Abstract
After isolation of tobacco (Nicotiana tabacum) leaf mitochondria, alternative oxidase (AOX) is predominantly present as the disulfide-linked, less-active “oxidized” form. In an in organello assay, significant AOX activity was dependent upon both the reduction of the regulatory disulfide bond (such as occurs by dithiothreitol) and upon the presence of the activator pyruvate. However, AOX activity in these assays was substantially affected when mitochondria were isolated in the presence of pyruvate. First, pyruvate protects against the oxidation of the regulatory sulfhydryl during isolation, such that subsequent in organello AOX activity is not dependent upon dithiothreitol. Second, pyruvate stabilizes AOX activity, such that mitochondria kept in the presence of pyruvate have higher maximum rates of AOX activity than mitochondria kept for some time in the absence of pyruvate. The ability of pyruvate to protect against AOX oxidation was exploited to assess the in vivo status of the regulatory sulfhydryl/disulfide system. In both tobacco suspension cells and tobacco leaves with high levels of AOX protein, the protein is predominantly present as the “reduced” active form in vivo under a range of respiratory conditions. Experiments also indicate that, while the presence of reduced protein may be a necessary prerequisite for significant AOX activity, it is not sufficient for activity and other factors must also be critical.
In plant mitochondria, there are two paths of respiratory electron transport from ubiquinol to O2 (Day et al., 1980; Lambers, 1985; Siedow and Umbach, 1995). Electron transfer through the Cyt pathway is coupled (through the generation of a proton motive force) to ATP synthesis, and the terminal oxidase (Cyt oxidase) is inhibited by CN. Alternatively, electron flow from ubiquinol to alternative oxidase (AOX) is not coupled to ATP production. AOX is CN-resistant but sensitive to substituted hydroxamic acids such as salicylhydroxamic acid and n-propyl gallate. It is postulated that the AOX pathway functions to modulate respiratory carbon flow (Lambers, 1985). One important consequence of this modulation could be to alleviate the generation of harmful reactive oxygen species in the mitochondrion by preventing over-reduction of particular electron transport chain components (Purvis and Shewfelt, 1993; Popov et al., 1997; Purvis, 1997; Wagner and Moore, 1997).
The partitioning of electrons to AOX appears to be dependent upon a covalent redox modulation of the protein, which is capable of existing in the inner mitochondrial membrane as either a non-covalently linked or a covalently linked dimer (Umbach and Siedow, 1993). The dimer, when covalently linked by an intermolecular disulfide bond between the two subunits, is a less-active form of AOX (as determined by in organello assays), while reduction of the disulfide bond to its component sulfhydryls produces a more active form (Umbach and Siedow, 1993). The two forms can be interconverted by treatment with the reductant dithiothreitol (DTT) and the oxidant diamide, and can be visualized by non-reducing SDS-PAGE and immunoblot analysis, in which the oxidized form has an apparent molecular mass twice that of the reduced form (Umbach and Siedow, 1993).
In tobacco (Nicotiana tabacum) AOX, Cys-126 is the residue involved in this sulfhydryl/disulfide regulatory system (Vanlerberghe et al., 1998). Reduction of tobacco AOX to its active form is mediated in isolated mitochondria by the oxidation of specific tricarboxylic acid cycle substrates, notably isocitrate and malate (Vanlerberghe et al., 1995). Presumably, intramitochondrial reducing power generated by the oxidation of these organic acids supports the reduction of AOX. Because of the organic acid specificity of this effect, reduction may be mediated by a thioredoxin system specifically requiring NADPH. Such a system has been identified in plant mitochondria but has not yet been ascribed any specific function (Moller and Rasmusson, 1998).
AOX activity is also strongly dependent upon the presence of particular α-keto acids, most notably pyruvate (Millar et al., 1993; Day et al., 1994). Significant AOX activity in isolated tobacco mitochondria is dependent upon both reduction of the regulatory disulfide bond and the presence of pyruvate (Vanlerberghe et al., 1995). Studies on soybean AOX suggest that pyruvate action is due to its interaction with a Cys sulfhydryl to form a thiohemiacetal, since the activation is mimicked by iodoacetate (Umbach and Siedow, 1996). It has also been shown that pyruvate acts to increase the Vmax of AOX without any significant effect on its affinity for ubiquinol (Hoefnagel et al., 1997; Millar et al., 1997).
With an appreciation that in organello AOX activity depends on the redox state of a sulfhydryl/disulfide system and the availability of pyruvate, it has been shown that the AOX pathway can compete with the Cyt pathway for electrons in isolated mitochondria (Hoefnagel et al., 1995; Ribas-Carbo et al., 1995). In this study, we detail further the interacting effects of the sulfhydryl/disulfide system and pyruvate on in organello and in vivo AOX activity, and provide evidence of the importance of these regulatory mechanisms for activity in vivo.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tobacco (Nicotiana tabacum cv Petit Havana SR1) mitochondrial AOX is encoded by the nuclear gene Aox1 (Vanlerberghe and McIntosh, 1994). The transgenic tobacco line B9 contains Aox1 in the sense orientation under the transcriptional control of the cauliflower mosaic virus 35S promoter and contains high levels of mitochondrial AOX protein (Vanlerberghe et al., 1998). Primary transformants of B9 were grown at 23°C to 27°C in Magenta boxes (Magenta Corporation, Chicago) on a modified Murashige and Skoog medium (Vanlerberghe et al., 1994) supplemented with 100 μg mL−1 kanamycin and under continuous fluorescent light.
Suspension cells were derived from B9 leaf tissue (Vanlerberghe et al., 1998). Cultures (200 mL) were routinely grown in the dark on a rotary shaker (140 rpm, 28°C) and were subcultured every 7 d by 14-fold dilution of the cells in fresh medium. The medium (Linsmaier and Skoog, 1965) contains 3% (w/v) Suc as carbon source and was supplemented with 75 μg mL−1 kanamycin. Wild-type tobacco (N. tabacum L. cv Bright Yellow) suspension cells were grown as above but in the absence of kanamycin.
Mitochondrial Isolation
Mitochondria were isolated from leaves and suspension cells as previously described (Vanlerberghe et al., 1995, 1998) except that in many cases the isolation was done in the presence of pyruvate. In this case, fresh pyruvate was added to each of the isolation media just prior to use. Mitochondrial protein was quantified by a modified Lowry assay (Larson et al., 1986).
In Organello AOX Activity
O2 uptake by leaf mitochondria (approximately 0.2–0.25 mg of protein in a final assay volume of 1 mL) and determination of AOX activity were done using a Clark-type O2 electrode cuvette, as previously described (Vanlerberghe et al., 1995, 1998). Typically, O2 uptake was initiated by the addition of 2 mm NADH followed by 1 mm ADP. Subsequently, the Cyt pathway was inhibited by the addition of 16 μm myxothiazol. O2 uptake in the presence of myxothiazol and sensitive to 100 μm n-propyl gallate is defined as AOX activity. The effect of pyruvate (2 mm) and DTT (10 mm) on AOX activity was determined by the addition of freshly made stocks of these compounds after the addition of myxothiazol. Higher concentrations of these compounds gave no further response. Typically, there was no O2 consumption after the addition of both myxothiazol and n-propyl gallate. The O2 concentration in air-saturated water at 25°C was assumed to be 240 μm. Further details of the assays are outlined in the table and figure legends.
Rapid Cellular Protein Extraction
In some experiments, cells were treated with various compounds (antimycin A [AA], p-trifluoromethoxycarbonylcyanide [FCCP], and H2O2) under standard growth culture conditions prior to the rapid extraction of total cellular protein from the cells and subsequent analysis of AOX protein in the extract. The details of the cell treatments involved are found in the figure legends. To carry out the extraction, an aliquot of cells (approximately 80 mg dry weight) was quickly separated from the culture medium by vacuum filtration onto a filter (no. 1, Whatman, Clifton, NJ). The cells were quickly rinsed once with 12 mL of ice-cold distilled water and transferred to a mortar that had been prechilled with liquid N2. Additional liquid N2 was then added to the cells to ensure that they were rapidly frozen. Then, 1 mL of ice-cold extraction buffer (4% [w/v] SDS, 20% [v/v] glycerol, 5 mm pyruvate, 1 mm PMSF, and 84 mm Tris, pH 6.8) was added and the frozen slurry was ground with a pestle as it thawed. The extract was then centrifuged at 3,000g for 2 min at 4°C. In some cases, the supernatant (cellular protein extract) was then boiled for 5 min, cooled on ice, centrifuged (5 min, 16,000g, 4°C), and an aliquot immediately loaded on an SDS-PAGE gel. In other cases, the supernatant was immediately frozen in liquid N2 and stored at −80°C prior to boiling and loading onto an SDS-PAGE gel. Whether the samples were loaded onto a gel immediately or stored at −80°C prior to analysis had no effect on the relative levels of the oxidized and reduced forms of AOX protein. SDS-PAGE and immunoblot analysis of the AOX protein were as described below.
AOX Protein Analysis
Reducing and non-reducing SDS-PAGE and immunoblot analyses were as previously described (Vanlerberghe et al., 1998) using a monoclonal antibody (AOA) raised against the Sauromatum guttatum AOX (Elthon et al., 1989). Treatment of mitochondria with freshly prepared diamide (3 mm) was as previously described (Vanlerberghe et al., 1998). Relative amounts of AOX protein were quantified using a scanning densitometer (GS-700, Bio-Rad Laboratories, Hercules, CA) with Molecular Analyst software (Bio-Rad).
RESULTS
Isolation of Mitochondria in the Presence of Pyruvate Has Effects on AOX Activity
Previously, we showed that after isolation of leaf mitochondria from wild-type tobacco plants or from transgenic plants expressing high levels of AOX protein, the majority of the AOX protein was present in the less-active oxidized form. Therefore, significant in organello AOX activity in the presence of pyruvate was dependent upon the conversion of AOX to its more active reduced form, such as by the addition of DTT, citrate, or malate (Vanlerberghe et al., 1995). This was illustrated by the AOX assay in Table I (Run no. 1), showing that pyruvate addition alone resulted in only low rates of AOX activity and that subsequent addition of DTT generated a much higher rate. If DTT was added prior to pyruvate, activity remained low until pyruvate was added, indicating that both pyruvate and DTT are required (Vanlerberghe et al., 1995).
Table I.
O2 uptake by transgenic tobacco (B9) leaf mitochondria isolated in the absence of pyruvate
| Run | Sequential Additions | O2 Uptake |
|---|---|---|
| nmol O2 mg−1 protein min−1 | ||
| 1 | NADH | 49 ± 2 |
| ADP | 139 ± 8 | |
| Myxothiazol | 5 ± 2 | |
| Pyruvate | 10 ± 1 | |
| DTT | 49 ± 3 | |
| n-Propyl gallate | 0 ± 0 | |
| 2 | Pyruvate | NDa |
| NADH | 57 ± 4 | |
| ADP | 143 ± 10 | |
| Myxothiazol | 14 ± 1 | |
| DTT | 52 ± 7 | |
| n-Propyl gallate | 0 ± 0 |
Rates of O2 uptake were determined in an O2 electrode cuvette after sequential addition of the compounds indicated. Note that in run no. 1, pyruvate was added after several minutes of NADH oxidation, while in run no. 2, pyruvate was added to the mitochondria 1 min prior to the addition of NADH. Compounds were added at the following final concentrations: 2 mm NADH, 1 mm ADP, 16 μm myxothiazol, 2 mm pyruvate, 10 mm DTT, and 100 μm n-propyl gallate. Data are the average ± sd (n = 3) using mitochondria from the same isolation. Separate mitochondrial isolations yielded similar results.
ND, Not determined.
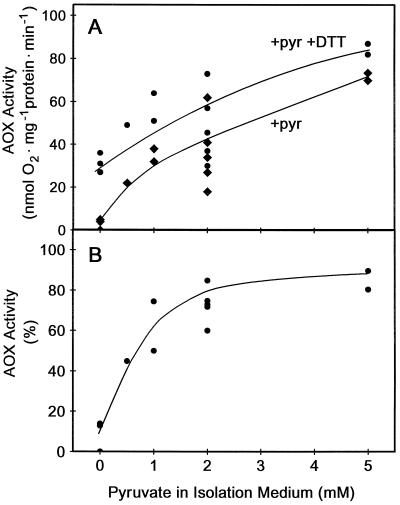
We now find that when transgenic (B9) tobacco leaf mitochondria with high levels of AOX protein are isolated in the presence of pyruvate (i.e. 0.5–5 mm pyruvate present in all of the isolation media), high rates of in organello AOX activity could be achieved in the absence of DTT, once saturating levels of pyruvate were added to the assay (Fig. 1). For example, when mitochondria were isolated without pyruvate, AOX activity measured after pyruvate addition to the AOX assay was very low (Fig. 1A), and only about 10% of that achieved following the subsequent addition of DTT (Fig. 1B). Alternatively, when mitochondria were isolated in the presence of 2 mm pyruvate, AOX activity measured after pyruvate addition to the AOX assay was high (Fig. 1A), near that which could be achieved following the subsequent addition of DTT (Fig. 1B). Note also that as the concentration of pyruvate in the isolation medium was increased, not only did AOX activity become less dependent upon DTT (Fig. 1B), but the maximum rate of AOX activity achieved (with pyruvate + DTT) also increased (Fig. 1A), although this effect was more variable.
Figure 1.
AOX activity in leaf mitochondria isolated in the presence of different concentrations of pyruvate. Transgenic tobacco (B9) mitochondria were isolated with pyruvate (0–5 mm) present in all of the isolation media and analyzed for AOX activity in an assay medium using an O2 electrode, as described in “Materials and Methods.” A, Effect of pyruvate concentration in the mitochondrial isolation medium on subsequent AOX activity in the presence of saturating (2 mm) pyruvate in the assay medium (+pyr, ♦) and after subsequent addition of 10 mm DTT to the assay medium (+pyr +DTT, ●). Each set of points (one +pyr, one +pyr +DTT) represents data from a separate mitochondrial isolation. B, Effect of pyruvate concentration in the mitochondrial isolation medium on DTT-independent AOX activity. Activity (in the presence of 2 mm pyruvate in the assay medium) is expressed as a percentage of activity with 2 mm pyruvate and 10 mm DTT in the assay medium. Data are derived from those in A.
The ability of pyruvate in the isolation medium to effectively substitute for DTT in promoting AOX activity could not be achieved simply by the addition of pyruvate to the assay medium prior to the addition of the substrate NADH (Table I, Run no. 2). In this case, high rates of AOX activity were still dependent upon the addition of DTT. Hence, pyruvate had to be present during the mitochondrial isolation in order for it to substitute for DTT.
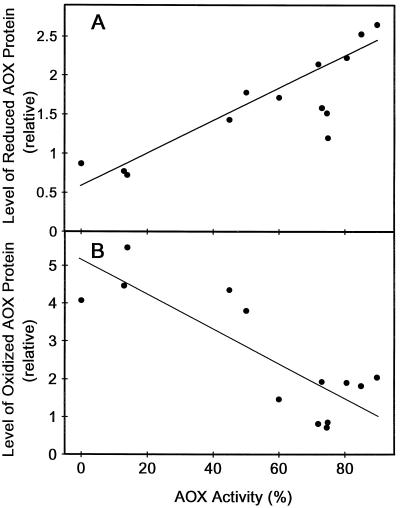
One possible explanation for the decreased dependence upon DTT noted above would be that the inclusion of pyruvate in the isolation medium alters the level of oxidized versus reduced AOX protein present after the isolation. In this case, increased AOX activity might correlate with a decrease in the oxidized form and an increase in the reduced form of the AOX protein present after isolation. Figure 2 shows that this was indeed the case. When AOX activity in the presence of pyruvate (but in the absence of DTT) was very low (mitochondria isolated without pyruvate), the majority of the AOX protein after isolation was in the oxidized form. However, as AOX activity in the presence of pyruvate (but absence of DTT) increases (mitochondria isolated with increasing concentrations of pyruvate), the level of oxidized protein declines and the level of reduced protein increases (Fig. 2). Therefore, the ability of pyruvate in the isolation medium to effectively substitute for DTT in the AOX activity assay correlates closely with its effect on the distribution of AOX protein between its oxidized (inactive) and reduced (active) forms.
Figure 2.
AOX activity in leaf mitochondria isolated in the presence of different concentrations of pyruvate ranging from 0–5 mm as a function of the level of the reduced (A) or oxidized (B) forms of the AOX protein present. AOX activity was measured and is expressed as described in Figure 1B. AOX protein levels were determined as described in “Materials and Methods.” Each set of points (one in A and one in B) are data from a separate mitochondrial isolation. The data are from the same mitochondria as in Figure 1.
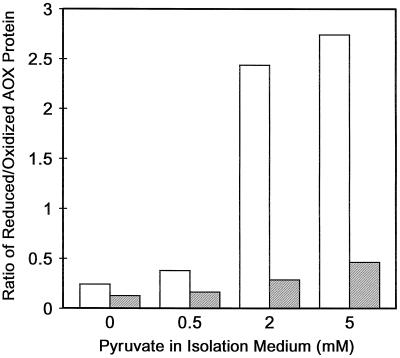
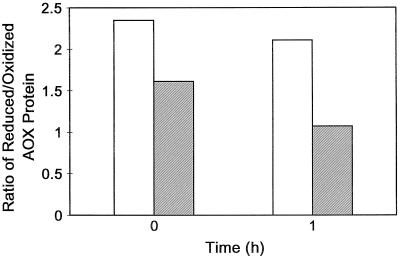
To further convince ourselves of this shift in the distribution of oxidized versus reduced protein, we utilized the sulfhydryl oxidant diamide. Figure 3 shows that when mitochondria were isolated without pyruvate, the ratio of reduced to oxidized protein was low and diamide treatment had little effect on the ratio. However, when mitochondria were isolated with 2 mm pyruvate, the ratio of reduced to oxidized protein was approximately 10-fold higher, and diamide treatment of these mitochondria could dramatically lower this ratio (Fig. 3).
Figure 3.
Effect of pyruvate concentration in the mitochondrial isolation media and subsequent treatment of isolated leaf mitochondria with diamide on the ratio of the levels of reduced and oxidized forms of the AOX protein present. Diamide treatment of mitochondria and quantification of AOX protein levels were described in “Materials and Methods.” The data are the average from 3 (0 mm pyruvate), 1 (0.5 mm pyruvate), 2 (2 mm pyruvate), and 1 (5 mm pyruvate) separate mitochondrial isolations. White bars, No diamide; shaded bars, plus diamide.
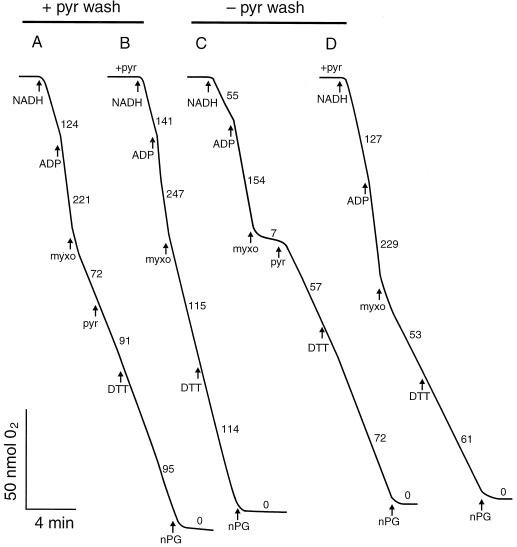
In another type of experiment, mitochondria were isolated with 5 mm pyruvate and subsequently washed twice in wash medium either containing 5 mm pyruvate or lacking pyruvate prior to analysis. Figure 4A shows that the mitochondria isolated and washed with 2 mm pyruvate displayed high rates of AOX activity after myxothiazol addition. This was due to significant carryover of pyruvate (0.25 mm) from the wash medium to the assay medium. After the addition of saturating pyruvate, the rate increased further and, as expected, was similar to that achieved following the subsequent addition of DTT. Similar rates of pyruvate-saturated AOX activity and dependence (or lack thereof) on DTT was seen whether pyruvate was added 1 min prior to or several minutes after NADH (compare Fig. 4, A and B).
Figure 4.
The effect of pyruvate on AOX activity. Leaf mitochondria were isolated from transgenic tobacco (B9) with 5 mm pyruvate present in all of the isolation media. These mitochondria were subsequently washed in media either also containing 5 mm pyruvate (traces A and B) or lacking pyruvate (traces C and D) prior to the O2 electrode analysis shown above. Note that in traces B and D, 2 mm pyruvate was added to the assay medium containing mitochondria 1 min prior to the addition of NADH. Additions were made at the following final concentrations: 2 mm NADH, 1 mm ADP, 16 μm myxothiazol (myxo), 2 mm pyruvate (pyr), 10 mm DTT, and 100 μm n-propyl gallate (nPG). Numbers on traces refer to rates of O2 uptake (nmol O2 mg−1 protein min−1). Representative results are shown.
When the mitochondria isolated with 5 mm pyruvate were washed in (−)-pyruvate medium prior to analysis, very little AOX activity was present prior to pyruvate addition (Fig. 4C), indicating that the pyruvate had been effectively removed. Once pyruvate was added, a high rate of activity was achieved. In this case, however, activity was more dependent upon DTT than in the (+)-pyruvate washed mitochondria (although not to the same extent as with mitochondria isolated without pyruvate). Again, similar rates of pyruvate-saturated AOX activity and dependence upon DTT were evident whether pyruvate was added prior to or after NADH (compare Fig. 4, C and D). Note also, however, that the maximum AOX activity achieved (with pyruvate + DTT) was less in the mitochondria given the (−)-pyruvate wash (Fig. 4, C and D) than in those given the (+)-pyruvate wash (Fig. 4, A and B).
For the experiment described above, we determined whether the increased dependence of AOX activity upon DTT in the (−)-pyruvate washed mitochondria compared with the (+)-pyruvate washed mitochondria (Fig. 4) was correlated with a change in the level of oxidized and reduced AOX protein present. Indeed, we found that even immediately after the wash (time 0), the ratio of reduced to oxidized AOX protein was significantly less in the (−)-pyruvate washed mitochondria than in the (+)-pyruvate washed mitochondria (Fig. 5). Also, while the ratio remained stable over time (on ice) in the (+)-pyruvate washed mitochondria, the ratio continued to decline with time in the (−)-pyruvate washed mitochondria. Nonetheless, 1 h after the wash, the ratio in (−)-pyruvate washed mitochondria was still well above that seen in mitochondria isolated without pyruvate (compare Fig. 5 to Figs. 2 and 3).
Figure 5.
The effect of pyruvate on the ratio of the reduced to oxidized forms of the AOX protein present in transgenic tobacco (B9) leaf mitochondria. Mitochondria were isolated with 5 mm pyruvate present in all of the isolation media and subsequently washed in media either also containing 5 mm pyruvate or lacking pyruvate. Immediately following the wash (time 0) and 1 h (on ice) after the wash (time 1 h), the ratio of the level of reduced to oxidized form of AOX protein present was determined. These data are from the same mitochondria analyzed in Figure 4. Representative results are shown. White bars, (+)-Pyruvate washed; shaded bars, (−)-pyruvate washed.
The in Vivo Status of the Regulatory Sulfhydryl/Disulfide System
The apparent ability of pyruvate to protect against AOX oxidation during mitochondrial isolation suggests that the inclusion of pyruvate in isolation media may be a means to determine the in vivo status of the AOX regulatory sulfhydryl/disulfide system under different respiratory conditions. To test this hypothesis, we isolated mitochondria (in the presence or absence of 5 mm pyruvate) from untreated tobacco suspension cells or from cells pretreated with 10 μm AA (an inhibitor of complex III of the respiratory chain). The experiments were done on a transgenic cell line (B9) that constitutively produces large amounts of AOX protein such that the level of KCN-resistant, SHAM-sensitive respiration (AOX capacity) of the cells is similar to the respiration rate of the cells (Vanlerberghe et al., 1998). Our rationale for these experiments was as follows.
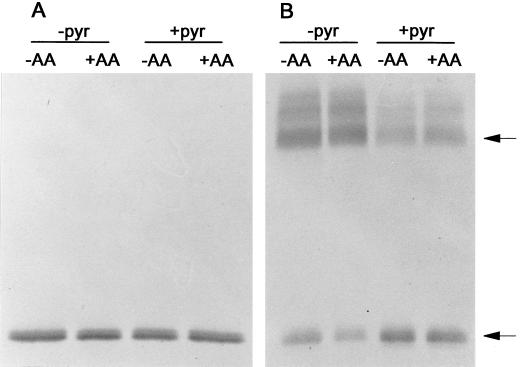
In untreated cells, it is likely that the high-capacity AOX pathway is not being fully utilized, so there may be a mixture of oxidized and reduced AOX protein present. However, upon addition of AA, the AOX pathway would quickly become highly utilized due to inhibition of the Cyt pathway, resulting in an increase in the level of reduced AOX protein and a decrease in the level of oxidized AOX protein. Figure 6 illustrates typical results. We found that pyruvate did indeed protect AOX against oxidation during the isolation of mitochondria from suspension cells, just as we had seen with leaf mitochondria. For mitochondria isolated without pyruvate, the ratio of reduced to oxidized AOX protein measured by densitometry was low (typically about 0.1), while for mitochondria isolated in the presence of pyruvate, the ratio was high (typically 2–3). However, the relative amounts of oxidized versus reduced AOX protein was not significantly altered by the AA treatment. One possible explanation for these results is that the AOX protein in these cells is already largely present in the reduced form, such that it is difficult to show further increases in the level of the reduced form after AA treatment. Also, it is possible that pyruvate is not completely effective at protecting AOX against oxidation during the mitochondrial isolation, such that small differences in AOX protein form that may have existed between untreated and AA-treated cells were still being lost during the isolation.
Figure 6.
Immunoblot of the AOX protein present in mitochondria isolated from transgenic (B9) tobacco suspension cells d 3 after subculture. In some cases, cells were pretreated for 1 h with 10 μm AA prior to the mitochondrial isolation. Media used in the mitochondrial isolation were supplemented with 5 mm pyruvate (+pyr) or were not supplemented (−pyr). Mitochondrial proteins (100 μg) were resolved by either reducing (A) or non-reducing (B) SDS-PAGE prior to the immunoblot analysis shown. Arrows indicate the position of the oxidized (upper arrow) and reduced (lower arrow) forms of the AOX protein. Representative results are shown.
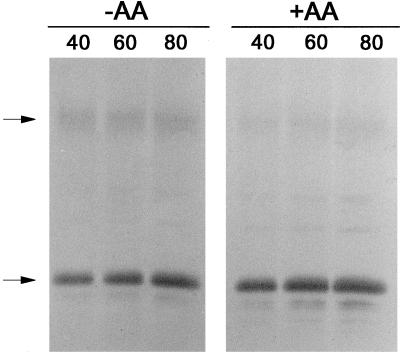
Given the above results, we took another approach to establishing whether a correlation exists between the AOX protein form in vivo and AOX activity in vivo. We found that in the B9 suspension cells, the AOX protein level was high enough that it could be visualized by immunoblot analysis of an extract of total cellular protein. This has the advantage of bypassing the lengthy mitochondrial isolation step, when the level of AOX protein forms could change. Pyruvate was also included in the buffer used to carry out these rapid extractions as a means to further stabilize the AOX protein form. Figure 7 illustrates typical results of such an experiment. The vast majority of the AOX protein in these cells under our standard growth conditions (−AA) was present in the reduced (active) form. Nonetheless, using the rapid protein extraction approach, we did detect a further shift from the oxidized to the reduced form after a short-term incubation with AA (Fig. 7), resulting in a 1.5- to 2-fold increase in the densitometer signal associated with the reduced form of AOX. While we could readily visualize AOX in a total cellular extract from B9 suspension cells, this approach was not successful for a B9 leaf extract; therefore, further experiments of this type utilized suspension cells.
Figure 7.
Immunoblot of the AOX protein present in a cellular protein extract from transgenic (B9) tobacco suspension cells d 3 after subculture. Protein extracts were obtained from untreated cells (−AA) or from cells pretreated for 1 h with 10 μm AA (+AA). Different volumes of extract (40, 60, or 80 μL) were subsequently analyzed by non-reducing SDS-PAGE and immunoblot analysis. Representative results are shown.
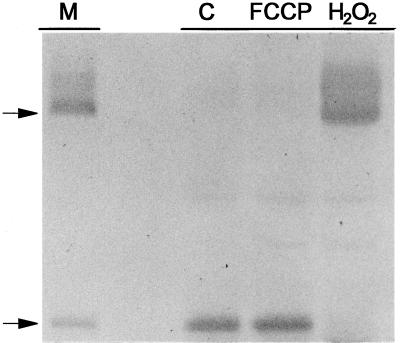
Using the rapid protein extraction approach, we were also able to demonstrate that treatment of B9 suspension cells with the respiratory uncoupler FCCP had no apparent effect on the form of AOX protein present, but that treatment with H2O2 resulted in a rapid and large shift toward the oxidized form (Fig. 8). This shift did not occur in a transgenic suspension cell line (C12, Vanlerberghe et al., 1998) that overexpresses a mutated AOX protein in which the redox-modulated regulatory Cys (Cys-126) is changed to Ala (data not shown). This confirms that the apparent high-Mr form of AOX visualized after H2O2 treatment is indeed due to the oxidation of the Cys-126 sulfhydryl and not to some unrelated phenomenon.
Figure 8.
Immunoblot of the AOX protein present in a cellular protein extract from transgenic (B9) tobacco suspension cells d 3 after subculture. Protein extracts were obtained from either untreated control cells (C), cells treated for 15 min with 1 μm FCCP, or cells treated for 10 min with 20 mm H2O2, and 40 μL of extract was subsequently analyzed by non-reducing SDS-PAGE and immunoblot analysis as described in “Materials and Methods.” The lane marked “M” is a sample of protein from isolated mitochondria to clearly show the position of the oxidized and reduced forms of the AOX protein. The arrows at left indicate the position of the oxidized (upper arrow) and reduced (lower arrow) forms of the AOX protein. Representative results are shown.
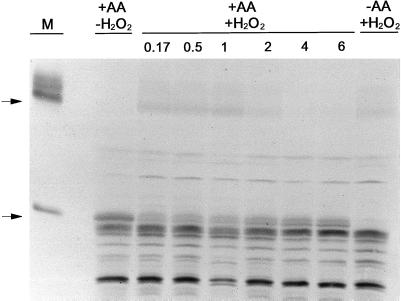
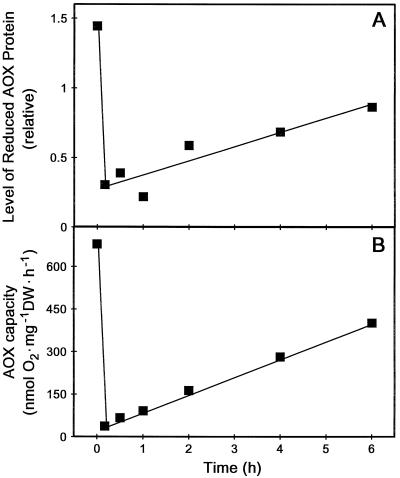
We felt that the ability of H2O2 to rapidly convert the AOX protein toward its oxidized form represented a good opportunity to assess how the change in form would affect in vivo AOX capacity. Unfortunately, we found that both the B9 and C12 suspension cells were very sensitive to H2O2 addition. A cell viability assay utilizing Evans blue and respiration assays using the O2 electrode both indicated that the cells were rapidly killed by the low concentrations of H2O2 required (data not shown). Therefore, to carry out this experiment, we needed to use a wild-type tobacco suspension cell line (cv Bright Yellow) that is considerably less sensitive to H2O2. We have previously used these cells and shown that a 5 mm H2O2 treatment for 8 h had little effect on control respiration rates (Vanlerberghe and McIntosh, 1996). However, in order to visualize the AOX protein in a total cellular protein extract from these wild-type cells, it was first necessary to induce large amounts of AOX protein in these cells. This was readily accomplished by a 24-h incubation of the cells with AA prior to the H2O2 treatment. We have previously shown that AA dramatically elevates the level of AOX protein in these cells (Vanlerberghe and McIntosh, 1992, 1994). Figure 9 illustrates typical results of such an experiment. The 24-h pretreatment with AA did increase AOX protein to a level that could be visualized by immunoblot analysis of total cellular protein. As expected, initially (prior to H2O2 addition), AOX was almost exclusively present as the reduced (active) form (Fig. 9); however, within 10 min of H2O2 addition, the level of reduced protein had decreased dramatically while the level of oxidized protein had increased (Figs. 9 and 10A). Associated with the large drop in reduced protein was a large drop in the KCN-resistant, SHAM-sensitive O2 uptake of the cells (AOX capacity) (Fig. 10B). Over the following 6 h, there was a gradual recovery in the level of reduced AOX protein (Figs. 9 and 10A) and this correlated closely with a recovery in AOX capacity (Fig. 10B).
Figure 9.
Immunoblot of the AOX protein present in a cellular protein extract from wild-type tobacco suspension cells. Cells d 3 after subculture were either left untreated (−AA) or were treated for 24 h with 10 μm AA (+AA) to induce large amounts of AOX protein in the cells. Then, the cells in culture were treated with 5 mm H2O2 (+H2O2). At different times following the H2O2 addition (ranging from 0.17 to 6 h), a cellular protein extract was obtained from an aliquot of the cell culture, and 40 μL of the extract was subsequently analyzed by non-reducing SDS-PAGE and immunoblot analysis. The lane marked “+AA, −H2O2” is a protein extract from cells just prior to the H2O2 addition. The lane marked “−AA, + H2O2” is an extract from cells not given the pretreatment with AA prior to the 10-min incubation with H2O2. The lane marked “M” is a protein sample from isolated mitochondria to clearly show the position of the oxidized and reduced forms of the AOX protein. The arrows at left indicate the position of the oxidized (upper arrow) and reduced (lower arrow) forms of the AOX protein.
Figure 10.
Relationship between the level of the reduced form of the AOX protein present in wild-type tobacco suspension cells and the AOX capacity of the cells. The first point (time 0) was obtained just prior to treatment of the cells with 5 mm H2O2, and the second point 10 min after H2O2 addition. A, Effect of H2O2 addition on the level of the reduced form of AOX protein present in cells. Data were obtained by densitometry analysis of the immunoblot shown in Figure 9. B, Effect of H2O2 addition on the AOX capacity of cells. O2 uptake by an aliquot of cells was monitored in an O2 electrode cuvette during the sequential addition of 1 μm FCCP, 1 mm KCN, and 2 mm SHAM. AOX capacity is defined as the KCN-resistant, SHAM-sensitive O2 uptake of the cells. Residual respiration (in the presence of KCN and SHAM) was 27.2 ± 11.8 nmol O2 mg−1 dry weight (DW) h−1 over the course of the experiment. This analysis was done in parallel with those in A. Representative results are shown.
DISCUSSION
The presence of pyruvate during the isolation of mitochondria from tobacco leaves has dramatic effects on subsequent in organello AOX activity. The results of Figures 1 to 5 indicate at least two important consequences of the presence of pyruvate. First, pyruvate protects against oxidation of the AOX regulatory sulfhydryl/disulfide system, such that the ratio of reduced to oxidized AOX protein is substantially higher after isolation with pyruvate (Figs. 2 and 3). This makes AOX activity in the in organello assay less dependent upon DTT (Fig. 1B).
A study with soybean suggested that the activation of AOX by pyruvate was due to its interaction with a Cys sulfhydryl to form a thiohemiacetal because activation was mimicked by iodoacetate (Umbach and Siedow, 1996). Since evidence suggests that pyruvate activation takes place from within the mitochondrial matrix (Day et al., 1994; Millar et al., 1996), there are two potential Cys residues for such an interaction (Umbach and Siedow, 1993; Vanlerberghe and McIntosh, 1997). However, the more C-terminal of these two residues (Cys-176 in tobacco) is clearly not involved. In both tobacco (Vanlerberghe et al., 1998) and Arabidopsis (Rhoads et al., 1998), replacement of this residue by an Ala generates an AOX enzyme that still displays normal activation by pyruvate or iodoacetate. In each of these studies, it was also shown that the more N-terminal Cys residue (Cys-126 in tobacco) is the residue responsible for the regulatory sulfhydryl/disulfide system (Rhoads et al., 1998; Vanlerberghe et al., 1998).
Given the above results, it is possible that the more N-terminal Cys is the residue not only responsible for the sulfhydryl/disulfide system but also directly responsible for the pyruvate activation. If pyruvate interaction with this residue were dependent on the presence of a sulfhydryl group (such as for the formation of a thiohemiacetal), then it would be expected that the oxidized form of native AOX is not responsive to pyruvate but that the reduced form (with its component sulfhydryls) is pyruvate stimulated, as has been observed (Vanlerberghe et al., 1995). If the more N-terminal Cys is the residue that interacts with pyruvate, it would explain the substantial loss of AOX activity that occurred in tobacco (Vanlerberghe et al., 1998) and Arabidopsis (Rhoads et al., 1998) when this Cys was changed to Ala, because in the absence of pyruvate stimulation, the reduced native AOX shows little in organello activity (Vanlerberghe et al., 1995).
Rhoads et al. (1998) have provided more direct evidence that pyruvate interacts with the more N-terminal Cys residue to form a thiohemiacetal. They substituted the more N-terminal Cys residue with the acidic residue Glu. This residue might be expected to substitute for the thiohemiacetal if the carboxyl group on the thiohemiacetal is the activating moiety. Indeed, they found that the resultant AOX enzyme displayed significant activity in the absence of pyruvate when expressed in Escherichia coli. Our finding that pyruvate is able to effectively protect the tobacco Cys-126 sulfhydryl against oxidation during mitochondrial isolation provides further evidence that pyruvate interacts directly with this Cys sulfhydryl.
Hoefnagel and Wiskich (1998) showed that turnover of AOX from either Arum italicum or soybean in the absence of pyruvate led to inactivation of the enzyme by its product (ubiquinone), remaining bound to the enzyme and effectively decreasing the amount of active enzyme. However, the presence of pyruvate during turnover prevented this inhibition, indicating that the stimulating effect of pyruvate was due to its ability to maintain a large pool of active AOX enzyme (Hoefnagel and Wiskich, 1998). Given those results, we investigated whether inactivation of the tobacco enzyme by turnover in the absence of pyruvate was occurring in our in organello assays. If so, this might alter our interpretation of why mitochondria isolated in the presence of pyruvate respond differently than those isolated in its absence. This question was investigated in mitochondria isolated in the absence of pyruvate (Table I).
The results indicate that the low AOX activity seen after pyruvate addition to these mitochondria (Table I, Run no. 1) was not due to an inactivation of the AOX enzyme during the assay period prior to pyruvate addition. Even if pyruvate was added prior to any other assay component (Run no. 2), the AOX activity with saturating pyruvate was still low. It is only after DTT addition to reduce the regulatory sulfhydryl/disulfide system that AOX becomes significantly active. Our results are not inconsistent with those of Hoefnagel and Wiskich (1998), who showed that AOX inactivation did not occur with NADH as the substrate.
The second consequence of the presence of pyruvate in the mitochondrial isolation medium was that it had some stabilizing effect on AOX activity, such that mitochondria kept in the presence of pyruvate generally had higher rates of maximum AOX activity (with pyruvate + DTT) than mitochondria kept for some time in the absence of pyruvate. The ability of pyruvate to stabilize AOX activity has also been reported during solubilization and partial purification of the enzyme from A. italicum, A. maculatum, and soybean (Zhang et al., 1996; Hoefnagel et al., 1997).
To further investigate this stabilizing effect, mitochondria were isolated with pyruvate and then divided into two aliquots, one of which was washed with pyruvate and one without pyruvate prior to AOX activity analysis (Figs. 4 and 5). The results indicated that the maximum AOX activity (with pyruvate + DTT) was significantly compromised when mitochondria were isolated with pyruvate, but that the pyruvate was subsequently washed out (compare Fig. 4, A and B, to Fig. 4, C and D). Therefore, the stabilizing effect of pyruvate appears to not only be important during mitochondrial isolation, but also during any subsequent incubation of the sample on ice. The results of these experiments also indicated that the high ratio of reduced to oxidized AOX protein achieved when mitochondria were isolated with pyruvate declines significantly if pyruvate is subsequently washed out (Fig. 5), which results in an increased dependence upon DTT for high activity (Fig. 4). At least a portion of the AOX protein present in tobacco mitochondria is very susceptible to oxidation in the absence of pyruvate. Our interpretation of the data is not hindered by an inactivation of the AOX enzyme during turnover in the absence of pyruvate, since the rate of pyruvate-saturated AOX activity in either set of mitochondria was not dependent upon whether pyruvate was added prior to or after NADH (compare Fig. 4, A to B, and Fig. 4, C to D).
The in vivo relationship between the redox state of the AOX regulatory sulfhydryl/disulfide system and AOX activity has yet to be firmly established. Umbach and Siedow (1997) showed that characterization of the in vivo redox status of AOX was problematic. They found that both the Sauromatum guttatum and soybean AOX regulatory sulfhydryl underwent oxidation during mitochondrial isolation, just as would appear to be occurring in tobacco. Further, they found that the inclusion of sulfhydryl reagents (iodoacetate and N-ethylmaleimide) in the mitochondrial isolation media, while preventing this oxidation, also led to a reduction of the oxidized form. Despite these critical problems, a few studies involving the isolation of mitochondria do suggest a correlation between the AOX protein form and AOX activity in vivo.
For both the S. guttatum appendix (Umbach and Siedow, 1993) and pea leaves (Lennon et al., 1995), the level of oxidized and reduced AOX protein present after mitochondrial isolation was dependent upon the developmental stage of the tissue and the protein form correlated with predicted changes in the in vivo activity of AOX in these tissues during development. Recently, Millar et al. (1998) were able to analyze the AOX protein in a total cellular protein extract from soybean root, thus bypassing the mitochondrial isolation step. They also found a similar correlation between AOX protein form and AOX activity (measured by oxygen isotope discrimination) during root development. Similar whole root extracts indicated that the AOX protein in 6- to 7-week-old Poa annua plants was almost exclusively present in the reduced form (Millenaar et al., 1998). To our knowledge, the only study that has reported short-term changes in the AOX protein form is that of Mizutani et al. (1998). They isolated mitochondria from either untreated rice roots or roots treated for 5 h with the complex III inhibitor SSF126. They found a significant level of oxidized AOX protein in untreated root, but almost entirely reduced protein in the SSF126-treated roots with high in vivo AOX activity due to inhibition of the Cyt pathway.
We took several approaches in an attempt to evaluate the in vivo redox status of tobacco AOX. First, we compared results obtained after mitochondrial isolations with those obtained by a rapid, whole-cell protein extraction procedure. Second, we included pyruvate in both the mitochondrial and whole-cell protein extraction buffers, since pyruvate appears to protect against the oxidation of Cys-126. Third, we examined the AOX redox status after short-term treatments capable of quickly altering in vivo AOX capacity or activity. In part, these experiments were meant to evaluate whether the AOX sulfhydryl/disulfide system is a mechanism that could modulate AOX activity in the short term, as opposed to more long-term regulation, which may be linked to tissue developmental changes.
Our results indicate that the tobacco AOX sulfhydryl/disulfide system is predominantly in the reduced form in vivo and we were unable to identify any short-term physiological conditions (with the exception of H2O2 treatment of cells; see below) in which AOX was significantly more oxidized (Figs. 6–10 and data not shown). This apparent stability against oxidation may relate to our above-noted effects of pyruvate. If a single Cys residue (Cys-126) is involved in both the sulfhydryl/disulfide regulatory system and the interaction with pyruvate, then both the mitochondrial redox state (the redox state of the NAD[P]H pool) and the level of pyruvate would be expected to influence in an interactive way the redox state of the AOX sulfhydryl/disulfide system. For example, it may be that once the regulatory disulfide is reduced by a highly reduced mitochondrial pyridine nucleotide pool, the sulfhydryl is effectively kept reduced by its interaction with the mitochondrial pool of pyruvate. In this case, it may be that a massive depletion of pyruvate and other α-keto acids (such as might occur during mitochondrial isolation) is necessary to bring about significant oxidation of the enzyme.
An example of this interaction was illustrated by the experiment with FCCP (Fig. 8). FCCP treatment approximately doubled the respiration rate (O2 consumption) of the cells (data not shown), indicating that it had been restricted by the availability of ADP (Dry et al., 1987). The increased availability of ADP after FCCP addition might be expected to decrease the reduction state of the mitochondrial pyridine nucleotide pool (Neuburger et al., 1984; Day et al., 1987) and result in the activation of pyruvate kinase, causing an increase in pyruvate (Turpin et al., 1990; Plaxton, 1996). While the more oxidized pyridine nucleotide pool expected in the presence of FCCP would not favor AOX reduction (Vanlerberghe et al., 1995), the expected increase in pyruvate would favor the reduced form and may explain why FCCP brought about no significant change in the AOX protein form (Fig. 8). Therefore, both the redox status and the carbon status of the mitochondrion may regulate the redox state of the AOX sulfhydryl/ disulfide system.
Our results indicate that AOX is present predominantly in the reduced active form in both transgenic tobacco leaf mitochondria and transgenic suspension cells expressing high levels of AOX protein. Nonetheless, both inhibitor-based studies on transgenic tobacco suspension cells expressing high levels of AOX protein (Vanlerberghe et al., 1994) and isotope discrimination studies on transgenic tobacco leaves expressing high levels of AOX protein (R.D. Guy and G.C. Vanlerberghe, unpublished data) suggest that only low levels of AOX activity occur in these systems under normal growth conditions. Therefore, it is clear that factors other than the AOX protein level and form are critical to ensure high AOX activity in vivo. Such factors include the level of pyruvate and the concentration and redox state of the ubiquinone pool (Siedow and Umbach, 1995; Ribas-Carbo et al., 1997).
In isolated tobacco mitochondria, the oxidized form of AOX would appear to have little potential for catalytic activity with pyruvate compared with the reduced form (Vanlerberghe et al., 1995). However, the relationship between the AOX protein form and its potential for catalytic activity (AOX capacity) has not been examined in vivo. Interestingly, the tobacco AOX Cys-126 sulfhydryl appears to be very susceptible in vivo to oxidation by H2O2 to produce the intermolecular disulfide bond. We took advantage of this observation to examine in vivo the relationship between the AOX protein form and AOX capacity. Prior to H2O2 addition, the level of KCN-resistant, SHAM-sensitive respiration (AOX capacity) of the cells was very high when the high level of AOX protein was predominantly in the reduced form (Figs. 9 and 10). However, after conversion to the oxidized form as a result of H2O2 addition, most of this capacity was lost. Capacity then recovered over time and was correlated with an increase in the reduced and decrease in the oxidized form of the AOX protein present. The same results were obtained with cells in which both cycloheximide and actinomycin D were added prior to H2O2 to prevent RNA and protein synthesis (data not shown). We believe, therefore, that the gradual recovery of AOX protein form and capacity is not the result of the synthesis of new AOX protein but rather the change in form of existing protein. We have previously shown the effectiveness of cycloheximide and actinomycin D in inhibiting AOX synthesis in these cells (Vanlerberghe and McIntosh, 1992).
Our previous study (Vanlerberghe et al., 1995) showed that in isolated mitochondria, the conversion of AOX from its inactive to active form (in response to malate or isocitrate oxidation) was a conversion capable of occurring rapidly, within the few minutes of an in organello assay. This suggests that the sulfhydryl/disulfide system is capable of providing short-term regulation of AOX activity. The relatively slow recovery of AOX to its reduced form (i.e. several hours) in the above experiment with H2O2 might be taken as evidence that this conversion is too slow to provide such short-term regulation of activity in vivo. However, this experiment should be interpreted with caution, since the recovery time may relate more to a recovery of the whole-cell redox state (perturbed by the H2O2 addition) than simply to the recovery of a component of the mitochondrial redox state required for AOX reduction. Nonetheless, the primary observation of this experiment is that AOX capacity in vivo correlates very closely with AOX protein form, which is consistent with only the reduced form being capable of significant activity in vivo.
Our data suggest that the presence of reduced AOX protein is a necessary prerequisite for significant AOX activity in vivo, but that the presence of reduced protein alone is not sufficient for AOX activity, that other factors (such as intramitochondrial pyruvate concentration) are also critical. Our data also support the assumption of Rhoads et al. (1998) that a single Cys residue (Cys-126 in tobacco) is involved in both the redox modulation and pyruvate activation of AOX.
ACKNOWLEDGMENTS
We thank Dr. Joseph T. Wiskich and Dr. Marcel Hoefnagel (both at the University of Adelaide, Australia) for helpful discussions during the course of this study.
Footnotes
This work was funded by a research grant from the Natural Sciences and Engineering Research Council of Canada to G.C.V.
LITERATURE CITED
- Day DA, Arron GP, Laties GG. Nature and control of respiratory pathways in plants: the interaction of cyanide-resistant respiration with the cyanide-sensitive pathway. In: Davies DD, editor. The Biochemistry of Plants, A Comprehensive Treatise. 2. Metabolism and Respiration. New York: Academic Press; 1980. pp. 581–611. [Google Scholar]
- Day DA, Millar AH, Wiskich JT, Whelan J. Regulation of alternative oxidase activity by pyruvate in soybean mitochondria. Plant Physiol. 1994;106:1421–1427. doi: 10.1104/pp.106.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Wiskich JT, Bryce JH, Dry IB. Regulation of ADP-limited respiration in isolated plant mitochondria. In: Moore AL, Beechy RB, editors. Plant Mitochondria: Structural, Functional and Physiological Aspects. New York: Plenum Press; 1987. pp. 59–66. [Google Scholar]
- Dry IB, Bryce JH, Wiskich JT. Regulation of mitochondrial respiration. In: Davies DD, editor. The Biochemistry of Plants: A Comprehensive Treatise. 11. Biochemistry of Metabolism. New York: Academic Press; 1987. pp. 213–251. [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Millar AH, Wiskich JT, Day DA. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch Biochem Biophys. 1995;318:394–400. doi: 10.1006/abbi.1995.1245. [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Rich PR, Zhang Q, Wiskich JT. Substrate kinetics of the plant mitochondrial alternative oxidase and the effects of pyruvate. Plant Physiol. 1997;115:1145–1153. doi: 10.1104/pp.115.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Wiskich JT. Activation of the plant alternative oxidase by high reduction levels of the Q-pool and pyruvate. Arch Biochem Biophys. 1998;355:262–270. doi: 10.1006/abbi.1998.0737. [DOI] [PubMed] [Google Scholar]
- Lambers H. Respiration in intact plants and tissues: its regulation and dependence on environmental factors, metabolism and invaded organisms. In: Douce R, Day DA, editors. Encyclopedia of Plant Physiology, New Series. 18. Higher Plant Cell Respiration. New York: Springer-Verlag; 1985. pp. 418–473. [Google Scholar]
- Larson E, Howlett B, Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986;155:243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Lennon AM, Pratt J, Leach G, Moore AL. Developmental regulation of respiratory activity in pea leaves. Plant Physiol. 1995;107:925–932. doi: 10.1104/pp.107.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirement of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Millar AH, Atkin OK, Menz RI, Henry B, Farquhar G, Day DA. Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol. 1998;117:1083–1093. doi: 10.1104/pp.117.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Finnegan PM, Whelan J, Drevon JJ, Day DA. Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ. 1997;20:1273–1282. [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996;111:613–618. doi: 10.1104/pp.111.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H. The role of alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol. 1998;118:599–607. doi: 10.1104/pp.118.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A, Miki N, Nanba K. Defense mechanism of rice plant to respiratory inhibition by a promising candidate blasticide, SSF126. Pestic Biochem Physiol. 1998;60:187–194. [Google Scholar]
- Moller IM, Rasmusson AG. The role of NADP in the mitochondrial matrix. Trends Plant Sci. 1998;3:21–27. [Google Scholar]
- Neuburger M, Day DA, Douce R. The regulation of malate oxidation in plant mitochondria by the redox state of endogenous pyridine nucleotides. Physiol Veg. 1984;22:571–580. [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Popov VN, Simonian RA, Skulachev VP, Starkov AA. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997;415:87–90. doi: 10.1016/s0014-5793(97)01099-5. [DOI] [PubMed] [Google Scholar]
- Purvis AC. Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol Plant. 1997;100:165–170. [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Sweet CR, Lennon AM, Rauch GS, Siedow JN. Regulation of the cyanide-resistant alternative oxidase of plant mitochondria: identification of the cysteine residue involved in the α-keto acid stimulation and intersubunit disulfide bond formation. J Biol Chem. 1998;273:30750–30756. doi: 10.1074/jbc.273.46.30750. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol. 1995;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN. The regulation of electron partitioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN, Umbach AL. Plant mitochondrial electron transfer and molecular biology. Plant Cell. 1995;7:821–831. doi: 10.1105/tpc.7.7.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin DH, Botha FC, Smith RG, Feil R, Horsey AK, Vanlerberghe GC. Regulation of carbon partitioning to respiration during dark ammonium assimilation by the green alga Selenastrum minutum. Plant Physiol. 1990;93:166–175. doi: 10.1104/pp.93.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Covalent and non-covalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxidase with sulfhydryl reagents suggests that α-keto acid activation involves the formation of a thiohemiacetal. J Biol Chem. 1996;271:25019–25026. doi: 10.1074/jbc.271.40.25019. [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Changes in the redox state of the alternative oxidase regulatory sulfhydryl/disulfide system during mitochondrial isolation: implications for inferences of activity in vivo. Plant Sci. 1997;123:19–28. [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, Mc- Intosh L. Alternative oxidase activity in tobacco leaf mitochondria: dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol. 1995;109:353–361. doi: 10.1104/pp.109.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Coordinate regulation of cytochrome and alternative pathway respiration in tobacco. Plant Physiol. 1992;100:1846–1851. doi: 10.1104/pp.100.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression: studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L, Yip JYH. Molecular localization of a redox-modulated process regulating plant mitochondrial electron transport. Plant Cell. 1998;10:1551–1560. doi: 10.1105/tpc.10.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L. Molecular genetic alteration of plant respiration: silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol. 1994;106:1503–1510. doi: 10.1104/pp.106.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Moore AL. Structure and function of the plant alternative oxidase: its putative role in the oxygen defense mechanism. Biosci Rep. 1997;17:319–333. doi: 10.1023/a:1027388729586. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hoefnagel MHN, Wiskich JT. Alternative oxidase from Arum and soybean: its stabilization during purification. Physiol Plant. 1996;96:551–558. [Google Scholar]