Summary
Periodontal disease is a bacterial biofilm-associated inflammatory disease that has been implicated in many systemic diseases. A new preventive method for periodontal disease needs to be developed in order to promote the health of the elderly in a super-aged society. The gingival epithelium plays an important role as a mechanical barrier against bacterial invasion and a part of the innate immune response to infectious inflammation in periodontal tissue. The disorganization of cell–cell interactions and subsequent inflammation contribute to the initiation of periodontal disease. These make us consider that regulation of host defensive functions, epithelial barrier and neutrophil activity, may become novel preventive methods for periodontal inflammation. Based on this concept, we have found that several agents regulate the barrier function of gingival epithelial cells and suppress the accumulation of neutrophils in the gingival epithelium. We herein introduce the actions of irsogladine maleate, azithromycin, amphotericin B, and Houttuynia cordata (dokudami in Japanese), which is commonly used in traditional medicine, on the epithelial barrier and neutrophil migration in gingival epithelial cells in vivo and in vitro, in order to provide support for the clinical application of these agents to the prevention of periodontal inflammation.
Keywords: Junctional epithelium, Gingival epithelial cell, Prevention of periodontal disease, Epithelial barrier, Neutrophil migration, Interleukin-8
1. Introduction
Periodontal disease is a bacterial biofilm-associated inflammatory disease that is caused by a shift in local microbial ecology [1], [2]. Since periodontal disease has been implicated in many systemic diseases, such as diabetes mellitus, cardiovascular diseases, aspiration pneumonia, and dementia etc, its prevention is essential for the health promotion of the elderly in a super-aged society of Japan. Periodontal disease may be prevented via two strategies: (1) the removal of periodontal pathogens (bacterial biofilm) from periodontal tissue and (2) the regulation of host defensive mechanisms in periodontal tissue against periodontal pathogens. At present, the most effective and safest method for the prevention of periodontal disease is the mechanical removal of biofilms by self-care. However, physiological or psychological conditions in the elderly make this difficult. The chemical or biological methods to remove or eliminate selectively periodontal pathogens from the oral cavity have not yet to be established. On the other hand, fluoride treatment has beneficial effects like prevention of dental caries by shifting the balance from demineralization to remineralization. Based on the same concept as fluoride treatments, we have been developing an approach to the regulation of host defenses in periodontal tissue for the prevention of periodontal disease.
The gingival junctional epithelium is located at a strategically important interface at the bottom of the gingival sulcus and actively contributes to inflammatory processes. It plays a crucial role as a mechanical barrier against bacterial invasion and in the innate immune response to infectious inflammation in periodontal tissue [3], [4], [5]. Thus, the interaction between epithelial cells and periodontopathogenic bacteria has been suggested to be critically involved in the initiation of periodontal disease. The regulation of function of gingival epithelial cells may prevent the onset of periodontal disease.
Irsogladine maleate acts on gastric mucosal epithelium as an anti-gastric ulcer agent. Azithromycin has immunomodulatory properties with beneficial effects for lung diseases such as diffuse panbronchiolitis and cystic fibrosis. Amphotericin B has also shown to modulate host immune responses against fungal infections. Houttuynia cordata (dokudami in Japanese) is used internally and externally as a traditional medicine and has many pharmacological activities. These agents may have the potential to regulate gingival epithelial cell function for prevention of periodontal disease. Based on this concept, we demonstrated the role of the gingival junctional epithelium in periodontal disease and the mechanisms regulating the functions of gingival epithelial cell by several agents such as irsogladine maleate, amphotericin B, azithromycin, and H. cordata. In this review, we propose a novel concept for the prevention of periodontal disease.
2. Role of the junctional epithelium in the initiation of periodontal disease
Epithelial cells function as a mechanical barrier against invasion by pathogenic organisms and promote intercellular communication through cell–cell junction complexes, followed by the production of inflammatory cytokines and anti-microbial peptides. Multi-protein cell junction complexes are symmetrical structures that form between cells and are crucial for maintaining the physical and functional integrity of tissues. Epithelial cells are generally interconnected by tight junctions, adherence junctions, desmosomes, and gap junctions. However, the junctional epithelium in clinically healthy periodontal tissue is only interconnected by desmosomes and occasionally by gap junctions, and has wide intercellular spaces [3], [5].
The initiation of periodontal disease is attributed to cleavage within the second or third cell layer of DAT cells (directly attached to the tooth) in the coronal-most portion of the junctional epithelium facing biofilms, and not to the detachment of DAT cells from the tooth [6], [7]. Following the cleavage of the junctional epithelial surface, the secretion of cytokines and chemokines such as interleukin (IL)-8 results in the accumulation of neutrophils in the junctional epithelium, while proteases secreted by neutrophils disrupts the epithelial barrier of the junctional epithelium [8]. The accumulation of activated neutrophils in lesion areas is observed in all diseases, and has been suggested to play a role in the onset of inflammation. The disorganization of cell–cell contacts and subsequent breakdown of the tissue architecture are often the result of pathophysiological conditions. Therefore, regulation of the epithelial barrier and the blockade of neutrophil activity have potential as candidate therapeutic strategies for inflammation in gingival junctional epithelium.
3. Molecular factors associated with the function of the junctional epithelium
3.1. Claudin-1
Tight junctions are essential for the tight sealing of cellular sheets, which, in turn, controls the paracellular ion flux and maintains tissue homeostasis. Claudins are tight junction-associated tetraspan proteins with relatively short cytoplasmic amino and carboxy termini flanking the first extracellular loop of 53 amino acids and the second shorter loop of 24 amino acids [9]. Claudins have been suggested to play an important role in regulating the epithelial barrier at tight junctions [10]. As described above, the junctional epithelium in clinically healthy periodontal tissue is interconnected by desmosomes and gap junctions, and not by tight junctions. Therefore, tight junctions do not appear to contribute to barrier function in the junctional epithelium [11]. Although we reported that claudins exist in the junctional epithelium of rat or porcine gingiva [12], [13], their roles in the junctional epithelium have not been elucidated. Furthermore, microarray and immunohistochemical analyses demonstrated that the expression of claudin-1 in the junctional epithelium following a chronic lipopolysaccharide (LPS) challenge was weaker than that in healthy subjects [14]. This suggests that claudin-1 is present in healthy junctional epithelium, and be crucially involved in epithelial barrier function with or without tight junctions. The expression of claudin-1 may decrease when the junctional epithelium is exposed to commensal or periodontopathic bacteria. Further studies to investigate the role of claudin-1 in the junctional epithelium are warranted.
3.2. E-cadherin
E-cadherin, a subclass of cadherin found in the stratified squamous epithelium, plays a crucial role in maintaining the structural integrity and function of adherens and desmosomal epithelial intercellular junctions [15], [16]. In the junctional epithelium, E-cadherin plays an important role against bacterial invasion, however, E-cadherin levels were shown to be reduced in inflamed gingival tissue [17], [18]. Furthermore, major periodontal pathogens such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans decreased the expression of E-cadherin in cultured gingival epithelial cells [19], [20]. In the gastric mucosal epithelium, the disruption of E-cadherin appears to increase epithelial permeability [21]. Thus, it may be possible that E-cadherin plays an important role against bacterial invasion in the gingival junctional epithelium.
3.3. Connexin 43 and gap junctional intercellular communication
Gap junctions are clusters of transmembranous hydrophilic channels that allow the direct exchange of molecules of up to 1200 Da in weight, including ions, sugars, and small peptides, between adjacent cells [22], [23]. Connexins are structural proteins of these gap junctions. Gap junctional intercellular communication (GJIC) plays a critical role in cellular coordination in tissue homeostasis. The expression of connexin gap junction proteins, connexin 26 and 43 was found to be weaker in a diseased gingival junctional epithelium than in that of healthy subjects. In cultured human gingival epithelial cells, A. actinomycetemcomitans, outer membrane protein (omp)-29 from A. actinomycetemcomitans and IL-1β reduced connexin 43 levels and GJIC [24], [25], [26]. Thus, GJIC responses of gingival epithelial cells following periodontal pathogens exposure may be involved in the initial process of periodontal disease elicited by the bacteria.
3.4. Smad2 and transforming growth factor-β signaling
Apoptosis in epithelial cells triggers the destruction of epithelial barrier function, which is important for the onset and progression of periodontitis [8], [27]. Previous studies reported that a large number of TUNEL-positive cells were present in the gingival epithelium of patients with periodontitis [27], [28]. Smad2 is a well-known downstream signaling molecule of TGF-β receptors, and plays a key role in TGF-β-mediated apoptosis in epithelial cells [29], [30]. An increase in the number of apoptosis-positive cells was recently reported in the gingival junctional epithelium of smad2 transgenic mice [31]. Furthermore, the overexpression of smad2 reduced the proliferation of the junctional epithelium [32] and induced alveolar bone loss by up-regulating TNF-α and receptor activator of nuclear factor kappa-B ligand (RANKL) in transgenic mice [33]. On the other hand, we demonstrated that A. actinomycetemcomitans induced apoptosis in gingival epithelial cells by activating the TGF-β receptor I-smad2-caspase-3 signaling pathway [34]. Furthermore, omp29 from A. actinomycetemcomitans binds to fibronectin in order to facilitate fibronectin/integrinβ1/FAK signaling-dependent TGF-β release from the extracellular matrix, thereby inducing gingival epithelial cell apoptosis via the TGF-β receptor/smad2 pathway [35]. Thus, the marked acceleration of TGF-β receptor/smad2 signaling by microbial pathogens may induce apoptosis and disrupt the gingival epithelial barrier, and this is followed by alveolar bone loss. The blockade of TGF-β/smad2 signaling has potential for the prevention of periodontal disease by regulating the gingival epithelial barrier.
3.5. IL-8
IL-8 is a member of the α-subfamily of chemokines that is characterized by a conserved tri-peptide near the N-terminus, containing cysteines (C-X-C), and an 8–10 kDa protein found in two isomeric forms, the 77 amino acid isoform and more biologically active 72 amino acid isoform. IL-8 is a bioactive chemokine that is present in the inflammatory environment and during cancer progression [36], [37]. In addition to exhibiting leukocyte chemotactic activity, IL-8 has been shown to induce DNA synthesis, migration, and the down-regulation of cleaved caspase-3 in cultured human gingival epithelial cells [38]. Furthermore, IL-8 reduced GJIC and CX43 expression in human gingival epithelial cells [24]. A previous study reported that CXCR-1/CXCR-2, which are IL-8 receptors, are expressed in the gingival epithelium [39]. Periodontopathogenic bacteria were found to increase the expression of IL-8 or cytokine-induced neutrophil chemoattractant (CINC)-2α in gingival epithelial cells in vivo and in vitro [24], [40], [41]. IL-8 levels in periodontal tissue and gingival crevicular fluid have been correlated with disease severity [42]. Furthermore, the expression of IL-8 in diseased tissue, particularly in the gingival epithelium, has been correlated with the migration of polymorphonuclear leukocytes [43], [44]. Therefore, as suggested by Graves et al. [45], the development of periodontal diseases may be related to the progression of inflammatory cell infiltration in periodontal tissues.
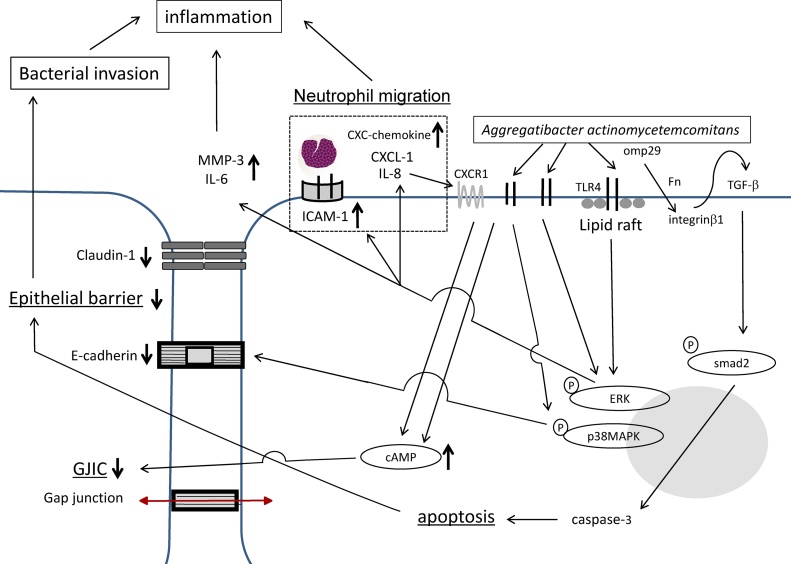
Fig. 1 shows the roles of gingival junctional epithelial cells in innate responses to a bacterial attack by periodontal disease.
Figure 1.
Schematic diagram showing the role of the gingival junctional epithelium in the innate response to a bacterial attack. A bacterial attack has a negative impact on the gingival epithelial barrier by disrupting the junctional complex and induces the migration of neutrophils through the activity of chemokines.
4. Regulation of the function on the junctional epithelium
4.1. Irsogladine maleate (an anti-gastric ulcer agent)
Irsogladine maleate has been clinically used as an anti-gastric ulcer agent [46], [47]. It has been shown to prevent gastric mucosal damage in several experimental animal models without inhibiting gastric secretion. This prevention is related to the inhibition of decreases in mucosal blood flow due to the disturbance of nitric oxide synthesis [48], [49]. Irsogladine maleate enhances GJIC in cultured rabbit gastric epithelial and pancreatic cancer cells through the augmentation of cyclic AMP [50], [51]. Its mucosal protective effects have been attributed to the enhancement of GJIC via the activation of muscarinic acetylcholine receptors and accumulation of cyclic AMP in gastric epithelial cells [50], [52], [53].
Irsogladine maleate also countered the reduction of GJIC and the levels of CX43 in cultures of human gingival epithelial cells stimulated by A. actinomycetemcomitans, omp29 from A. actinomycetemcomitans, or IL-1β [24], [25], [26]. In addition, irsogladine maleate prevented these reductions through increased cyclic AMP in human gingival epithelial cells. Irsogladine maleate has been shown to restore A. actinomycetemcomitans-induced reductions in E-cadherin in vivo and in vitro [54]. In addition, a pretreatment with irsogladine maleate reduced TNF-α-induced increases in gingival epithelial permeability by preventing the disruption of E-cadherin and claudin-1, suggesting that irsogladine maleate regulates the gingival epithelial cell barrier [55]. Collectively, these findings indicate that irsogladine maleate enhances epithelial barrier function by regulating intercellular junctional complexes through structural proteins in the junctional complex.
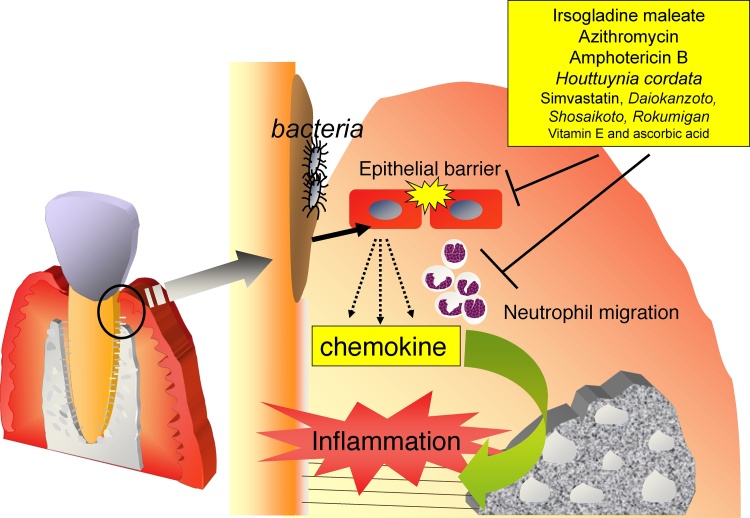
Irsogladine maleate has been shown to inhibit A. actinomycetemcomitans-induced inflammatory responses in the gingival epithelium by suppressing neutrophil migration in vivo and in vitro [54]. Consistent with this finding, irsogladine maleate reduced the A. actinomycetemcomitans- or P. gingivalis-induced increases in IL-8 and CXCL-1 levels in cultures of human gingival epithelial cells [54], [56]. The regulation of IL-8 levels by irsogladine maleate is partially involved in abrogation of the reduction of GJIC and CX43 expression by A. actinomycetemcomitans [24]. Furthermore, a DNA microarray analysis showed that irsogladine maleate down-regulated increases in the expression of CXC-chemokines, such as CXCL-1, CXCL-2, CXCL-3, CXCL-6, and CXCL-8 in A. actinomycetemcomitans-stimulated human gingival epithelial cells [54]. Irsogladine maleate also suppressed several inflammation-related proteins, such as matrix metalloproteinase (MMP)-3, IL-6, and ICAM-1 in A. actinomycetemcomitans-stimulated epithelial cells [57]. These regulatory mechanisms are mediated by the regulation of the p38 mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase (ERK) cascade in gingival epithelial cells by irsogladine maleate. Based on these findings, irsogladine maleate appears to control inflammation in the gingival epithelium by regulating the function of gingival epithelial cells (Table 1; Fig. 2).
Table 1.
List of references indicating regulation of the cellular function on gingival epithelial cells.
| Epithelial barrier |
Neutrophil migration |
Cytokine | |||||
|---|---|---|---|---|---|---|---|
| Permeability | E-cadherin | GJIC | IL-8 | ICAM-1 | |||
| Irsogladine maleate | ↑ | ↓ | Fujita et al. [24] | ||||
| ↑ | ↓ | Uchida et al. [25] | |||||
| ↑ | Fujita et al. [26] | ||||||
| ↑ | ↓ | Fujita et al. [54] | |||||
| ↓ | ↑ | Fujita et al. [55] | |||||
| ↓ | ↓ | IL-6↓ MMP3↓ | Miyagawa et al. [57] | ||||
| ↓ | Savitri et al. [56] | ||||||
| Azithromycin | ↓ | ↑ | ↓ | Miyagawa et al. [69] | |||
| Amphotericin B | ↓ | IL-6↓ | Imai et al. [77], [82] | ||||
| Simvastatin | ↓ | IL-6↓ | Sakoda et al. [78] | ||||
| Houttuynia cordata | ↓ | IL-6↓ | Kabir et al. [91] | ||||
| Daiokanzoto | ↓ | IL-6↓ | Fournier-Larente et al. [92] | ||||
| Shosaikoto | Calprotectin↑ | Hiroshima et al. [93] | |||||
| Rokumigan | IL-6↓ | Liao et al. [94] | |||||
| Vitamin E and ascorbic acid | ↓ | ↑ | Abe-Yutori et al. [95] | ||||
Figure 2.
Schematic diagram showing potential pathways to prevent periodontal disease. Irsogladine maleate, azithromycin, amphotericin B, and Houttuynia cordata (Dokudami) suppress inflammation in the gingival epithelium by regulating cellular functions such as the induction of chemokines.
4.2. Azithromycin (an antimicrobial agent)
Azithromycin is a macrolide antibiotic delivered through phagocytes with a long half-life, broad antibacterial spectrum, and the ability to inhibit biofilm formation. Previous studies reported that the use of azithromycin as an adjuvant treatment for periodontitis improved clinical and microbiological parameters over those achieved by conventional treatments alone [58], [59]. Its antibacterial properties are well documented, and it appears to be active against Gram-negative organisms, including periodontopathic bacteria such as P. gingivalis and A. actinomycetemcomitans [60], [61]. In addition to its anti-microbial properties, azithromycin possesses immunomodulatory properties with beneficial effects for lung diseases such as diffuse panbronchiolitis and cystic fibrosis [62], [63]. Several in vitro studies have attempted to elucidate the mechanisms responsible for these beneficial effects. Azithromycin was found to suppress inflammatory cytokines including IL-1β, IL-6, and IL-8 in human bronchial epithelial cells and nasal epithelial cells [64], [65]. In the human epithelial cell line, KB cells, azithromycin inhibited LPS-induced increases in IL-8 [66]. Furthermore, it maintained epithelial integrity in human airway epithelial cells [67], [68]. Similar to other epithelial cells, azithromycin restored transepithelial electrical resistance and E-cadherin expression in human gingival epithelial cells and suppressed the secretion of IL-8 in human gingival epithelial cells stimulated with TNF-α [69]. Furthermore, azithromycin inhibited the phosphorylation of ERK and p38 MAP kinase in TNF-α-stimulated epithelial cells. These findings suggest that azithromycin increases gingival integrity and suppresses inflammatory responses under inflammatory conditions, and as such, exerts protective effects in gingival epithelial cells and may contribute to suppressing the inflammation in gingival tissue (Table 1).
4.3. Drugs that regulate lipid or cholesterol
Amphotericin B is a major antifungal drug that is used to treat severe fungal infections because of its high efficiency and potency against many fungi [70]. Amphotericin B kills fungi by binding to the fungal membrane, ergosterol, which forms pores that cause lethal leaks in the fungal membrane [71]. However, many side effects are associated with amphotericin B including rigor, fever, and hypertension/hypotension, with the most well-known chronic toxicity being nephrotoxicity. Amphotericin B also binds to cholesterol in mammalian cell membranes, and this is mainly responsible for its toxic potential [71]. This agent has also been reported to induce the production of pro-inflammatory cytokines in host cells including gingival fibroblasts [72], [73]. Furthermore, amphotericin B modulates host immune responses against fungi infections [74], [75]. Lipid formulations of amphotericin B down-regulate inflammatory cytokines in monocytes [76].
In human gingival epithelial cells, amphotericin B also suppressed A. actinomycetemcomitans-induced increases in IL-6 and IL-8 through p38 MAP kinase and ERK [77]. Similar to A. actinomycetemcomitans-stimulated cells, amphotericin B down-regulated the increases in inflammatory-related genes in TNF-α-stimulated gingival epithelial cells [77]. These findings suggest that amphotericin B does not act on a particular receptor of virulence factors from A. actinomycetemcomitans, and may regulate a post-receptor event or intracellular signaling. In addition, simvastatin, which inhibits the synthesis of cholesterol, attenuated IL-1β-induced increases in IL-6 and IL-8 levels in epithelial cells [78]. Therefore, the direct or indirect regulation of cholesterol may represent a target for the suppression of inflammatory mediators.
Lipid rafts are unique biophysical entities that are enriched in glycosphingolipids, cholesterol, and sphingomyelin, and function as platforms in protein sorting and signal transduction [79]. Therefore, lipid rafts play roles in innate immunity in the gingival epithelium such as the activation of toll-like receptors. Methyl-β-cyclodextrin (MβCD) treatments were previously shown to be a rapid and efficient method for selectively removing cholesterol from the plasma membranes of cultured cells [80], [81], and MβCD has been extensively used as a cholesterol-depleting reagent. The depletion of cholesterol or disruption of lipid rafts by MβCD has been shown to ameliorate the TLR4-mediated inflammatory responses induced by A. actinomycetemcomitans in gingival epithelial cells [82]. In gastric or lung epithelial cells, the regulation of TLR-4 mobilization into lipid rafts suppresses inflammatory responses [83], [84]. Thus, molecules that inhibit the translocation of TLR4 may also suppress of inflammatory responses in gingival epithelial cells. For example, glycyrrhizin has been shown to inhibit the LPS-induced translocation of TLR4 into lipid rafts, thereby attenuating LPS-mediated inflammatory responses [85]. In addition to the regulation of the epithelial barrier, lipid rafts play a role in bacterial invasion into gingival epithelial cells. Thus, the regulation of lipid rafts may control host-bacteria interactions in the gingival epithelium (Table 1).
4.4. Oriental medicine and others
In order to protect the gingival epithelium from bacterial attack, the medication described herein (irsogladine maleate, azithromycin, amphotericin B) needs to be taken every day. However, the use of these medications every day is not adequate for systemic conditions. Therefore, the development of a new preventive material for periodontal disease that may be popularized and taken daily is required, such as a natural product or oriental medicine.
H. cordata is a medicinal herb that is generally used as a traditional oriental medicine. It has been shown to a broad range of pharmacological activities, including anti-viral, anti-leukemic, anti-oxidative, anti-anaphylactic, and anti-cancer effects. Thirty-eight known compounds have been identified by comparisons of their physical and spectroscopic data with those of corresponding authentic samples or literature values [86]. Quercitrin exhibits strong anti-oxidant activity by scavenging reactive oxidative species. Cepharadione, norcepharadione, and aristolactams exhibit anti-viral and anti-tyrosinase activities. Recent studies provided evidence to support and explain the anti-inflammatory activities of H. cordata [87], [88], [89], [90]. In cultured human gingival epithelial cells, A. actinomycetemcomitans facilitated the mRNA expression of MMP-3, IL-8, IL-6, and ICAM-1 in human gingival epithelial cells, while these mRNA levels were attenuated by a treatment with H. cordata [91]. Furthermore, the ERK signaling cascade, which was previously reported to be involved in A. actinomycetemcomitans-induced increases in MMP-3, IL-8, or ICAM-1, was disturbed in gingival epithelial cells following a treatment with H. cordata. H. cordata also inhibited TNF-α-induced enhancements in these genes in human gingival epithelial cells [91].
Daiokanzoto (TJ-84), which is composed of crude extracts of Rhubarb rhizomes and Glycyrrhiza roots, reduces virulence factor gene expression in P. gingivalis and exerts anti-inflammatory effects in oral epithelial cells [92]. Shosaikoto (TJ-9) has been prescribed for chronic liver diseases such as hepatitis, liver fibrosis, and cirrhosis and also for respiratory tract diseases. It consists of aqueous extracts from seven herbs including Bupleurum root, Pinellia tuber, Scutellaria root, Jujube fruit, Ginseng root, Glycyrrhiza root and Ginger rhizome, and contains more than 15 active ingredients. Shosaikoto increases the expression of calprotectin and S100A7 through partially IL-1α in oral epithelial cells [93]. Rokumigan, which consists six different herbs, rehmanniae radix, dioscoreae rhizoma, corni fructus, hoelen, moutan cortex, and alismatis rhizoma, is one of the most common herbal formulas, and has been shown to inhibit IL-6 secretion in cultured gingival epithelial cells [94]. In addition to oriental medicine, Vitamin E and l-ascorbic acid 2-phosphate magnesium salt inhibit decreases in E-cadherin expression, the penetration of P. gingivalis-LPS and increases in TNF-α [95] (Table 1).
5. Conclusion
Regulation of the functions on gingival epithelial cells may become a new method for the prevention of periodontal disease.
Conflict of interest
No potential conflicts of interest are disclosed.
Acknowledgements
We thank Nippon Shinyaku Co., Ltd., (Kyoto, Japan), for donating irsogladine maleate. We are grateful to Drs. Hiromichi Yumoto, Yuushi Uchida, Hideki Shiba, Hitoshi Komatsuzawa, and Shinya Murakami for their experimental support.
References
- 1.Darveau R.P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 2.Berezow A.B., Darveau R.P. Microbial shift and periodontitis. Periodontol 2000. 2011;55(1):36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimono M., Ishikawa T., Enokiya Y., Muramatsu T., Matsuzaka K., Inoue T. Biological characteristics of the junctional epithelium. J Electron Microsc (Tokyo) 2003;52(6):627–639. doi: 10.1093/jmicro/52.6.627. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder H.E., Listgarten M.A. The junctional epithelium: from strength to defense. J Dent Res. 2003;82(3):158–161. doi: 10.1177/154405910308200302. [DOI] [PubMed] [Google Scholar]
- 5.Bosshardt D.D., Lang N.P. The junctional epithelium: from health to disease. J Dent Res. 2005;84(1):9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 6.Takata T., Donath K. The mechanism of pocket formation: a light microscopic study on undecalcified human material. J Periodontol. 1988;59(4):215–221. doi: 10.1902/jop.1988.59.4.215. [DOI] [PubMed] [Google Scholar]
- 7.Hillmann G., Vipismakul V., Donath K. Development of plaque-induced gingival pockets in an animal experiment. Dtsch Zahnarztl Z. 1990;45(5):264–266. [PubMed] [Google Scholar]
- 8.Schroeder H.E., Listgarten M.A. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 9.Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 10.Furuse M., Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Saito I., Watanabe O., Kawahara H., Igarashi Y., Yamamura T., Shimono M. Intercellular junctions and the permeability barrier in the junctional epithelium: a study with freeze-fracture and thin sectioning. J Periodontal Res. 1981;16(5):467–480. doi: 10.1111/j.1600-0765.1981.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T., Hayashida K., Shiba H., Kishimoto A., Matsuda S., Takeda K. The expressions of claudin-1 and E-cadherin in junctional epithelium. J Periodontal Res. 2010;45(4):579–582. doi: 10.1111/j.1600-0765.2009.01258.x. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh M., Kurashige Y., Nishimura M., Yamazaki M., Igarashi S., Kaku T. Expression of claudin-4 and -7 in porcine gingival junctional epithelium. Med Mol Morphol. 2009;42(4):212–215. doi: 10.1007/s00795-009-0464-9. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T., Firth J.D., Kittaka M., Ekuni D., Kurihara H., Putnins E.E. Loss of claudin-1 in lipopolysaccharide-treated periodontal epithelium. J Periodontal Res. 2012;47(2):222–227. doi: 10.1111/j.1600-0765.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- 15.Wheelock M.J., Jensen P.J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J Cell Biol. 1992;117(2):415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niessen C.M. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 17.Ye P., Chapple C.C., Kumar R.K., Hunter N. Expression patterns of E-cadherin, involucrin, and connexin gap junction proteins in the lining epithelia of inflamed gingiva. J Pathol. 2000;192(1):58–66. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH673>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Hatakeyama S., Yaegashi T., Oikawa Y., Fujiwara H., Mikami T., Takeda Y. Expression pattern of adhesion molecules in junctional epithelium differs from that in other gingival epithelia. J Periodontal Res. 2006;41(4):322–328. doi: 10.1111/j.1600-0765.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 19.Katz J., Sambandam V., Wu J.H., Michalek S.M., Balkovetz D.F. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68(3):1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noguchi T., Shiba H., Komatsuzawa H., Mizuno N., Uchida Y., Ouhara K. Syntheses of prostaglandin E2 and E-cadherin and gene expression of beta-defensin-2 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans. Inflammation. 2003;27(6):341–349. doi: 10.1023/b:ifla.0000006702.27906.e9. [DOI] [PubMed] [Google Scholar]
- 21.Wessler S., Backert S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 2008;16(8):397–405. doi: 10.1016/j.tim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Unwin P.N., Ennis P.D. Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol. 1983;97(5 Pt. 1):1459–1466. doi: 10.1083/jcb.97.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowenstein W.R. Regulation of cell-to-cell communication by phosphorylation. Biochem Soc Symp. 1985;50:43–58. [PubMed] [Google Scholar]
- 24.Fujita T., Ashikaga A., Shiba H., Uchida Y., Hirono C., Iwata T. Regulation of IL-8 by Irsogladine maleate is involved in abolishment of Actinobacillus actinomycetemcomitans-induced reduction of gap-junctional intercellular communication. Cytokine. 2006;34(5–6):271–277. doi: 10.1016/j.cyto.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Uchida Y., Shiba H., Komatsuzawa H., Hirono C., Ashikaga A., Fujita T. Irsogladine maleate influences the response of gap junctional intercellular communication and IL-8 of human gingival epithelial cells following periodontopathogenic bacterial challenge. Biochem Biophys Res Commun. 2005;333(2):502–507. doi: 10.1016/j.bbrc.2005.05.147. [DOI] [PubMed] [Google Scholar]
- 26.Fujita T., Ashikaga A., Shiba H., Kajiya M., Kishimoto A., Hirata R. Irsogladine maleate counters the interleukin-1 beta-induced suppression in gap-junctional intercellular communication but does not affect the interleukin-1 beta-induced zonula occludens protein-1 levels in human gingival epithelial cells. J Periodontal Res. 2008;43(1):96–102. doi: 10.1111/j.1600-0765.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 27.Abuhussein H., Bashutski J.D., Dabiri D., Halubai S., Layher M., Klausner C. The role of factors associated with apoptosis in assessing periodontal disease status. J Periodontol. 2014;85(8):1086–1095. doi: 10.1902/jop.2013.130095. [DOI] [PubMed] [Google Scholar]
- 28.Tonetti M.S., Cortellini D., Lang N.P. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect Immun. 1998;66(11):5190–5195. doi: 10.1128/iai.66.11.5190-5195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.H., Wan X.H., Song J., Kang J.J., Chung W.S., Lee E.H. TGF-beta-induced apoptosis and reduction of Bcl-2 in human lens epithelial cells in vitro. Curr Eye Res. 2002;25(3):147–153. doi: 10.1076/ceyr.25.3.147.13475. [DOI] [PubMed] [Google Scholar]
- 30.Mithani S.K., Balch G.C., Shiou S.R., Whitehead R.H., Datta P.K., Beauchamp R.D. Smad3 has a critical role in TGF-beta-mediated growth inhibition and apoptosis in colonic epithelial cells. J Surg Res. 2004;117(2):296–305. doi: 10.1016/S0022-4804(03)00335-4. [DOI] [PubMed] [Google Scholar]
- 31.Fujita T., Alotaibi M., Kitase Y., Kota Y., Ouhara K., Kurihara H. Smad2 is involved in the apoptosis of murine gingival junctional epithelium associated with inhibition of Bcl-2. Arch Oral Biol. 2012;57(11):1567–1573. doi: 10.1016/j.archoralbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Alotaibi M.K., Kitase Y., Shuler C.F. Smad2 overexpression reduces the proliferation of the junctional epithelium. J Dent Res. 2014;93(9):898–903. doi: 10.1177/0022034514543016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alotaibi M.K., Kitase Y., Shuler C.F. Smad2 overexpression induces alveolar bone loss and up regulates TNF-α, and RANKL. Arch Oral Biol. 2016;71:38–45. doi: 10.1016/j.archoralbio.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto T., Fujita T., Ouhara K., Kajiya M., Imai H., Shiba H. Smad2 is involved in Aggregatibacter actinomycetemcomitans-induced apoptosis. J Dent Res. 2014;93(11):1148–1154. doi: 10.1177/0022034514550041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto T., Fujita T., Kajiya M., Ouhara K., Matsuda S., Komatsuzawa H. Aggregatibacter actinomycetemcomitans outer membrane protein 29 (Omp29) induces TGF-β-regulated apoptosis signal in human gingival epithelial cells via fibronectin/integrinβ1/FAK cascade. Cell Microbiol. 2016;18(12):1723–1738. doi: 10.1111/cmi.12607. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani P., Lasagni L., Annunziato F., Serio M., Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25(4):201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 38.Fujita T., Yoshimoto T., Matsuda S., Kajiya M., Kittaka M., Imai H. Interleukin-8 induces DNA synthesis, migration and down-regulation of cleaved caspase-3 in cultured human gingival epithelial cells. J Periodontal Res. 2015;50(4):479–485. doi: 10.1111/jre.12230. [DOI] [PubMed] [Google Scholar]
- 39.Sfakianakis A., Barr C.E., Kreutzer D.L. Localization of the chemokine interleukin-8 and interleukin-8 receptors in human gingiva and cultured gingival keratinocytes. J Periodontal Res. 2002;37(2):154–160. doi: 10.1034/j.1600-0765.2002.00024.x. [DOI] [PubMed] [Google Scholar]
- 40.Silva T.A., Garlet G.P., Fukada S.Y., Silva J.S., Cunha F.Q. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86(4):306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- 41.Uchida Y., Shiba H., Komatsuzawa H., Takemoto T., Sakata M., Fujita T. Expression of IL-1 beta and IL-8 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans. Cytokine. 2001;14(3):152–161. doi: 10.1006/cyto.2001.0863. [DOI] [PubMed] [Google Scholar]
- 42.Tsai C.C., Ho Y.P., Chen C.C. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66(10):852–859. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 43.Garlet G.P., Avila-Campos M.J., Milanezi C.M., Ferreira B.R., Silva J.S. Actinobacillus actinomycetemcomitans-induced periodontal disease in mice: patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 2005;7(4):738–747. doi: 10.1016/j.micinf.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Miyauchi M., Kitagawa S., Hiraoka M., Saito A., Sato S., Kudo Y. Immunolocalization of CXC chemokine and recruitment of polymorphonuclear leukocytes in the rat molar periodontal tissue after topical application of lipopolysaccharide. Histochem Cell Biol. 2004;121(4):291–297. doi: 10.1007/s00418-004-0636-6. [DOI] [PubMed] [Google Scholar]
- 45.Graves D.T., Delima A.J., Assuma R., Amar S., Oates T., Cochran D. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69(12):1419–1425. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- 46.Hiraishi H., Haruma K., Miwa H., Goto H. Clinical trial: irsogladine maleate, a mucosal protective drug, accelerates gastric ulcer healing after treatment for eradication of Helicobacter pylori infection—the results of a multicentre, double-blind, randomized clinical trial (IMPACT study) Aliment Pharmacol Ther. 2010;31(8):824–833. doi: 10.1111/j.1365-2036.2010.04250.x. [DOI] [PubMed] [Google Scholar]
- 47.Murakami K., Okimoto T., Kodama M., Tanahashi J., Mizukami K., Shuto M. Comparison of the efficacy of irsogladine maleate and famotidine for the healing of gastric ulcers after Helicobacter pylori eradication therapy: a randomized, controlled, prospective study. Scand J Gastroenterol. 2011;46(3):287–292. doi: 10.3109/00365521.2010.531485. [DOI] [PubMed] [Google Scholar]
- 48.Ueda F., Aratani S., Mimura K., Kimura K., Nomura A., Enomoto H. Effect of 2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate (MN-1695) on gastric mucosal damage induced by various necrotizing agents in rats. Arzneimittelforschung. 1984;34(4):478–484. [PubMed] [Google Scholar]
- 49.Kyoi T., Oka M., Noda K., Ukai Y. Irsogladine prevents monochloramine-induced gastric mucosal lesions by improving the decrease in mucosal blood flow due to the disturbance of nitric oxide synthesis in rats. J Pharmacol Sci. 2003;93(3):314–320. doi: 10.1254/jphs.93.314. [DOI] [PubMed] [Google Scholar]
- 50.Ueda F., Kyoi T., Mimura K., Kimura K., Yamamoto M. Intercellular communication in cultured rabbit gastric epithelial cells. Jpn J Pharmacol. 1991;57(3):321–328. doi: 10.1254/jjp.57.321. [DOI] [PubMed] [Google Scholar]
- 51.Kawasaki Y., Tsuchida A., Sasaki T., Yamasaki S., Kuwada Y., Murakami M. Irsogladine malate up-regulates gap junctional intercellular communication between pancreatic cancer cells via PKA pathway. Pancreas. 2002;25(4):373–377. doi: 10.1097/00006676-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Ueda F., Watanabe M., Hirata Y., Kyoi T., Kimura K. Changes in cyclic AMP content of rat gastric mucosa induced by ulcerogenic stimuli—in relation to the antiulcer activity of irsogladine maleate. Jpn J Pharmacol. 1991;55(4):493–499. doi: 10.1254/jjp.55.493. [DOI] [PubMed] [Google Scholar]
- 53.Ueda F., Ban K., Ishima T. Irsogladine activates gap-junctional intercellular communication through M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther. 1995;274(2):815–819. [PubMed] [Google Scholar]
- 54.Fujita T., Kishimoto A., Shiba H., Hayashida K., Kajiya M., Uchida Y. Irsogladine maleate regulates neutrophil migration and E-cadherin expression in gingival epithelium stimulated by Aggregatibacter actinomycetemcomitans. Biochem Pharmacol. 2010;79(10):1496–1505. doi: 10.1016/j.bcp.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Fujita T., Yumoto H., Shiba H., Ouhara K., Miyagawa T., Nagahara T. Irsogladine maleate regulates epithelial barrier function in tumor necrosis factor-α-stimulated human gingival epithelial cells. J Periodontal Res. 2012;47(1):55–61. doi: 10.1111/j.1600-0765.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 56.Savitri I.J., Ouhara K., Fujita T., Kajiya M., Miyagawa T., Kittaka M. Irsogladine maleate inhibits Porphyromonas gingivalis-mediated expression of toll-like receptor 2 and interleukin-8 in human gingival epithelial cells. J Periodontal Res. 2015;50(4):486–493. doi: 10.1111/jre.12231. [DOI] [PubMed] [Google Scholar]
- 57.Miyagawa T., Fujita T., Ouhara K., Matsuda S., Kajiya M., Hayashida K. Irsogladine maleate regulates the inflammatory related genes in human gingival epithelial cells stimulated by Aggregatibacter actinomycetemcomitans. Int Immunopharmacol. 2013;15(2):340–347. doi: 10.1016/j.intimp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Hirsch R., Deng H., Laohachai M.N. Azithromycin in periodontal treatment: more than an antibiotic. J Periodontal Res. 2012;47(2):137–148. doi: 10.1111/j.1600-0765.2011.01418.x. [DOI] [PubMed] [Google Scholar]
- 59.Muniz F.W., de Oliveira C.C., de Sousa Carvalho R., Moreira M.M., de Moraes M.E., Martins R.S. Azithromycin: a new concept in adjuvant treatment of periodontitis. Eur J Pharmacol. 2013;705(1–3):135–139. doi: 10.1016/j.ejphar.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 60.Pajukanta R., Asikainen S., Saarela M., Alaluusua S., Jousimies-Somer H. In vitro activity of azithromycin compared with that of erythromycin against Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1992;36(6):1241–1243. doi: 10.1128/aac.36.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pajukanta R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol Immunol. 1993;8(5):325–326. doi: 10.1111/j.1399-302x.1993.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 62.Yousef A.A., Jaffe A. The role of azithromycin in patients with cystic fibrosis. Paediatr Respir Rev. 2010;11(2):108–114. doi: 10.1016/j.prrv.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Schultz M.J. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004;54(1):21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki H., Shimomura A., Ikeda K., Furukawa M., Oshima T., Takasaka T. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope. 1997;107(12 Pt. 1):1661–1666. doi: 10.1097/00005537-199712000-00016. [DOI] [PubMed] [Google Scholar]
- 65.Khair O.A., Devalia J.L., Abdelaziz M.M., Sapsford R.J., Davies R.J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8(9):1451–1457. [PubMed] [Google Scholar]
- 66.Matsumura Y., Mitani A., Suga T., Kamiya Y., Kikuchi T., Tanaka S. Azithromycin may inhibit interleukin-8 through suppression of Rac1 and a nuclear factor-kappa B pathway in KB cells stimulated with lipopolysaccharide. J Periodontol. 2011;82(11):1623–1631. doi: 10.1902/jop.2011.100721. [DOI] [PubMed] [Google Scholar]
- 67.Asgrimsson V., Gudjonsson T., Gudmundsson G.H., Baldursson O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 2006;50(5):1805–1812. doi: 10.1128/AAC.50.5.1805-1812.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halldorsson S., Gudjonsson T., Gottfredsson M., Singh P.K., Gudmundsson G.H., Baldursson O. Azithromycin maintains airway epithelial integrity during Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2010;42(1):62–68. doi: 10.1165/rcmb.2008-0357OC. [DOI] [PubMed] [Google Scholar]
- 69.Miyagawa T., Fujita T., Yumoto H., Yoshimoto T., Kajiya M., Ouhara K. Azithromycin recovers reductions in barrier function in human gingival epithelial cells stimulated with tumor necrosis factor-α. Arch Oral Biol. 2016;62:64–69. doi: 10.1016/j.archoralbio.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Ostrosky-Zeichner L., Marr K.A., Rex J.H., Cohen S.H. Amphotericin B: time for a new gold standard. Clin Infect Dis. 2003;37(3):415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 71.Johnson R.H., Einstein H.E. Amphotericin B and coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:434–441. doi: 10.1196/annals.1406.019. [DOI] [PubMed] [Google Scholar]
- 72.Tamai R., Sugamata M., Kiyoura Y. Amphotericin B up-regulates lipid A-induced IL-6 production via caspase-8. J Dent Res. 2012;91(7):709–714. doi: 10.1177/0022034512446486. [DOI] [PubMed] [Google Scholar]
- 73.Sau K., Mambula S.S., Latz E., Henneke P., Golenbock D.T., Levitz S.M. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem. 2003;278(39):37561–37568. doi: 10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Ami R., Lewis R.E., Kontoyiannis D.P. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis. 2008;47(2):226–235. doi: 10.1086/589290. [DOI] [PubMed] [Google Scholar]
- 75.Dotis J., Simitsopoulou M., Dalakiouridou M., Konstantinou T., Panteliadis C., Walsh T.J. Amphotericin B formulations variably enhance antifungal activity of human neutrophils and monocytes against Fusarium solani: comparison with Aspergillus fumigatus. J Antimicrob Chemother. 2008;61(4):810–817. doi: 10.1093/jac/dkn036. [DOI] [PubMed] [Google Scholar]
- 76.Simitsopoulou M., Roilides E., Dotis J., Dalakiouridou M., Dudkova F., Andreadou E. Differential expression of cytokines and chemokines in human monocytes induced by lipid formulations of amphotericin B. Antimicrob Agents Chemother. 2005;49(4):1397–1403. doi: 10.1128/AAC.49.4.1397-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai H., Fujita T., Kajiya M., Ouhara K., Miyagawa T., Matsuda S. Amphotericin B down-regulates Aggregatibacter actinomycetemcomitans-induced production of IL-8 and IL-6 in human gingival epithelial cells. Cell Immunol. 2014;290(2):201–208. doi: 10.1016/j.cellimm.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Sakoda K., Yamamoto M., Negishi Y., Liao J.K., Node K., Izumi Y. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res. 2006;85(6):520–523. doi: 10.1177/154405910608500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 80.Klein U., Gimpl G., Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34(42):13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- 81.Hansen G.H., Niels-Christiansen L.L., Thorsen E., Immerdal L., Danielsen E.M. Cholesterol depletion of enterocytes: effect on the Golgi complex and apical membrane trafficking. J Biol Chem. 2000;275(7):5136–5142. doi: 10.1074/jbc.275.7.5136. [DOI] [PubMed] [Google Scholar]
- 82.Imai H., Fujita T., Kajiya M., Ouhara K., Yoshimoto T., Matsuda S. Mobilization of TLR4 into lipid rafts by Aggregatibacter actinomycetemcomitans in gingival epithelial cells. Cell Physiol Biochem. 2016;39(5):1777–1786. doi: 10.1159/000447877. [DOI] [PubMed] [Google Scholar]
- 83.Lu D.Y., Chen H.C., Yang M.S., Hsu Y.M., Lin H.J., Tang C.H. Ceramide and Toll-like receptor 4 are mobilized into membrane rafts in response to Helicobacter pylori infection in gastric epithelial cells. Infect Immun. 2012;80(5):1823–1833. doi: 10.1128/IAI.05856-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abate W., Alghaithy A.A., Parton J., Jones K.P., Jackson S.K. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J Lipid Res. 2010;51(2):334–344. doi: 10.1194/jlr.M000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Y., Zhou E., Wei Z., Song X., Liu Z., Wang T. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochim Biophys Acta. 2014;1840(6):1755–1764. doi: 10.1016/j.bbagen.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 86.Chou S.C., Su C.R., Ku Y.C., Wu T.S. The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull (Tokyo) 2009;57(11):1227–1230. doi: 10.1248/cpb.57.1227. [DOI] [PubMed] [Google Scholar]
- 87.Li W., Zhou P., Zhang Y., He L. Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. J Ethnopharmacol. 2011;133(2):922–927. doi: 10.1016/j.jep.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W., Fan T., Zhang Y., Zhou P., Niu X., He L. Houttuynia cordata Thunb. volatile oil exhibited anti-inflammatory effects in vivo and inhibited nitric oxide and tumor necrosis factor-α production in LPS-stimulated mouse peritoneal macrophages in vitro. Phytother Res. 2013;27(11):1629–1639. doi: 10.1002/ptr.4905. [DOI] [PubMed] [Google Scholar]
- 89.Lu H.M., Liang Y.Z., Yi L.Z., Wu X.J. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol. 2006;104(1–2):245–249. doi: 10.1016/j.jep.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am J Chin Med. 2005;33(3):415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- 91.Kabir M., Fujita T., Ouhara K., Kajiya M., Matsuda S., Shiba H. Houttuynia cordata suppresses the Aggregatibacter actinomycetemcomitans-induced increase of inflammatory-related genes in cultured human gingival epithelial cells. J Dent Sci. 2015;10(1):88–94. [Google Scholar]
- 92.Fournier-Larente J., Azelmat J., Yoshioka M., Hinode D., Grenier D. The Daiokanzoto (TJ-84) Kampo formulation reduces virulence factor gene expression in Porphyromonas gingivalis and possesses anti-inflammatory and anti-protease activities. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hiroshima Y., Bando M., Kataoka M., Shinohara Y., Herzberg M.C., Ross K.F. Shosaikoto increases calprotectin expression in human oral epithelial cells. J Periodontal Res. 2010;45(1):79–86. doi: 10.1111/j.1600-0765.2009.01203.x. [DOI] [PubMed] [Google Scholar]
- 94.Liao J., Azelmat J., Zhao L., Yoshioka M., Hinode D., Grenier D. The Kampo medicine Rokumigan possesses antibiofilm, anti-inflammatory, and wound healing properties. Biomed Res Int. 2014;2014 doi: 10.1155/2014/436206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abe-Yutori M., Chikazawa T., Shibasaki K., Murakami S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J Periodontal Res. 2017;52(1):42–50. doi: 10.1111/jre.12367. [DOI] [PubMed] [Google Scholar]