Abstract
We previously reported that ghrelin prevented l-dopa (LD)-induced inhibition of gastric emptying (GE) of a non-nutrient solution in rats. Parkinson’s disease treatment involves the combined administration of l-dopa with the enzyme l-amino acid decarboxylase inhibitor, carbidopa (CD) to reduce peripheral formation of dopamine. We investigated the effect LD/CD given orogastrically (og) on GE of a non-nutrient or nutrient meal and whether og pretreatment with rikkunshito, a kampo medicine clinically used to treat gastroparesis, influenced LD/CD effect on GE and postprandial antral and duodenal motility in conscious rats. LD/CD (20/2 mg kg−1) decreased significantly GE to 26.3 ± 6.0% compared to 61.2 ± 3.2% in og vehicle monitored 20-min after a non-nutrient meal and to 41.9 ± 5.8% compared to 72.9 ± 5.2% in og vehicle monitored 60 min after a nutrient meal. Rikkunshito (0.5 or 1.0 g kg−1) reduced the LD/CD (20/2 mg kg−1) inhibition of GE of non-nutrient meal (36.9 ± 7.4% and 46.6 ± 4.8% respectively vs. 12.1 ± 7.4% in og vehicle plus LD/CD) while having no effect alone (56.6 ± 8.5%). The ghrelin antagonist, [d-Lys3]-GHRP-6 (1 mg kg−1) injected intraperitoneally partially reversed rikkunshito preventive effect on LD/CD-inhibited GE. Rikkunshito (1.0 g kg−1) blocked LD/CD (20/2 mg kg−1)-induced delayed GE of a nutrient meal and the reduction of postprandial antral motility. In 6-hydroxydopamine-induced Parkinson’s disease rat model, rikkunshito (1.0 g kg−1, og) also prevented LD/CD-inhibited gastric emptying of a nutrient meal and enhanced fasting plasma levels of acylated ghrelin. These data indicate that oral rikkunshito alleviates the delayed GE induced by LD/CD in naïve and PD rat model in part through ghrelin-related mechanisms.
Keywords: Gastric motility, Ghrelin, l-dopa, Parkinson’s disease, Rats, Rikkunshito
1. Introduction
Oral administration of l-dopa (levodopa), a metabolic precursor of dopamine, is regarded as the “gold standard” for the treatment and management of Parkinson’s disease (PD), a pathological condition associated with progressive degeneration of nigrostriatal dopamine pathway [37]. Unlike dopamine, l-dopa crosses the blood brain barrier via a saturable transporter and is converted to dopamine in the brain by the enzyme, l-amino acid decarboxylase (AADC) to produce its therapeutic effect [8]. However, the high pre-systemic metabolism of l-dopa to dopamine within the gut including the stomach by AADC can reduce up to 70% the initial oral dose of l-dopa that will undergo active transport and absorption by the small intestine [8,36]. Therefore, l-dopa administered orally is given in combination with a peripheral AADC inhibitor, commonly, carbidopa (α-methyldopahydrazine) at a ratio of 10/1 or 4/1 to curtail the gastrointestinal conversion of l-dopa to dopamine and consequently maximize l-dopa entry into the brain [11].
One determinant factor that also influences the l-dopa bioavailability is the gastric emptying rate. Clinical studies provided evidence of a relationship between l-dopa pharmacokinetics and gastric emptying in PD patients [15,32]. The oral administration of l-dopa/carbidopa (LD/CD) given in the fasting state or before a low protein meal inhibits gastric emptying in healthy young or elderly volunteers [40–42,57] as well as in PD patients who have already developed delayed solid gastric emptying [12,19,24]. In contrast to clinical studies, there is a paucity of experimental studies on the effects of l-dopa on gastric motor function [53,60] and the influence of peripheral AADC inhibitors administered orally in conjunction with l-dopa is still unknown in rodents.
To improve the management of altered gastric emptying in PD patients treated with anti-parkinsonian drug therapy, a few gastric prokinetic agents such as the serotonin receptor 4 (5-HT4) agonist, cisapride have been shown effective [3,38]. However, the cardiac arrhythmia side effects of cisapride lead to its withdrawal from the market and limited its clinical use [38]. Dopamine antagonists such as domperidone, a dopamine D2 receptor antagonist that does not readily cross the blood brain barrier [23], has been reported to accelerate gastric emptying of a solid meal [39,46] and to increase plasma l-dopa concentration [35] in PD patients treated with l-dopa/AACD inhibitors. However domperidone is not licensed in every country and safety issue has been recently pointed out due to potential cardiotoxic effects at high dose in elderly patients [26]. Another potential candidate is ghrelin (acylated, “active” form), a gut peptide hormone that has potent gastric prokinetic effects [9]. Our recent preclinical studies indicate that ghrelin prevented oral administration of l-dopa-induced delayed gastric emptying of a non-nutrient solution in rats [60]. Stable ghrelin agonists have been reported to improve delayed gastric emptying in various experimental and clinical conditions associated with diabetes, postoperative and morphine-induced ileus and immune challenge [4,7,33,48,56,58]. Likewise, rikkunshito, a Japanese Kampo medicine acting as a ghrelin enhancer [50], alleviates gastroparesis [1,52], dyspepsia [1,25,52,54,61], post-operative gastric ileus [27,62], and gastroesophageal reflux disease [31,34,54] in experimental or clinical studies. A recent pilot clinical study also indicates that rikkunshito can ameliorate gastroparesis in PD patients [14].
Therefore, the objectives of the present study were first to test the influence of orogastric (og) administration of l-dopa in conjunction with carbidopa on gastric emptying of non-nutrient and nutrient meals, and postprandial antro-duodenal motility in conscious rats. Second, to examine whether orally administered rikkunshito ameliorates LD/CD-induced alterations of gastric motor function and whether rikkunshito action involves ghrelin signaling using the receptor antagonist, [d-Lys3]-GHRP-6 [2]. Lastly, we used the 6-hydroxydopamine (6-OHDA) experimental PD model [45] treated with LD/CD to assess the effects of rikkunshito on gastric emptying and plasma ghrelin levels.
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, USA, weighting 280–320 g and Charles River Laboratories Japan, Yokohama, Japan, weighting 230–290 g) were housed 2–4 animals/cage under controlled illumination (12:12 h light/dark cycle) and temperature (22 ± 2 °C) and acclimatized for at least one week before the experiments. Animals were fed standard rodent diet (Pro-lab RMH 2500, LabDiet, PMI Nutrition, Brentwood, MO, USA and MF, Oriental Yeast, Tokyo, Japan) and tap water ad libitum. In other studies, 6-OHDA and vehicle microinjected rats were purchased from Japan SLC (Shizuoka, Japan) 5 weeks after treatment. Eight-weeks old Sprague-Dawley male rats (Japan SLC) were microinjected into the right striatum with either vehicle (0.2% ascorbic acid/saline, 2 µL × 4 sites) or 6-OHDA (Sigma-Aldrich, USA, 3.5 µg µL−1 in 0.2% ascorbic acid/saline, 2 µL × 4 sites) using the following coordinates from bregma: anterior-posterior (+1.3, +0.4, −0.4, −1.3 mm), mediolateral (−2.6, −3.0, −4.2, −4.5 mm) dorsoventral (−5.0 mm). After surgery, all rats were kept one per cage and 4 weeks later, the 6-OHDA rat were tested for behavioral manifestations of PD assessed by more than seven rotations/min in response to a subcutaneous injection of apomorphine (0.5 mg kg−1). One week after the apomorphine test, 6-OHDA and control rats were received at the experimental facilities. They were housed 2/cages and acclimated to similar conditions as the naïve rats for another week before the experiments, and their respective body weight was 380–440 g and 370–460 g.
Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol #06015-08) and Experimental Animal Ethics Committee of Tsumura & Co (animal protocol #12–028 and #12–128).
2.2. Compounds
Rikkunshito, powdered extract consisting of Atractylodis lanceae rhizoma (4 g, 18.6%), Ginseng radix (4 g, 18.6%), Pinelliae tuber (4 g, 18.6%), Hoelen (4 g, 18.6%), Zizyphi fructus (2 g, 9.3%), Aurantii nobilis pericarpium (2 g, 9.3%), Glycyrrhizae radix (1 g, 4.7%), and Zingiberis rhizoma (0.5 g, 2.3%) (Tsumura & Co., Tokyo, Japan) was suspended in water. l-dopa (l-3,4-dihydroxyphenylalanine methyl ester hydrochloride) and s-(−)-carbidopa (CD) were dissolved in vehicle composed of 10% dimethyl sulfoxide, 5% Tween-80 and 85% saline, all from Sigma-Aldrich. [d-Lys3]-GHRP-6 (Phoenix Pharmaceuticals, CA, USA) was dissolved in sterile saline.
2.3. Gastric motor function assessment
2.3.1. Gastric emptying of a non-nutrient meal
Gastric emptying of a non-nutrient meal (1.5% methylcellulose and 0.05% phenol red viscous solution) was determined as described in our previous studies [60]. In brief, rats were fasted overnight (1 rat/cage) for 18–20 h with access to water up to the start of the experiments conducted between 9:00 AM and 1:00 PM. Animals received an orogastric gavage (og) of the viscous solution (1.5 mL) and were euthanized 20 min later by CO2 inhalation followed by thoracotomy. The stomach was removed and homogenized in 100 mL of 0.1 N NaOH using a Polytron (Brinkman Instruments, Westbury, NY). Five milliliters of the supernatant were added to 0.5 mL 20% trichloroacetic acid, centrifuged at 3000 rpm at 4 °C for 20 min and 3 mL of the supernatant added to 4 mL of 0.5 N NaOH. The absorbance of the samples was read at 560 nm (Shimadzu 260 Spectrophotometer). Gastric emptying was calculated as percent emptying = (1 − absorbance of test sample/absorbance of standard) × 100. Phenol red recovered from stomach of rats euthanized immediately after gavage of the same volume of solution served as standard.
2.3.2. Gastric emptying of a nutrient meal
Gastric emptying of nutrient meal was performed as previously described [30]. Twenty four hours-fasted rats (2–4 rats/cage), with free access to water up to the start of the experiments conducted between 1:00 and 4:00 PM, were gavaged with 1 mL of the meal composed of standard powdered chow (32 g, MF; Oriental Yeast, Tokyo, Japan) and 40 g of glass bead (0.2-mm diameter, BZ-02; AS One, Osaka, Japan) in 80 mL of distilled water. Rats were euthanized under isoflurane anesthesia 1 h after the gavage of the meal. The stomach was removed and gastric content recovered, dried and weighed. The gastric emptying was calculated as percent emptying = (1 − dried weight of gastric content/dried weight of 1 mL test meal) × 100.
2.3.3. Antroduodenal motility recording
The procedure was essentially as previously described [17]. Rats were food restricted overnight (two chow pellets ~6 g) and anesthetized with sodium pentobarbital (50 mg kg−1 body weight, Kyoritsu Seiyaku, Tokyo, Japan). After laparotomy, a strain-gauge force transducer (F-08IS; Star Medical, Tokyo, Japan) was sutured to the serosal surface of the antral and duodenal serous membranes to monitor circular muscle contractions. The wire of the transducer was then exteriorized from the back of the neck and protected by Nelaton’s catheter and protective wire. Afterwards, rats were single housed for a 6-day recovery period. Then, antro-duodenal motility was recorded in freely-moving rats that were overnight food restricted to one chow (~3 g) before the study conducted between 10:00 AM and 6:00 PM. The strain-gauge force transducer (previously calibrated by application of 10 or 20 g weights by the manufacturer), was connected to a preamplifier (FS-04 M; Star Medical), through a bridge box (FB-01; Star Medical). Electric signals were recorded into the computer using a MP150 (BIOPAC Systems, Goleta, CA). The system was calibrated before each experiment using the calibrator (Star Medical Equipment, Inc., Japan) and contractions were expressed in grams. The motility index (MI) was determined as the area under the curve (AUC) in the antrum and duodenum for a 60 min period. The percentage of change in MI (%MI) = 100 × (AUC post treatment/AUC pre-treatment).
2.4. Experimental protocols
2.4.1. Influence of LD/CD at various doses on gastric motor function in naïve rats
2.4.1.1. Non-nutrient meal
Overnight fasted rats received an orogastric gavage (0.3 mL/rat) of vehicle or LD/CD (10/1.0, 15/1.5 or 20/2 mg kg−1). Then, 10 min later, all groups received by gavage (1.5 mL/rat) the methylcellulose/phenol red viscous solution and were euthanized 20 min later to assess the % of gastric emptying. LD/CD doses were selected based on previous studies in rats [6,16,60].
2.4.1.2. Nutrient meal
Twenty four hours-fasted rats received an orogastric gavage (0.3 mL/rat) of vehicle or LD/CD (15/1.5, 20/2 and 50/5 mg kg−1). Then, 10 min later all, groups were gavaged with the test meal (1 mL/rat) and 60 min thereafter, euthanized to determine the % of gastric emptying. Based on the dose-response, LD/CD at 20/2 mg kg−1 was selected for all further studies.
2.4.1.3. Postprandial antral-duodenal motility
In rats that were food restricted (~3 g for overnight), a 2-h basal antral and duodenal motility was recorded, then the consecutive treatments were given as illustrated in Fig. 1. First, the nutrient meal (1 mL/rat, og), identical to that used in gastric emptying, was given (1st test meal) followed by 1-h recording of the antro-duodenal phasic contractile activity; the motility index was analyzed as “pre-treatment value”. More than 2 h after the 1st test meal, LD/CD (20/2 mg kg−1) or vehicle (0.3 mL), was administered orally and 10 min later, the 2nd test meal given and post-treatment measurement was recorded for 1 h (“post-treatment value”). The %MI was calculated using “pre-treatment value” and “post-treatment value” and compared between LD/CD treated rats and vehicle-treated rats.
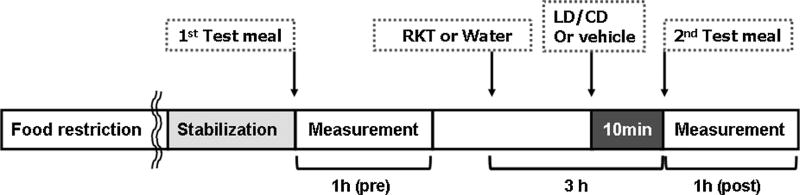
Fig. 1.
Experimental protocol of antral and duodenal motility recording by strain gauge in conscious rats. Rats were implanted with strain gauges on the antrum and duodenum and singly housed for 6 days thereafter. Rats were food-restricted for overnight on the previous day of measurement. The antro-duodenal phasic contractile activity was recorded for 1 h after the 1st test meal administration, then, more than 2 h after, rikkunshito (1.0 g kg−1) or vehicle (water, 10 mL kg−1) was administered orogastrically, followed 2 h 50 min later by that of l-dopa/carbidopa (LD/CD, 20/2 mg kg−1) or vehicle (0.3 mL), and 10 min later, a 2nd test meal was administered and post-treatment measurement was recorded for 1 h.
2.4.2. Influence of rikkunshito on LD/CD-induced alterations of gastric motor function in naïve rats
2.4.2.1. Gastric emptying
Rats received an orogastric gavage of rikkunshito (0.5 or 1.0 g kg−1) or distilled water (3 mL/rat) at 2 h 50 min before that of LD/CD (20/2 mg kg−1) or vehicle (0.3 mL/rat), and the same protocols as previously described in Section 2.4.1. were followed to measure emptying of non-nutrient or nutrient meal. The rikkunshito doses used were based on previous reports in rats showing the prevention N(G)-nitro arginine induced delayed gastric emptying or cisplatin-induced anorexia [21,51]. Based on data obtained under our conditions, the maximal effective dose of 1 g kg−1 was used in all subsequent studies.
2.4.2.2. Postprandial antral-duodenal motility
Rikkunshito (1.0 g kg−1) or vehicle (water) was given per orogastric gavage (10 mL kg−1) more than 2h after the 1st test meal administration, followed 2 h 50 min later by that of LD/CD (20/2 mg kg−1) or vehicle (0.3 mL), and after 10 min a 2nd test meal and post-treatment antral and duodenal contractile activity was recorded for 1 h (Fig. 1). The %MI was compared between water + LD/CD treated rats and rikkunshito + LD/CD treated rats.
2.4.3. Effect of ghrelin receptor antagonist on rikkunshito action in naïve rats
[d-Lys3]-GHRP-6 (1 mg kg−1) or vehicle (sterile saline) was injected intraperitoneally (1 mL kg−1) at the same time as rikkunshito (1.0 g kg−1, og) or distilled water (3 mL/rat, og) and 2 h 50 min later, LD/CD (20/2 mg kg−1) or vehicle (0.3 mL/rat) was gavaged. The methylcellulose/phenol red solution (1.5 mL) was given 10 min later in all groups and gastric emptying was measured 20 min thereafter. The regimen of ghrelin receptor antagonist administration was based on our previous studies showing the blockade of ip ghrelin-induced stimulation of gastric emptying [47].
2.4.4. Effect of rikkunshito on gastric emptying of nutrient meal and plasma ghrelin levels in 6-OHDA rats treated acutely with LD/CD
2.4.4.1. Gastric emptying
In 24-h fasted rats microinjected with 6-OHDA or vehicle into the striatum 6 weeks before, rikkunshito (1.0 g kg−1) or distilled water (10 mL kg−1) was administered og 2 h 50 min before that of LD/CD (20/2 mg kg−1) or vehicle (0.3 mL/rat). Then 10 min later, all groups were gavaged with the nutrient meal (1 mL/rat) and 60 min thereafter, euthanized for the determination of gastric emptying.
2.4.4.2. Plasma ghrelin
After 24-h food deprivation, rats received orogastric gavage of rikkunshito (1.0 g kg−1) or vehicle (distilled water, 10 mL kg−1), 2 h 50 min later, LD/CD (20/2 mg kg−1) or vehicle (0.3 mL/rat) was administered orally and 30 min after, rats were decapitated and trunk blood was collected in tubes containing EDTA-2K, 1.6 mg mL−1 (Dojindo Laboratories, Kumamoto, Japan) and protease inhibitor cocktail (Sigma-Aldrich, P2714). Samples were promptly centrifuged at 4 °C, and the supernatant was acidified with 1 N HCl (1/10 volume) and stored at −80 °C until use. The plasma acyl ghrelin level was determined using the Active ghrelin ELISA Kit (Mitsubishi Chemical Medience, Tokyo, Japan).
2.5. Statistical analysis
Data are expressed as mean ± SEM and analyzed by one-way or two-way ANOVA followed by Student-Newman-Keuls post hoc test or Student’s t-test after the F-test. Differences in multiple groups’ mean values were assessed by Dunnett’s test after Bartlett test. A p value <0.05 was considered statistically significant.
3. Results
3.1. LD/CD decreases dose-dependently gastric emptying in naïve rats
Oral administration of LD/CD (15/1.5 or 20/2 mg kg−1) 10 min before that of the non-nutrient meal reduced dose-dependently the percentage of gastric emptying compared to vehicle as monitored 20 min later (40.0 ± 4.8% and 26.3 ± 6.0% respectively vs. 61.2 ± 3.2%, n = 9–11, p < 0.05) while a lower dose 10/1 mg kg−1 had no effect (64.5 ± 2.5%, n = 3; Fig. 2A). Likewise, LD/CD (20/2 and 50/5 mg kg−1, og) administered 10 min before a nutrient meal, dose-dependently decreased the percentage of gastric emptying monitored 60 min later compared with og vehicle (41.9 ± 5.8% and 28.9 ± 5.6% respectively vs. vehicle 72.9 ± 5.2%, n = 8–11, p < 0.05; Fig. 2B) while the lower dose 15/1.5 mg kg−1 did not result in a significant change (gastric emptying: 58.1 ± 4.1%, n = 10). Based on the dose-response, LD/CD at 20/2 mg kg−1 administered og was selected for all subsequent studies.
Fig. 2.
Inhibition of gastric emptying of non-nutrient or nutrient meal by l-dopa/carbidopa in overnight fasted rats. l-dopa/carbidopa was administered at different doses by orogastric gavage (0.3 mL) and 10 min later, the viscous non-nutrient solution was administered by orogastric gavage (1.5 mL) and 20 min later gastric emptying was measured (A); or a powdered chow + beads were administered per gavage (1.0 mL) and 60 min later, gastric emptying was measured (B). Data are mean ± SEM of animal numbers indicated at the bottom of each column. *p < 0.05 vs. respective vehicles (0/0).
3.2. Rikkunshito prevents LD/CD-induced delayed gastric emptying in naïve rats
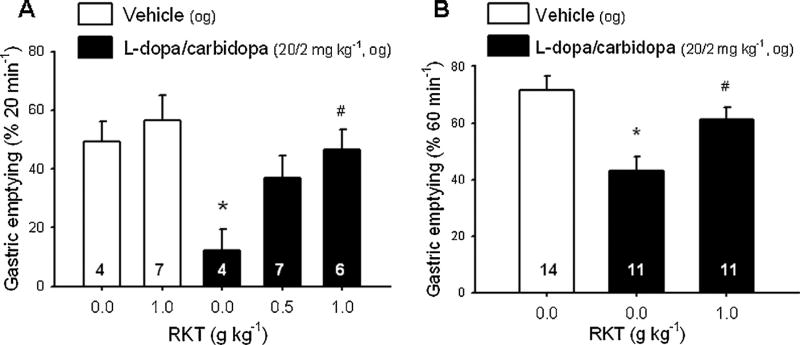
In vehicle pretreated (og) rats, LD/CD significantly decreased the percentage of gastric emptying of a non-nutrient meal compared to og vehicles (12.1 ± 7.4% vs. 49.3 ± 7.0%, n = 4, p < 0.05; Fig. 3A). Rikkunshito given og prevented the LD/CD inhibitory effect as shown by the trend to increase gastric emptying values to 36.9 ± 7.4% (p > 0.05) at 0.5 g kg−1 and the significant increase to 46.6 ± 6.8% at 1.0 g kg−1 (n = 6–7/group, p < 0.05) compared to vehicle plus LD/CD (Fig. 3A). Rikkunshito at 1.0 g kg−1 had no effect on basal gastric emptying (56.6 ± 8.5%, n = 7; Fig. 3A). Two-way ANOVA showed significant influence of LD/CD (F1.17 = 7.98; p < 0.05) and RKT at 1.0 g kg−1 (F1.17 = 6.23; p < 0.05). Likewise, in og vehicle-pretreated group, LD/CD reduced the percentage of gastric emptying of a nutrient meal to 40.9 ± 5.2% compared to 70.5 ± 5.2%, in og vehicles (n = 11–14, p < 0.05). Rikkunshito pretreatment (1.0 g kg−1, og) prevented LD/CD inhibitory effect (gastric emptying: 60.0 ± 4.3%, n = 11, p < 0.05; Fig. 3B). Rikkunshito at 1.0 g kg−1, og, was used in all subsequent studies.
Fig. 3.
Rikkunshito (RKT) prevents l-dopa/carbidopa-induced delayed gastric emptying of non nutrient meal (A) and nutrient meal (B) in fasted rats. Orogastric gavages (og) of RKT (0.5 or 1.0 g kg−1) or vehicle (distilled water) was performed in overnight fasted rats 2 h 50 min before that of l-dopa/carbidopa (20/2 mg kg−1) or vehicle and 10 min later, the viscous non-nutrient solution was administered (1.5 mL, og) and after 20 min, gastric emptying was measured (A); or a powdered chow + beads were administered per gavage (1.0 mL) and 60 min later, gastric emptying was monitored (B). Data are mean ± SEM of animal numbers indicated at the bottom of each bar, *p < 0.05 vs. distilled water (0.0)-vehicle and # p < 0.05 vs. distilled water-l-dopa/carbidopa.
3.3. Rikkunshito prevents LD/CD-induced decreased postprandial antral and duodenal motility in naïve rats
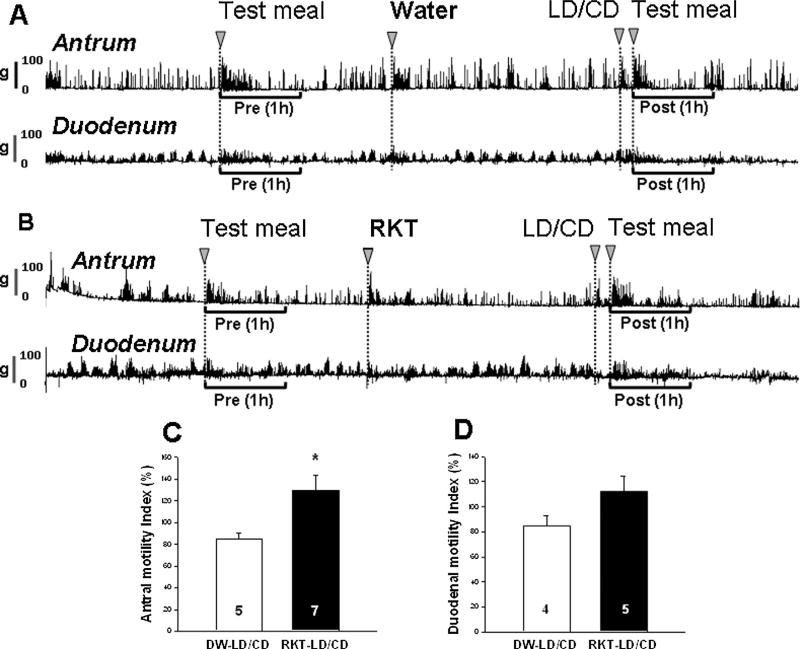
The contractile activity recorded from strain gauge transducers in the antrum and duodenum in food restricted freely moving rats consisted of cyclic waves of Phase III-like contractions (Fig. 4A and B). The 1st test meal administration induced transient contractions in the antrum followed by fed motor activities in the antrum and duodenum which returned to the fasted motor activities 2 h after test meal administration (Fig. 4A and B). LD/CD significantly decreased the %MI (86.1 ± 3.3% vs. 99.7 ± 3.9%, n = 5–7/group, p < 0.05; data not shown). Rikkunshito pretreatment significantly increased %MI in the antrum compared with vehicle pretreatment in LD/CD treated rats (130.2 ± 13.4% vs. 84.7 ± 5.5%, n = 4–5/group, p < 0.05; Fig. 4A and C). Likewise, LD/CD gavage reduced the duodenal %MI compared to vehicle (86.6 ± 6.4% vs. 116.0 ± 7.0%, n = 3/group, p < 0.05; data not shown), and rikkunshito pretreatment tends to attenuate the inhibition by LD/CD compared to vehicle pretreatment although it did not reach statistical significance (112.6 ± 11.5% vs. 85.0 ± 7.7%, n = 4/group, p = 0.09; Fig. 4B and D).
Fig. 4.
Rikkunshito (RKT) prevents l-dopa/carbidopa (LD/CD)-induced reduction of postprandial antral and duodenal motility in conscious rats measured by strain gauge transducer. Single housed rats with strain gauge implanted 6 days earlier, were food restricted (~3 g/rat) overnight before the experiment. After the recording was stabilized (time 0), the 1st test meal was gavaged and 1 h pretreatment motility was recorded. Vehicle or RKT (1.0 g kg−1) was gavaged at 2 h 50 min before that of LD/CD (20/2 mg kg−1) and 10 min later the 2nd test meal was given and postprandial motility was recorded for 1 h: representative original traces in the antrum and duodenum in vehicle (A) or RKT pretreated (B), LD/CD treated rats; motility index (%MI) in the antrum (C; n = 5–7) and duodenum (D; n = 4–5) showed significant increase by RKT compared with vehicle (distilled water, DW) before LD/CD. Data are mean ± SEM. *p < 0.05 vs. each DW group.
3.4. Ghrelin antagonist blunts rikkunshito effect on LD/CD-induced delayed gastric emptying in naïve rats
The ghrelin antagonist, [d-Lys3]-GHRP-6 (1 mg kg−1, ip) did not modify basal gastric emptying of non-nutrient meal compared with ip vehicle + og vehicle treated rats (62.0 ± 5.1% vs. 61.1 ± 3.5%, n = 5 and 9, p > 0.05; Fig. 5). LD/CD inhibited significantly gastric emptying in rats pretreated with ip vehicles (22.5 ± 7.4%, n = 7, p < 0.05) and this effect was prevented by rikkunshito (50.3 ± 4.1%, n = 6, p < 0.05). [d-Lys3]-GHRP-6 injected ip partially reversed rikkunshito-induced prevention of the delayed gastric emptying induced by LD/CD (32.2 ± 6.3%, p < 0.05 vs. 50.3 ± 4.1% ip vehicle-rikkunshito-LD/CD; n = 6/group, Fig. 5).
Fig. 5.
Ghrelin antagonist (GA), [d-Lys3]-GHRP-6 blunts rikkunshito (RKT) preventive effect on l-dopa/carbidopa (LD/CD)-induced delayed gastric emptying of non-nutrient viscous solution in fasted rats. [d-Lys3]-GHRP-6 (1 mg kg−1) or vehicle (saline, 1 mL kg−1) was injected intraperitoneally immediately before oral gavage of rikkunshito (1.0 g kg−1) or vehicle and 2 h 50 min later LD/CD (20/2 mg kg−1) or vehicle was gavaged. Ten minutes thereafter, the viscous non-nutrient solution was administered by orogastric gavage and gastric emptying was measured 20 min later. Animal numbers per group are shown at the bottom of each bar. Data are mean ± SEM. *p < 0.05 vs. vehicle-DW-vehicle; #p < 0.05 between vehicle-RKT-LD/CD and GA-RKT-LD/CD. §p < 0.05 vs. vehicle-RKT-LD/CD.
3.5. Rikkunshito prevents the delayed gastric emptying and increased plasma ghrelin levels in 6-OHDA PD rats treated with LD/CD
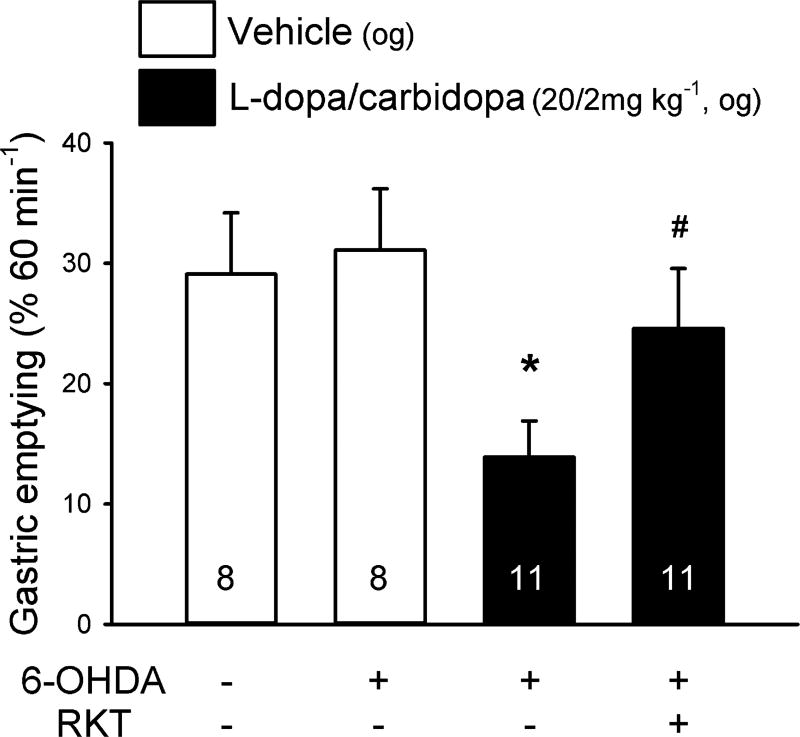
In vehicle pretreated (og) rats, the 60 min gastric emptying of nutrient meal was not different between vehicle and 6-OHDA microinjected rats (29.1% ± 5.1% vs. 31.1 ± 5.1%, n = 8/group, Fig. 6). LD/CD (20/2 mg kg−1, og) decreased gastric emptying of a nutrient meal compared to og vehicle in 6-OHDA rats (13.9 ± 3.0% vs. 31.1 ± 5.1%, n = 8–11/group, p < 0.05, Fig. 6). Rikkunshito prevented the LD/CD inhibitory effect on gastric emptying (24.6 ± 4.0%, n = 11/group, p < 0.05 vs. vehicle plus LD/CD) in the 6-OHDA rats (Fig. 6) while not influencing basal gastric emptying compared to og vehicle in 6-OHDA treated rats (water: 48.6 ± 4.6% vs. rikkunshito: 46.5 ± 4.4%, n = 9–10; data not shown).
Fig. 6.
Rikkunshito (RKT) prevents l-dopa/carbidopa-induced delayed gastric emptying of nutrient meal in fasted 6-OHDA induced Parkinson’s disease model rats. At 6 weeks after 6-OHDA or vehicle microinjection into the right striatum, orogastric gavages (og) of RKT (1.0 g kg−1) or distilled water (DW) was performed in 24-h fasted rats 2 h 50 min before that of l-dopa/carbidopa (20/2 mg kg−1) or vehicle followed 10 min later by the og nutrient meal. Gastric emptying was monitored at 60 min. Data are mean ± SEM of animal numbers indicated at the bottom of each bar. *p < 0.05 vs. distilled water (DW)-vehicle and #p < 0.05 vs. DW-LD/CD.
In vehicle pretreated (og) rats, fasting plasma levels of acylated ghrelin were not different between vehicle and 6-OHDA microinjected rats (49.3 ± 3.4 fmol mL−1 vs. 44.4 ± 4.1 fmol mL−1, Fig. 7). LD/CD (20/2 mg kg−1, og) did not alter plasma acylated ghrelin levels assessed 30 min later in 6-OHDA microinjected rats (46.5 ± 2.4 fmol mL−1, Fig. 7). Rikkunshito pretreatment significantly increased plasma acylated ghrelin levels by 28.9% compared to vehicle in LD/CD administered 6-OHDA rats (59.8 ± 3.6 fmol mL−1, p < 0.05 vs. vehicle plus LD/CD).
Fig. 7.
Rikkunshito (RKT) enhanced plasma acylated ghrelin levels in l-dopa/carbidopa-administered 6-OHDA induced Parkinson’s disease model in 24-h fasted rats. Orogastric gavages of RKT (1.0 g kg−1) or distilled water (DW) was performed in 24-h fasted rats 2 h 50 min before that of l-dopa/carbidopa (20/2 mg kg−1) or vehicle followed 30 min later by decapitation for trunk blood collection. Data are mean ± SEM of animal numbers indicated at the bottom of each bar, *:p < 0.05 vs. distilled water (DW)-LD/CD.
4. Discussion
In this study, we demonstrated that LD/CD delayed gastric emptying of non-nutrient and nutrient meal and reduced postprandial antro-duodenal motility in naïve rats. We also showed that rikkunshito prevented LD/CD-induced inhibition of gastric propulsive motor function and its action involved in part ghrelin receptor signaling as shown by the use of ghrelin antagonist. In addition, in the 6-OHDA PD rat model, rikkunshito prevented LD/CD delayed gastric emptying and induces a small but significant elevation of circulating ghrelin.
The orogastric co-administration of LD/CD (15/1.5 or 20/2 mg kg−1) 10 min before a non-nutrient meal in overnight fasted rats reduced dose-dependently the 20-min gastric emptying by 35% and 57% respectively from that of vehicle-treated rats, while the dose of 10/1.0 mg kg−1 was ineffective. Previously, we reported that l-dopa given alone at 5 or 15 mg kg−1 inhibited gastric emptying of a non-nutrient meal by 33% and 61% respectively from that of vehicle group when tested under otherwise similar conditions [60]. Taken together these data indicate that the coadministration of CD with l-dopa reduces the potency of l-dopa to suppress gastric emptying of non-nutrient meal. This is likely to reflect the decreased formation of dopamine established to suppress gastric emptying through activation of peripheral D2/D3 receptors in rats [20,22,64]. Indeed, the peripheral AADC inhibitor, CD given in conjunction with l-dopa reduces plasma dopamine levels while increasing those of l-dopa measured 30–90 min after their oral administration at 10/1.5 ratio (200/25 mg kg−1) in rats [44] and in humans [43]. Moreover, in humans the dopamine D2 receptor antagonists, metoclopramide and domperidone inhibit LD/CD-induced delayed gastric emptying of a meal in healthy subjects and PD patients [5,39,46].
We also provide the first experimental evidence that oral administration of LD/CD (15/1.5, 20/2.0 or 50/5.0 mg kg−1) suppressed dose-dependently the gastric emptying of a nutrient meal by 20%, 43% and 60% respectively from that of vehicle-treated rats. This is associated with the reduction of postprandial antral motility in freely moving rats which is likely to contribute in delaying emptying. Previous studies in dogs showed that l-dopa infused intravenously during the postprandial state suppressed gastric body, antrum and pylorus contractile activity within 10 min, an effect reversed by D2 antagonist [53]. The higher dose (50/5 vs. 20/2 mg kg−1) required for LC/CD to induce similar magnitude of gastric emptying suppression of nutrient vs. non-nutrient meal may be related to the longer duration (60 vs. 20 min) needed for the evaluating the transit of a nutrient meal. Consistent with these experimental studies, in several clinical reports, CD used at a similar dose as in the present study (1.3 mg kg−1) that induces 80% inhibition of l-dopa decarboxylation in healthy young human subjects [42,43], also delayed gastric emptying of a solid meal.
In addition, we showed in the 6-OHDA rats which is an established PD model [45], that orogastric LD/CD (20/2 mg kg−1) treatment also reduced significantly gastric emptying of solid nutrient by 56% from that in og vehicle. It is to note that in either vehicle or 6-OHDA rats, basal gastric emptying is lower than those in naïve rats. It may be related to the prior brain surgery linked with microinjection of vehicle or 6-OHDA into the striatum and subsequent single housing for 4 weeks which are known stressors to delay gastric emptying [49]. The reduction of gastric emptying by LD/CD in our PD model is consistent with clinical studies in PD patients treated with LD/CD [12,19,24]. There is also evidence that LD/CD can alter gastric propulsive motility per se in addition to the already impaired gastric transit in PD patients [13,19]. However in our 6-OHDA rat PD model which had motor symptom of dopamine alterations [45], the gastric emptying assessed 60 min after of nutrient meal was not different from that of vehicle microinjected group. We previously reported in α-synuclein over-expressing mice, a genetic PD model that there was no change in gastric emptying of nutrient or non nutrient meal while the animals display constipation-like feature of PD [59]. However recent studies indicate that the 6-OHDA rat PD model showed delayed gastric emptying of solid food after a fast [65,66]. This discrepancy may be related to differences in the experimental protocols of assessing gastric emptying between our and their studies (powder vs. regular chow, 1 h vs. 2–4 h monitoring) or the mode and site of 6-OHDA administration (unilateral 28 µg into the striatum vs. bilateral 4 µg/site or unilateral 24 µg into the substantia nigra). However collectively, these data showed that the oral use of LD/CD at 20/2 mg kg−1 impaired gastric propulsive motor function in both naïve and 6-OHDA PD rats providing a relevant experimental model to study the effects of drugs that may circumvent the altered gastric transit associated with antiparkinsonian treatments.
We showed that og pretreatment with rikkunshito (1 g kg−1) normalized gastric emptying of nutrient and non-nutrient meal that was inhibited by LD/CD in naïve rats. At the 0.5 g kg−1 og dose, rikkunshito effect did not reach significance as previously reported under conditions of intraperitoneal injection of dopamine-induced delayed gastric emptying in rats [55]. The normalization of gastric emptying by rikkunshito pretreatment is likely to be related to improve antral motility impaired by LD/CD. This is supported by the restoration of postprandial contractile activity prominently in the antrum reduced by the oral administration of LD/CD. In other experimental studies, oral administration of rikkunshito was reported to prevent the reduction of gastric emptying of a non-nutrient solution induced by oral administration of nitric oxide synthase inhibitor [21] and intraperitoneal injection of 5-HT reuptake inhibitors in naïve rats [17]. Our data also indicate that in 6-OHDA PD model, rikkunshito can normalize the delayed gastric emptying of a nutrient meal induced by LD/CD. This data may have translational application as in a recent pilot, open-label clinical study, rikkunshito was reported to ameliorate gastro-paresis in 20 PD patients of whom 16 were treated with LD/CD [14].
The underlying mechanisms through which rikkunshito improves gastric propulsive motor functions inhibited by LD/CD may be mediated in part by increasing ghrelin signaling, a well established prokinetic peptide [9]. Convergent evidence indicates that orally administered rikkunshito increases ghrelin availability. This is achieved by stimulating basal or inhibited ghrelin secretion in rodents [29,51], dogs [63] as well as in humans [29], and by inhibiting the deacylation of the peptide leading to an increase ratio of active acylated vs. inactive deacylated form of the peptide in rodents and humans [51]. In addition, in vitro studies indicate that rikkunshito enhances ghrelin receptor binding and signaling [18]. In the present study, we showed that the peripheral injection of the ghrelin antagonist, [d-Lys3]-GHRP-6 partially blocked rikkunshito effect to normalize gastric emptying in LD/CD treated naïve rats indicative of an action dependent from ghrelin receptor. We previously found that l-dopa injected intravenously at a dose reducing gastric emptying, did not alter plasma levels of acylated ghrelin monitored at 30 and 60 min post injection [60]. Likewise, in the present study, LD/CD did not alter fasted levels of acyl ghrelin in 6-OHDA rats. However we recently reported that peripheral injection of ghrelin prevents l-dopa-induced delay gastric emptying in rats establishing the ability of enhanced ghrelin receptor activation to block l-dopa inhibitory effect [60]. In the present study, rikkunshito significantly increased by 28.6% plasma acylated ghrelin levels in LD/CD administered 6-OHDA PD rats. However of note since the restorative effect of rikkunshito on LD/CD induced delayed gastric emptying in naïve rats was only partly abolished by ghrelin receptor antagonist, and the rise in circulating ghrelin induced by rikkunshito is rather modest, it is likely that rikkunshito action may involve additional mechanisms. A recent report indicates that Atractylodes lancea rhizome, one of the component contained in rikkunshito, prevents the delayed gastric emptying induced by ip dopamine in mice [22] suggesting an interaction with peripheral D2 receptors which remained to be explored.
In summary, we provided evidence that og administration of LD/CD impaired gastric propulsive motor function in naïve rats and 6-OHDA PD models in rats. The og retreatment with rikkunshito prevented LD/CD inhibitory effect through an action that in part medicated through increased ghrelin signaling and other mechanisms yet to be elucidated. In the context of limited therapeutic options to treat gastroparesis in PD patients [28] and the negative impact of delayed gastric emptying on the absorption of l-dopa in PD and related response fluctuations [10,15,32], rikkunshito warrants further investigations as adjunct therapy in PD patients in randomized double blind clinical studies [14].
Acknowledgments
This work was supported by Tsumura & Co., NIHDDK-41303 (Animal core, YT, LW), Fox Foundation Target Validation grant (LW, YT), and Veterans Administration Research Career Scientist Award (YT). We are grateful to Mrs. Honghui Liang for excellent technical support and we thank Ms. Eugenia Hu for reviewing the manuscript.
Abbreviations
- AUC
area under the curve
- AADC
l-amino acid decarboxylase
- DW
distilled water
- GE
gastric emptying
- LD/CD
l-dopa/carbidopa
- og
orogastric or orogastrically
- %MI
percentage change in motility index
- 6-OHDA
6-hydroxydopamine
- PD
Parkinson’s disease
Footnotes
Conflict of interest
Drs. S. Mogami, C. Yamada and T. Hattori are employees of Tsumura & Co.
Author contributions
Conceived and designed the experiments: LW, SM, TH, KY, YT. Performed the experiments: LW, HK, CY, SY. Analyzed the data: LW, SM, HK, YT. Contributed reagents/materials/analysis tools: SM, TH, YT. Wrote the paper: LW, YT; Reviewed the paper: SM, HK, KY, TH.
References
- 1.Arai M, Matsumura T, Tsuchiya N, Sadakane C, Inami R, Suzuki T, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology. 2012;59:62–6. doi: 10.5754/hge11246. [DOI] [PubMed] [Google Scholar]
- 2.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675–80. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 3.Asai H, Udaka F, Hirano M, Minami T, Oda M, Kubori T, et al. Increased gastric motility during 5-HT4 agonist therapy reduces response fluctuations in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11:499–502. doi: 10.1016/j.parkreldis.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25:720–32. doi: 10.1111/nmo.12193. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz DM, McCallum RW. Interaction of levodopa and metoclopramide on gastric emptying. Clin Pharmacol Ther. 1980;27:414–20. doi: 10.1038/clpt.1980.55. [DOI] [PubMed] [Google Scholar]
- 6.Bredberg E, Lennernas H, Paalzow L. Pharmacokinetics of levodopa and carbidopa in rats following different routes of administration. Pharm Res. 1994;11:549–55. doi: 10.1023/a:1018970617104. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6:343–52. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. 2010;257:S253–61. doi: 10.1007/s00415-010-5728-8. [DOI] [PubMed] [Google Scholar]
- 9.de Smet B, Mitselos A, Depoortere I. Motilin and ghrelin as prokinetic drug targets. Pharmacol Ther. 2009;123:207–23. doi: 10.1016/j.pharmthera.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Deleu D, Ebinger G, Michotte Y. Clinical and pharmacokinetic comparison of oral and duodenal delivery of levodopa/carbidopa in patients with Parkinson’s disease with a fluctuating response to levodopa. Eur J Clin Pharmacol. 1991;41:453–8. doi: 10.1007/BF00626368. [DOI] [PubMed] [Google Scholar]
- 11.Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson’s disease. Clin Pharmacokinet. 2002;41:261–309. doi: 10.2165/00003088-200241040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Djaldetti R, Baron J, Ziv I, Melamed E. Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology. 1996;46:1051–4. doi: 10.1212/wnl.46.4.1051. [DOI] [PubMed] [Google Scholar]
- 13.Djaldetti R, Ziv I, Melamed E. Impaired absorption of oral levodopa: a major cause for response fluctuations in Parkinson’s disease. Isr J Med Sci. 1996;32:1224–7. [PubMed] [Google Scholar]
- 14.Doi H, Sakakibara R, Sato M, Hirai S, Masaka T, Kishi M, et al. Dietary herb extract rikkunshi-to ameliorates gastroparesis in Parkinson’s disease: a pilot study. Eur Neurol. 2014;71:193–5. doi: 10.1159/000355608. [DOI] [PubMed] [Google Scholar]
- 15.Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, et al. Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J Neurol Sci. 2012;319:86–8. doi: 10.1016/j.jns.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 16.El Moursy SA, Shawky HM, Abdel WZ, Rashed L. The effect of memantine and levodopa/carbidopa on the responses of phrenic nerve-diaphragm preparations from aged rats. Med Sci Monit. 2009;15:BR339–48. [PubMed] [Google Scholar]
- 17.Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, et al. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry. 2009;65:748–59. doi: 10.1016/j.biopsych.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. doi: 10.1038/tp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041–7. doi: 10.1002/mds.1203. [DOI] [PubMed] [Google Scholar]
- 20.Kashyap P, Micci MA, Pasricha S, Pasricha PJ. The D2/D3 agonist PD128907 (R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4-oxazin-9-ol) inhibits stimulated pyloric relaxation and spontaneous gastric emptying. Dig Dis Sci. 2009;54:57–62. doi: 10.1007/s10620-008-0335-6. [DOI] [PubMed] [Google Scholar]
- 21.Kido T, Nakai Y, Kase Y, Sakakibara I, Nomura M, Takeda S, et al. Effects of rikkunshito, a traditional Japanese medicine, on the delay of gastric emptying induced by N(G)-nitro-l-arginine. J Pharmacol Sci. 2005;98:161–7. doi: 10.1254/jphs.fpj04056x. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Sumiyoshi M. Effects of an Atractylodes lancea rhizome extract and a volatile component beta-eudesmol on gastrointestinal motility in mice. J Ethnopharmacol. 2012;141:530–6. doi: 10.1016/j.jep.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Kohli JD, Glock D, Goldberg LI. Selective DA2 versus DA1 antagonist activity of domperidone in the periphery. Eur J Pharmacol. 1983;89:137–41. doi: 10.1016/0014-2999(83)90618-0. [DOI] [PubMed] [Google Scholar]
- 24.Kurlan R, Rothfield KP, Woodward WR, Nutt JG, Miller C, Lichter D, et al. Erratic gastric emptying of levodopa may cause random fluctuations of parkinsonian mobility. Neurology. 1988;38:419–21. doi: 10.1212/wnl.38.3.419. [DOI] [PubMed] [Google Scholar]
- 25.Kusunoki H, Haruma K, Hata J, Ishii M, Kamada T, Yamashita N, et al. Efficacy of rikkunshito, a traditional Japanese medicine (Kampo), in treating functional dyspepsia. Intern Med. 2010;49:2195–202. doi: 10.2169/internalmedicine.49.3803. [DOI] [PubMed] [Google Scholar]
- 26.Lertxundi U, Domingo-Echaburu S, Soraluce A, Garcia M, Ruiz-Osante B, Aguirre C. Domperidone in Parkinson’s disease: a perilous arrhythmogenic or the gold standard. Curr Drug Saf. 2013;8:63–8. doi: 10.2174/1574886311308010009. [DOI] [PubMed] [Google Scholar]
- 27.Li RX, Zhou Y, Li JL, Li J, Chen Y. Clinical study on application of Chinese herbs during the perioperative period of laparoscopic cholecystectomy. Chin J Integr Med. 2007;13:59–61. doi: 10.1007/s11655-007-0059-z. [DOI] [PubMed] [Google Scholar]
- 28.Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23–32. doi: 10.1002/mds.25708. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura T, Arai M, Yonemitsu Y, Maruoka D, Tanaka T, Suzuki T, et al. The traditional Japanese medicine rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol. 2010;45:300–7. doi: 10.1007/s00535-009-0166-z. [DOI] [PubMed] [Google Scholar]
- 30.Mogami S, Suzuki H, Fukuhara S, Matsuzaki J, Kangawa K, Hibi T. Reduced ghrelin production induced anorexia after rat gastric ischemia and reperfusion. Am J Physiol Gastrointest Liver Physiol. 2012;302:G359–64. doi: 10.1152/ajpgi.00297.2011. [DOI] [PubMed] [Google Scholar]
- 31.Morita T, Furuta K, Adachi K, Ohara S, Tanimura T, Koshino K, et al. Effects of rikkunshito (TJ-43) on esophageal motor function and gastroesophageal reflux. J Neurogastroenterol Motil. 2012;18:181–6. doi: 10.5056/jnm.2012.18.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller T, Erdmann C, Bremen D, Schmidt WE, Muhlack S, Woitalla D, et al. Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin Neuropharmacol. 2006;29:61–7. doi: 10.1097/00002826-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–8. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahata M, Muto S, Oridate N, Ohnishi S, Nakagawa K, Sadakane C, et al. Impaired ghrelin signaling is associated with gastrointestinal dysmotility in rats with gastroesophageal reflux disease. Am J Physiol Gastrointest Liver Physiol. 2012;303:G42–53. doi: 10.1152/ajpgi.00462.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa N, Nagai M, Tsujii T, Iwaki H, Yabe H, Nomoto M. Coadministration of domperidone increases plasma levodopa concentration in patients with Parkinson disease. Clin Neuropharmacol. 2012;35:182–4. doi: 10.1097/WNF.0b013e3182575cdb. [DOI] [PubMed] [Google Scholar]
- 36.Nissinen E, Tuominen R, Perhoniemi V, Kaakkola S. Catechol-O-methyl-transferase activity in human and rat small intestine. Life Sci. 1988;42:2609–14. doi: 10.1016/0024-3205(88)90330-x. [DOI] [PubMed] [Google Scholar]
- 37.Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010;5:229–38. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quigley EM. Cisapride: what can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011;12:147–56. doi: 10.1111/j.1751-2980.2011.00491.x. [DOI] [PubMed] [Google Scholar]
- 39.Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol. 2007;102:2036–45. doi: 10.1111/j.1572-0241.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 40.Robertson DR, Higginson I, Macklin BS, Renwick AG, Waller DG, George CF. The influence of protein containing meals on the pharmacokinetics of levodopa in healthy volunteers. Br J Clin Pharmacol. 1991;31:413–7. doi: 10.1111/j.1365-2125.1991.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson DR, Renwick AG, Macklin B, Jones S, Waller DG, George CF, et al. The influence of levodopa on gastric emptying in healthy elderly volunteers. Eur J Clin Pharmacol. 1992;42:409–12. doi: 10.1007/BF00280127. [DOI] [PubMed] [Google Scholar]
- 42.Robertson DR, Renwick AG, Wood ND, Cross N, Macklin BS, Fleming JS, et al. The influence of levodopa on gastric emptying in man. Br J Clin Pharmacol. 1990;29:47–53. doi: 10.1111/j.1365-2125.1990.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson DR, Wood ND, Everest H, Monks K, Waller DG, Renwick AG, et al. The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol. 1989;28:61–9. doi: 10.1111/j.1365-2125.1989.tb03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose S, Jenner P, Marsden CD. The effect of carbidopa on plasma and muscle levels of l-dopa, dopamine, and their metabolites following l-dopa administration to rats. Mov Disord. 1988;3:117–25. doi: 10.1002/mds.870030203. [DOI] [PubMed] [Google Scholar]
- 45.Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res. 2007;11:151–67. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 46.Soykan I, Sarosiek I, Shifflett J, Wooten GF, McCallum RW. Effect of chronic oral domperidone therapy on gastrointestinal symptoms and gastric emptying in patients with Parkinson’s disease. Mov Disord. 1997;12:952–7. doi: 10.1002/mds.870120618. [DOI] [PubMed] [Google Scholar]
- 47.Stengel A, Goebel-Stengel M, Wang L, Luckey A, Hu E, Rivier J, et al. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol Motil. 2011;23:e294–308. doi: 10.1111/j.1365-2982.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stengel A, Taché Y. Ghrelin: new insight to mechanisms and treatment of postoperative gastric ileus. Curr Pharm Des. 2011;17:1587–93. doi: 10.2174/138161211796196990. [DOI] [PubMed] [Google Scholar]
- 49.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, et al. Rikkun-shito as aghrelin enhancer. Methods Enzymol. 2012;514:333–51. doi: 10.1016/B978-0-12-381272-8.00021-0. [DOI] [PubMed] [Google Scholar]
- 51.Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, et al. Rikkun-shito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–13. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 52.Tatsuta M, Iishi H. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther. 1993;7:459–62. doi: 10.1111/j.1365-2036.1993.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 53.Tazawa S, Masuda N, Koizumi T, Kitazawa M, Nakane T, Miyata H. KDR-5169, a new gastrointestinal prokinetic agent, enhances gastric contractile and emptying activities in dogs and rats. Eur J Pharmacol. 2002;434:169–76. doi: 10.1016/s0014-2999(01)01543-6. [DOI] [PubMed] [Google Scholar]
- 54.Tominaga K, Arakawa T. Kampo medicines for gastrointestinal tract disorders: a review of basic science and clinical evidence and their future application. J Gastroenterol. 2013;48:452–62. doi: 10.1007/s00535-013-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tominaga K, Kido T, Ochi M, Sadakane C, Mase A, Okazaki H, et al. The traditional japanese medicine rikkunshito promotes gastric emptying via the antagonistic action ofthe5-HT(3) receptor pathway in rats. Evid Based Complement Alternat Med. 2011;2011:248481. doi: 10.1093/ecam/nep173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–52. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 57.Waller DG, Roseveare C, Renwick AG, Macklin B, George CF. Gastric emptying in healthy volunteers after multiple doses of levodopa. Br J Clin Pharmacol. 1991;32:691–5. [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St Pierre DH, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–20. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24:e425–36. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Murphy NP, Stengel A, Goebel-Stengel M, St Pierre DH, Maidment NT, et al. Ghrelin prevents levodopa-induced inhibition of gastric emptying and increases circulating levodopa in fasted rats. Neurogastroenterol Motil. 2012;24:e235–45. doi: 10.1111/j.1365-2982.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao Y, Liu YY, Yu KQ, Ouyang MZ, Luo R, Zhao XS. Chinese herbal medicine liu jun zi tang and xiang sha liu jun zi tang for functional dyspepsia: meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:936459. doi: 10.1155/2012/936459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yagi M, Homma S, Kubota M, Iinuma Y, Kanada S, Kinoshita Y, et al. The herbal medicine rikkunshito stimulates and coordinates the gastric myoelectric activity in post-operative dyspeptic children after gastrointestinal surgery. Pediatr Surg Int. 2004;19:760–5. doi: 10.1007/s00383-003-1053-y. [DOI] [PubMed] [Google Scholar]
- 63.Yanai M, Mochiki E, Ogawa A, Morita H, Toyomasu Y, Ogata K, et al. Intragastric administration of rikkunshito stimulates upper gastrointestinal motility and gastric emptying in conscious dogs. J Gastroenterol. 2013;48:611–9. doi: 10.1007/s00535-012-0687-8. [DOI] [PubMed] [Google Scholar]
- 64.Yoshikawa T, Yoshida N. The possible involvement of dopamine D3 receptors in the regulation of gastric emptying in rats. Life Sci. 2010;87:638–42. doi: 10.1016/j.lfs.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 65.Zheng LF, Wang ZY, Li XF, Song J, Hong F, Lian H, et al. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res. 2011;1420:59–67. doi: 10.1016/j.brainres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Zhu HC, Zhao J, Luo CY, Li QQ. Gastrointestinal dysfunction in a Parkinson’s disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci. 2012;47:15–25. doi: 10.1007/s12031-011-9560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]