Introduction/Abstract
The liver plays critical roles in both homeostasis and pathology. It is the major site of drug metabolism in the body and, as such, a common target for drug-induced toxicity and is susceptible to a wide range of diseases. In contrast to other solid organs, the liver possesses the unique ability to regenerate. The physiological importance and plasticity of this organ make it a crucial system of study to better understand human physiology, disease, and response to exogenous compounds.
The purpose of this review is to inform the reader of the significance and available methods for replicating human liver physiology and pathology ex vivo. First, the physiologic roles of the liver and its cellular constituents will be discussed. Second, we will discuss the need for developing an ex vivo liver system. Third, the advantages and disadvantages of different cell sources used to populate the system will be mentioned. Fourth, the benefits of currently employed ex vivo liver culture systems (both commercially available and used in research laboratories) will be discussed. Finally, future directions to advance these systems, including complexing liver culture systems with other organ-based culture systems (“organ chips”), will be proposed.
Keywords: Microphysiologic systems, organoids, 3D culture systems
Physiology
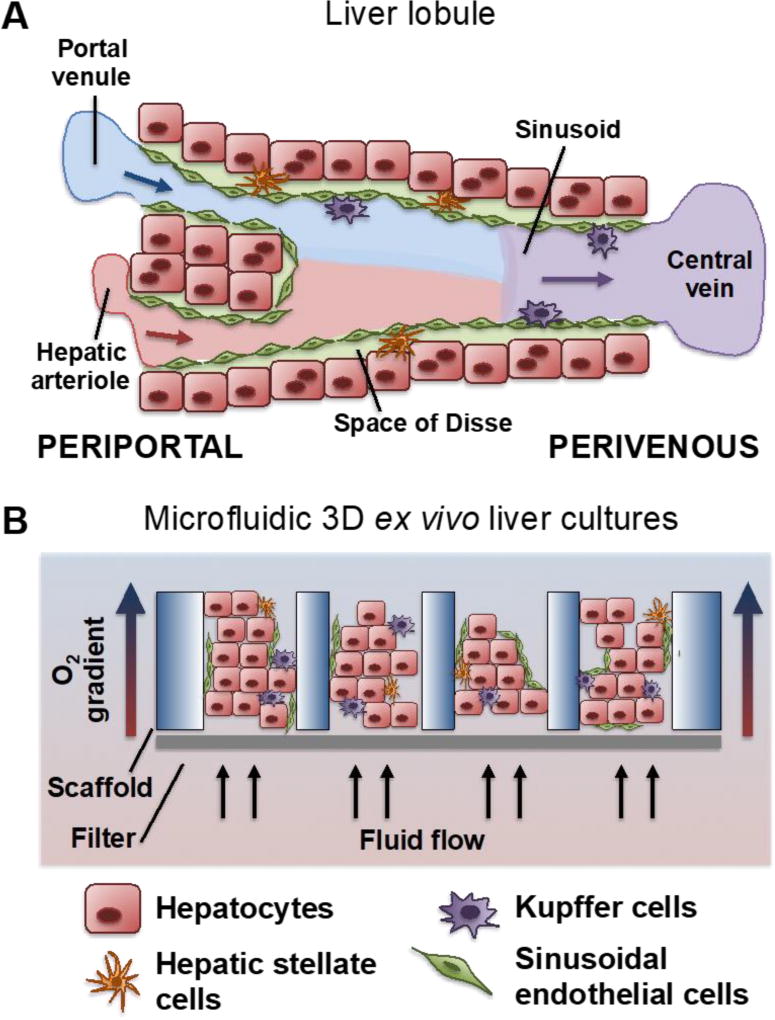
The liver is a complex organ that is integral to a multitude of whole body functions and is extensively reviewed in standard medical textbooks. For the purposes of this review, a few salient aspects are emphasized. The liver is organized into lobules, functional units of the liver, which are subsequently organized into larger lobes. Lobules consist of multiple hepatic sinusoids, whose structure is illustrated in Figure 1A. The flow of blood from the portal triad to the central vein contributes to a zonation based on decreasing oxygen tension. This affects both the parenchymal (hepatocytes) and non-parenchymal cells (NPC) that will be discussed herein.
Figure 1.
(A) The architecture of the hepatic sinusoid. Blood enters the portal triad region of the liver through a hepatic arteriole and portal venule and traverses the hepatic sinusoid to the central vein, whereby it is drained into the larger hepatic central veins. The sinusoidal endothelial cells mediate blood flow and are fenestrated to allow rapid diffusion of nutrients, signaling factors, and drug compounds. The hepatocytes compose the parenchyma of the liver and sit deep to the endothelium. The hepatic stellate cells reside in the Space of Disse, a zone between the endothelium and hepatocytes. Finally, the Kupffer cells line the inside of the sinusoid and mediate antigen sensing and intercellular communication. (B) A schematic of the three dimensional liver tissues as presented within the LiverChip, that are subject to fluid flow in micro-scale pores of a scaffold, which facilitates shear stresses and establishment of a physiological oxygen gradient.
The parenchymal cells, mainly hepatocytes, comprise 60% of the cell population and are responsible for the liver’s metabolic activity (including the cytochrome P450 (CYP450) isoforms), the production and release of acute phase proteins, production of plasma proteins (such as albumin and clotting factors), and the integration of various components of glucose, lipid, nitrogen, and oxidative metabolism1,2. These hepatocytes mature postnatally, causing different CYP450 isoforms and assemblies thereof to be expressed in fetal, neonatal and adult hepatocytes3. For example, CYP3A7 is very highly expressed in fetal hepatocytes, whereas this isoform is replaced by CYP3A4 expression in adult hepatocytes. Moreover, pediatric hepatocytes typically exhibit increased clearance of CYP450-metabolized drugs as compared to adult hepatocytes4, despite no differences in intrinsic CYP450 expression between the two cell sources5. Thus, hepatocyte functioning is key to any ex vivo system that replicates the in vivo liver.
Oxygen tension plays an important role in regulating liver zonation and functionality, with the liver uniquely receiving both arterial (hepatic artery, ~25%) and venous (portal circulation, ~75%) blood. The partial pressure of oxygen drops as one progresses across the liver sinusoid, the functional unit of the liver, from periportal to perivenous hepatocytes6. This oxygen differential regulates the response of the liver to metabolic and toxic stimuli by facilitating differential metabolism, termed “liver zonation.” For example, the relatively hypoxic perivenous hepatocytes are responsible for the majority of substrate metabolism through the CYP450 system whereas the relatively oxygen-rich periportal hepatocytes boast mainly oxidative metabolic functions6. Liver zonation is also observed in in vitro cultures7,8. Thus, the organization of the parenchyma motivates careful engineering to replicate hepatic function and toxicity and capture the full panoply seen in vivo.
Blood flow carried through vessels lined by the fenestrated endothelium of the sinusoids is imperative for liver functions. Hepatic blood flow dictates the metabolic zonation of the liver6 and alterations in flow can reduce liver function and drug clearance9. Blood flow carries oxygen, nutrients, and chemicals that are distributed to the liver tissue. In the absence of adequate blood flow, these factors are consumed by the proximate cells and do not reach more distant populations. Reciprocally, juxtavascular cells can contribute factors to the circulation. Locally, the potency of hepatocyte-secreted factors is a function of the blood-to-cell volume ratio, which is approximately 20.5 mL blood per mL tissue for healthy adult livers10. Assuming a hepatocyte volume of 3.4 pL11, this corresponds to roughly 14 million hepatocytes being supplied by 1 mL of blood. This consideration is particularly important for in vitro and ex vivo culture systems, as media flow is used by some to model in vivo blood flow (see “Engineered Culture Systems”).
The non-parenchymal cells (NPC) compose the remaining 40% of the cell population and play a significant role in tissue architecture and in mediating responses of the tissue to metabolic and toxic stimuli, as well as supporting the hepatocyte function2,12. These cell types include liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), hepatic stellate cells (HSCs), and pit cells (natural killer cells, NKs). Inclusion of NPCs in hepatocyte culture systems has shown beneficial effects. For example, 3-dimensional (3D) liver tissue models show increased hepatocyte functions when nonparenchymal cells are incorporated13. Additionally, KCs play a significant role in the response of the liver to injury through the production of cytokines and reactive oxygen species14. Moreover, HSCs respond to injury both by adopting a myofibroblast phenotype that remodels the liver extracellular matrix15 and increasing the CYP450 activity of hepatocytes16. Finally, LSEC proliferation in response to injury has been suggested to aid the liver’s potent regenerative capacity17. Thus, the NPCs complement the synthetic and metabolic functions of hepatocytes by contributing pro-regenerative, pro-inflammatory, and pro-fibrotic stimuli.
Modeling the Liver Microenvironment
Current preclinical models for hepatotoxicity involve human cell culture and animal models. Recently, efforts to develop ex vivo hepatic culture systems, “liver-on-a-chip,” have been undertaken by many research groups and biotech companies due to the liver’s capacity for drug metabolism, excretion, vulnerability to drug-induced damage, and as a primary organ in many diseases. Drug-induced liver injury remains a major reason for drugs being withdrawn from the market, and causes both morbidity and mortality for patients. Importantly, humans metabolize and respond to agents differently from other mammals; to the point, most all species present unique xenobiotic handling18. In fact, one-third of toxicities observed in humans are not predicted in any of the species commonly employed for drug safety testing19, possibly due to their failure to model reactive metabolites generated through human-specific metabolic pathways20. Moreover, individual animal models have a success rate of as low as 40% in predicting hepatotoxic compounds21, resulting in 26% of clinical trial failures being due to hepatotoxicity22.
Current liver tissue culture systems exist on a spectrum of complexity. Historic hepatocyte culture systems involved collagen-sandwich culture or 2D Micro-Patterned Co-culture (MPCC) systems using primary rat hepatocytes and 3T3-J2 fibroblasts. Systems have progressed to include 3D static spheroid models and perfusion culture devices, which introduce nutrient and oxygen gradients and shear stress that are important for hepatocyte functions23. The systems discussed below offer distinct advantages and disadvantages for investigating the response of hepatic micro-tissues to different drugs and other stimuli.
Cell Sourcing
The complex physiology of the liver and need for its accurate representation in engineered systems requires careful selection of cell type(s) and their origin. As previously discussed, hepatic tissue is composed of hepatocytes (60% of liver cells) and a complex complement of NPCs (40% of liver cells). Integration of both cell fractions is often needed to adequately reflect pharmacokinetics, pharmacodynamics, toxicity of drugs, and liver disease progression, given the intercommunication between the different liver cell types. Four sources of hepatocytes will be discussed: primary human cells, primary animal cells, immortalized human cell lines, and pluripotent stem cells. Each of these cell sources has its advantages and disadvantages, and each will be discussed below. A qualitative summary of cell selection parameters can be seen in Table 1.
Table 1.
A summary of properties from different hepatocyte sources: animal, human cell line, primary human, and iPSC-derived.
| Hepatocyte Source |
Animal | HepG2 | HepaRG | Primary Human |
Cryopreserved Human |
iPSC- derived |
|---|---|---|---|---|---|---|
| Viability | Medium to High* | High | High | Medium to High* | Medium | High |
| Attachment to ECM | High | High | High | High | Medium | High |
| CYP450 Activity | Medium | Low | Medium | High | High | Low to Medium |
| Longevity in Culture | Medium | High | High | Medium | Low to Medium | Low |
| 1OATP/NTCP | High | Low | Medium | High | Medium | Low to Medium |
| Cost | Medium | Low | Low | High | High | High |
| Availability | Medium | High | High | Low | Medium | Medium |
| References | 45–47, 49 | 52, 53, 55 | 53, 55, 56, 58–60 | 23–25 | 35, 36, 39, 41 | 69–71 |
Dependent on efficiency and time of isolation procedure.
OATP (organic anion transporting polypeptide), NTCP (sodium taurocholate co-transporting polypeptide).
Primary human hepatocytes
Functional primary human hepatocytes obtained through collagenase perfusion and dissociation have been the gold standard in drug discovery for 25 years due to their ability to most accurately reflect in vivo metabolism and toxicity24–26. Primary human hepatocytes have been isolated from livers with benign (processes not affecting the hepatocytes) and hepatocyte-specific pathologies. Some examples include end stage primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), alcoholic liver disease (ALD), and resections for colorectal metastases or benign growths27. Several groups have found patient-specific factors to play a role in the yield of hepatocytes during isolation or in their functionality. While patient gender and type of disease had no effect on hepatocyte yield or functionality28,29, cholestasis (signified by elevated gamma glutamyl transferase level – GGT)30,31, severe steatosis31,32, and older age29,31,33,34 negatively influenced isolation outcomes. Although some patients have been exposed to chemotherapy, hepatocyte function and viability are not altered35. While an ideal source, limited availability of cells and reliance on patient surgeries are major reasons why primary hepatocytes are not more commonly used. Moreover, many donors are pediatric patients, whose hepatocytes may have metabolic dysfunction or exhibit differential clearance when compared to adult human hepatocytes (see “Physiology”).
Advances in cryopreservation have allowed for easier distribution and commercialization of primary human hepatocytes. Previous work has demonstrated cryopreserved primary human hepatocytes to still exhibit 94% of the clearance of model drugs (e.g. diclofenac) after 1 year in liquid nitrogen, as compared to freshly isolated human hepatocytes36. With proper cryopreservation, viabilities >90% can be achieved37. Additionally, minimal changes in metabolic functions have been demonstrated even after 14 years in liquid nitrogen38. While cryopreserved primary human hepatocytes exhibit high viability and comparable CYP450 activity to fresh cells, they exhibit suboptimal attachment to extracellular matrix molecules, due to downregulation and degradation of the adhesion molecules β1-integrin and E-cadherin respectively during cryopreservation39. Additionally, decreased expression of uptake transporters such as OATP (organic anion-transporting polypeptide) and NTCP (sodium-taurocholate co-transporting polypeptide) family members results in under-prediction of drug clearance rates40. Finally, high viability methods often involve centrifugation to remove dead and damaged hepatocytes, which also reduces the cell yield.
Cryopreserved primary human hepatocytes have been used in numerous studies to investigate CYP450 induction41, drug clearance42, and hepatobiliary transport43. In contrast to human hepatic cell lines or animal primary cells, cryopreserved primary human hepatocytes can probe the influence of genetic background on hepatocyte metabolism or drug clearance. For example, a collection of cryopreserved hepatocytes from 64 donors has been used to investigate gender-specific activities of CYP450 isoforms in vitro, which corroborated with in vivo findings, namely a higher expression of CYP3A4, the most dominant human isoform, in females44. Thus, while human cell lines and animal hepatocytes are more accessible, primary human hepatocytes will likely continue to play an important role in drug development due to their ability to most effectively reflect in vivo metabolism, clearance, and response to toxins by patients.
Animal-derived hepatocytes
Animal-based testing has formed the foundation for translating in vitro studies to clinical trials. Before moving to humans, the Federal Drug Administration (FDA) requires pharmacokinetic, toxicity, and efficacy studies in at least one rodent (mouse, rat) and one non-rodent (dog, rabbit) species. Such studies are designed to examine integrated organ responses and are able to suggest, at least initially, the influence of genetic diversity on drug responses45. Additionally, these species are readily commercially available, thus serving as sources of fresh primary cells. However, animal studies frequently do not elicit the pharmacokinetic behavior seen in humans due to species differences in drug metabolism and clearance, in particular differential CYP450 metabolic activity46,47. Differences in CYP450 induction by model drugs between human and animal (rat, dog) are well established48. Improvements in predicting human-specific metabolism and clearance from animal studies have been made through retrospective analyses49. Yet, sourcing cells from animals may be cost prohibitive, ethically controversial, and futile if interspecies differences prevent human-relevant data from being collected50. Moreover, since animal hepatocytes express different CYP450 isoforms than humans, extrapolating of gender-specific differences in drug metabolism is challenging51.
Human hepatic cell lines
Human hepatic cell lines, either cancer-derived or immortalized hepatocytes, can be effectively propagated in culture over multiple passages. Some of the most commonly used hepatic cell lines include HepG2, Huh7, Hep3B, and SK-Hep-1, all derived from hepatocellular carcinoma (HCC), and HepaRG, an HCC cell line that constitutes a mix of both hepatocytes and biliary-like cells52,53.
One issue with using human hepatic cell lines is that they exhibit lower and variable CYP450 expression than primary human hepatocytes53. For example, CYP3A4 is not expressed in HepG2 or Hep3B cells whereas CYP2D6 is expressed at less than 5% the level of primary human hepatocytes. Studies in HepG2 cells demonstrated that CYP450 expression may also vary according to culture duration and passage number, some isoforms varying up to 200-fold across the first 10 passages54. Some cell lines hold more promising results. For example, induction of CYP450 enzymes and the drug transporter MDR1 was seen in a novel hepatic cell line, Fa2N-4, in response to rifampin treatment55.
Another issue with human hepatic cell lines is that they exhibit reduced expression of both sinusoidal and canalicular transporters56. These transporters function to shuttle drug compounds from the blood to the bile canaliculi. While hydrophobic compounds can diffuse across the hepatocyte plasma membrane, hydrophilic compounds require active transport. Reduced transport activity and decreased CYP450-catalyzed metabolism may result in inaccurate pharmacokinetic and toxicity predictions.
The HepaRG cell line is a promising substitute for primary human hepatocytes. When seeded at a low density, HepaRG cells are capable of proliferating and differentiating to confluency to form colonies of hepatocytes surrounded by biliary epithelial cells that exhibit CYP450 expression levels comparable to primary human hepatocytes57. HepaRG cells have also been found to functionally express both sinusoidal and canalicular transporters58,59. Additionally, HepaRG cells can identify drugs likely to induce liver injury, albeit using doses up to 100-fold higher than would be seen clinically60. Moreover, HepaRG cells exhibit a more robust response to inflammatory stimuli (e.g. IL-6, TNFα) on liver drug metabolism and elimination than primary human hepatocytes, potentially due to genetic variation in the latter61.
While hepatocyte cell lines are effective in predicting the metabolism and elimination of some drugs, their overall sensitivity to detect toxic compounds is lower than primary human hepatocytes (13% for HepaRG compared to 44% for PHHs)52. Further, being derived from a single female donor, these cells do not account for genetic variation in drug responses that often uncover limiting toxicities in a subset of persons. This limitation is also seen with primary hepatocytes, as testing is often performed on a limited number of donors. Importantly, since human hepatic cell lines are derived from primary hepatic tumors, they may not accurately represent normal cellular responses. These weakness ultimately require the use of complementary approaches (i.e. normal primary cells)23.
Induced and Embryonic Pluripotent Stem Cells (iPSC and ESC)
Stem cells are defined as cells capable of self-renewal and differentiation into mature cells of a particular tissue type62. Stem cells encompass both those derived from blastocysts (ESC) and also those derived from mature cells through reprogramming (iPSC) using viral vectors, Cre-lox expression cassettes, or mRNA/miRNA transfection63. While iPSC generation and differentiation is time consuming, it allows for an easily sharable cell population with theoretically limitless growth potential that can mimic preliminary clinical trials in vitro, hopefully improving the success in subsequent human studies.
Many protocols demonstrate differentiation of iPSC64,65 or ESC66–68 into hepatocyte-like cells. Protocols that try to replicate liver embryogenesis can achieve hepatocyte-like cell yields of up to 80%68. Additionally, the expression of microRNAs (mir), specifically mir-122, during the differentiation process can further improve hepatocyte fidelity69. However, many differentiation protocols result in hepatocytes that express immature markers (e.g. alpha-feto protein; AFP), secrete less albumin, and exhibit dramatically reduced CYP450 activity than primary human hepatocytes70–72. Indeed, hepatocyte-like cells differentiated from iPSC using multiple sources and two different protocols demonstrated a phenotype akin to fetal hepatocytes73.
An advantage of iPSCs is the potential to study a broad spectrum of hepatocyte lines with different genetic, epigenetic backgrounds71,73 and disease backgrounds. Additionally, iPSCs exhibit sensitivity comparable to primary human hepatocytes for detecting drugs that cause drug induced liver injury (65% vs 70%)74. While a promising technology, current iPSC technical limitations include epigenetic memory, genomic instability, state of maturation to adult cells and variability among cell lines. Strategies to address these weaknesses include using small molecule treatment, avoiding targeting p53 during reprograming, and use of multiple iPSCs from different genetic backgrounds, respectively75. Namely, platforms aimed to analyze the influence of small molecules on hepatocyte expansion and differentiation of iPSCs to mature hepatocytes have shown promising results76. However, a steady supply of hepatocyte-like cells derived from iPSC is often not available to many researchers, which limits consistent use to labs with experience in their differentiation. Encouragingly, some companies are starting to commercialize iPSC-derived hepatocytes.
Non-parenchymal Cells (NPCs)
The NPC component of the liver is critical to recreating physiologic liver functioning and response to injurious stimuli. As such, inclusion of NPCs is desirable to replicate metabolism and signaling pathways observed in vivo. Addition of NPCs in hepatic culture systems has shown to alter signaling networks present in the microenvironment, enhance hepatocyte synthetic functions, prevent hepatocyte de-differentiation in culture, and enhance hepatocyte metabolic response to drug treatment1,77,78. Both primary and immortalized cell types are available and will be discussed below.
Primary NPCs are isolated in a similar manner to primary hepatocytes, by liver enzymatic perfusion. These cells can either be harvested alone or simultaneously with primary hepatocytes. When harvested alone, the NPCs of the liver can be enriched, at the expense of hepatocyte recovery, by using a pronase-collagenase perfusion method79,80. For isolating both cell components, collagenase perfusion followed by density centrifugation are used to collect and separate the hepatocytes from the NPCs, respectively. Several methods have been used to purify the NPCs, which include iodixanol or percol density gradients and magnetic activated cell sorting81–83. Importantly, the disease state of the human donor tissue can greatly influence the quality and quantity of cells obtained84,85. To avoid the need for donor tissue, others have demonstrated generation of NPCs from human iPSCs86.
While primary NPCs are the most effective at reproducing the microenvironment seen physiologically, their main disadvantages are non-specific activation and availability. Using primary NPCs requires having surgical specimens. Cryopreserved NPCs can be purchased commercially either as a collective or for specific NPC types. Due to the high cost of primary cells, various cell lines or alternative cell types have been employed in co-cultures with hepatocytes to provide similar trophic signals as primary NPCs. The next several sections will illustrate approaches to incorporate each NPC type into hepatic culture systems.
Endothelial Cells
Liver sinusoidal endothelial cells (LSECs) are a unique endothelial cell type displaying abundant fenestrations, high endocytotic ability, and ability to provide ideal trophic support to hepatocytes. These cells are often unstable in vitro87–90 and are difficult to cryopreserve91. As such, some groups have instead used human umbilical vein endothelial cells (HUVECs) that, in co-culture with hepatocytes, improve hepatocyte specific function, such as CYP450 activity and albumin synthesis92–94. However, as HUVECs are a unique cell type that lack appreciable levels of key cell surface receptors CXCR395 and EGFR96, other primary human endothelial cells have been employed with variable results94,97.
Immortalized endothelial cell lines have also been used in hepatocyte co-culture. HMEC-1 (human foreskin endothelium) cells have been shown to enhance hepatocyte albumin secretion98 and can provide inflammatory cues that affect both the hepatocytes as well as metastatic cancer cells99. TMNK-1 cells are an immortalized LSEC cell line that has been used in hepatocyte co-culture to investigate paracrine signaling100, influence on cancer cell phenotype99, and promote hepatocyte-specific functions in ESC-derived hepatocytes101. However, immortalized LSECs phenotypically resemble activated endothelial cells implicated in pathologic vessel formation in chronic liver disease102, and may not emulate normal physiology, in particular due to their lack of fenestrations.
Kupffer Cells
KCs are implicated in host defense and are important producers of cytokines in inflammatory responses and liver diseases. Immortalized KCs have been generated in mouse and rat103, but not human. THP-1 monocytes have been differentiated into macrophages and used in co-culture applications with both primary hepatocytes and human hepatic cell lines. Inflammatory responses were noticed in hepatocytes as a result of macrophage-secreted products104 and direct co-culture105. To demonstrate the importance of KC inclusion in hepatocyte cultures, LPS stimulation of a primary hepatocyte and KC perfusion co-culture system demonstrated no effect on hydrocortisone metabolism, similar to that seen clinically106. However, immortalized KCs or activated macrophage cell lines do not replicate physiologic KC behavior. KCs are typically quiescent and tolerogenic due to high basal expression of TGFβ and PD1 and expression of IL-10 upon LPS stimulation107. As such, alternatives to primary KCs may promote more inflammatory-related changes than seen in vivo. Of benefit to labs without access to fresh primary cells, cryopreserved KC are commercially available for use.
Stellate Cells
Hepatic stellate cells (HSCs) are important cells for the storage of retinoids and other lipids and are implicated in hepatocyte proliferation after partial hepatectomy, hepatic fibrosis, and portal hypertension. Freshly isolated primary HSCs quickly activate when cultured on plastic108 and their gene expression in vitro does not fully reproduce that observed in vivo109. HSC quiescence can be preserved by culturing on laminin-rich gels or in suspension on a non-adherent surface110. Several immortalized HSC lines exist for study, including hTERT-HSC, GREF-X, LI90, TWNT-1 and more recently, LX-1 and LX-2. However, only the LX-2 cell line can be maintained in serum-free media and transfected with relatively high efficiency (30%)111. Importantly, while immortalized cell lines express many of the same markers as primary HSCs, their response to stimuli and basal level of activation differ from that observed in vivo112. Co-culture of HSCs with hepatocytes has been used to maintain hepatocyte differentiation in vitro by cell contact mediated signals and transfer of lipids113.
NPC Considerations
It is clear that including NPCs in culture systems influences hepatocyte functions and the tissue microenvironment. ECs can enhance albumin synthesis and CYP450 activity in co-culture with hepatocytes. KCs can elicit inflammatory changes in response to stimuli that resemble those seen in vivo. Finally, HSCs are important for storing lipids that can be utilized by the hepatocytes and preserving hepatocyte differentiation. While primary non-parenchymal cells are the gold standard, they are difficult to obtain in sufficient numbers. NPC yield is low when isolated concurrently with hepatocytes and subsequent purification steps can alter NPC function. Several immortalized cell lines can serve comparable (though not equivalent) roles for each NPC type, and also offer the benefits of being easily distributable and homogeneous.
Engineered Culture Systems
The overarching goal of all liver culture systems is to provide either a high throughput, low cost, easy to operate, and reliable system that recapitulates a reasonable fraction of human physiology, or a medium to low throughput system that reliably recapitulates liver structure and function (liver biomimetic). This depends on the ability of the system to generate an environment that reflects the in vivo phenotype of the hepatocytes and NPCs, which is highly dependent on both chemical and mechanical cues. A critical issue with routine 2D primary human hepatocyte culture systems is that hepatocyte functionality rapidly declines within days114. To overcome this, hepatocytes have been cultured using the collagen-sandwich method. This involves seeding a monolayer of hepatocytes on a gelled layer of rat tail collagen and overlaying another gelled collagen layer on top. This culture method allows hepatocytes to maintain synthetic function (as determined by albumin mRNA) for at least six weeks115. However, collagen sandwich cultures do not replicate the complex multi-cellular nature of the liver and does not incorporate fluid flow, which is important for preserving in vivo hepatocyte function116. Several of the most recent engineering advances to improve hepatocyte function and viability in culture will be discussed in this review. These systems are demarcated into the following categories: 2D Micro-Patterned Systems, 3D Spheroid Culture Systems, and Perfusion Culture Systems. For a comprehensive review of all advancements made in the realm of hepatocyte culture, the reader is referred to other references25,117,118.
Non-Perfusion Systems
Both static and perfusion systems exist for hepatocyte culture. Static culture systems include 2D micro-patterned systems and 3D spheroid culture systems. A summary of the properties of these systems can be found in Table 2.
Table 2.
A summary of properties of different non-perfusion hepatocyte culture systems.
| System | Reference | System Type |
Cell Type(s) | Culture Time |
CYP450 | Urea | Albumin | Throughput |
|---|---|---|---|---|---|---|---|---|
| HepatocPac™ by Hepregen, Corp. | 119 | 2D | PHH or PRH + 3T3 | 4–6 Weeks | Stable | Stable | Stable | Low |
| PDMS Stencil | 125 | 2D | PRH + 3T3 | 7 Days | N.D. | Stable | Stable | Low |
| Micropatterned 96-well Plate | 74 | 2D | PHH or iHep + 3T3 | 4 Weeks | Stable | Stable | Stable | High |
| Spheroid Microarray Chip | 131 | 3D Spheroid | PRH + 3T3 | 14 Days | N.D. | Stable | Stable | High |
| Micropatterned Fibrous Mat | 93 | 3D Spheroid | PRH + 3T3 + HUVEC | 15 Days | Stable | ↓ | Stable | Low |
| PEG-encapsulated Spheroid | 133 | 3D Spheroid | PRH + 3T3 | 50 Days | Stable | N.D. | Stable | High |
| Human Microspheroids | 134 | 3D Spheroid | PHH + HNPC | 35 Days | Stable | N.D. | Stable | High |
| RegeneTox™ by Regenemed, Inc. | 135 | 3D Spheroid | PHH or PRH + HNPC or RNPC | 90 Days | Stable | Stable | Stable | Medium |
| GravityTRAP™ by InSphero AG | 136 | 3D Spheroid | HepaRG | 21 Days | Stable | ↑ | Stable | High |
PHH = Primary Human Hepatocyte, PRH = Primary Rat Hepatocyte, 3T3 = 3T3 Fibroblasts, iHep = iPSC-derived human hepatocytes, HUVEC = Human Umbilical Vein Endothelial Cells, HNPC = Human Non-Parenchymal Cells, RNPC = Rat Non-Parenchymal Cells, N.D = Not determined.
2D Micro-Patterned Systems
The use of 2D micro-patterned culture systems are an improvement upon standard sandwich culture as they fine-tune tissue architecture by controlling the size, geometry, and functionality of culture chambers. One commercialized system, HepatoPac™ by Hepregen, Corp. consists of micro-patterned 2D plates seeded with either rat or human hepatocytes and 3T3-J2 stromal cells. Cultures maintain hepatocyte specific functions for 4–6 weeks119–121, notably albumin and urea secretion, phase I/II metabolism, and formation of canalicular networks. Publications have also reported sensitivities of 66% and 100% for corroborating hepatotoxins when tested with one or at least two cell donors, respectively120. The extended hepatocyte functionality may allow for accurate predictions of in vivo drug transporter activity122. Additionally, this system can be used to model CYP450 enzyme induction121,123. However, this system’s low throughput make it less suitable than traditional monolayer culture for toxicity screening studies124.
Similar systems have used micro-patterned hepatocyte islands to enhance hepatocyte functioning in 2D culture. For example, Cho and colleagues used polydimethylsiloxane (PDMS) stencils to co-culture rat hepatocytes and 3T3 fibroblasts and demonstrated enhanced hepatocyte functions when co-cultured in a layered format (hepatocytes seeded on an island of fibroblasts) as compared to a co-planar format125. Ware and colleagues used micro-patterned hepatocyte-murine fibroblast co-cultures to predict hepatotoxicity using both primary human hepatocytes and iPSC-derived hepatocyte-like cells74. However, these systems do not take advantage of the positive influence of LSECs on hepatocyte functions, as previously shown with hepatocyte co-culture with HUVECs126.
Since these systems involve a monolayer of cells, they are amenable to high content image analysis without confocal optics. However, these systems have not been fully characterized for co-culture with liver-specific NPCs and are patterned using rat tail collagen I rather than liver specific matrix proteins23. Previous studies have shown that culturing hepatocytes on liver-specific ECM leads to higher attachment efficiency and lower expression of the dedifferentiation markers vimentin and cytokeratin 18127. Moreover, supplementation of liver-specific ECM digests in hepatocyte cultures showed improved albumin synthesis and CYP450 activity128,129. Enhanced albumin secretion and cellular connectivity is also observed with less stiff culture substrates, such as heparin gels, with the least physiological morphology observed on collagen coated glass130. These findings suggest culture materials that mimic physiologic liver extracellular matrix promote maintenance of in vivo hepatocyte functions.
3D Spheroid Culture Systems
Three dimensional culture systems add an additional layer of complexity by more accurately representing the tissue architecture of a whole liver. Micro-patterning and functionalizing surfaces can facilitate the formation of 3D hepatocyte spheroids. For example, Fukuda and colleagues micro-patterned cylindrical culture wells with collagen and polyethyleneglycol, which facilitated rat hepatocyte spheroid formation. These spheroids demonstrated enhanced albumin secretion and ammonia detoxification compared to monolayer culture for up to 14 days in culture131. They also used a photo-crosslinkable chitosan hydrogel to incorporate 3T3 fibroblasts into their spheroids132.
Liu et al micro-patterned electrospun fibrous mats for the co-culture of rat hepatocytes, HUVECs, and 3T3 fibroblasts lasting up to 15 days93. They observed the formation of hepatocyte spheroids and noted co-culture with both fibroblasts and endothelial cells enhanced albumin secretion, urea synthesis, and CYP450 expression as compared to monoculture or co-culture with either fibroblasts or endothelial cells alone. While this system incorporates all three cell types on the same culture surface, each cell type is seeded in a specific area, limiting direct contact but allowing paracrine communication93.
Alternatively, primary rat hepatocytes and 3T3 fibroblasts can initially be co-cultured on 2D micro-patterned surfaces and the resulting cell aggregates detached and encapsulated in PEG to form 3D spheroids133. These spheroids are capable of showing dose-dependent acetaminophen toxicity responses, as well as CYP450 induction by model compounds, such as rifampin and phenobarbital. Additionally, the small size of these spheroids (~100µm) prevents the formation of a necrotic core. However, this system has not yet been characterized using human hepatocytes.
Bell and colleagues have generated spheroids from primary human cryopreserved hepatocytes and NPCs that demonstrate preserved metabolic function and viability up to five weeks in culture134. Beneficially, this system uses all human cells, is highly scalable (made in 96 well plates), and demonstrates long term response to hepatotoxins. However, the cell-to-media ratio is quite low, which may reduce the impact of paracrine factors secreted by the spheroids.
There are two 3D spheroid systems commercially available. First, RegeneTox™ marketed by Regenemed Inc. allows for the co-culture of NPCs and hepatocytes through use of a transwell insert. NPCs are seeded above two interconnected nylon scaffolds and cultured for a week prior to hepatocyte isolation and seeding. The media are changed three times a week and the co-cultures can last more than three months in the system, as demonstrated by albumin, urea, transferrin, and fibrinogen secretion, and stable CYP1A1, 3A4, and 2C9 activity. Moreover, the liver tissue was responsive to inflammatory stimuli by releasing mediators such as TNFα and IL-8135. However, this system is relatively low throughput (24-well plate equivalent) and utilizes a culture surface orders of magnitude stiffer than native liver tissue.
GravityTRAP™ marketed by InSphero AG uses a hanging drop platform to seed cells in a concentrated suspension to grow liver tissue in 3D. These tissues can be transferred into a specialized 96-well plate for culture and analysis, remaining viable for at least 4 weeks. Spheroids demonstrate robust CYP3A4 expression and albumin production compared to 2D cultures. Spheroid size is highly reproducible but may be time consuming without automation. Additionally, spheroid size is limited, as spheroids larger than 200µm develop central necrosis due to hypoxia136.
While the dimensionality of these systems allow for more complex intercellular communication than 2D culture systems, they do not incorporate flow, which is known to be important for maintaining liver-cell specific functions.
Perfusion Culture Systems
Perfusion culture systems allow for the incorporation of physiological “blood” flow (media), which is an important factor for hepatocyte detoxification of drugs137 and establishment of the physiological oxygen and chemical gradients. Such systems will be grouped by their size (both volume and cell number), designated as either macroscale or microscale. A summary of the properties of the discussed systems can be found in Table 3.
Table 3.
A summary of properties of different perfusion hepatocyte culture systems.
| System | References | System Type |
Cell Type(s) | Culture Time |
Flow Rate (µL/min) |
Total Volume |
CYP450 | Urea | Albumin | Throughput |
|---|---|---|---|---|---|---|---|---|---|---|
| Spinning Bioreactor Spheroids | 138 | Macroscale Perfusion | PHH | 3–4 Weeks | 41.6 | 300mL | Stable | ↓ | ↑ | High |
| Radial Flow Bioreactor | 139 | Macroscale Perfusion | PRH + 3T3 | 5–11 Days | 830 | 54mL | N.D. | ↓ | ↓ | Medium |
| Multichamber Modular Bioreactor | 140 | Macroscale Perfusion | PHH | 7–21 Days | 250–500 | 5mL | Stable or ↓ | Stable | Stable | Low |
| LiverChip™ by CN Bio Innovations Ltd. | 77 | Microscale Perfusion | PHH + HNPC | 15–29 Days | 60 | 1.6mL | Stable | Stable | Stable | High |
| Metabolomics on a Chip | 147) | Microscale Perfusion | HepG2/C3A | 48 Hours | 10 | 3mL | Stable | N.D. | Stable | Low |
| Hollow Fiber Perfusion Bioreactor | 148 | Microscale Perfusion | PHH + HNPC | 10 Days | 3000 | 0.5mL | ↓ | ↓ | N.D. | Low |
| Artificial Liver Sinusoid | 150, 151 | Microscale Perfusion | PHH or PRH or HepG2/C3A | 7–8 Days | 0.01 – 0.02 | 0.075mL | N.D. | N.D. | Stable | Low |
| HµREL® biochips by HµREL Corp. | 116, 152 | Microscale Perfusion | cPHH + HNPC | 6 Days | 4.5 | 0.1mL | Stable or ↓ | N.D. | N.D. | Medium |
| Human Liver Sinusoid | 153, 157 | Microscale Perfusion | PHH & cPHH + EA.hy926 + U937 + LX-2 | 28 Days | 0.08 to 0.25 | 100 µl | Stable | Stable | Stable | Medium |
PHH = Primary Human Hepatocyte, cPHH = cryopreserved Primary Human Hepatocyte, PRH = Primary Rat Hepatocyte, 3T3 = 3T3 Fibroblasts, HNPC = Human Non-Parenchymal Cells, N.D = Not determined, EA.hy926 = human endothelial cell line, U937 = human monocyte cell line, LX-2 = human hepatic stellate cell line
Macroscale
A few groups have applied large scale perfusion bioreactor devices to the culture of hepatocyte spheroids for drug discovery applications. For example, Tostoes and colleagues demonstrated the culture of primary human hepatocyte spheroids in a spinning perfusion bioreactor for up to 3–4 weeks138. The working volume was 300mL and 20% of this was replaced daily through medium perfusion. They demonstrated CYP450 induction and the metabolism of a model substrate, 7-methoxycoumarin. While albumin synthesis increased and stabilized over the culture period, urea synthesis significantly decreased with culture, likely due to spheroid hypoxia. Additionally, this culture system has only been tested for hepatocyte monoculture.
Another system uses a radial flow bioreactor (RFB), where the bed of matrix housing cells separates the media inlet and outlet, which facilitates nutrient and oxygen diffusion across the tissue. Park and colleagues co-cultured rat hepatocytes and 3T3-J2 fibroblasts on a series of stacked glass substrates, with microfabricated grooves and a collagen coating, to improve oxygen delivery to the tissue139. However, while RFBs culture large number of hepatocytes (2–5×107), the high flow rates used cause loss of hepatic-specific functions after a few days due to unphysiological shear stresses139.
Vinci and colleagues cultured primary human hepatocytes on coverslips for 7 days then transferred the coverslips into either their multichamber bioreactor or a new dish for continued static culture. They found that the expression of metabolic (CYP450) and transport (OATP, MDR1) genes were much higher under perfusion conditions and that higher flow rates affected this enhanced expression140. The use of primary human hepatocytes makes this system amenable to the investigation of human-specific drug interactions. However, the working volume (5mL) is quite large compared to the cell seeding number (200k), which reduces the influence of secreted factors.
While macroscale perfusion culture systems allow for the influences of shear stress and nutrient supply on drug detoxification to be extrapolated, they carry several weaknesses. First, the number of cells required is substantially higher than smaller-scale culture systems, which may prove challenging given the limited availability of hepatocytes. Second, the media volume to cell volume ratio is much higher than is seen in vivo. Third, many of these systems replace the circulating media with fresh media on a continuous basis. As such, these systems may demonstrate reduced signaling from soluble factors released by the hepatic tissue.
Microscale
In contrast to macroscale (5–300mL) perfusion culture systems, the use of microscale (0.1–3mL) systems allows for higher cell-to-media volume ratios, which approach those seen in vivo. The LiverChip by CNBio Innovations (originally Zyoxel) models the liver tissue with a perfusion system on a larger volume scale than most micro-perfusion systems (1.6mL)2,77,141,142. Earlier generations of this system utilized alternative well geometry143,144. Hepatocytes and a full complement of donor matched NPCs are seeded on polystyrene (mechanically stiff) scaffolds at a 1:1 ratio. Fluid flow is driven by pneumatic pumps at a rate of 60 µL/min through the scaffolds, providing direct, but physiologically representative shear stresses and oxygen gradients on the resident tissue cells (Figure 1B). Tissues are established and remain functional up to one month, as measured by blood urea nitrogen levels, fibrinogen, alpha-1 antitrypsin (A1AT), and CYP450 activity. The culture chamber allows for a 3D hepatic tissue to form, which provides an advantage over 2D culture systems by facilitating cell junction formation. This culture system has been used to study metastasis of cancer cells in the context of the liver microenvironment2,77,141,142 and probe the influence of LPS on hydrocortisone metabolism145. This system offers a unique opportunity to study cancer drug efficacy and hepatotoxic effects in a single system. However, there is currently no effective way to physically monitor tissue formation during the culture period.
Choucha-Snouber and colleagues designed a liver biochip to investigate metabolic responses to the anticancer drug flutamide146. Their chip is fabricated from PDMS and contains fibronectin-coated microstructures for cell seeding. HepG2/C3a cells are seeded in the system and kept in culture for a total of 48 hours, 24 hours of which is for perfusion and drug treatment. In comparison to the LiverChip, this system uses a lower flow rate of 10µL/min. The low volume of the biochip (40µL) is desirable to achieve a physiologic ratio of media supply to cell volume. However, this system has not tested co-culture with NPCs. Additionally, using PDMS as the fabrication substrate allows for gasses to readily equilibrate between the external environment and the cell culture chamber, which may limit modeling of a physiologically hypoxic liver microenvironment. Additionally, small hydrophobic compounds adsorb to PDMS, which limits the drugs this system can accurately assess without extensive calibration and testing. This design was extended to investigate kidney-liver co-culture systems147.
Hoffmann and colleagues designed a hollow-fiber based microscale perfusion culture system, with a volume of 0.5mL148. A unique feature of this system is the incorporation of three different supply channels to the cultured tissue – two media circuits and one gas circuit. The authors demonstrate robust tissue formation, which includes NPCs. Moreover, NPCs were noted to form vascular-like structures. While the culture volume is low, the flow rate and fresh media replenishment rate are quite high in comparison (3mL/min and 0.5mL/hour respectively). This may prove a limiting factor for analyzing long-term effects of secreted factors. Additionally, the expression and activity of several CYP450 (3A4 and 2B6) isoforms decreases dramatically between days 3 and 10 of culture148. Since 50% of currently tested drugs are metabolized by CYP3A4149, the decrease in its activity over the culture period may make this system not suitable for long term toxicity or metabolic assays.
CellAsic designed a hepatic sinusoid cord-like culture system, in which a central cord of cells is enveloped by a fluid transport channel. Communication between the cells and fluid channel was accomplished by small (1µm by 2µm) canals, limiting transport to diffusion. The system is primed with fluid and cells are subsequently seeded. After cell seeding, the chip is placed in the incubator on an incline to facilitate gravity-driven continuous flow, at rates of 10–20nL/min, to supply the tissue. The system can maintain hepatocytes for over 1 week, as determined by albumin secretion, and exhibit dose-dependent hepatotoxicity of diclofenac as seen in vivo150,151. Thirty two independent culture systems can be run simultaneously on a device the size of a 96-well plate. While this system generates 3D tissue, an improvement over 2D culture systems, it has not been investigated for culture periods longer than 7 days.
HµREL® biochips by Hurel Corp. allow up to 8 microfluidic circuits to operate in parallel152. These chips contain two cell culture compartments connected in series, one of which was designed to house liver tissue. Cells are seeded onto the polystyrene biochip either in monoculture or co-culture, using human hepatocytes and NPCs. Flow was generated by use of a peristaltic pump with a flow rate of 4.5µL/min/chip. Hepatocyte co-cultures in the system subjected to flow demonstrated increased metabolic activity when compared to static monocultures or co-cultures and remained viable for up to 6 days116. One advantage of this system is that the HµREL tubing exhibits limited adsorption of hydrophobic drug molecules. Additionally, the polystyrene housing reduces gas permeability of the device, facilitating formation of a hypoxic microenvironment. However, the tissues formed in this system are not 3D and the maximum culture period is limited compared to other systems. Moreover, CYP450 isoforms exhibit varying activities in the system – while CYP3A4 activity increases over the 6 day culture duration, CYP2D6 activity decreases after 2 days of culture116.
Finally, the liver acinus microphysiology system (LAMPS) is a microfluidic platform to investigate hepatic physiology, drug safety, and disease models8 that evolved from the first generation liver MPS (SQL-SAL)153. The LAMPS incorporates either fresh or cryopreserved human hepatocytes, as well as a full complement of non-parenchymal cells: human endothelial (primary or TMNK-1), immune (THP-1 or U937), and stellate (LX-2) cells in a Nortis, Inc microfluidic device. A porcine liver extracellular matrix (LECM), ca. 10µm thick, is layered between the endothelial cells and the hepatocytes. Approximately 20% of the hepatocytes and stellate cells contain fluorescent biosensors that measure apoptosis and reactive oxygen species for real-time fluorescence readouts154, along with analysis of the secretome and metabolic activity. Distinct zone 1 and zone 3 microenvironments are created that allow the direct determination of zone-specific physiological and disease functions155. This system is viable for at least 28 days under continuous perfusion flow and exhibits concentration- and time-dependent toxicity profiles to drugs (e.g. troglitazone) in a similar manner to that seen clinically156. Structurally, the endothelial cells are separated from the hepatocytes by a biomimetic of the Space of Disse forming a three-dimensional layer of cells and LECM. The immune cells permit the system to be LPS responsive, releasing TNFα upon stimulation and in combination with some drugs causes immune-mediated hepatotoxicity. Moreover, the LX-2 cells can be activated in response to methotrexate treatment, resulting in the expression of smooth muscle actin (SMA) and the production of collagen 1A2, reflecting a fibrosis phenotype. Most importantly, this system is amenable to real-time transmitted light and fluorescence imaging, allowing for investigating toxicity kinetics and disease progression.
The platform is continually being evolved to replace the NPCs with either primary human cells or iPSC-derived cells, since the use of immortalized NPCs may not reflect full physiological capabilities. This platform contains PDMS which requires the presence of carrier proteins for hydrophobic drugs and pre-measurement of the loss of drugs to the device before making any interpretations157. Finally, the media in this system is not recirculated in the current format. However, the small volumes and low flow rates allow the detection of paracrine signaling mediators secreted by the cells, although there are other advantages to having the media recirculated (see above).
Conclusions and Future Directions
Advanced liver culture systems hold the potential to transform drug discovery and development, through more accurate modeling of human in vivo pharmacokinetics and pharmacodynamics, as well as understanding mechanisms of disease progression and toxicity. Several factors affect the accuracy of these model systems in predicting pharmaceutical toxicity. The cells used in the culture system and the culture surface (e.g. plastic, hydrogel) allow for modeling cell-cell and cell-ECM interactions respectively. Systems that include media flow (“blood flow”) provide mechanical stimuli that benefit tissue function. Inclusion of cells derived from human tissues allows for human-specific pathways to be modeled. Special attention should be paid to the use of primary versus immortalized cells, as the latter may elicit signals not seen in physiologic conditions. The use of animal cells instead of human cells may encounter similar intractable weaknesses. Importantly, the expression levels of CYP450 isoforms and drug transporters over the culture period may influence toxicity predictions.
Future directions for improving these systems include incorporating lentiviral-induced fluorescent biosensors for an automated readout of various cellular functions154 and finer architectural control of liver microstructure. Importantly, the ability to image the devices in real time is highly advantageous. Fabrication of devices using materials other than PDMS – which adsorbs hydrophobic molecules and biologics – will improve the utility of these devices as pharmacokinetic models for drug discovery. Moreover, procurement of a universal, readily available cell source (potentially iPSC-derived hepatocytes) will improve the ease with which these systems are used for drug characterization studies. Networking of additional organ chips, such as gut and kidney, will contribute additional signals to the liver tissue, allow for modeling of drug absorption, and disposition after hepatic metabolism. Finally, further development and usage of quantitative systems pharmacology as a new paradigm in drug discovery and development involving the integration and iteration of experimental and computational models, in parallel with advanced culture systems will facilitate complex data analysis and may allow for predictive models to be generated158,159.
It is hoped that the development of validated human experimental model systems can be used in place of animal models to improve preclinical drug selection. By implementing more accurate human experimental model systems, it is expected that there will be increased efficacy and safety in clinical trials leading to improved therapeutics at a lower cost.
Acknowledgments
The authors thank the members of the Wells lab and those of our collaborators in organ systems development.
Funding
This chapter derives from funding from the US NIH (UH3TR000496 and UH3TR000503) and VA (Merit BX003368). CHB was supported by a CATER fellowship from NIH (EB001026) and F30CA199947 from the NCI, and SW by a fellowship from the DoD CDMRP in Breast Cancer (W81XWH-14-1-0062). None of these sources had any input or control over the contents of this communication.
Footnotes
Conflicts
LG and AW hold a patent position in the LiverChip commercialized by CNBio Innovations. DLT has a patent application in review on the liver MPS.
References
- 1.Bhatia S, Toner M, Foy B, Rotem A. Zonal liver cell heterogeneity: effects of oxygen on metabolic functions of hepatocytes. Cell Eng. 1996;1:125–135. [Google Scholar]
- 2.Wheeler SE, et al. All-human microphysical model of metastasis therapy. Stem Cell Res. Ther. 2013;(4 Suppl 1):S11. doi: 10.1186/scrt372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakkola J, Tanaka E, Pelkonen O. Developmental Expression of Cytochrome P450 Enzymes in Human Liver. Pharmacol. Toxicol. 1998;82:209. doi: 10.1111/j.1600-0773.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg G, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 2002;66:185–200. doi: 10.1093/toxsci/66.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Blanco JG, Harrison PL, Evans WE, Relling MV. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab. Dispos. 2000;28:379–82. [PubMed] [Google Scholar]
- 6.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 7.Buck LD, Inman SW, Rusyn I, Griffith LG. Co-regulation of primary mouse hepatocyte viability and function by oxygen and matrix. Biotechnol. Bioeng. 2014;111:1018–1027. doi: 10.1002/bit.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee-Montiel FT, et al. Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp. Biol. Med. 2017;242:1617–1632. doi: 10.1177/1535370217703978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin. Pharmacokinet. 1999;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi H, Masuyama M, Koyama H, Oguro A, Takahashi T. Quantitative measurement of human tissue hepatic blood volume by C15O inhalation with positron-emission tomography. Liver. 1996;16:258–62. doi: 10.1111/j.1600-0676.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 11.Lodish Harvey, Arnold Berk, Zipursky S Lawrence, Matsudaira Paul, Baltimore David, D J. Molecular Cell Biology. 4. W. H. Freeman; 2000. [Google Scholar]
- 12.Taylor DP, Clark A, Wheeler S, Wells A. Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Res. Treat. 2014;144:551–60. doi: 10.1007/s10549-014-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur A, et al. Human induced pluripotent stem cell-based microphysiological tissue models of myocardium and liver for drug development. Stem Cell Res. Ther. 2013;(4 Suppl. 1):S14. doi: 10.1186/scrt375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemasters JJ. The liver: biology and pathobiology. 2001:257–279. at < http://www.gastrohep.org/theliver/18ARIAS018.pdf>.
- 15.Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430:195–207. doi: 10.1007/BF01324802. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RJ, et al. The Effect of Three-Dimensional Co-Culture of Hepatocytes and Hepatic Stellate Cells on Key Hepatocyte Functions in vitro. Cells Tissues Organs. 2005;181:67–79. doi: 10.1159/000091096. [DOI] [PubMed] [Google Scholar]
- 17.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J. Clin. Invest. 2013;123:1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuber R, Anzenbacherová E, Anzenbacher P. Cytochromes P450 and experimental models of drug metabolism. J. Cell. Mol. Med. 6:189–98. doi: 10.1111/j.1582-4934.2002.tb00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baillie TA, Rettie AE. Role of Biotransformation in Drug-Induced Toxicity: Influence of Intra- and Inter-Species Differences in Drug Metabolism. Drug Metab. Pharmacokinet. 2011;26:15–29. doi: 10.2133/dmpk.dmpk-10-rv-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bale SS, et al. In vitro platforms for evaluating liver toxicity. Exp. Biol. Med. (Maywood) 2014 doi: 10.1177/1535370214531872. 1535370214531872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhushan A, et al. Towards a three-dimensional microfluidic liver platform for predicting drug efficacy and toxicity in humans. Stem Cell Res. Ther. 2013;(4 Suppl. 1):S16. doi: 10.1186/scrt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vunjak-Novakovic G, Bhatia S, Chen C, Hirschi K. HeLiVa platform: integrated heart-liver-vascular systems for drug testing in human health and disease. Stem Cell Res. Ther. 2013;(4 Suppl. 1):S8. doi: 10.1186/scrt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khetani SR, et al. Microengineered Liver Tissues for Drug Testing. J. Lab. Autom. 2015 doi: 10.1177/2211068214566939. [DOI] [PubMed] [Google Scholar]
- 24.Pichard L, et al. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab. Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 25.LeCluyse EL, et al. Isolation and culture of primary human hepatocytes. Methods Mol. Biol. 2005;290:207–229. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14:394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Bhogal RH, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: A one hundred liver experience. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandre E, et al. Cryopreservation of adult human hepatocytes obtained from resected liver biopsies. Cryobiology. 2002;44:103–13. doi: 10.1016/s0011-2240(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 29.Richert L, et al. Tissue collection, transport and isolation procedures required to optimize human hepatocyte isolation from waste liver surgical resections. A multilaboratory study. Liver Int. 2004;24:371–378. doi: 10.1111/j.1478-3231.2004.0930.x. [DOI] [PubMed] [Google Scholar]
- 30.Vondran FWR, et al. Isolation of primary human hepatocytes after partial hepatectomy: Criteria for identification of the most promising liver specimen. Artif. Organs. 2008;32:205–213. doi: 10.1111/j.1525-1594.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 31.Alexandre E, et al. Cryopreservation of adult human hepatocytes obtained from resected liver biopsies. Cryobiology. 2002;44:103–13. doi: 10.1016/s0011-2240(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 32.Donato M, Lahoz a, Jimenez N. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab. 2006;34:1556–1562. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd TDR, et al. Effect of patient, operative and isolation factors on subsequent yield and viability of human hepatocytes for research use. Cell Tissue Bank. 2004;5:81–87. doi: 10.1023/B:CATB.0000034079.10985.bd. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara T, et al. Factors affecting hepatocyte isolation, engraftment, and replication in an in vivo model. Liver Transpl. 2010;16:974–82. doi: 10.1002/lt.22099. [DOI] [PubMed] [Google Scholar]
- 35.Hewes JC, et al. A prospective study of isolated human hepatocyte function following liver resection for colorectal liver metastases: the effects of prior exposure to chemotherapy. J. Hepatol. 2006;45:263–70. doi: 10.1016/j.jhep.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Mcginnity DF, Soars MG, Urbanowicz Ra, Riley RJ. Evaluation of Fresh and Cryopreserved Hepatocytes As in Vitro Drug Metabolism Tools for the Prediction of Metabolic Clearance Abstract? Drug Metab. Dispos. 2004;32:1247–1253. doi: 10.1124/dmd.104.000026. [DOI] [PubMed] [Google Scholar]
- 37.Li AP. Human hepatocytes: Isolation, cryopreservation and applications in drug development. Chem. Biol. Interact. 2007;168:16–29. doi: 10.1016/j.cbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Sudo M, Nishihara M, Takahashi J, Asahi S. Long-Term Stability of Cryopreserved Human Hepatocytes: Evaluation of Phase I and II Drug-Metabolizing Enzyme Activities and CYP3A4/5 Induction for More than a Decade. Drug Metab. Dispos. 2017;45:734–736. doi: 10.1124/dmd.117.075234. [DOI] [PubMed] [Google Scholar]
- 39.Terry C, Hughes RD, Mitry RR, Lehec SC, Dhawan A. Cryopreservation-induced nonattachment of human hepatocytes: role of adhesion molecules. Cell Transplant. 2007;16:639–47. doi: 10.3727/000000007783465000. [DOI] [PubMed] [Google Scholar]
- 40.Lundquist P, et al. The impact of solute carrier (SLC) drug uptake transporter loss in human and rat cryopreserved hepatocytes on clearance predictions. Drug Metab. Dispos. 2014;42:469–80. doi: 10.1124/dmd.113.054676. [DOI] [PubMed] [Google Scholar]
- 41.Roymans D, et al. Determination of cytochrome P450 1A2 and cytochrome P450 3A4 induction in cryopreserved human hepatocytes. Biochem. Pharmacol. 2004;67:427–437. doi: 10.1016/j.bcp.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Brown HS, Griffin M, Houston JB. Evaluation of cryopreserved human hepatocytes as an alternative in vitro system to microsomes for the prediction of metabolic clearance. Drug Metab. Dispos. 2007;35:293–301. doi: 10.1124/dmd.106.011569. [DOI] [PubMed] [Google Scholar]
- 43.Bi Ya, Kazolias D, Duignan DB. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab. Dispos. 2006;34:1658–1665. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- 44.Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol. Appl. Pharmacol. 2004;199:193–209. doi: 10.1016/j.taap.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Harrill AH, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009;19:1507–1515. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin JH, et al. Species differences in the pharmacokinetics and metabolism of indinavir, a potent human immunodeficiency virus protease inhibitor. Drug Metab. Dispos. 1996;24:1111–1120. [PubMed] [Google Scholar]
- 47.Pearce R, Greenway D, Parkinson a. Species differences and interindividual variation in liver microsomal cytochrome P450 2A enzymes: Effects on coumarin, dicumarol, and testosterone oxidation. Arch. Biochem. Biophys. 1992;298:211–225. doi: 10.1016/0003-9861(92)90115-d. [DOI] [PubMed] [Google Scholar]
- 48.Lu C, Li AP. Species comparison in P450 induction: Effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem. Biol. Interact. 2001;134:271–281. doi: 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 49.Naritomi Y, et al. Prediction of Human Hepatic Clearance From in Vivo Animal Experiments and in Vitro Metabolic Studies With Liver Microsomes From Animals and Humans Abstract? Drug Metab. Dispos. 2001;29:1316–1324. [PubMed] [Google Scholar]
- 50.Huh D, Hamilton Ga, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 52.Gerets HHJ, et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012;28:69–87. doi: 10.1007/s10565-011-9208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo L, et al. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab. Dispos. 2011;39:528–538. doi: 10.1124/dmd.110.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003;31:1035–42. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 55.Mills JB, Rose Ka, Sadagopan N, Sahi J, de Morais SMF. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J. Pharmacol. Exp. Ther. 2004;309:303–309. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Scialis RJ, Feng B, Leach K. Detection of statin cytotoxicity is increased in cells expressing the OATP1B1 transporter. Toxicol. Sci. 2013;134:73–82. doi: 10.1093/toxsci/kft085. [DOI] [PubMed] [Google Scholar]
- 57.Guillouzo A, et al. The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem. Biol. Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Kotani N, et al. Expression and transport function of drug uptake transporters in differentiated HepaRG cells. Mol. Pharm. 2012;9:3434–3441. doi: 10.1021/mp300171p. [DOI] [PubMed] [Google Scholar]
- 59.Le Vee M, Noel G, Jouan E, Stieger B, Fardel O. Polarized expression of drug transporters in differentiated human hepatoma HepaRG cells. Toxicol. Vitr. 2013;27:1979–1986. doi: 10.1016/j.tiv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Tomida T, Okamura H, Satsukawa M, Yokoi T, Konno Y. Multiparametric assay using HepaRG cells for predicting drug-induced liver injury. Toxicol. Lett. 2015;236:16–24. doi: 10.1016/j.toxlet.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Klein M, et al. A systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG cells. Drug Metab. Dispos. 2015;43:273–83. doi: 10.1124/dmd.114.060962. [DOI] [PubMed] [Google Scholar]
- 62.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 63.Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013;997:23–33. doi: 10.1007/978-1-62703-348-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li HY, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011;32:5994–6005. doi: 10.1016/j.biomaterials.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Takayama K, et al. Efficient Generation of Functional Hepatocytes From Human Embryonic Stem Cells and Induced Pluripotent Stem Cells by HNF4α Transduction. Mol. Ther. 2012;20:127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duan Y, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 68.Chistiakov Da, Chistiakov Pa. Strategies to produce hepatocytes and hepatocyte-like cells from pluripotent stem cells. Hepatol. Res. 2012;42:111–119. doi: 10.1111/j.1872-034X.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 69.Szkolnicka D, Zhou W, Lucendo-Villarin B, Hay DC. Pluripotent stem cell-derived hepatocytes: potential and challenges in pharmacology. Annu. Rev. Pharmacol. Toxicol. 2013;53:147–59. doi: 10.1146/annurev-pharmtox-011112-140306. [DOI] [PubMed] [Google Scholar]
- 70.Bukong TN, Lo T, Szabo G, Dolganiuc A. Novel developmental biology-based protocol of embryonic stem cell differentiation to morphologically sound and functional yet immature hepatocytes. Liver Int. 2012;32:732–741. doi: 10.1111/j.1478-3231.2011.02743.x. [DOI] [PubMed] [Google Scholar]
- 71.Yi F, Liu G-H, Belmonte JCI. Human induced pluripotent stem cells derived hepatocytes: rising promise for disease modeling, drug development and cell therapy. Protein Cell. 2012;3:246–250. doi: 10.1007/s13238-012-2918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnology Advances. 2014;32:504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baxter M, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 2015;62:581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ware BR, Berger DR, Khetani SR. Prediction of Drug-Induced Liver Injury in Micropatterned Co-cultures Containing iPSC-Derived Human Hepatocytes. Toxicol. Sci. 2015;145:252–62. doi: 10.1093/toxsci/kfv048. [DOI] [PubMed] [Google Scholar]
- 75.Kim K, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shan J, et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat. Chem. Biol. 2013;9:514–20. doi: 10.1038/nchembio.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wheeler SE, et al. Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br. J. Cancer. 2014;111:2342–2350. doi: 10.1038/bjc.2014.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasmall SC, West DA, Olsen K, Roberts RA. Role of hepatic non-parenchymal cells in the response of rat hepatocytes to the peroxisome proliferator nafenopin in vitro. Carcinogenesis. 2000;21:2159–65. doi: 10.1093/carcin/21.12.2159. [DOI] [PubMed] [Google Scholar]
- 79.Riccalton-Banks L, Bhandari R, Fry J, Shakesheff KM. A simple method for the simultaneous isolation of stellate cells and hepatocytes from rat liver tissue. Mol. Cell. Biochem. 2003;248:97–102. doi: 10.1023/a:1024184826728. [DOI] [PubMed] [Google Scholar]
- 80.Chang W, et al. Isolation and culture of hepatic stellate cells from mouse liver. Acta Biochim. Biophys. Sin. (Shanghai) 2014;46:291–8. doi: 10.1093/abbs/gmt143. [DOI] [PubMed] [Google Scholar]
- 81.Werner M, et al. All-In-One: Advanced preparation of Human Parenchymal and Non-Parenchymal Liver Cells. PLoS One. 2015;10:e0138655. doi: 10.1371/journal.pone.0138655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Damm G, et al. Human parenchymal and non-parenchymal liver cell isolation, culture and characterization. Hepatol. Int. 2013;7:951–8. doi: 10.1007/s12072-013-9475-7. [DOI] [PubMed] [Google Scholar]
- 83.Pfeiffer E, et al. Featured Article: Isolation, characterization, and cultivation of human hepatocytes and non-parenchymal liver cells. Exp. Biol. Med. (Maywood) 2015;240:645–56. doi: 10.1177/1535370214558025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kegel V, et al. Protocol for Isolation of Primary Human Hepatocytes and Corresponding Major Populations of Non-parenchymal Liver Cells. J. Vis. Exp. 2016:e53069. doi: 10.3791/53069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Damm G, et al. Human parenchymal and non-parenchymal liver cell isolation, culture and characterization. Hepatol. Int. 2013;7:951–8. doi: 10.1007/s12072-013-9475-7. [DOI] [PubMed] [Google Scholar]
- 86.Koui Y, et al. An In Vitro Human Liver Model by iPSC-Derived Parenchymal and Non-parenchymal Cells. Stem Cell Reports. 2017;9:490–498. doi: 10.1016/j.stemcr.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krause P, et al. Hepatocyte-supported serum-free culture of rat liver sinusoidal endothelial cells. J. Hepatol. 2000;32:718–26. doi: 10.1016/s0168-8278(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 88.Nelson LJ, et al. Acetaminophen cytotoxicity is ameliorated in a human liver organotypic co-culture model. Sci. Rep. 2015;5:17455. doi: 10.1038/srep17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hang T-C, Lauffenburger DA, Griffith LG, Stolz DB. Lipids promote survival, proliferation, and maintenance of differentiation of rat liver sinusoidal endothelial cells in vitro. AJP Gastrointest. Liver Physiol. 2012;302:G375–G388. doi: 10.1152/ajpgi.00288.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwa AJ, et al. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564–79. doi: 10.1096/fj.06-7473com. [DOI] [PubMed] [Google Scholar]
- 91.Griffith LG, Wells A, Stolz DB. Engineering liver. Hepatology. 2014;60:1426–34. doi: 10.1002/hep.27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leclerc E, Sakai Y, Fujii T. Perfusion culture of fetal human hepatocytes in microfluidic environments. Biochem. Eng. J. 2004;20:143–148. [Google Scholar]
- 93.Liu Y, Li H, Yan S, Wei J, Li X. Hepatocyte cocultures with endothelial cells and fibroblasts on micropatterned fibrous mats to promote liver-specific functions and capillary formation capabilities. Biomacromolecules. 2014;15:1044–1054. doi: 10.1021/bm401926k. [DOI] [PubMed] [Google Scholar]
- 94.Nelson LJ, et al. Acetaminophen cytotoxicity is ameliorated in a human liver organotypic co-culture model. Sci. Rep. 2015;5:17455. doi: 10.1038/srep17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. IP-10 induces dissociation of newly formed blood vessels. J. Cell Sci. 2009;122:2064–77. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–80. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 97.Kang YBA, Rawat S, Cirillo J, Bouchard M, Noh HM. Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: toward engineering the liver sinusoid. Biofabrication. 2013;5:45008. doi: 10.1088/1758-5082/5/4/045008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Yu-Shih, Ke Ling-Yi, Huang Ying-Chen LC-H. 3D circulatory perfusion-culture system by using high efficiency proportional cell contact. Proc MicroTAS. 2012:1018–1020. [Google Scholar]
- 99.Taylor DP, Clark A, Wheeler S, Wells A. Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Res. Treat. 2014;144:551–60. doi: 10.1007/s10549-014-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawasaki T, et al. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J. Hepatol. 2010;53:648–54. doi: 10.1016/j.jhep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 101.Soto-Gutiérrez A, et al. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat. Protoc. 2007;2:347–56. doi: 10.1038/nprot.2007.18. [DOI] [PubMed] [Google Scholar]
- 102.Huebert RC, et al. Immortalized liver endothelial cells: a cell culture model for studies of motility and angiogenesis. Lab. Invest. 2010;90:1770–81. doi: 10.1038/labinvest.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kitani H, et al. Establishment of c-myc-immortalized Kupffer cell line from a C57BL/6 mouse strain. Results Immunol. 2014;4:68–74. doi: 10.1016/j.rinim.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melino M, et al. Macrophage secretory products induce an inflammatory phenotype in hepatocytes. World J. Gastroenterol. 2012;18:1732–44. doi: 10.3748/wjg.v18.i15.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matak P, et al. Activated macrophages induce hepcidin expression in HuH7 hepatoma cells. Haematologica. 2009;94:773–80. doi: 10.3324/haematol.2008.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarkar U, et al. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab. Dispos. 2015;43:1091–9. doi: 10.1124/dmd.115.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J. Hepatol. 2014;61:660–71. doi: 10.1016/j.jhep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 108.Yin C, Evason KJ, Asahina K, Stainier DYR. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Invest. 2013;123:1902–10. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Minicis S, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 110.Friedman SL. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu L, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Friedman SL. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krause P, Saghatolislam F, Koenig S, Unthan-Fechner K, Probst I. Maintaining hepatocyte differentiation in vitro through co-culture with hepatic stellate cells. In Vitro Cell. Dev. Biol. Anim. 45:205–12. doi: 10.1007/s11626-008-9166-1. [DOI] [PubMed] [Google Scholar]
- 114.Soldatow VY, LeCluyse EL, Griffith LG, Rusyn I. In vitro models for liver toxicity testing. Toxicol. Res. 2013;2:23–39. doi: 10.1039/C2TX20051A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dunn JCY, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: Evidence for transcriptional and translational regulation. J. Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem. Pharmacol. 2010;79:1036–1044. doi: 10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godoy P, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives of Toxicology. 2013:87. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guguen-Guillouzo C, Guillouzo Ain. 2010:1–40. doi: 10.1007/978-1-60761-688-7_1. [DOI] [PubMed] [Google Scholar]
- 119.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 120.Khetani SR, et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol. Sci. 2013;132:107–117. doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 121.Ukairo O, et al. Long-Term Stability of Primary Rat Hepatocytes in Micropatterned Cocultures. J. Biochem. Mol. Toxicol. 2013;27:204–212. doi: 10.1002/jbt.21469. [DOI] [PubMed] [Google Scholar]
- 122.Chan TS, et al. Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac. Drug Metab. Dispos. 2013;41:2024–32. doi: 10.1124/dmd.113.053397. [DOI] [PubMed] [Google Scholar]
- 123.Dixit V, Moore A, Tsao H, Hariparsad N. Application of Micropatterned Cocultured Hepatocytes to Evaluate the Inductive Potential and Degradation Rate of Major Xenobiotic Metabolizing Enzymes. Drug Metab. Dispos. 2016;44:250–61. doi: 10.1124/dmd.115.067173. [DOI] [PubMed] [Google Scholar]
- 124.Dixit V, Moore A, Tsao H, Hariparsad N. Application of Micropatterned Cocultured Hepatocytes to Evaluate the Inductive Potential and Degradation Rate of Major Xenobiotic Metabolizing Enzymes. Drug Metab. Dispos. 2016;44:250–61. doi: 10.1124/dmd.115.067173. [DOI] [PubMed] [Google Scholar]
- 125.Cho C, et al. Layered patterning of hepatocytes in co-culture systems using microfabricated stencils. Biotechniques. 2010;48:47–52. doi: 10.2144/000113317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zinchenko YS, Culberson CR, Coger RN. Contribution of non-parenchymal cells to the performance of micropatterned hepatocytes. Tissue Eng. 2006;12:2241–1251. doi: 10.1089/ten.2006.12.2241. [DOI] [PubMed] [Google Scholar]
- 127.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J. Cell. Physiol. 2014;229:984–9. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 128.Skardal A, et al. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials. 2012;33:4565–75. doi: 10.1016/j.biomaterials.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loneker AE, Faulk DM, Hussey GS, D’Amore A, Badylak SF. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. J. Biomed. Mater. Res. Part A. 2016;104:957–965. doi: 10.1002/jbm.a.35636. [DOI] [PubMed] [Google Scholar]
- 130.You J, et al. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng. Part A. 2013;19:2655–63. doi: 10.1089/ten.tea.2012.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fukuda J, Sakai Y, Nakazawa K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials. 2006;27:1061–1070. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 132.Fukuda J, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 133.Li CY, et al. Micropatterned cell-cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Eng. Part A. 2014;0:1–13. doi: 10.1089/ten.tea.2013.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bell CC, et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016;6:25187. doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kostadinova R, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2013;268:1–16. doi: 10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 136.Gunness P, et al. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies. Toxicol. Sci. 2013;133:67–78. doi: 10.1093/toxsci/kft021. [DOI] [PubMed] [Google Scholar]
- 137.Roy P, Washizu J, Tilles AW, Yarmush ML, Toner M. Effect of flow on the detoxification function of rat hepatocytes in a bioartificial liver reactor. Cell Transplant. 2001;10:609–614. [PubMed] [Google Scholar]
- 138.Tostões RM, et al. Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology. 2012;55:1227–1236. doi: 10.1002/hep.24760. [DOI] [PubMed] [Google Scholar]
- 139.Park J, et al. Radial flow hepatocyte bioreactor using stacked microfabricated grooved substrates. Biotechnol. Bioeng. 2008;99:455–467. doi: 10.1002/bit.21572. [DOI] [PubMed] [Google Scholar]
- 140.Vinci B, et al. Modular bioreactor for primary human hepatocyte culture: Medium flow stimulates expression and activity of detoxification genes. Biotechnol. J. 2011;6:554–564. doi: 10.1002/biot.201000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clark AM, et al. A microphysiological system model of therapy for liver micrometastases. Exp. Biol. Med. (Maywood) 2014 doi: 10.1177/1535370214532596. 1535370214532596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clark AM, et al. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip. 2017;17:156–168. doi: 10.1039/c6lc01171c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Powers MJ, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol. Bioeng. 2002;78:257–69. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 144.Domansky K, et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–8. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarkar U, et al. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab. Dispos. 2015;43:1091–9. doi: 10.1124/dmd.115.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Snouber LC, et al. Metabolomics-on-a-chip of hepatotoxicity induced by anticancer drug flutamide and its active metabolite hydroxyflutamide using hepg2/c3a microfluidic biochips. Toxicol. Sci. 2013;132:8–20. doi: 10.1093/toxsci/kfs230. [DOI] [PubMed] [Google Scholar]
- 147.Choucha-Snouber L, et al. Investigation of ifosfamide nephrotoxicity induced in a liver-kidney co-culture biochip. Biotechnol. Bioeng. 2013;110:597–608. doi: 10.1002/bit.24707. [DOI] [PubMed] [Google Scholar]
- 148.Hoffmann Sa, et al. Analysis of drug metabolism activities in a miniaturized liver cell bioreactor for use in pharmacological studies. Biotechnol. Bioeng. 2012;109:3172–3181. doi: 10.1002/bit.24573. [DOI] [PubMed] [Google Scholar]
- 149.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 150.Zhang MY, et al. Microfluidic environment for high density hepatocyte culture. Biomed. Microdevices. 2008;10:117–121. doi: 10.1007/s10544-007-9116-9. [DOI] [PubMed] [Google Scholar]
- 151.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007;97:1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 152.Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem. Pharmacol. 2009;78:625–632. doi: 10.1016/j.bcp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vernetti LA, et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. (Maywood) 2016;241:101–14. doi: 10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Senutovitch N, et al. Fluorescent protein biosensors applied to microphysiological systems. Exp. Biol. Med. (Maywood) 2015;240:795–808. doi: 10.1177/1535370215584934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soto-Gutierrez A, Gough A, Vernetti LA, Taylor DL, Monga SP. Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems. Exp. Biol. Med. (Maywood) 2017;242:1605–1616. doi: 10.1177/1535370217707731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gough A, Vernetti L, Bergenthal L, Shun TY, Taylor DL. The Microphysiology Systems Database for Analyzing and Modeling Compound Interactions with Human and Animal Organ Models. Appl. Vitr. Toxicol. 2016;2:103–117. doi: 10.1089/aivt.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vernetti L, et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep. 2017;7:42296. doi: 10.1038/srep42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu J, et al. Quantitative Systems Pharmacology Approaches Applied to Microphysiological Systems (MPS): Data Interpretation and Multi-MPS Integration. CPT pharmacometrics Syst. Pharmacol. 2015;4:585–94. doi: 10.1002/psp4.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stern AM, Schurdak ME, Bahar I, Berg JM, Taylor DL. A Perspective on Implementing a Quantitative Systems Pharmacology Platform for Drug Discovery and the Advancement of Personalized Medicine. J. Biomol. Screen. 2016;21:521–34. doi: 10.1177/1087057116635818. [DOI] [PMC free article] [PubMed] [Google Scholar]