Abstract
Purpose
The aim of this study was to evaluate the capacity of single and combined applications of the bark of the stems and roots of Magnolia officinalis Rehd. et Wils. (Magnoliae Cortex) and Zea mays L. (maize) to modulate inflammation in RAW 264.7 cells stimulated with Porphyromonas gingivalis.
Methods
RAW 264.7 cells were stimulated with P. gingivalis, and Magnoliae Cortex and/or maize was added. Cytotoxicity and the capacity to modulate inflammation were determined with a methylthiazol tetrazolium (MTT) assay, nitrite production, enzyme-linked immunosorbent assay (ELISA), and western blotting.
Results
Treatment with Magnoliae Cortex and/or maize inhibited nuclear transcription factor κB (NF-κB) pathway activation and nuclear p44/42 mitogen-activated protein kinase (MAPK) and inducible nitric oxide synthase (iNOS) protein expression in P. gingivalis-stimulated RAW 264.7 cells. Moreover, the treatments suppressed cytokines (prostaglandin E2 [PGE2], interleukin [IL]-1β, and IL-6) and nitrite production.
Conclusions
Both Magnoliae Cortex and maize exerted an anti-inflammatory effect on P. gingivalis-stimulated RAW 264.7 cells, and this effect was more pronounced when the extracts were combined. These findings show that these extracts may be beneficial for slowing the progression of periodontal disease.
Keywords: Cytokines, Magnolia, Mitogen-activated protein kinase 3, Periodontal diseases, Porphyromonas gingivalis, Zea mays
Graphical Abstract

INTRODUCTION
Periodontal disease results from undesired immune reactions in response to periodontal pathogens such as Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans [1]. Removing oral pathogens by mechanical or chemical means is a key component of controlling periodontal disease. Moreover, as an adjunctive treatment of periodontal disease, subantimicrobial-dose doxycycline (SDD) suppresses host-derived matrix metalloproteinases, which modulate the host immune reaction [2]. Additionally, patients with aggressive periodontitis may need additional modulation of their hyper-inflammatory status, as well as the removal of oral pathogens [3]. The modulation of inflammatory mediators may additionally help to control periodontal disease, along with the mechanical removal of oral pathogens [4].
Inflammatory cytokines and other soluble mediators are expressed in stimulated macrophages through the activation of mitogen-activated protein kinase (MAPK) and nuclear transcription factor κB (NF-κB) [5]. The interaction of Toll-like receptors (TLRs) on macrophages with bacterial components such as lipopolysaccharide (LPS), lipoproteins, and endotoxin result in the activation of NF-κB [5,6]. NF-κB is a key transcription factor for pro-inflammatory mediators, including cytokines and chemokines such as interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2), as well as nitric oxide (NO) [6,7]. In addition, TLR engagement initiates signal transduction cascades such as MAPK, extracellular signal-regulated kinase 1/2 (ERK 1/2), c-Jun N-terminal kinase 1/2 (JNK 1/2), and p38 MAP kinase pathways [8].
Magnoliae Cortex, the bark of the stems and roots of Magnolia officinalis Rehd. et Wils., is used to treat acute diarrhea, cramping abdominal pain, regurgitation, vomiting, and dyspepsia [9]. Honokiol and magnolol are phenolic compounds that have been isolated from Magnoliae Cortex and are known to suppress the production of LPS-mediated cellular responses such as TNF-α, PGE2, and NO expression [10]. Honokiol inhibits TNF-α and IL-6 in a dose-dependent manner [11]. Additionally, honokiol has anti-inflammatory effects on activated macrophages by inhibiting TNF-α and NO expression through inhibition of the MAPK, protein kinase C-α (PKC-α), and NF-κB pathways [12]. Magnolol inhibits the IL-6-induced Janus kinase (JAK)/signal transduction and activator of transcription (STAT) 3 signaling pathway by reducing STAT3 binding activity in endothelial cells [13].
The corn silk and corn kernels of Zea mays L. (maize) contain zeatin, flavonoids, alkaloids, allantoin, saponins, volatile oils, vitamins, starch, fats, cellulose, and β-sitosterol [14]. Corn kernels and corn silk have been found to exhibit pharmacological effects, including anti-hepatoma [15] and anti-fatigue [16] properties. Corn bran inhibits NO production and inducible nitric oxide synthase (iNOS) expression in a dose-dependent manner [17]. In addition, an unsaponifiable fraction of maize reduced gingival inflammation and tooth mobility in patients with periodontal disease [18]. In previous studies, maize and Magnoliae Cortex had an anti-microbial effect on periodontal pathogens [19], promoted bone tissue regeneration in rats [20], and facilitated clinical improvement in a dog model of experimental periodontitis [21]. However, the underlying mechanisms have not yet been established.
It is possible to combine more than one substance to modulate multi-targeted inflammation processes and functions. In such cases, the combination can produce stable and synergistic effects. The aim of this study was to evaluate the capacity of Magnoliae Cortex and maize to modulate inflammation in RAW 264.7 cells stimulated with P. gingivalis TLR ligands. Magnoliae Cortex and maize were separately or simultaneously applied to cells, and the induced inflammatory reactions were measured as the amount of NO, PGE2, IL-1β, IL-6, p44/42 MAPK, iNOS, and NF-κB produced.
MATERIALS AND METHODS
Sample preparation
The soft 75% ethanol Magnoliae Cortex extract was provided by Dongbang FTL (Seoul, Korea). The titrated, unsaponifiable maize extract fraction was provided by Dongkook Pharmaceutical Co., Ltd. (Seoul, Korea). Ibuprofen (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control. Dimethyl sulfoxide (DMSO) was used as a solvent. The final soft 75% ethanol Magnoliae Cortex extract concentration was 60 μg/mL in 1% DMSO; this was denoted as M. The final concentration of the titrated unsaponifiable maize extract fraction was 300 μg/mL in 1% DMSO; this was denoted as Z. The combined treatment of M and Z was denoted as MZ. The final ibuprofen concentration was 10 mM in 1% DMSO; this was denoted as IBU.
Cell culture
RAW 264.7 cells (murine) were obtained from Korean Cell Line Bank (KCLB, Seoul, Korea) and cultured in a 5% CO2 atmosphere at 37°C in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Invitrogen) (complete DMEM). The cells were inoculated in 10-cm dishes at a density of 1.9×104/cm2, 24-well plates at a density of 5×104/cm2, and 96-well plates at a density of 3.3×104/cm2 and cultured for 24 hours. The cells were pre-treated with M, Z or MZ, with or without Pam3CSK4 (InvivoGen, San Diego, CA, USA), a synthetic TLR ligand. IBU was used as a positive control.
Nitrite production measurements
RAW 264.7 cells were inoculated into 24-well plates and incubated for 24 hours in complete DMEM as follows: with medium alone; with medium and Pam3CSK4 (10 μg/mL); with the soft 75% ethanol Magnoliae Cortex extract (0.6, 6, or 60 μg/mL) and Pam3CSK4 (10 μg/mL); with the titrated, unsaponifiable maize extract fraction (3, 30, or 300 μg/mL) and Pam3CSK4 (10 μg/mL); with a mixture of one of the extracts and Pam3CSK4 (10 μg/mL); or with IBU (10 mM) and Pam3CSK4 (10 μg/mL). To each well, 80 μL of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-[1-naphthyl] ethylenediamine dihydrochloride in water; Sigma-Aldrich) was added, and the mixture was incubated for 5 minutes in the dark. The total nitrite level was measured and calculated on the basis of absorbance at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell viability measurements using the MTT assay
RAW 264.7 cells were inoculated into a 96-well plate and incubated for 24 hours in complete DMEM. The culture medium was subsequently discarded and replenished as follows: with medium alone; with the soft 75% ethanol Magnoliae Cortex extract (0.6, 6, 60, or 600 μg/mL); with the titrated, unsaponifiable maize extract fraction (3, 30, 300, or 3000 μg/mL); or with a mixture of one of the extracts and IBU (10 mM) for 24 hours. Filtered methylthiazol tetrazolium (MTT) solution in DMEM was added to each well (5 mg MTT/mL), and the cells were incubated at 37°C for 4 hours. The unreacted dye was then removed. The insoluble MTT formazan crystals were allowed to dissolve in DMSO at room temperature for 15 minutes, and absorbance was measured at 570 nm using a microplate reader (Molecular Devices).
ELISA for PGE2, IL-1β, and IL-6 release measurements
Cytokines were measured by enzyme-linked immunosorbent assay (ELISA). RAW 264.7 cells were plated in a 24-well plate and cultured in DMEM containing 10% FBS for 24 hours. These cells were then serum-starved overnight in 0.5% FBS-containing medium. For PGE2 release measurement, the cells were pretreated for 30 minutes before treatment with Pam3CSK4 (10 μg/mL) for 24 hours in a 5% CO2 incubator at 37°C with medium alone; with the soft 75% ethanol Magnoliae Cortex extract (6 or 60 μg/mL); with the titrated, unsaponifiable maize extract fraction (30 or 300 μg/mL); or with a mixture of one of the extracts and IBU (10 mM). The collected media were stored at −70°C for cytokine analysis. The supernatant was assessed using PGE2-specific ELISA kits (R&D system, Minneapolis, MN, USA) according to the manufacturer's instructions. The absorbance was read in a microplate reader (Molecular Devices). For IL-1β and IL-6 release measurements, the cells were pretreated with M (60 μg/mL), Z (300 μg/mL), MZ (60 μg/mL M and 300 μg/mL Z), or IBU (10 μg/mL) for 30 minutes prior to treatment with Pam3CSK4 (10 μg/mL) for 24 hours in a 5% CO2 incubator at 37°C. The collected media were stored at −70°C for cytokine analysis. The supernatant was evaluated using a bead-based multiplex cytokine assay for IL-1β and IL-6 (Milliplex, Millipore, Billerica, MA, USA) and analyzed with a Luminex 200 System (Luminex, Austin, TX, USA).
iNOS, COX2, and p44/42 MAPK measurements by western blotting
RAW 264.7 cells were plated in 10-cm dishes and cultured in DMEM containing 10% FBS for 24 hours and then serum-starved overnight in 0.5% FBS medium. The cells were pretreated with M (60 μg/mL), Z (300 μg/mL), MZ (60 μg/mL M and 300 μg/mL Z), or IBU (10 μg/mL) for 30 minutes prior to treatment with Pam3CSK4 (10 μg/mL) for 24 hours (iNOS and COX-2) or 30 minutes (p44/42 MAPK) in a 5% CO2 incubator at 37°C. The cells were washed with ice-cold phosphate-buffered saline and harvested with protein extraction solution (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 150 mM NaCl, 1% NP-40, and 1 mM PMSF) (Elpisbio, Daejeon, Korea) according to the manufacturer's protocol. Total cell lysates (10 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin (BSA) for 1 hour, and primary antibodies against iNOS (Cell Signaling Technology, Danvers, MA, USA), cyclooxygenase-2 (COX-2; Cell Signaling Technology, Danvers, MA, USA), phospho-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA), ERK1/2 (Cell Signaling Technology, Danvers, MA, USA), and β-actin (Sigma-Aldrich) were added to the Tris-buffered saline (TBS)-T solution containing 5% BSA and incubated overnight at 4°C. After 3 washes with TBS-T buffer, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Cell Signaling Technology, Danvers, MA, USA) in TBS-T-containing 5% BSA for 1 hour. The membranes were subsequently washed 3 times with TBS-T buffer and analyzed with enhanced chemiluminescence detection reagents (Dogen, Innotech, Daejeon, Korea).
NF-κB activity measurements
NF-κB activity was measured using an NF-κB p65 ActiveELISA kit (Imgenex Corp, San Diego, CA, USA) according to the manufacturer's instructions. Absorbance at 405 nm was determined using a microplate reader (Molecular Devices).
Statistical analysis
Data were analyzed using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as means±standard deviations. The statistical analyses were conducted with the Kruskal-Wallis test, followed by the Mann-Whitney test. Differences were considered to be significant when the P values were <0.05.
RESULTS
Effects of M and Z on Pam3CSK4-induced NO production by RAW 264.7 cells
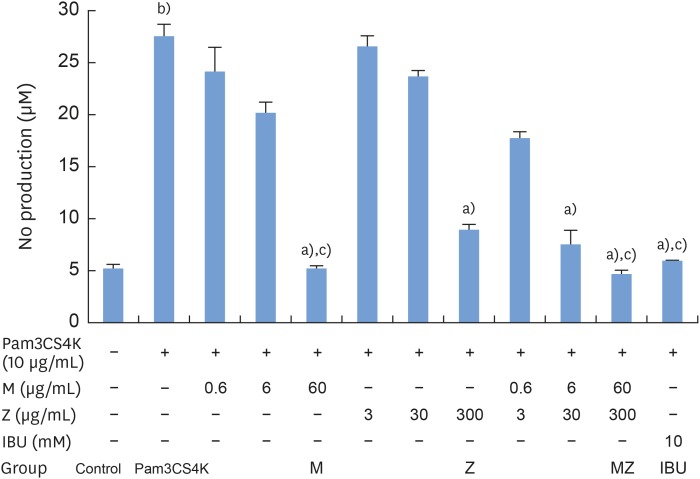
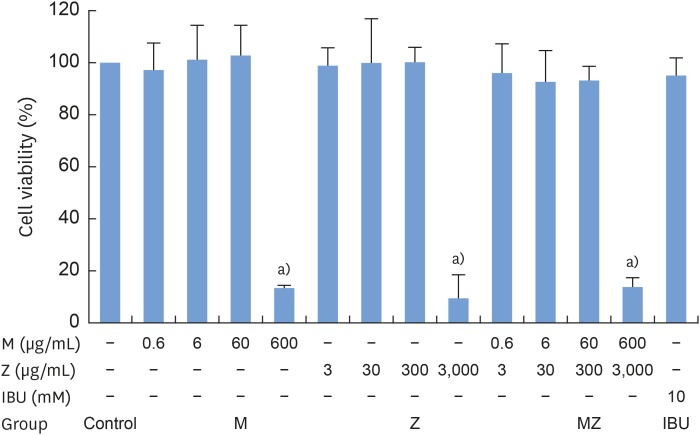
To evaluate the potential anti-inflammatory effects of M and Z on Pam3CSK4-stimulated RAW 264.7 cells, NO production was measured. Pam3CSK4 treatment alone dramatically induced NO production in comparison with the control group, whereas the M and Z treatments significantly suppressed NO production in Pam3CSK4-induced RAW 264.7 cells. Interestingly, M and MZ were more effective at suppressing NO production than Z, and the combination was as effective as IBU (Figure 1). Because concentrations of 3,000 μg/mL of the maize extract and/or 600 μg/mL of the Magnoliae Cortex extract exhibited significant cytotoxicity (Figure 2), these concentrations were excluded from the evaluations of NO and other cytokines.
Figure 1. Effects of M and Z on Pam3CSK4-induced NO production in RAW 264.7 cells. Cells (5×104/cm2) were treated with M, Z, MZ, or IBU and 10 μg/mL Pam3CSK4 for 24 hours at 37°C. In experiments without Pam3CSK4, the medium alone was used as a negative control, and IBU was used as a positive control. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, NO: nitric oxide, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control; c)P<0.05 in comparison with Z.
Figure 2. Cytotoxicity of M and Z treatments in RAW 264.7 cells. Cells (3.3×104/cm2) were treated with M, Z, or IBU for 24 hours at 37°C. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide, MZ: the combination treatment of M and Z.
a)P<0.05 in comparison with the control.
Effects of M and Z on PGE2, IL-1β, and IL-6 production in Pam3CSK4-induced RAW 264.7 cells
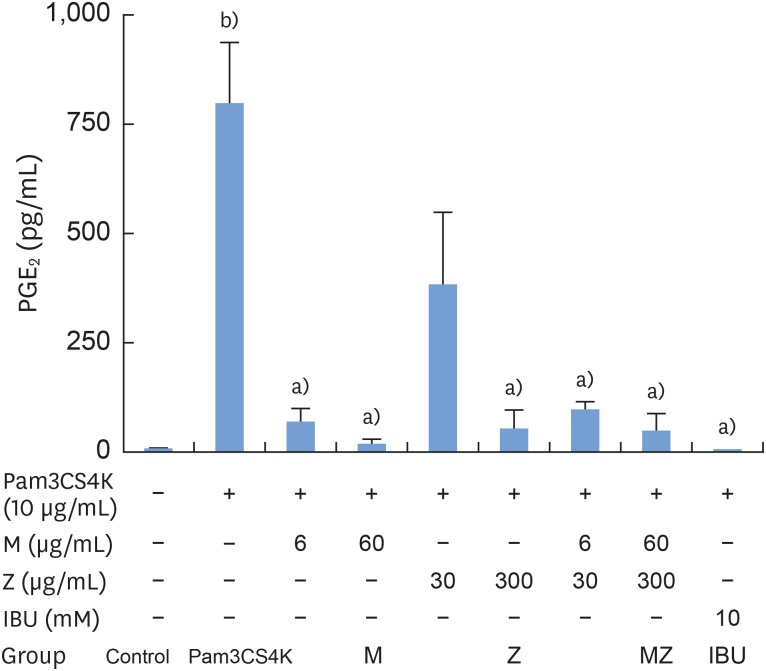
To determine the effects of M and Z on PGE2 formation, cells were treated with M, Z, MZ, or IBU for 30 minutes and then stimulated with 10 μg/mL Pam3CSK4 for 24 hours. PGE2 was significantly induced in Pam3CSK4-stimulated cells. However, the addition of M and Z significantly suppressed PGE2 production in Pam3CSK4-induced RAW 264.7 cells. Moreover, M and Z inhibited PGE2 production in a manner similar to that of IBU treatment (Figure 3).
Figure 3. Effects of M and Z on Pam3CSK4-induced PGE2 formation in RAW 264.7 cells. Cells (5×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes before treatment with 10 μg/mL Pam3CSK4 for 24 hours at 37°C. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, PGE2: prostaglandin E2, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control.
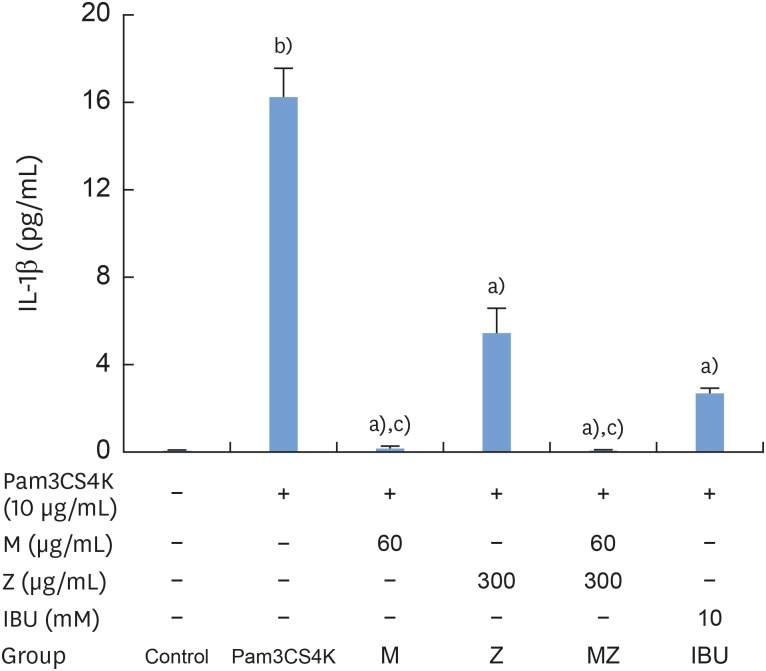
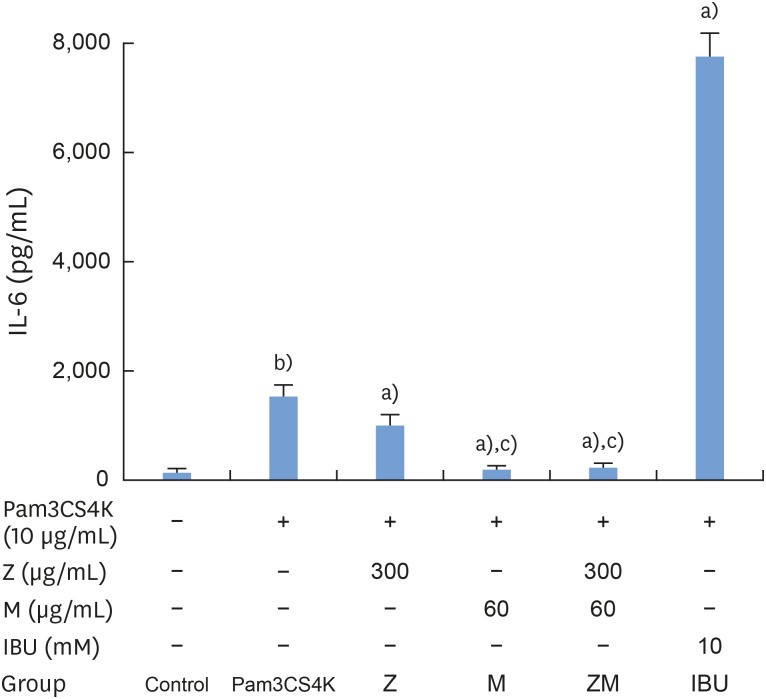
According to our results, Pam3CSK4 significantly elevated IL-1β and IL-6 levels in RAW 264.7 cells (Figures 4 and 5), but IL-1β and IL-6 production was strongly reduced when the cells were treated with M and Z. Treating the cells with M and MZ significantly suppressed IL-1β production, a trend that was more pronounced than with Z and IBU (Figure 4). This result shows that with regard to IL-1β, M treatment inhibited inflammation more effectively than Z. However, Z and MZ treatments more significantly inhibited IL-6 levels than M and IBU (Figure 5), indicating that with regard to IL-6, Z was more efficient at suppressing inflammation, even when considering the possibility of a synergistic effect of MZ on IL-6 suppression. Interestingly, IBU treatment promoted IL-6 production to a significantly greater degree than Pam3CSK4 treatment alone.
Figure 4. Effects of M and Z on Pam3CSK4-induced IL-1β formation in RAW 264.7 cells. Cells (5×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes before treatment with 10 μg/mL Pam3CSK4 for 24 hours at 37°C. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, IL-1β: interleukin-1β, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control; c)P<0.05 in comparison with both Z and IBU.
Figure 5. Effects of M and Z on Pam3CSK4-induced IL-6 formation in RAW 264.7 cells. Cells (5×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes before treatment with 10 μg/mL Pam3CSK4 for 24 hours at 37°C. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, IL-6: interleukin-6, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control; c)P<0.05 in comparison with M.
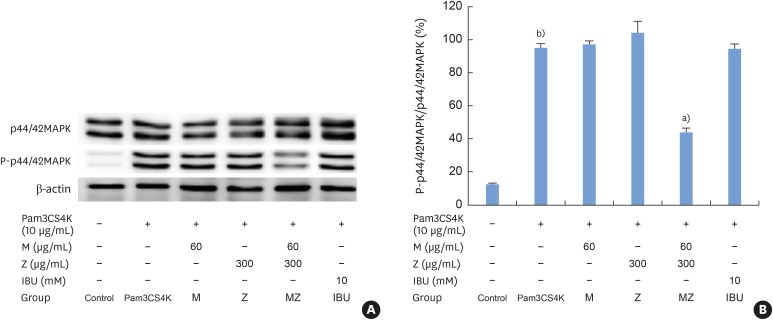
Effects of M and Z on p44/42 MAPK activation in Pam3CSK4-induced RAW 264.7 cells
Pam3CSK4 treatment led to significant increases in the levels of phosphorylated p44/42 MAPK; however, MZ inhibited p44/42 MAPK activation in Pam3CSK4-induced RAW 264.7 cells (Figure 6A and B).
Figure 6. Effects of M and Z on nuclear p44/42 MAPK expression levels in Pam3CSK4-induced RAW 264.7 cells. The levels of p44/42 MAPK and β-actin expression were detected by western blotting using specific antibodies. Cells (1.9×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes before being treated with 10 μg/mL Pam3CSK4. To detect p44/42 MAPK expression, Pam3CSK4 was added for 30 minutes at 37°C. The blots shown here are representative of 3 independent experiments. Each sample contained 10 μg of total protein. (A) The levels of p44/42 MAPK, P-p44/42 MAPK and β-actin expression; (B) ratio of P-p44/42 MAPK to p44/42 MAPK expressed as a percentage.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), MAPK: mitogen-activated protein kinase, Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control.
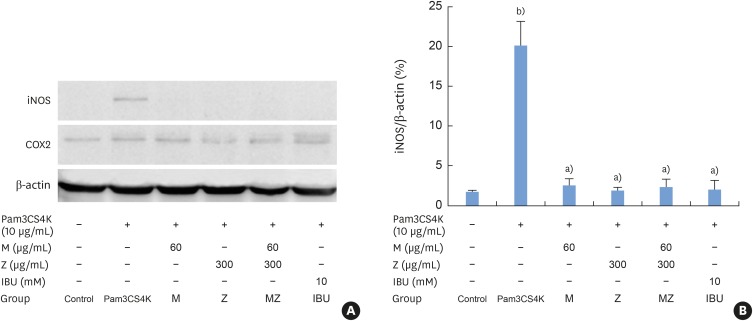
Effects of M and Z on iNOS, and COX-2 expression in Pam3CSK4-induced RAW 264.7 cells
iNOS expression increased after Pam3CSK4 stimulation in RAW 264.7 cells, but M, Z, MZ, and IBU significantly inhibited iNOS expression (Figure 7A and B). There was no change in COX-2 expression following treatment with Pam3CSK4, M, Z, or IBU.
Figure 7. Effects of M and Z on nuclear iNOS and COX-2 expression levels in Pam3CSK4-induced RAW 264.7 cells. The levels of iNOS, COX-2, and β-actin expression were detected by western blotting using specific antibodies. Cells (1.9×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes before being treated with 10 μg/mL Pam3CSK4. To detect iNOS and COX-2 expression, Pam3CSK4 was added for 24 hours at 37°C. The blots shown here are representative of 3 independent experiments. Each sample contained 10 μg of total protein. (A) The levels of iNOS, COX-2, and β-actin expression; (B) ratio of iNOS to β-actin expressed as a percentage.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), iNOS: inducible nitric oxide synthase, COX-2: cyclooxygenase-2, Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control.
Effects of M and Z on Pam3CSK4-induced NF-κB transactivation in RAW 264.7 cells
To determine whether M and Z could affect the level of p65 expression in RAW 264.7 cells, cells were treated with M, Z, MZ, or IBU for 30 minutes and then stimulated with 10 μg/mL Pam3CSK4 for 2 hours. M reduced the level of p65 and MZ significantly inhibited NF-κB transactivation to the control level (Figure 8). These results show a synergistic effect of M and Z on Pam3CSK4-induced RAW 264.7 cells with respect to NF-κB inhibition.
Figure 8. Effects of M and Z on Pam3CSK4-induced NF-κB transactivation in RAW 264.7 cells. Cells (5×104/cm2) were treated with M, Z, MZ, or IBU for 30 minutes prior to treatment with 10 μg/mL Pam3CSK4 for 2 hours at 37°C. The data are expressed as mean±standard deviation, as obtained from 6 separate experiments.
M: soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, NF-κB: nuclear transcription factor κB, MZ: the combination treatment of M and Z, IBU: ibuprofen (10 mM in 1% DMSO), DMSO: dimethyl sulfoxide, p65: transcription factor p65.
a)P<0.05 in comparison with Pam3CSK4; b)P<0.05 in comparison with the control; c)P<0.05 in comparison with M and Z.
DISCUSSION
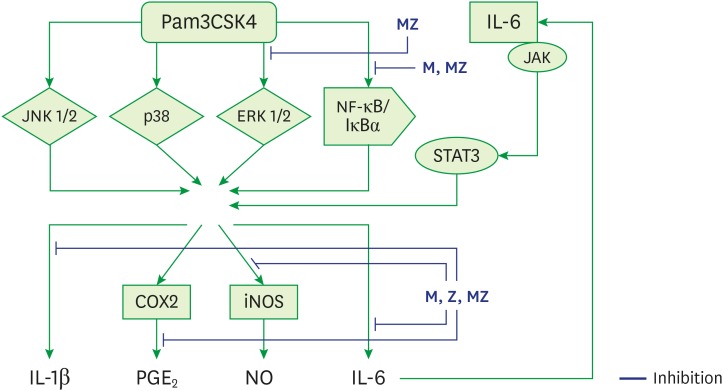
The primary etiology of periodontal disease is infection by periodontal pathogens such as P. gingivalis, P. intermedia, and A. actinomycetemcomitans [1,2,4,22]. Conversely, periodontal disease progresses in response to the host immune inflammatory reaction to periodontal pathogens and their products [2,4,22]. TLR ligands from pathogens such as P. gingivalis stimulate immune cells, such as neutrophils, monocytes, and macrophages, and immune cells produce cytokines by multiple processes (Figure 9) [4,23]. These inflammatory mediators, such as IL-1β, IL-6, and TNF-α, cause local tissue destruction and alveolar bone loss [2,4,22].
Figure 9. Schematic model for the anti-inflammatory mechanism of M and Z in Pam3CSK4-induced RAW 264.7 cells.
ML soft 75% ethanol Magnoliae Cortex extract (60 μg/mL in 1% DMSO), Z: titrated unsaponifiable maize extract fraction (300 μg/mL in 1% DMSO), Pam3CSK4: synthetic Toll-like receptors of Porphyromonas gingivalis, MZ: the combination treatment of M and Z, JNK 1/2: c-Jun N-terminal kinase 1/2, p38: p38 mitogen-activated protein kinase, ERK 1/2: extracellular signal-regulated kinase 1/2, NF-κB/IκBα: nuclear transcription factor κB pathway, JAK/STAT3: Janus kinase/signal transduction and activator of transcription 3 signaling pathway, iNOS: inducible nitric oxide synthase, COX-2: cyclooxygenase-2, IL: interleukin; PGE2: prostaglandin E2, NO: nitric oxide, DMSO: dimethyl sulfoxide.
Aggressive periodontitis generally involves rapid attachment loss and bone destruction inconsistent with the amount of the microbial deposit [3,22,24]. Single nucleotide polymorphisms on genes encoding cytokines, receptors, and metabolic modulators and proteins result in increased disease susceptibility or overexpression of the host innate inflammatory reaction [22]. Modulators of inflammatory mediators, such as SDD, may be especially beneficial for slowing the progression of periodontal disease, especially aggressive periodontitis, along with the mechanical removal of oral pathogens [2,4,25].
Magnolol and honokiol, the main components of Magnoliae Cortex, have been reported to suppress inflammatory reactions both in vitro and in vivo [12,26]. One of the main components of maize, β-sitosterol, has been shown to exhibit anti-inflammatory effects that suppress monocyte activation by IL-6 and TNF-α and might be useful as a treatment for rheumatoid arthritis [27]. Although anti-inflammatory effects of Magnoliae Cortex and maize have been presented in previous studies, details regarding the mechanism through which these effects occur have not been reported [17,18,26,28]. Herein, we demonstrated that M and/or Z can modulate the inflammatory response by suppressing TLR ligand-induced signaling and pro-inflammatory molecule production.
NO, an inflammation mediator, is a reactive molecule that has a variety of effects depending on its relative concentration [29]. Small amounts of NO are produced by constitutive nitric oxide synthase (NOS) enzymes (i.e., endothelial NOS and neural NOS), and are essential for physiological homeostasis. In contrast, the large amounts of NO produced by inducible NOS have been closely linked to the pathophysiology of numerous inflammatory diseases [30]. In the present study, various M, Z, MZ, and IBU concentrations were examined, and the optimal concentration with minimal cytotoxic effects and maximum inhibition of NO production was selected. The high level of NO production observed in Pam3CSK4-stimulated macrophages was strikingly suppressed by M and Z, with an effectiveness that was almost the same as that of IBU. NO inhibition by iNOS downregulation in macrophages modulates host immune inflammation induced by bacterial infection, and the results presented herein correspond well with those of earlier studies. For example, previous studies demonstrated that honokiol and magnolol, the major active components of Magnoliae Cortex, inhibited NO release and iNOS expression in a dose-dependent manner [10,12,26]. In addition, a study of corn bran showed that 80% of an ethanolic corn bran extract inhibited NO production and iNOS expression in macrophages stimulated with bacterial products [17].
The NF-κB pathway, which is considered to be a prototypical pro-inflammatory signaling cascade, is activated by TLRs that recognize microbial molecular patterns [31]. NF-κB binds to the iNOS gene promoter, thereby promoting iNOS expression and NO production [29]. However, some drugs, such as salicylates, only inhibit iNOS expression without affecting NF-κB-mediated iNOS mRNA expression [32]. In the present study, M and MZ inhibited Pam3CSK4-induced iNOS expression at the transcriptional level by suppressing NF-κB activation, whereas Z inhibited iNOS expression without suppressing NF-κB activation. These findings are different from those of Kim et al. [17], who reported that corn phenolic amides contributed to the inhibition of NO production by downregulating the level of NF-κB-mediated iNOS gene expression. These results indicate that the various components of corn interact with inflammatory processes via different pathways. In the present study, MZ significantly suppressed NF-κB activation, and this activity was more pronounced than that of M, suggesting that M and Z exerted a synergistic effect with respect to NF-κB inhibition.
In addition to the NF-κB signaling pathway, JAK-STAT signaling regulates inflammatory processes [29,33]. Cytokines such as interleukins and interferons activate the Janus family of tyrosine kinases (JAK1, JAK2, JAK3, and tyrosine kinase 2 [Tyk2]), and activated JAK kinases phosphorylate members of the STAT family [33]. In addition to interleukins and interferons, other cytokines, such as IL-6, also activate JAK1 and STAT3 [13]. Suppression of IL-6-mediated JAK-STAT signaling is important for controlling inflammation [13]. In the present study, M and Z significantly suppressed Pam3CSK4-induced IL-6 production in RAW 264.7 cells, and Z was more effective at inhibiting IL-6 secretion than was M. These results suggest that Z does not suppress the NF-κB pathway, but instead suppresses the JAK-STAT pathway, and that M suppresses both pathways to exert its anti-inflammatory effects. These results are consistent with those of an earlier study reporting that magnolol extracted from Magnoliae Cortex suppressed IL-6-induced STAT3 phosphorylation and downstream target gene expression in endothelial cells [13]. Interestingly, the results of our study show that IBU significantly increased Pam3CSK4-induced IL-6 production, but not IL-6 production without Pam3CSK4 stimulation (data not shown). These findings correspond well with those of earlier studies, in which ibuprofen significantly increased Pam3CSK4-induced IL-6 production [34] but had no inhibitory effects on IL-6 bioactivity [35]. Although IBU suppressed inflammation, further studies are needed to reveal the precise mode of action with regard to inflammatory suppression.
Parallel to the NF-κB and JAK-STAT pathways, the ERK 1/2 pathway is also key for regulating inflammation at the transcriptional level. Recognition of bacterial components by TLRs leads to the phosphorylation of interleukin-1 receptor-associated kinase (IRAK), which in turn activates tumor necrosis factor receptor-activated factor 6 (TRAF6) [5]. TRAF6 activates IκB kinase and MAPK, which phosphorylate downstream kinases. Activated IκB kinase and MAPK then activate p38 and ERK 1/2 as well as NF-κB, leading to IL-1β, TNF-α, and NO transcription and secretion [5]. In the present study, MZ significantly reduced phospho-p44/42 MAPK expression in Pam3CSK4-stimulated macrophages, but neither M nor Z could inhibit the ERK 1/2 pathway alone. These results indicate that M and Z exerted a synergistic effect in inhibiting the ERK 1/2 pathway, suggesting that they may control inflammation more effectively when used together.
The increased release of active IL-1β is a hallmark of auto-inflammatory diseases and chronic inflammatory diseases. IL-1β entering the circulation from a local inflammation site induces IL-1 itself as well as systemic inflammation [36,37]. Both IL-1β bursts and IL-1β polymorphisms are closely related to the slowly progressive inflammatory processes that occur in periodontitis, coronary heart disease, osteoarthritis, and type 2 diabetes [36,37,38]. As a result, blocking IL-1β has been the focus of auto-inflammatory and chronic inflammatory disease treatments [37]. In the present study, M and Z efficiently blocked IL-1β secretion in Pam3CSK4-stimulated RAW 264.7 cells. Additionally, M significantly and more effectively blocked IL-1β secretion than IBU. These results suggest that M and Z can modulate not only chronic inflammatory reactions but also auto-immune inflammatory reactions.
In conclusion, both the Magnoliae Cortex and maize extracts modulated the downstream targets of multiple inflammatory pathways, and decreased Pam3CSK4-induced inflammatory reactions by inhibiting NO, PGE2, ERK 1/2, iNOS, IL-1β, IL-6, and NF-κB levels. However, a difference in their modes of action was observed, in that Magnoliae Cortex was more effective at decreasing NO, IL-1β, and NF-κB levels, while maize was more effective at reducing IL-6 levels. In all experiments, the combination of Magnoliae Cortex and maize showed either the same or enhanced results relative to the use of Magnoliae Cortex or maize alone. Additionally, greater efficacy was obtained with respect to NF-κB and ERK 1/2 inhibition when both extracts were added simultaneously. Overall, Magnoliae Cortex and maize may be beneficial for slowing periodontal disease progression. Further study is needed to elucidate the precise anti-inflammatory signaling pathways targeted by each of the components of Magnoliae Cortex and maize.
Footnotes
Funding: This work was supported by DongKook Pharmaceutical Co., LTD., Seoul, Korea.
Author Contributions: Conceptualization: In-Chul Rhyu; Formal analysis: Jae-Yoon Kim, Kyoung-Hwa Kim, Yang Jo Seol; Investigation: Jae-Yoon Kim, Kyoung-Hwa Kim, Eun-Hye Kwag; Methodology: Jae-Yoon Kim, Kyoung-Hwa Kim, Yong Moo Lee; Project Administration: In-Chul Rhyu; Validation: Kyoung-Hwa Kim, Eun-Hye Kwag, Tae-Il Kim, Young Ku; Writing - original draft: Jae-Yoon Kim, Kyoung-Hwa Kim, Eun-Hye Kwag; Writing - review & editing: Jae-Yoon Kim, Kyoung-Hwa Kim, Tae-Il Kim, Yang Jo Seol, Yong Moo Lee, Young Ku, In-Chul Rhyu.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(Suppl 4S):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 2.Yağan A, Kesim S, Liman N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2014;85:478–489. doi: 10.1902/jop.2013.130138. [DOI] [PubMed] [Google Scholar]
- 3.Nibali L. Aggressive periodontitis: microbes and host response, who to blame? Virulence. 2015;6:223–228. doi: 10.4161/21505594.2014.986407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paquette DW, Williams RC. Modulation of host inflammatory mediators as a treatment strategy for periodontal diseases. Periodontol 2000. 2000;24:239–252. doi: 10.1034/j.1600-0757.2000.2240112.x. [DOI] [PubMed] [Google Scholar]
- 5.Zawawi KH, Kantarci A, Schulze-Späte U, Fujita T, Batista EL, Jr, Amar S, et al. Moesin-induced signaling in response to lipopolysaccharide in macrophages. J Periodontal Res. 2010;45:589–601. doi: 10.1111/j.1600-0765.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- 7.Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HY, Hua KF, Wu WC, Hsu J, Weng ST, Lin TL, et al. Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T cells. J Cell Physiol. 2008;215:15–26. doi: 10.1002/jcp.21144. [DOI] [PubMed] [Google Scholar]
- 9.Moon B. Molecular authentication of Magnoliae Cortex and its adulterant Machilus Cortex based on psbA-trnH DNA barcode. Korean Herb Med Inform. 2014;2:67–75. [Google Scholar]
- 10.Kim BH, Cho JY. Anti-inflammatory effect of honokiol is mediated by PI3K/Akt pathway suppression. Acta Pharmacol Sin. 2008;29:113–122. doi: 10.1111/j.1745-7254.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 11.Munroe ME, Arbiser JL, Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179:753–763. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 12.Chao LK, Liao PC, Ho CL, Wang EI, Chuang CC, Chiu HW, et al. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-kappaB pathway to reduce LPS-induced TNFalpha and NO expression. J Agric Food Chem. 2010;58:3472–3478. doi: 10.1021/jf904207m. [DOI] [PubMed] [Google Scholar]
- 13.Chen SC, Chang YL, Wang DL, Cheng JJ. Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and gene expression in endothelial cells. Br J Pharmacol. 2006;148:226–232. doi: 10.1038/sj.bjp.0706647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milind P, Isha D. Zea maize: a modern craze. Int Res J Pharm. 2013;4:39–43. [Google Scholar]
- 15.Yang J, Li X, Xue Y, Wang N, Liu W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014;64:276–280. doi: 10.1016/j.ijbiomac.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Hu QL, Zhang LJ, Li YN, Ding YJ, Li FL. Purification and anti-fatigue activity of flavonoids from corn silk. Int J Phys Sci. 2010;5:321–326. [Google Scholar]
- 17.Kim EO, Min KJ, Kwon TK, Um BH, Moreau RA, Choi SW. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem Toxicol. 2012;50:1309–1316. doi: 10.1016/j.fct.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Son S. Influence of standard extract of the unsaponifiable fraction of Zea mays L on periodontal disease. Quintessence Int. 1982;13:895–901. [Google Scholar]
- 19.Kim TI, Choi EJ, Chung CP, Han SB, Ku Y. Antimicorbial effect of Zea Mays L. and Magnoliae cortex extract mixtures on periodontal pathogen and effect on human gingival fibroblast cellular activity. J Korean Acad Periodontol. 2002;32:249–255. [Google Scholar]
- 20.Kim TI, Rhyu IC, Ku Y, Lee YM, Chung CP. The effect of zea Mays L. and Magnoliae Cortex extracts mixture on the rat calvarial defects: in vivo study of bone regenerative activity. J Korean Acad Periodontol. 2002;32:403–414. [Google Scholar]
- 21.Kim TI, Chung CP, Ku Y. The effects of Magnoliae Cortex and Zea Mays L. extract mixtures on experimentally induced periodontitis of beagle dog. J Korean Acad Periodontol. 2002;32:847–855. [Google Scholar]
- 22.Gonçalves PF, Harris TH, Elmariah T, Aukhil I, Wallace MR, Shaddox LM. Genetic polymorphisms and periodontal disease in populations of African descent: A review. J Periodontal Res. 2018;53:164–173. doi: 10.1111/jre.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocgozlu L, Elkaim R, Tenenbaum H, Werner S. Variable cell responses to P. gingivalis lipopolysaccharide. J Dent Res. 2009;88:741–745. doi: 10.1177/0022034509341166. [DOI] [PubMed] [Google Scholar]
- 24.Lang N, Bartold PM, Cullinan M, Jeffcoat M, Mombelli A, Murakami S, et al. Consensus report: aggressive periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 25.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda H, Kageura T, Oda M, Morikawa T, Sakamoto Y, Yoshikawa M. Effects of constituents from the bark of Magnolia obovata on nitric oxide production in lipopolysaccharide-activated macrophages. Chem Pharm Bull (Tokyo) 2001;49:716–720. doi: 10.1248/cpb.49.716. [DOI] [PubMed] [Google Scholar]
- 27.Ulbricht CE. An evidence-based systematic review of beta-sitosterol, sitosterol (22,23-dihydrostigmasterol, 24-ethylcholesterol) by the Natural Standard Research Collaboration. J Diet Suppl. 2016;13:35–92. [PubMed] [Google Scholar]
- 28.Owoyele BV, Negedu MN, Olaniran SO, Onasanwo SA, Oguntoye SO, Sanya JO, et al. Analgesic and anti-inflammatory effects of aqueous extract of Zea mays husk in male Wistar rats. J Med Food. 2010;13:343–347. doi: 10.1089/jmf.2008.0311. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 30.Chen JN, de Mejia EG, Wu JS. Inhibitory effect of a glycoprotein isolated from golden oyster mushroom (Pleurotus citrinopileatus) on the lipopolysaccharide-induced inflammatory reaction in RAW 264.7 macrophage. J Agric Food Chem. 2011;59:7092–7097. doi: 10.1021/jf201335g. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu YS, Lee JH, Seok JH, Hong JH, Lee YS, Lim JH, et al. Acetaminophen inhibits iNOS gene expression in RAW 264.7 macrophages: differential regulation of NF-kappaB by acetaminophen and salicylates. Biochem Biophys Res Commun. 2000;272:758–764. doi: 10.1006/bbrc.2000.2863. [DOI] [PubMed] [Google Scholar]
- 33.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 34.Sironi M, Gadina M, Kankova M, Riganti F, Mantovani A, Zandalasini M, et al. Differential sensitivity of in vivo TNF and IL-6 production to modulation by anti-inflammatory drugs in mice. Int J Immunopharmacol. 1992;14:1045–1050. doi: 10.1016/0192-0561(92)90149-f. [DOI] [PubMed] [Google Scholar]
- 35.Kang BS, Chung EY, Yun YP, Lee MK, Lee YR, Lee KS, et al. Inhibitory effects of anti-inflammatory drugs on interleukin-6 bioactivity. Biol Pharm Bull. 2001;24:701–703. doi: 10.1248/bpb.24.701. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]