Abstract
Purpose
This study investigated the validity of the Charlson comorbidity index (CCI) as a predictor of periodontal disease (PD) over a 12-year period.
Methods
Nationwide representative samples of 149,785 adults aged ≥60 years with PD (International Classification of Disease, 10th revision [ICD-10], K052–K056) were derived from the National Health Insurance Service-Elderly Cohort during 2002–2013. The degree of comorbidity was measured using the CCI (grade 0–6), including 17 diseases weighted on the basis of their association with mortality, and data were analyzed using multivariate Cox proportional-hazards regression in order to investigate the associations of comorbid diseases (CDs) with PD.
Results
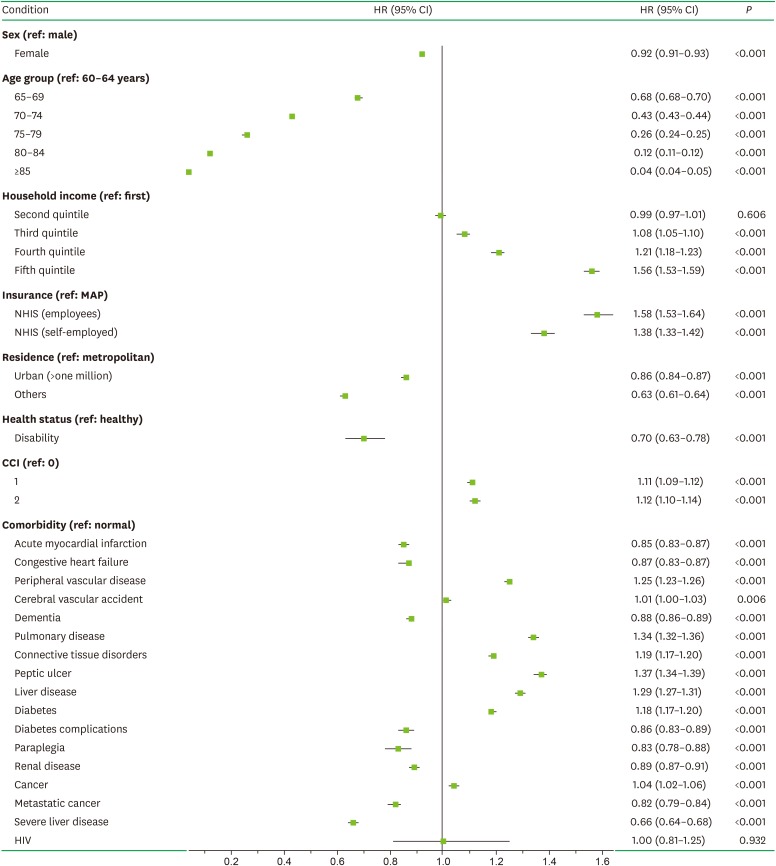
The multivariate Cox regression analysis with adjustment for sociodemographic factors (sex, age, household income, insurance status, residence area, and health status) and CDs (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorders, peptic ulcer, liver disease, diabetes, diabetes complications, paraplegia, renal disease, cancer, metastatic cancer, severe liver disease, and human immunodeficiency virus [HIV]) showed that the CCI in elderly comorbid participants was significantly and positively correlated with the presence of PD (grade 1: hazard ratio [HR], 1.11; P<0.001; grade ≥2: HR, 1.12, P<0.001).
Conclusions
We demonstrated that a higher CCI was a significant predictor of greater risk for PD in the South Korean elderly population.
Keywords: Comorbidity, Periodontal disease, Risk factors
Graphical Abstract
INTRODUCTION
Chronic periodontal disease (PD) is a bacteria-induced chronic inflammatory disease. Although several aspects of this disease remain controversial, it is known to share proinflammatory cytokines and mediators with various lifestyle-related comorbid diseases (CDs), such as cardiovascular disease, pulmonary disease, diabetes mellitus, rheumatoid arthritis, vasculogenic erectile dysfunction, osteoporosis, and cancer [1,2,3,4,5]. These CDs may act as risk factors for PD, and PD can simultaneously be a risk indicator or risk factor for these comorbid conditions [6].
Many recent studies have found associations between PD and mortality from CDs. LaMonte et al. [7] reported that the presence of severe PD in elderly women increased their risk of total mortality by 12% (hazard ratio [HR], 1.12; 95% confidence interval [CI], 1.05–1.21; P=0.002). Sharma et al. [8] further reported that the 10-year all-cause mortality rates of patients with chronic kidney disease were 32% and 41% without and with PD, respectively. Moreover, Saremi et al. [9] reported a 3.2-fold increase (95% CI, 1.1–9.3) in cardiorenal mortality (ischemic heart disease and diabetic nephropathy combined) when patients with PD were followed up for an average of 11 years, indicating that PD may be a major predictor of mortality due to ischemic heart disease and diabetic nephropathy. These findings indicate that PD is very common in elderly patients with CDs and may also be associated with health decline and mortality.
Evaluating systemic diseases in a patient before initiating treatment can be useful for predicting treatment results, such as the re-admission, re-operation, complication, and mortality rates; the validity of these factors has been evaluated in various clinical areas, and new evaluation methods are continuously being developed. Several methods, such as the Charlson comorbidity index (CCI), the Cumulative Illness Rating Scale, the Index of Coexistent Disease, and the Kaplan-Feinstein Scale, have been developed for the comprehensive and systematic assessment of CDs [10]. The CCI is the most widely used tool for estimating the prognosis of patients with CDs [11]. The use of this index with weights that vary (from 1 to 6) according to the severity of different diseases was shown to be effective in predicting the 1-year mortality rate based on the following 19 conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes without end-organ damage, hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, tumor without metastases, leukemia, lymphoma, moderate or severe liver disease, metastatic solid tumor, and acquired immunodeficiency syndrome (AIDS) [11].
Although results from many studies have consistently supported using the CCI as a significant and independent prognostic indicator in various cancers and inflammatory diseases, the prognostic significance of this index has not been validated in PD patients, including elderly PD patients [12,13]. Therefore, the aim of the present study was to determine the validity of the CCI as a predictor of PD in elderly participants using the database of the nationwide population-based National Health Insurance Service-Elderly Cohort (NHIS-EC), which was newly released in 2016.
MATERIALS AND METHODS
Study design and data collection
This study selected 5.1 million elderly people (older than 60 years at the end of December 2002) during 2002 and 2003 as the entire population, from which 10% (n=558,147) were sampled by simple stratification by the National Health Insurance Service (NHIS) Big Data Steering Department. This database covered the following parameters: sociodemographic and socioeconomic information, insurance status, health check-up examinations, and records of patients' medical and dental history. These parameters were stratified to cover 12 years (2002–2013) and anonymized to protect individuals' privacy within the cohort study.
This study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational cohort studies (www.strobe-statement.org) and was approved by the Institutional Review Board of Daejeon Dental Hospital, Wonkwang University (approval No. W1704/001-001).
Definition of sociodemographic factors
The following potential confounding sociodemographic factors of PD were collected from the NHIS-EC database and assessed: sex, age (6 groups: those aged 60–84 years in 5-year intervals, and those aged ≥85 years), household income (5 groups: those with area-based [10 groups] and job-based [10 groups] health insurance were divided into 5 quintiles, with the Medical Aid Program [MAP] included in the first quintile), insurance status (3 groups: classified into the MAP group and NHIS [employees and self-employed] groups), residence area (3 groups: classified into metropolitan [≥10 million], urban [≥1 million], and others), and health status (3 groups: those classified as healthy, and with major and minor disabilities according to the Handicapped Welfare Law). All participants with responses regarding sociodemographic factors with either missing or unknown parameters were excluded from the current analysis.
Assessment of the CCI and PD
Elderly patients diagnosed with PD and CDs corresponding to the CCI prior to 2002 were excluded from the analysis, and the baseline was then established. Among the patients who had been diagnosed with CDs, we assessed the CCI during the follow-up period from 2002 to 2003. The CCI with International Classification of Disease, 10th revision (ICD-10) codes has been verified by Deyo et al. [14] and Sundararajan et al. [15], and we defined CDs using the ICD-10 codes provided by Sundararajan et al. [15]. The CCI assigns a weight of 1–6 points to each CD as follows, with the sum of individual scores serving as a measure of the overall comorbidity of a participant. If the weight was 0, the CDs listed below were not diagnosed (Table 1):
Table 1. ICD-10 codes and weights for the CCI scoring system.
| Condition | Weighting | Codes (ICD-10) |
|---|---|---|
| Acute myocardial infarctiona) | 1 | I21, I22, I252 |
| Congestive heart failure | 1 | I50 |
| Peripheral vascular disease | 1 | I71, I739, I790, R02, Z958, Z959 |
| Cerebral vascular accident | 1 | I60–69, G450–452, G454, G458, G459, G46 |
| Dementia | 1 | F00–F02, F051 |
| Pulmonary disease | 1 | J40–J47, J61–J67 |
| Connective tissue disorders | 1 | M32–M35, M058–M060, M063, M069 |
| Peptic ulcer | 1 | K25–K28 |
| Liver disease | 1 | K702, K703, K717, K73, K740, K742–K746 |
| Diabetes | 1 | E101, E105, E109, E111, E115, E119, E131, E135, E139, E141, E145, E149 |
| Diabetes complications | 2 | E102–104, E112–114, E132–134, E142–144 |
| Paraplegia | 2 | G041, G81–G822 |
| Renal disease | 2 | N01, N03, N052–N056, N072, N18, N19, N25 |
| Cancer | 2 | C0–C96 |
| Metastatic cancer | 3 | C77–C80 |
| Severe liver disease | 3 | K721, K729, K766, K767 |
| HIV | 6 | B20–B24 |
ICD-10: International Classification of Disease, 10th revision, CCI: Charlson comorbidity index, HIV: human immunodeficiency virus.
a)Including coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and angina pectoris.
1 point was assigned for a history of acute myocardial infarction (including coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and angina pectoris; codes I21, I22, and I252, respectively), congestive heart failure (I50), peripheral vascular disease (I71, I739, I790, R02, Z958, and Z959), cerebral vascular accident (I60–I69, G450–G452, G454, G458, G459, and G46), dementia (F00–F02 and F051), pulmonary disease (J40–J47 and J61–J67), connective tissue disorders (M32–M35, M058–M060, M063, and M069), peptic ulcer (K25–K28), liver disease (K702, K703, K717, K73, K740, and K742–K746), and diabetes without end-organ damage (E101, E105, E109, E111, E115, E119, E131, E135, E139, E141, E145, and E149).
2 points were assigned for diabetes complications (E102–104, E112–114, E132–134, and E142–144), paraplegia (G041 and G81–G822), renal disease (N01, N03, N052–N056, N072, N18, N19, and N25), and cancer (C0–C96).
3 points were assigned for metastatic cancer (C77–C80) and severe liver disease (K721, K729, K766, and K767).
6 points were assigned for human immunodeficiency virus [HIV] (B20–B24).
Participants who were diagnosed with PD prior to 2003 were excluded, and we included participants who were newly diagnosed with PD (acute periodontitis [ICD-10, K052], chronic periodontitis [K053], periodontosis [K054], other periodontal disease [K055], and unspecified periodontal disease [K056]) and who received periodontal treatment (including NHIS prescription codes: U1010, subgingival curettage; U1020, excisional new attachment procedure; U1051–1052, simple/complex periodontal flap operation; U1071–1072, bone graft for alveolar bone defects; or U1081–1083, guided tissue regeneration) based on the guidelines of the Centers for Disease Control and American Academy of Periodontology using dental history questionnaires, clinical signs, and an oral examination and radiographic evaluation by a registered general dentist or periodontist who was qualified to perform a dental check-up [16,17]. To increase the validity of the PD diagnoses obtained in this study, we only selected PD participants who had been diagnosed and treated at least twice from 2004 to 2013.
Statistical analysis
We calculated HRs with 95% CIs using multivariate Cox proportional-hazards regression analysis, adjusting for sociodemographic factors (sex, age, household income, insurance status, residence area, and health status) and CDs (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorders, peptic ulcer, liver disease, diabetes, diabetes complications, paraplegia, renal disease, cancer, metastatic cancer, severe liver disease, and HIV). The cumulative incidence of PD in participants with CDs was estimated using a Kaplan-Meier curve, with differences assessed using the log-rank test. All statistical tests were 2-sided, and a P value of <0.05 was considered to indicate statistical significance. Analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC, USA) by the Department of Health Insurance Research, Ilsan Hospital, NHIS.
RESULTS
Results for the CCI and the incidence of PD in participants with CDs
Table 2 presents the data for the incidence of CDs in study subjects with PD. The incidence of CDs in participants with PD was highest for pulmonary disease (n=123,556, 82.5%), followed by peptic ulcer (n=123,191, 82.2%), liver disease (n=105,157, 70.2%), diabetes (n=91,047, 60.8%), peripheral vascular disease (n=80,058, 53.4%), cerebral vascular accident (n=72,245, 48.2%), connective tissue disorders (n=45,401, 30.3%), cancer (n=44,294, 29.6%), congestive heart failure (n=38,669, 25.8%), dementia (n=30,678, 20.5%), acute myocardial infarction (n=15,929, 10.6%), renal disease (n=9,723, 6.5%), severe liver disease (n=9,403, 6.3%), metastatic cancer (n=5,945, 4.0%), diabetes complications (n=5,185, 3.5%), paraplegia (n=1,452, 1.0%), and HIV (n=130, 0.1%).
Table 2. Incidence of comorbid diseases corresponding to the CCI together with periodontal disease in the study subjects.
| Condition | Weighting | Males (n=65,817) No. (%) |
Females (n=83,968) No. (%) |
Pa) |
|---|---|---|---|---|
| Acute myocardial infarction | 1 | 7,768 (11.8) | 8,161 (9.7) | <0.001 |
| Congestive heart failure | 1 | 14,793 (22.5) | 23,876 (28.4) | <0.001 |
| Peripheral vascular disease | 1 | 31,753 (48.2) | 48,305 (57.5) | <0.001 |
| Cerebral vascular accident | 1 | 29,884 (45.4) | 42,361 (50.4) | <0.001 |
| Dementia | 1 | 10,930 (16.6) | 19,748 (23.5) | <0.001 |
| Pulmonary disease | 1 | 53,354 (81.1) | 70,202 (83.6) | <0.001 |
| Connective tissue disorders | 1 | 14,155 (21.5) | 31,246 (37.2) | <0.001 |
| Peptic ulcer | 1 | 52,309 (79.5) | 70,882 (84.4) | <0.001 |
| Liver disease | 1 | 45,961 (69.8) | 59,196 (70.5) | <0.001 |
| Diabetes | 1 | 38,838 (59.0) | 52,209 (62.2) | <0.001 |
| Diabetes complications | 2 | 2,172 (3.3) | 3,013 (3.6) | <0.001 |
| Paraplegia | 2 | 667 (1.0) | 785 (0.9) | <0.001 |
| Renal disease | 2 | 5,292 (8.0) | 4,431 (5.3) | <0.001 |
| Cancer | 2 | 25,718 (39.1) | 18,576 (22.1) | <0.001 |
| Metastatic cancer | 3 | 3,081 (4.7) | 2,864 (3.4) | <0.001 |
| Severe liver disease | 3 | 5,764 (8.8) | 3,639 (4.3) | <0.001 |
| HIV | 6 | 67 (0.1) | 63 (0.1) | <0.001 |
CCI: Charlson comorbidity index, HIV: human immunodeficiency virus.
a)All P values from the χ2 test are significantly statistics (P<0.05).
Associations of the CCI with incident PD in elderly participants
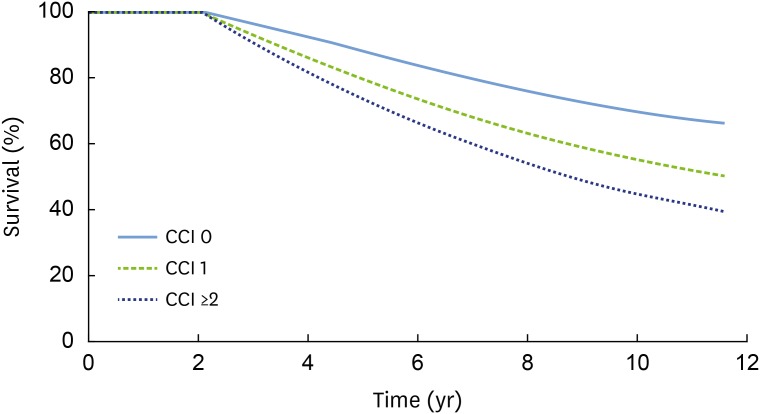
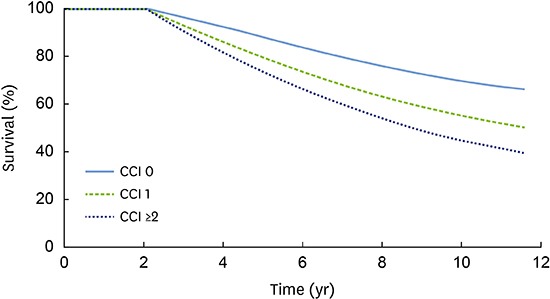
Figure 1 presents the adjusted Kaplan-Meier curve for elderly comorbid participants with PD and a known CCI. Higher CCIs were associated with a higher incidence of PD in elderly comorbid participants (P<0.001). As indicated in Figure 2, the multivariate Cox proportional-hazards regression analysis with adjustment for sociodemographic factors, CCI, and CDs showed that the incidence of PD in elderly comorbid participants had a significant positive relationship with household income, insurance status, the CCI, peripheral vascular disease, cerebral vascular accident, pulmonary disease, connective tissue disorders, peptic ulcer, liver disease, diabetes, and cancer.
Figure 1. The cumulative incidence of PD in patients with CDs was estimated using the Kaplan-Meier method and compared using the log-rank test. A higher CCI was associated with a higher incidence of PD in elderly participants with comorbidities (P<0.001).
PD: periodontal disease, CD: comorbid disease, CCI: Charlson comorbidity index.
Figure 2. Associations of comorbidities with PD in elderly participants in the multivariate regression analysis. The multivariate Cox proportional-hazards regression analysis adjusted for sex, age, household income, insurance status, residence area, health status, acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorders, peptic ulcer, liver disease, diabetes, diabetes complications, paraplegia, renal disease, cancer, metastatic cancer, severe liver disease, and HIV. Data are HRs with 95% CIs.
PD: periodontal disease, HIV: human immunodeficiency virus, HR: hazard ratio, CI: confidence interval, MAP: Medical Aid Program, NHIS: National Health Insurance Service, CCI: Charlson comorbidity index.
DISCUSSION
Targeting elderly individuals aged 60 years or older in South Korea, the present study is the first to quantify the usefulness of the CCI as a tool for evaluating CDs that are predictive factors for PD. CDs refer to chronic diseases that coexist or are present at the time of diagnosis of the disease under consideration. More than 50% of patients with cancer are generally reportedly to have CDs, including hypertension, diabetes mellitus, pulmonary disease, and cardiovascular disease [18]. The present study similarly found that 51.2% of participants with PD had CDs that were included in the CCI.
Female participants showed a slightly lower risk of PD than male participants (HR, 0.92; 95% CI, 0.91–0.93; P<0.001), which is consistent with the finding of a recent systematic review that male patients generally had a similar or a higher prevalence of PD than female patients [19]. Most studies have found that the morbidity rate of PD increased significantly with age [20,21]. However, the present study found that the HR for PD decreased with age, especially for the age range of 60–64 years; this might be attributable to fewer subjects visiting dentists and being diagnosed with PD compared to those visiting a hospital for CDs. In other words, this might have occurred because the proportion of participants who were diagnosed with PD by dentists decreased when they had severe concomitant physical or mental diseases that limited their access to dental services [22,23]. This is supported by the consistent observation of low HRs for PD among those with low income levels who lived in areas with a lower accessibility of dentists and had physical disorders.
The HR values for PD associated with CCIs of 1 and ≥2 were 1.11 (95% CI, 1.09–1.12; P<0.001) and 1.12 (95% CI, 1.10–1.14; P<0.001), respectively, meaning that PD appeared to be associated with an increase in the severity of — and mortality associated with — CDs. In particular, the results of the 12-year Kaplan-Meier analysis showed that the risk of PD increased significantly with the CCI. Analyzing the distribution of the CDs revealed the following distribution of increased risks for PD: 37% for peptic ulcer, 34% for pulmonary disease, 29% for liver disease, 25% for peripheral vascular disease, 19% for connective-tissue disorder, 18% for diabetes, 4% for cancer, and 1% for cerebral vascular accidents. Acute myocardial infarction, congestive heart failure, and dementia were CDs associated with lower risks of PD. Since acute myocardial infarction and congestive heart failure are diseases with high mortality rates, it can be postulated that individuals with those diseases would be unlikely to visit dentists to be diagnosed with PD, resulting in them showing lower risks in the present study [24]. Despite reports that tooth loss and PD worsen cognitive function, and the presence of shared inflammatory mediators with Alzheimer disease, a recent systematic review argued that the foundations for a direct correlation between PD and Alzheimer disease were insufficient and controversial [25].
Among the CDs included in the CCI, the HRs for peptic ulcer disease indicated that chronic gastritis, peptic ulcer, gastric carcinoma, and gastric mucosa-associated lymphoid tissue lymphoma showed the strongest associations with PD. Helicobacter pylori has been suspected to be one of the main causes of peptic ulcer disease [26]. Despite being easily treatable, H. pylori has a high re-infection rate, and the lifetime risk of peptic ulcer disease (once infection occurs) is as high as 10% to 20% [27]. The suspected major pathways of re-infection are dental plaque, poor oral hygiene, and PD [28]. Treating peptic ulcer disease in combination with periodontal treatment was approximately 30% more successful at eradicating gastric H. pylori than conventional triple therapy (antibiotics, antimicrobials, and proton-pump inhibitors) without periodontal treatment [29]. Therefore, elderly participants, who tend to have poor oral hygiene and limited access to periodontal treatment, may be more likely to develop chronic PD; it is postulated that these factors may further increase the risk of the recurrence of peptic ulcer disease. No studies have investigated the effects of peptic ulcer disease on PD, meaning that the underlying mechanisms remain to be investigated.
The CDs with the second highest risk — pulmonary diseases such as pneumonia and chronic obstructive pulmonary disease — have also been found to be risk factors for PD in many studies, including a systematic review [30,31]. As with peptic ulcer disease, potential respiratory pathogens have been found in dental plaque, and they have been considered major sources of respiratory infection [32]. Sumi et al. [32] reported that the respiratory pathogens Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Enterobacter cloacae accounted for most of the pathogens in the dental plaque of elderly individuals. In particular, pneumonia is a life-threatening infection that is a significant risk factor for mortality in the elderly [33]. We found that pulmonary diseases, which are major contributors to morbidity and mortality, increased the risk of PD in the elderly by 34%. Although the study of pulmonary disease as a risk factor for PD is very limited, these observations suggest that pulmonary disease has substantial implications not only for oral health, but also for the entire public health sector.
Many researchers have confirmed that CDs are important predictive factors for health [34,35]. Chronic PD is rapidly becoming a global epidemic disease with an increasing prevalence worldwide, particularly in elderly populations. According to a report of the Health Insurance Review and Assessment Service in 2016, PD has been the most expensive health problem in South Korea (amounting to about US$ 1 billion) and the second-most-frequent disease (about 26.3% [13 million] of the Korean population) among outpatients since 2011. In addition, consistent with the general trend in developed countries, the elderly population is rapidly growing in South Korea, which is predicted to be a super-aged society — defined as more than 20% of the population being aged 65 years or older — by 2020. In the present study, the risk factors for PD increased the incidence of CDs and healthcare costs to different extents, depending on the severity of the CDs, which suggests that more in-depth evaluation and management are required before establishing appropriate policies for dental health care. Thus, the risk of PD may be especially high for CDs associated with high CCI values (which are the most severe types of CDs), and so elderly participants with CDs should be actively informed of their elevated risk for PD and advised to seek appropriate medical and periodontal care.
This study was subject to several limitations. First, the analysis was unable to reflect various clinical outcomes due to the inherent limitations of the retrospective NHIS-EC data. It poorly reflected the detailed characteristics of the risk factors, particularly the severity and duration of PD and CDs. Second, another major weakness of this study is that smoking and remaining teeth were not assessed, although many previous studies have found these to be important confounding factors for PD [36]. Third, selection bias can occur due to the cohort design, and in particular the inclusion of only patients who visited dental and medical clinics and who were diagnosed with PD and CDs. Fourth, owing to the study design (which established the baseline as the period between 2002 and 2003), participants' sociodemographic and socioeconomic position could have changed during the follow-up period, which extended through 2013. We accept that this study failed to consider this point, making it a limitation of this study. Finally, the CCI used in this study was developed in 1986; it is currently recognized for its usefulness and validity, but new weights should continuously be developed as medical technology evolves. Quantifying the usefulness of the CCI as a predictor of PD will require the development of novel evaluation tools for CDs based on the most recent medical knowledge.
This 12-year longitudinal cohort study provides evidence that a high CCI was significantly associated with a higher risk of PD in the South Korean elderly population. Although the significant associations reported here must be confirmed by further studies, these observations have potentially important implications not only for oral health, but also for the entire public health sector.
ACKNOWLEDGEMENTS
This study used national sample cohort data of the National Health Insurance Service (NHIS-2017-2-401).
Footnotes
Funding: This research was supported by Wonkwang University in 2018.
Author Contributions: Conceptualization: Jae-Hong Lee, Seong-Ho Choi; Data curation: Jae-Hong Lee, Jung-Kyu Choi, Seong-Nyum Jeong, Seong-Ho Choi; Formal analysis: Jae-Hong Lee, Jung-Kyu Choi, Seong-Nyum Jeong; Funding acquisition: Jae-Hong Lee; Investigation: Jae-Hong Lee, Jung-Kyu Choi, Seong-Nyum Jeong, Seong-Ho Choi; Methodology: Jae-Hong Lee, Jung-Kyu Choi, Seong-Nyum Jeong, Seong-Ho Choi; Project administration: Jae-Hong Lee, Seong-Nyum Jeong, Seong-Ho Choi; Resources: Jae-Hong Lee, Jung-Kyu Choi, Seong-Ho Choi; Software: Jae-Hong Lee, Jung-Kyu Choi, Seong-Nyum Jeong; Supervision: Seong-Ho Choi; Validation: Jae-Hong Lee, Seong-Ho Choi; Writing - original draft: Jae-Hong Lee, Jung-Kyu Choi; Writing - review & editing: Seong-Nyum Jeong, Seong-Ho Choi.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Lee JH, Lee JS, Park JY, Choi JK, Kim DW, Kim YT, et al. Association of lifestyle-related comorbidities with periodontitis: a nationwide cohort study in Korea. Medicine (Baltimore) 2015;94:e1567. doi: 10.1097/MD.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Choi JK, Kim SH, Cho KH, Kim YT, Choi SH, et al. Association between periodontal flap surgery for periodontitis and vasculogenic erectile dysfunction in Koreans. J Periodontal Implant Sci. 2017;47:96–105. doi: 10.5051/jpis.2017.47.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Oh JY, Youk TM, Jeong SN, Kim YT, Choi SH. Association between periodontal disease and non-communicable diseases: a 12-year longitudinal health-examinee cohort study in South Korea. Medicine (Baltimore) 2017;96:e7398. doi: 10.1097/MD.0000000000007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JK, Kim YT, Kweon HI, Park EC, Choi SH, Lee JH. Effect of periodontitis on the development of osteoporosis: results from a nationwide population-based cohort study (2003–2013) BMC Womens Health. 2017;17:77. doi: 10.1186/s12905-017-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Kweon HH, Choi JK, Kim YT, Choi SH. Association between periodontal disease and prostate cancer: results of a 12-year longitudinal cohort study in South Korea. J Cancer. 2017;8:2959–2965. doi: 10.7150/jca.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour GJ. Relationship between periodontal infections and systemic disease - the oral systemic connection. Int J Antimicrob Agents. 2007;29:S57–S58. [Google Scholar]
- 7.LaMonte MJ, Genco RJ, Hovey KM, Wallace RB, Freudenheim JL, Michaud DS, et al. History of periodontitis diagnosis and edentulism as predictors of cardiovascular disease, stroke, and mortality in postmenopausal women. J Am Heart Assoc. 2017;6:e004518. doi: 10.1161/JAHA.116.004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Dietrich T, Ferro CJ, Cockwell P, Chapple IL. Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43:104–113. doi: 10.1111/jcpe.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Melfi C, Holleman E, Arthur D, Katz B. Selecting a patient characteristics index for the prediction of medical outcomes using administrative claims data. J Clin Epidemiol. 1995;48:917–926. doi: 10.1016/0895-4356(94)00202-2. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Jönsen A, Clarke AE, Joseph L, Belisle P, Bernatsky S, Nived O, et al. Association of the Charlson comorbidity index with mortality in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63:1233–1237. doi: 10.1002/acr.20506. [DOI] [PubMed] [Google Scholar]
- 13.Bar B, Hemphill JC., 3rd Charlson comorbidity index adjustment in intracerebral hemorrhage. Stroke. 2011;42:2944–2946. doi: 10.1161/STROKEAHA.111.617639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 17.1999 International International Workshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook, Illinois, October 30–November 2, 1999. Ann Periodontol. 1999;4:1–112. doi: 10.1902/annals.1999.4.1.i. [DOI] [PubMed] [Google Scholar]
- 18.Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44:745–753. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: a systematic review. J Periodontol. 2010;81:1379–1389. doi: 10.1902/jop.2010.100044. [DOI] [PubMed] [Google Scholar]
- 20.López R, Smith PC, Göstemeyer G, Schwendicke F. Ageing, dental caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S145–S152. doi: 10.1111/jcpe.12683. [DOI] [PubMed] [Google Scholar]
- 21.Tonetti MS, Bottenberg P, Conrads G, Eickholz P, Heasman P, Huysmans MC, et al. Dental caries and periodontal diseases in the ageing population: call to action to protect and enhance oral health and well-being as an essential component of healthy ageing - consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S135–S144. doi: 10.1111/jcpe.12681. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lee JS, Choi JK, Kweon HI, Kim YT, Choi SH. National dental policies and socio-demographic factors affecting changes in the incidence of periodontal treatments in Korean: A nationwide population-based retrospective cohort study from 2002-2013. BMC Oral Health. 2016;16:118. doi: 10.1186/s12903-016-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Oh JY, Choi JK, Kim YT, Park YS, Jeong SN, et al. Trends in the incidence of tooth extraction due to periodontal disease: results of a 12-year longitudinal cohort study in South Korea. J Periodontal Implant Sci. 2017;47:264–272. doi: 10.5051/jpis.2017.47.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freisinger E, Malyar NM, Reinecke H. Peripheral artery disease is associated with high in-hospital mortality particularly in males with acute myocardial infarction in a nationwide real-world setting. Vasa. 2016;45:169–174. doi: 10.1024/0301-1526/a000512. [DOI] [PubMed] [Google Scholar]
- 25.Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713–718. doi: 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YH, Lv ZF, Zhong Y, Liu DS, Chen SP, Xie Y. The internalization of Helicobacter pylori plays a role in the failure of H. pylori eradication. Helicobacter. 2017;22:e12324. doi: 10.1111/hel.12324. [DOI] [PubMed] [Google Scholar]
- 27.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 28.Anand PS, Nandakumar K, Shenoy KT. Are dental plaque, poor oral hygiene, and periodontal disease associated with Helicobacter pylori infection? J Periodontol. 2006;77:692–698. doi: 10.1902/jop.2006.050163. [DOI] [PubMed] [Google Scholar]
- 29.Zarić S, Bojić B, Janković L, Dapčević B, Popović B, Čakić C, et al. Periodontal therapy improves gastric Helicobacter pylori eradication. J Dent Res. 2009;88:946–950. doi: 10.1177/0022034509344559. [DOI] [PubMed] [Google Scholar]
- 30.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- 31.Manger D, Walshaw M, Fitzgerald R, Doughty J, Wanyonyi KL, White S, et al. Evidence summary: the relationship between oral health and pulmonary disease. Br Dent J. 2017;222:527–533. doi: 10.1038/sj.bdj.2017.315. [DOI] [PubMed] [Google Scholar]
- 32.Sumi Y, Miura H, Michiwaki Y, Nagaosa S, Nagaya M. Colonization of dental plaque by respiratory pathogens in dependent elderly. Arch Gerontol Geriatr. 2007;44:119–124. doi: 10.1016/j.archger.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Larsson J, Itenov TS, Bestle MH. Risk prediction models for mortality in patients with ventilator-associated pneumonia: A systematic review and meta-analysis. J Crit Care. 2017;37:112–118. doi: 10.1016/j.jcrc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 35.Laor A, Tal S, Guller V, Zbar AP, Mavor E. The Charlson comorbidity index (CCI) as a mortality predictor after surgery in elderly patients. Am Surg. 2016;82:22–27. [PubMed] [Google Scholar]
- 36.Gil-Montoya JA, de Mello AL, Barrios R, Gonzalez-Moles MA, Bravo M. Oral health in the elderly patient and its impact on general well-being: a nonsystematic review. Clin Interv Aging. 2015;10:461–467. doi: 10.2147/CIA.S54630. [DOI] [PMC free article] [PubMed] [Google Scholar]