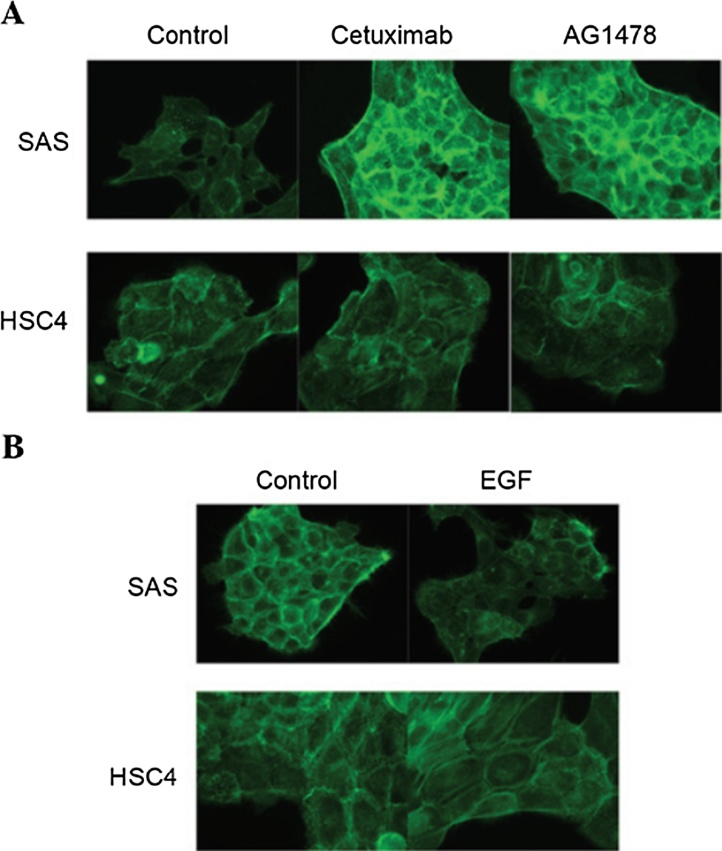
4.2. EGFR inhibitors promote, and EGF suppresses, the accumulation of actin filaments in SAS cells
As the remodeling of the actin network is considered to be involved in cell migration [49]. Analyzing the effects of cetuximab or AG1478 treatment on actin filament levels using phalloidin staining, the degree of staining was markedly increase following the treatment of SAS cells with cetuximab and AG1478, as compared with untreated cells (Fig. 9A). By contrast, the EGFR inhibitors cetuximab and AG1478 were not observed to affect the degree of phalloidin staining in HSC4 cells (Fig. 9A). EGFR is induced by EGF binding, and EGF affects actin organization in HSC4 and SAS cells. EGF treatment reduce the degree of palloidin staining in SAS cells, but not in HSC4 cells (Fig. 9B). Thus, the EGF-EGFR signaling pathway regulates actin filament turnover, and that it is necessary for cell motility. The cytoskeleton, which is composed of actin filaments, is a highly organized network that enables cellular motion [50]. It was hypothesized that the accumulation of actin filaments may produce irregular tension in the cytoskeletal system that is able to disrupt the orchestrated generation of force and interfere with directional cell migration. Therefore, the effects of EGFR inhibitors on the motility of SAS cells are possibly due to the accumulation of actin filaments in the cytoskeleton.
Figure 9.
Staining of actin fiber.
Cultured HSC4 and SAS cells were fixed in 3.5% (w/v) paraformaldehyde for 10 min at room temperature, permeabilized in 0.2% (v/v) Triton X-100 for 5 min at room temperature, and blocked in 2% (w/v) BSA for 30 min at room temperature. Fixed cells were incubated in 100 nM of Acti-stain™ 488 phalloidin in the dark for 30 min at room temperature. Phalloidin staining was observed under fluorescent microscopy (Olympus, Tokyo, Japan). The EGF-EGFR signaling pathway regulates the actin network. Fluorescent images of phalloidin staining of the actin filaments in HSC4 and SAS cells treated with (A) cetuximab or AG1478 and (B) EGF. Fixed cells were permeabilized and stained with Acti-stain™ 488.
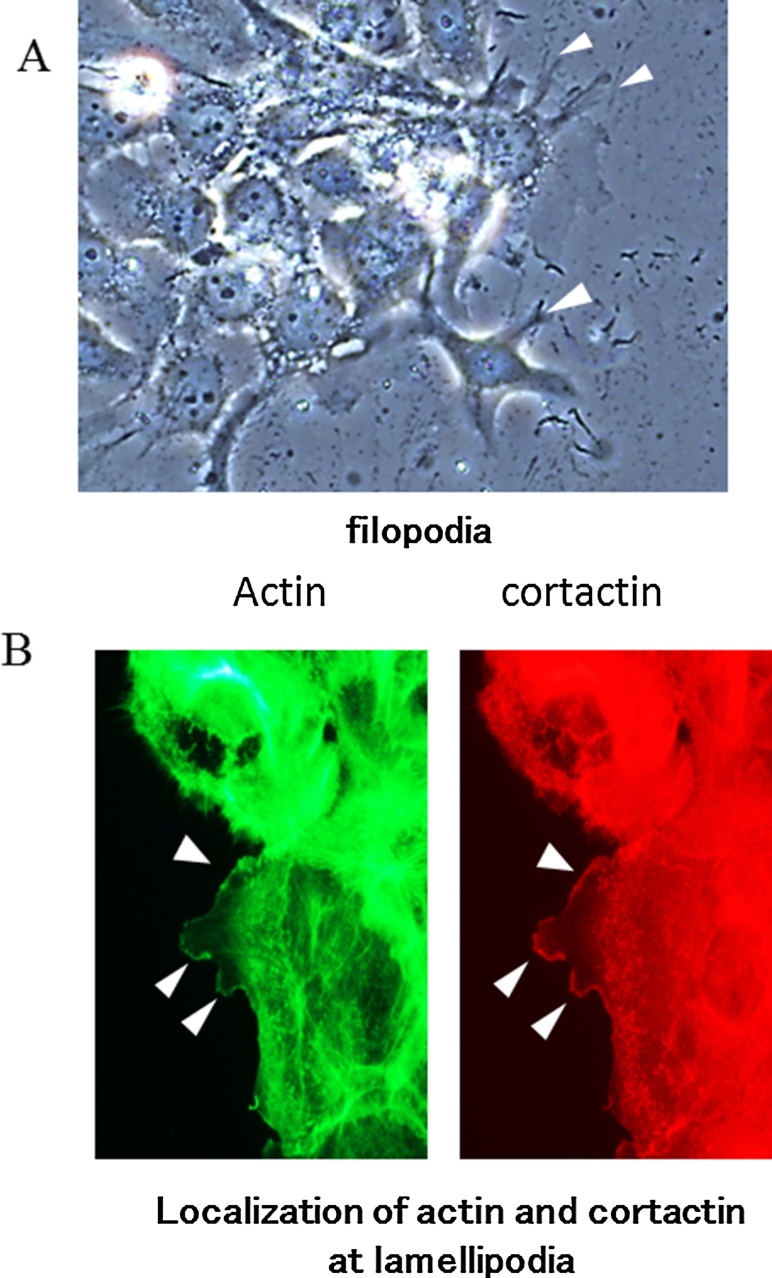
4.3. Filopodia and lamellipodia formation is induced by HGF/c-Met signalling in HSC4 and SAS cells
The cells promote actin polymerization toward the moving direction, and migrate by extruding the cell membrane from the inside. At this time, filopodia and lamellipodia are formed in the leading edge of the cells [51], [52]. To confirm the formation of lamellipodia in HSC4 cells, the index which determine the formation of lamellipodia as co-localization of actin and cortactin are used by immunocytochemistry [53]. Filopodia is thin cell processes and extends from the leading edge of migrating cells (Fig. 10A) and lamellipodia are thin, sheet-like membrane processes found at the leading edge of migrating cells (Fig. 10B). Examine the relationship to the formation of filopodia and lamellipodia, and EGFR and HGF/c-Met pathway in HSC4 and SAS cells, the effects of HGF/c-Met on filopodia and lamellipodia reduce significantly compared with untreated cells of SU11274 treatment [54], and filopodia formation in HSC4 and SAS cells was decreased by inhibition of c-Met signaling. Further, filopodia-forming HSC4 and SAS cells were increased of HGF treatment compared with the serum-free medium. Additionally, the ratios of HSC4 and SAS cells with lamellipodia was reduced significantly by SU11274 treatment compared with untreatment cells. The percentages of lamellipodia-forming HSC4 and SAS cells after HGF treatment did no differ from those of untreated cells were increased[54]. Thus, HGF/c-Met signalling is important for the formation of filopodia and lamellipodia in both the HSC4 and SAS cells, and that EGFR signalling plays an important role in filopodia and lamellipodia formation in SAS cells (Figure 11, Figure 12). These results are consistent with previous reports that HGF/c-Met signalling promoted cell migration through lamellipodia and filopodia formation in lung endothelial cells [55] and in some normal cells (30). Thus, it is possible that lamellipodia and filopodia formation is regulated by c-Met signalling, thereby promoting the migration of OSCC cells. ERK and PI3K/Akt serve as downstream effectors of c-Met signalling [55]. However, the molecules downstream of c-Met/ERK and c-Met/PI3K/Akt signalling that directly regulate filopodia and lamellipodia formation remain unknown. In this context, we showed that c-Met signalling was involved in the regulation of lamellipodin protein levels. Promotion of cell migration potency via the c-Met pathway is possibly regulated by increasing the level of lamellipodin, because upregulation of lamellipodin protein markedly promoted cell migration [56].
Figure 10.
The typical images of filopodia and lamellipodia. Cultured cells were fixed in 3.5% (w/v) paraformaldehyde, permeabilized in 0.2% (v/v) Triton X-100 and blocked in 2% (w/v) bovine serum albumin (BSA). The cells were incubated with anti-cortactin antibody at 4 °C overnight, followed by Alexa Fluor 594-conjugated IgG (Thermo Fisher Scientific, Yokohama, Japan) as the secondary antibody and Acti-stain 488 phalloidin (Cytoskeleton) for actin-fiber staining. After incubation, SlowFade gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen/Life Technologies) was added to the cells. The specimens were observed by fluorescence microscopy (Olympus IX73; Olympus, Tokyo, Japan). We determined lamellipodia formation by evaluating fluorescent actin fibers and cortactin co-localization at the cell periphery. (A) Arrow heads indicate filopodia which are slender cytoplasmic projection that extended beyond the leading edge in migrating cells. (B) It is known that actin and cortactin co-localizes at lamellipodia. These images show the immunofluorescence staining of actin or cortactin, in HSC4 cells. Arrow heads indicate lamellipodia.
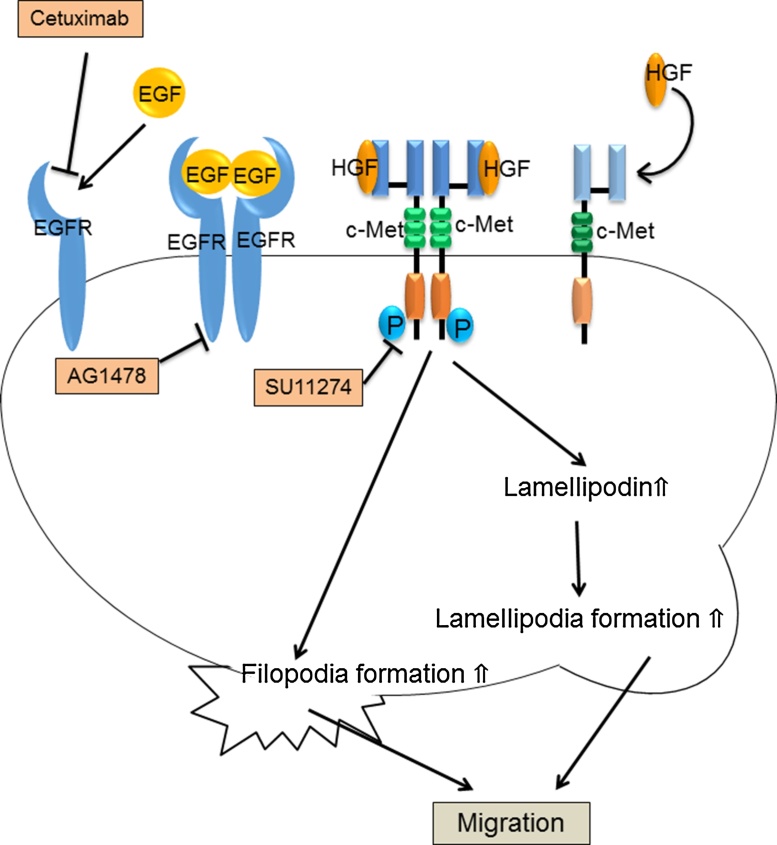
Figure 11.
The signaling relating to cell migration of HSC4. HGF/c-Met signaling increase the filopodia formation, the amount of lamellipodin and the lamellipodia formation. The addition of SU11274, which is a c-Met inhibitor, suppresses the HGF/c-Met signaling and reduces the cell migration of HSC4. On the other hand, EGF/EGFR signaling is not involved in the cell migration of HSC4.
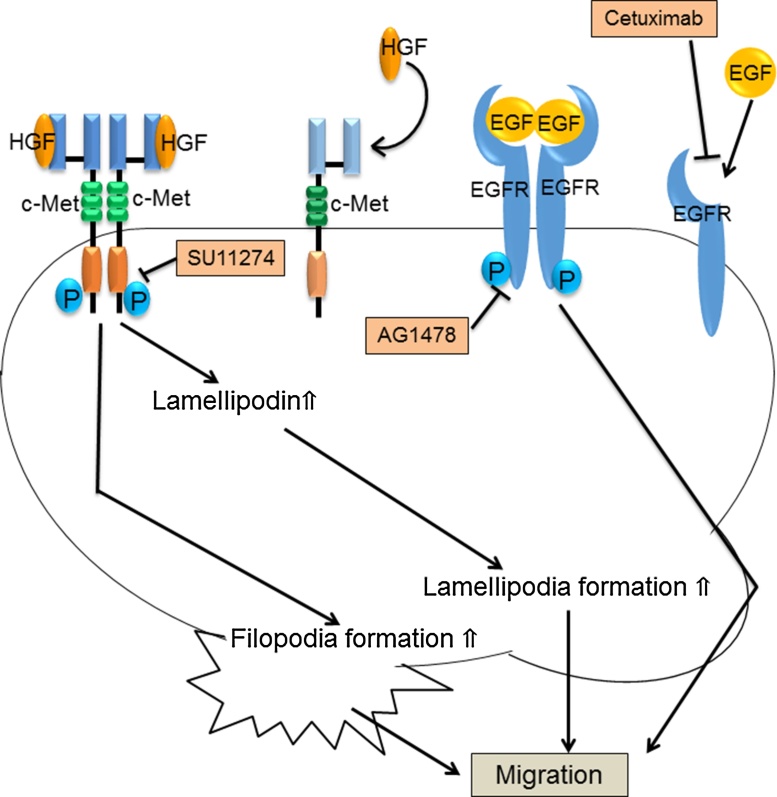
Figure 12.
The signaling relating to cell migration of SAS.
As well as HSC4, HGF/c-Met signaling increase the filopodia formation, the amount of lamellipodin and the lamellipodia formation, and continuing promotes the cell migration of SAS. Moreover, EGFR signaling also promotes the cell migration of SAS. However, the formation of such cell protrusion does not depend on EGFR signaling.
5. Conclusions
SAS cell migration is sensitive to the EGFR inhibitors cetuximab. Moreover, HGF/c-Met pathway also has an important role for OSCC cell migration. Therefore, cetuximab treatment in combination with HGF/c-Met inhibitor may be a candidate therapeutic target for the prevention of cancer stem cell dissociation from the primary tumor, migration into the surrounding tissues during the early stage and colonization at distant sites in OSCC metastasis.
Cancer cells possessing sphere formation ability survive and grow from single cells under anchorage-independent culture conditions. These characteristics are similar to the properties of circulating tumor cells (CTCs) during metastasis. CTCs are expected to be mesenchymal, since they enter the circulation from the primary tumor after epithelial-to-mesenchymal transition (EMT). However, CTCs consist of epithelial and mesenchymal subpopulations [57]. Both CTC subpopulations colonize in distant organs, where they are involved in secondary tumorigenesis as epithelial CSCs [58]. Thus, lapatinib can be used as a potential anticancer drug for both differentiated cancer cells of the primary tumor and for cells of the metastatic cancer.
Conflict of interest statement
There are no conflicts of interest associated with this review.
References
- 1.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Harris R.C., Chung E., Coffey R.J. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga C.L., Baselga J. Tyrosine kinase inhibitors: why does the current process of clinical development not apply to them? Cancer Cell. 2004;5:525–531. doi: 10.1016/j.ccr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Galer C.E., Corey C.L., Wang Z., Younes M.N., Gomez-Rivera F., Jasser S.A. Dual inhibition of epidermal growth factor receptor and insulin-like growth factor receptor I: reduction of angiogenesis and tumor growth in cutaneous squamous cell carcinoma. Head Neck. 2011;33:189–198. doi: 10.1002/hed.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 6.Ang K.K., Berkey B.A., Tu X., Zhang H.Z., Katz R., Hammond E.H. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 7.Chung C.H., Zhang Q., Hammond E.M., Trotti A.M., III, Wang H., Spencer S. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:331–338. doi: 10.1016/j.ijrobp.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galizia G., Lieto E., De Vita F., Orditura M., Castellano P., Troiani T. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–3660. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 9.Li S., Schmitz K.R., Jeffrey P.D., Wiltzius J.J., Kussie P., Ferguson K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Chung K.Y., Shia J., Kemeny N.E., Shah M., Schwartz G.K., Tse A. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Jonker D.J., O'Callaghan C.J., Karapetis C.S., Zalcberg J.R., Tu D., Au H.J. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 12.Sobrero A.F., Maurel J., Fehrenbacher L., Scheithauer W., Abubakr Y.A., Lutz M.P. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 13.Bokemeyer C., Bondarenko I., Makhson A., Hartmann J.T., Aparicio J., de Braud F. Fluorouracil, leucovorin and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E., Köhne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 15.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 16.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 17.Herbst R.S., Arquette M., Shin D.M., Dicke K., Vokes E.E., Azarnia N. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J., Trigo J.M., Bourhis J., Tortochaux J., Cortés-Funes H., Hitt R. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568–5577. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 19.Burtness B., Goldwasser M.A., Flood W., Mattar B., Forastiere A.A. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 20.Kokai Y., Myers J.N., Wada T., Brown V.I., Levea C.M., Davis J.G. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 21.Di Fiore P.P., Pierce J.H., Fleming T.P., Hazan R., Ullrich A., King C.R. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y., Li S., Hu Y.P., Wang J., Hauser J., Conway A.N. Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosis. Cancer Res. 2006;66:404–411. doi: 10.1158/0008-5472.CAN-05-2506. [DOI] [PubMed] [Google Scholar]
- 23.Pirker R., Pereira J.R., von Pawel J., Krzakowski M., Ramlau R., Park K. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 24.Dontu G., Abdallah W.M., Foley J.M., Jackson K.W., Clarke M.F., Kawamura M.J. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi Y., Yasui H., Kakudo K., Nozaki M. Cetuximab-resistant oral squamous cell carcinoma cells become sensitive in anchorage-independent culture conditions through the activation of the EGFR/AKT pathway. Int J Oncol. 2015;47:2165–2172. doi: 10.3892/ijo.2015.3215. [DOI] [PubMed] [Google Scholar]
- 26.Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisch S.M., Vuori K., Ruoslahti E., Chan-Hui P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meredith J.E., Jr., Fazeli B., Schwartz M.A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z., Li H., Wu X., Yoo B.H., Yan S.R., Stadnyk A.W. Detachment-induced upregulation of XIAP and cIAP2 delays anoikis of intestinal epithelial cells. Oncogene. 2006;25:7680–7690. doi: 10.1038/sj.onc.1209753. [DOI] [PubMed] [Google Scholar]
- 30.Rosen K., Coll M.L., Li A., Filmus J. Transforming growth factor-alpha prevents detachment-induced inhibition of c-Src kinase activity, Bcl-XL down-regulation, and apoptosis of intestinal epithelial cells. J Biol Chem. 2001;276:37273–37279. doi: 10.1074/jbc.M106424200. [DOI] [PubMed] [Google Scholar]
- 31.Liu P., Begley M., Michowski W., Inuzuka H., Ginzberg M., Gao D. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maa M.C., Leu T.H., McCarley D.J., Schatzman R.C., Parsons S.J. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 34.Wu W., Graves L.M., Gill G.N., Parsons S.J., Samet J.M. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation. J Biol Chem. 2002;277:24252–24257. doi: 10.1074/jbc.M200437200. [DOI] [PubMed] [Google Scholar]
- 35.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 36.Montesano R., Vassalli P., Orci L. Structural heterogeneity of endocytic membranes in macrophages as revealed by the cholesterol probe, filipin. J Cell Sci. 1981;51:95–107. doi: 10.1242/jcs.51.1.95. [DOI] [PubMed] [Google Scholar]
- 37.Santos N.C., Ter-Ovanesyan E., Zasadzinski J.A., Prieto M., Castanho M.A. Filipin-induced lesions in planar phospholipid bilayers imaged by atomic force microscopy. Biophys J. 1998;75:1869–1873. doi: 10.1016/S0006-3495(98)77627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Peng F., Wu D., Ingram A.J., Gao B., Krepinsky J.C. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi Y., Yasui H., Kakudo K., Nozaki M. Lapatinib-resistant cancer cells possessing epithelial cancer stem cell properties develop sensitivity during sphere formation by activation of the ErbB/AKT/cyclin D2 pathway. Oncol Rep. 2016;36:3058–3064. doi: 10.3892/or.2016.5073. [DOI] [PubMed] [Google Scholar]
- 40.Farnie G., Johnson R.L., Williams K.E., Clarke R.B., Bundred N.J. Lapatinib inhibits stem/progenitor proliferation in preclinical in vitro models of ductal carcinoma in situ (DCIS) Cell Cycle. 2014;13:418–425. doi: 10.4161/cc.27201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rusnak D.W., Lackey K., Affleck K., Wood E.R., Alligood K.J., Rhodes N. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 42.Spector N.L., Xia W., Burris H., III, Hurwitz H., Dees E.C., Dowlati A. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 43.Trowe T., Boukouvala S., Calkins K., Cutler R.E., Jr., Fong R., Funke R. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14:2465–2475. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 44.Kancha R.K., von Bubnoff N., Bartosch N., Peschel C., Engh R.A., Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creedon H., Gómez-Cuadrado L., Tarnauskaitė Ž., Balla J., Canel M., MacLeod K.G. Identification of novel pathways linking epithelial-to-mesenchymal transition with resistance to HER2-targeted therapy. Oncotarget. 2016;7:11539–11552. doi: 10.18632/oncotarget.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal H.C., Sharma S., Strickland L.R., Agarwal J., Athar M., Elmets C.A. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morozevich G.E., Kozlova N.I., Ushakova N.A., Preobrazhenskaya M.E., Berman A.E. Integrin α5β1 simultaneously controls EGFR-dependent proliferation and Akt-dependent pro-survival signaling in epidermoid carcinoma cells. Aging (Albany, NY) 2012;4:368–374. doi: 10.18632/aging.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohnishi Y., Yasui H., Kakudo K., Nozaki M. Regulation of cell migration via EGFR signaling in oral squamous cell carcinoma cells. Oncol Lett. 2016;13:930–936. doi: 10.3892/ol.2016.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 50.Small J.V., Resch G.P. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr Opin Cell Biol. 2005;17:517–523. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Hui A.Y., Meens J.A., Schick C., Organ S.L., Qiao H., Tremblay E.A. Src and FAK mediate cell–matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J Cell Biochem. 2009;107:1168–1181. doi: 10.1002/jcb.22219. [DOI] [PubMed] [Google Scholar]
- 52.Michael M., Vehlow A., Navarro C., Krause M. c-Abl, Lamellipodin, and Ena/VASP proteins cooperate in dorsal ruffling of fibroblasts and axonal morphogenesis. Curr Biol. 2010;20:783–791. doi: 10.1016/j.cub.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weed S.A., Karginov A.V., Schafer D.A., Weaver A.M., Kinley A.W., Cooper J.A. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasui H., Ohnishi Y., Nakajima M., Nozaki M. Migration of oral squamous cell carcinoma cells can be induced by HGF/c-Met signaling via lamellipodia and filopodia formation. Oncol Rep. 2017;37:3674–3680. doi: 10.3892/or.2017.5587. [DOI] [PubMed] [Google Scholar]
- 55.Usatyuk P.V., Fu P., Mohan V., Epshtein Y., Jacobson J.R., Gomez-Cambronero J. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J Biol Chem. 2014;289:13476–13491. doi: 10.1074/jbc.M113.527556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Law A.L., Vehlow A., Kotini M., Dodgson L., Soong D., Theveneau E. Lamellipodin and the Scar/WAVE complex cooperate to promote cell migration in vivo. J Cell Biol. 2013;203:673–689. doi: 10.1083/jcb.201304051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu M., Bardia A., Wittner B.S., Stott S.L., Smas M.E., Ting D.T. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beerling E., Seinstra D., de Wit E., Kester L., van der velden D., Maynard C. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 2016;14:2281–2288. doi: 10.1016/j.celrep.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakin A.V., Rinehart C., Tomlinson A.K., Arteaga C.L. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]