Abstract
Objectives
Iron deficiency anemia leads to long-term neurodevelopmental deficits by altering iron-dependent brain metabolism. The objective of the study was to determine if iron deficiency induces metabolomic abnormalities in the cerebrospinal fluid (CSF) in the preanemic stage and to ascertain the aspects of abnormal brain metabolism affected.
Methods
Standard hematological parameters [hemoglobin (Hgb), mean corpuscular volume (MCV), transferrin (Tf) saturation and zinc protoporphyrin/heme (ZnPP/H)] were compared at 2, 4, 6, 8 and 12 months in iron-sufficient (IS; n=7) and iron-deficient (ID; n=7) infant rhesus monkeys. Five CSF metabolite ratios were determined at 4, 8 and 12 months using 1H NMR spectroscopy at 16.4T and compared between groups and in relation to hematologic parameters.
Results
ID infants developed iron deficiency (Tf saturation <25%) by 4 months of age and all became anemic (Hgb<110 g/L and MCV<60 fL) at 6 months. Their heme indices normalized by 12 months. Pyruvate/glutamine and phosphocreatine/creatine (PCr/Cr) ratios in CSF were lower in the ID infants by 4 months (p<0.05). The PCr/Cr ratio remained lower at 8 months (p=0.02). ZnPP/H, an established blood marker of preanemic ID, was positively correlated with the CSF citrate/glutamine ratio (marginal correlation, 0.34; p<0.001; family-wise error rate = 0.001).
Discussion
Metabolomic analysis of the CSF is sensitive for detecting the effects of preanemic iron deficiency on brain energy metabolism. Persistence of a lower PCr/Cr ratio at 8 months, even as hematological measures demonstrated recovery from anemia, indicate that the restoration of brain energy metabolism is delayed. Metabolomic platforms offer a useful tool for early detection of the impact of iron deficiency on brain metabolism in infants.
Keywords: Cerebrospinal fluid, Energy metabolism, Infant, Iron, Iron deficiency, Metabolomics: Neurodevelopment, Rhesus monkey
Introduction
Iron deficiency (ID) is the most common micronutrient deficiency in the world, affecting over 2 billion people. It is particularly prevalent in children below the age of 3 years (1–3). Iron is important for energy metabolism, synaptogenesis, neurotransmission and myelination during brain development. Early life ID with anemia (IDA) has been associated with cognitive and behavioral impairments that persist into adulthood despite starting iron treatment soon after the diagnosis of anemia (4). Non-anemic ID, which is threefold more common than IDA, can also result in long-term neurodevelopmental effects (5, 6). Therefore, early detection and prompt treatment of ID-induced brain impairment is necessary to ensure normal neurodevelopment.
Currently, the American Academy of Pediatrics endorses broad-based population screening of children for ID by measuring the hemoglobin (Hgb) concentration at 12 months of age in order to detect anemia (7). This strategy is inadequate for the early detection of brain impairment, because anemia is the end-stage state of ID. During negative iron balance, available iron is prioritized to the red blood cells over all other tissues, including the brain (8–11). Studies in infant humans (8, 12), monkeys (13), sheep (9, 14) and rats (15) have demonstrated that brain iron concentration is compromised in order to maintain an adequate iron supply for erythropoiesis. Thus, the developing brain is already iron deficient by the time anemia is diagnosed. This risk to the brain is greatest during the fetal and early postnatal periods when the rapid phase of brain development competes with the brisk erythropoiesis that accompanies rapid somatic growth. Genetic models of non-anemic neuron-specific ID demonstrate that brain ID, independent of anemia, is responsible for the irreversible neurological deficits (11, 16, 17). Moreover, most hematological indices of ID in children are based on statistical cut-offs from population norms and do not have a validated relationship to brain iron concentrations or metabolic health.
Novel methods that can sensitively index abnormal brain metabolism may provide a better understanding of the relationship between commonly used serum biomarkers of ID and the risk of brain impairment early in the course of ID, so that iron supplementation in clinical practice can be based on the prevention of long-term adverse neurological effects, instead of only the correction of hematological anemia. Previous studies have demonstrated that in vivo ultra-high-field 1H NMR spectroscopy (MRS) is a robust tool for longitudinally tracking the negative metabolic consequences of ID on the developing brain (15, 16, 18, 19). However, repeated in vivo MRS is not feasible in human infants. Metabolomic analyses of biofluids (serum, cerebrospinal fluid [CSF] and urine) are useful for diagnosing, as well as for assessing the severity and progression of neurological disorders (20–22). Using these methods, metabolic pathways involved in tricarboxylic acid (TCA) cycle, glucose metabolism, mitochondrial function, neurotransmitter and amino acid metabolism, and phospholipid biosynthesis, several of which are altered by ID, can be assessed (20, 23–27). The metabolomic profile of CSF is directly dependent upon metabolite production in the brain parenchyma and thus closely approximates brain-specific changes (20, 28).
Using 1H NMR-based metabolomic analysis of CSF, a previous study found evidence of disrupted brain energy metabolism in infant rhesus monkeys with clinical IDA (29). Whether these metabolomic changes are present earlier in CSF before the onset of clinical anemia, and thus could serve as biomarkers of ID-induced brain metabolic dysfunction is unknown and was investigated in the present study. We hypothesized that brain metabolomic alterations indexing biologically relevant iron-dependent metabolic pathways would be present in the pre-anemic stage and that some iron-related metabolomic abnormalities would persist in the CNS, even as traditional iron and red blood cell measures conveyed that there was recovery from anemia. We employed a controlled, but naturalistic nonhuman primate (NHP) model of infantile ID where the infant monkey’s growth-related needs for iron is not entirely fulfilled by bioavailable iron in breast milk. In this model, signs of ID emerge at 4 months of age, and clinical anemia becomes evident in a subset of infants by 6 months of age (30). Further, it has been established in this primate model that the storage iron (H-ferritin) concentration is decreased and the expression of iron transporter proteins (divalent metal transporter-1 and transferrin [Tf]) is increased in the CSF of anemic infants (13). These differences in iron transport protein levels in CSF are also associated with an effect on functional activity. CSF obtained from anemic infant monkeys exert a larger chemoattractant effect on iron transport when tested in an ex vivo model of the blood-brain-barrier (31). Blood and CSF specimens were collected at 2–4 months intervals across this critical developmental trajectory, which parallels the time course for ID in human infants.
Methods
Subjects
The subjects were 14 infant rhesus monkeys (Macaca mulatta), 7 iron-sufficient (IS; 4 M, 3 F) and 7 ID (2 M, 5 F), born and reared in a long-established indoor breeding colony. All infants were full term vaginal births to different mothers, of which 3 were half-siblings (same father) across both groups. Infants were born over a 3-year period, and reared under standardized laboratory conditions. Husbandry and experimental procedures were approved by the institutional Animal Care and Use Committee.
Housing and diet
Mothers and their offspring were maintained on a commercial biscuit (iron concentration 225 mg/kg diet, Table 1), and were supplemented 3–4 times per week with fruits and vegetables. The diet provides adequate iron for a healthy adult monkey, but is not sufficiently fortified to entirely fulfill the additional maternal needs for iron during pregnancy and thus creates this experimental model of infantile IDA. Prior studies have demonstrated that between 30% and 40% of infants born to female monkeys consuming this diet will develop IDA by 6 months due to a combination of lower iron stores at birth and the high iron-needs for postnatal growth (13, 30). Infants lived with their mothers during the nursing phase through 6–7 months of age, and then were housed in small social groups with other weanlings through 12 months of age. The older infants were thus entirely on a solid diet comprised of the same commercial biscuits.
Table 1.
Ingredient composition and mineral and vitamin concentrations of the diet1
| Ingredient | Minerals | Vitamins | |||
|---|---|---|---|---|---|
| Protein (%) | 15.00 | Iron (mg/kg) | 225 | A (IU/kg) | 20.0 |
| Carbohydrate (%) | 69.09 | Zinc (mg/kg) | 110 | B12 (mg/kg) | 0.073 |
| Fat (%) | 5.00 | Copper (mg/kg) | 21 | C (mg/kg) | 500 |
| Ash (%) | 5.00 | ||||
| Fiber (crude) (%) | 6.00 | ||||
Harlow Primate Diet 5LFD (LabDiet, PMI Nutrition International, St. Louis, MO.)
Specimen collection
Blood samples were obtained at 2-month intervals to track their iron status, and CSF samples were collected at 4-month intervals to compare the metabolomic profiles of those that developed IDA or remained IS. The blood collection was purposefully spaced at 2-month intervals to minimize the chance that frequent phlebotomy could contribute to the observed metabolomic effects (32). The collected blood volume (2–3 mL) represents <5% of the total blood volume even at the youngest ages (infants weigh approximately 500 g at birth). The mothers were trained to enter a specially designed holding apparatus for sample collection. Infants were briefly removed and blood samples were collected by femoral venipuncture, after which the infant was returned to the mother. These samples were used for hematology (Hgb and mean corpuscular volume [MCV]) and iron panels (Tf saturation and zinc protoporphyrin/heme [ZnPP/H]). Hematological parameters were used to categorize infants as either IS or ID/IDA.
CSF samples were collected from all 14 infants at 4-month intervals (4, 8 and 12 months of age). Specimens (<1 mL) were collected from the cervical region under acute ketamine sedation (15 mg/kg), with meloxicam used to extend the post-procedural analgesia as in previous studies (13, 29, 33). Collection at this level of the intrathecal compartment enables CSF to be drawn down primarily from the brain, and thus lessens a potential influence of the rostral-to-caudal gradient found for some brain analytes with lumbar taps (34). Samples were centrifuged to remove cellular debris and kept frozen below −60 °C until analysis.
Determination of hematological parameters
The Hgb, MCV, and Tf saturation were determined at a CLIA certified clinical laboratory familiar with samples from nonhuman primates (Meriter Labs, Madison, WI). ZnPP/H, a proxy for inadequate bone marrow iron availability for incorporation by red blood cells, was determined on site using a hematofluorometer (AVIV). Criteria for IDA were: Hgb <110 g/L and MCV <60 fL as used in previous studies (13, 29, 30). Criteria for ID were %Tf saturation <25% and ZnPP/H >150 μM/M (30, 35).
Metabolomic analysis
CSF samples were analyzed using published methods (29) with minor modifications. Briefly, 500μl of frozen CSF was lyophilized and resuspended in 100μl D2O and 2μM TSP (pH 7.2±0.05). One-dimensional 1H NMR spectra (128 scans) were acquired using a 700 MHz NMR spectrometer (Bruker Avance, Billerica, MA) with a 1.7-mm TCI 1H-enhanced cryoprobe using a standard 1D-NOESY pulse sequence. Line broadening of 0.3 Hz was applied before Fourier transformation, autophasing and autobaseline corrections were applied using TopSpin (Bruker, Billerica, MA). Metabolites were identified by chemical shift in relation to the TSP resonance (δ 0.0 ppm), and quantified in relation to the TSP concentration peak using Chenomx NMR Suite version 8.0 (Chenomx Inc., Edmonton, Canada). Due to the potential sample loss during the process of lyophilization and subsequent variability in the volume of the CSF samples, metabolite ratios, instead of metabolite concentrations were used in the analysis.
Statistical analysis
Differences in hematological parameters and iron indices (Hgb, MCV, Tf saturation and ZnPP/H) were compared with analysis of variance, considering age as a repeated measure (2, 4, 6, 8 and 12 months) and iron status (IS and ID) as a between factor. When there was a significant interaction between age and iron status, the significance of the difference between the IS and ID groups was evaluated separately at each developmental time point with unpaired t tests. Differences in CSF metabolite ratios between groups were evaluated with unpaired t tests. The magnitude of the relationships between hematological parameters and metabolite ratios were quantified with marginal Pearson correlations to account for within-subject dependence. Marginal Pearson correlations were computed as described in Section 2.2 of (36), using R code provided by the authors, wherein the observations over time for each subject are considered as a dependent cluster. To adjust for multiple comparisons, the family-wise error rate (FWER) was computed using Holm’s step-down procedure (37). Statistical significance was set at p <0.05.
Results
Hematological parameters
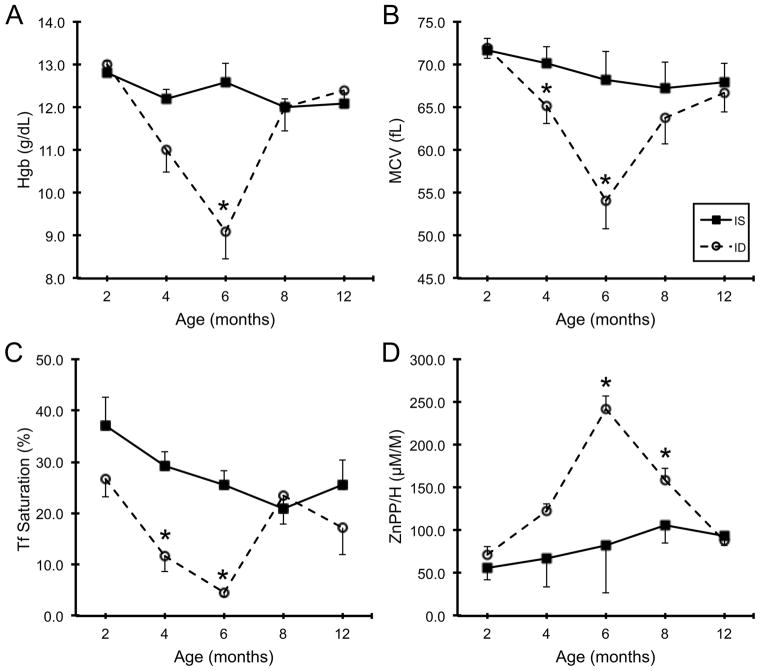
Developmental changes in hematological parameters and iron indices across the first year of life are shown in Fig 1. Dichotomizing the 14 infants into IS and ID based on the values at 6 months of age demonstrated that there was a significant interaction between Iron Status and Age (p<0.05) in all four parameters, with the maximal difference at 6 months. There was evidence of pre-anemic ID (Tf saturation, <25%) at 4 months (Fig 1C). One infant fulfilled the criterion for IDA (Hgb <110 g/L and MCV <60 fL) at 4 months, and two others had Hgb<110 g/L without microcytosis (MCV ≥60 fL). All 6-month-old infants categorized in the ID group reached clinical criteria for anemia. Both Hgb and MCV returned to the normal range by 8 months of age in the ID group, and thus hematological parameters were similar for the older infants between 8–12 months of age, corresponding to the period when all infants were consuming the same solid food exclusively. [Insert Fig 1 near here]
Fig 1.
Developmental changes in hemoglobin (A), mean corpuscular volume (B), serum transferrin saturation (C) and zinc protoporphyrin/heme (D) across the first year of age in iron sufficient and iron deficient infant rhesus monkeys. Values are mean±SEM; N=7 per group. There is a main effect of Age, Iron Status and Age × Iron Status for all four parameters (p<0.05). *p<0.05, iron sufficient vs. iron deficient. Abbreviations: IS, iron sufficient; ID, iron deficient; Hgb, hemoglobin; MCV, mean corpuscular volume; Tf, transferrin; ZnPP/H, zinc protoporphyrin/heme.
Metabolomic indices in the CSF
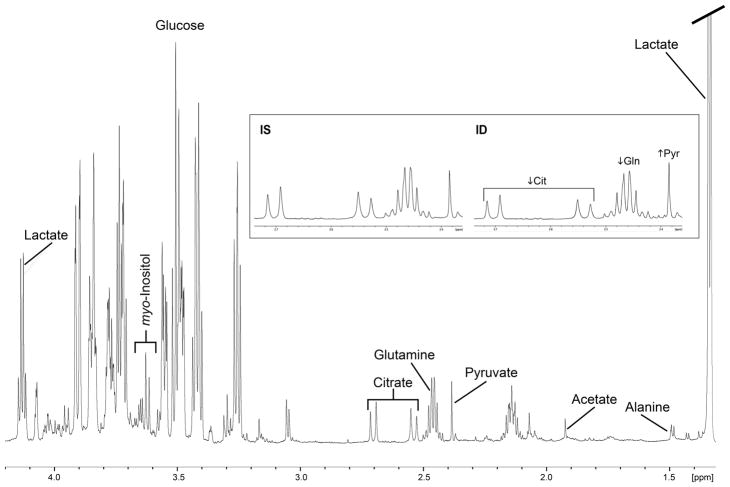
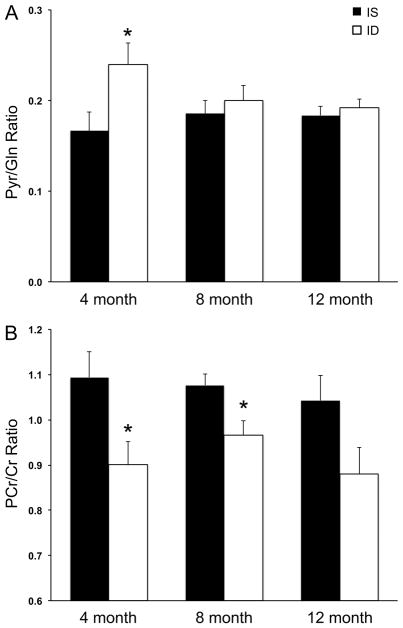
1H NMR spectra from the CSF of one iron sufficient and one iron deficient monkey at 4 months of age are shown in Fig 2. NMR spectra could not acquired from one 4-month-old ID animal due to technical issues related to sample preparation. Five metabolite ratios were generated from all other CSF spectra (N=7 at all three ages in the IS group, and N=6 at 4 months and N=7 at 8- and 12 months in the ID group): pyruvate/glutamine (Pyr/Gln), citrate/pyruvate (Cit/Pyr), citrate/lactate (Cit/Lac), citrate/glutamine (Cit/Gln) and phosphocreatine/creatine (PCr/Cr). The Pyr/Gln ratio was higher (Fig 3A) and the PCr/Cr ratio was lower (Fig 3B) in the ID group at 4 months of age (p<0.05). Iron deficiency that was already more emergent by 4 months of age (Hgb <110 g/L) had a larger effect on the CSF Pyr/Gln ratio (64% increase vs. 18% increase in those with Hgb ≥110 g/L at 4 months), but not on the PCr/Cr ratio (14% decrease in those with Hgb<110 g/L vs. 21% decrease in those with Hgb ≥110 g/L). The CSF PCr/Cr ratio remained 10% lower (p=0.02) at 8 months in the ID group. There were no significant differences between the IS and ID infants in the Cit/Pyr, Cit/Lac and Cit/Gln ratios at any age (Table 2). [Insert Fig 2, Fig 3 and Table 2 near here]
Fig 2.
1H NMR spectra with the typical metabolite peaks in the cerebrospinal fluid of 4-month-old monkeys. Representative spectra from one iron sufficient animal and one with iron deficiency are shown. Abbreviations: IS, iron sufficient; ID, iron deficient; Cit, citrate; Gln, glutamine; Pyr, pyruvate.
Fig 3.
Pyruvate/glutamine (A) and phosphocreatine/creatine (B) ratios in the cerebrospinal fluid across the first year of age in iron sufficient and iron deficient infant rhesus monkeys. Values are mean±SEM; N=7 per group, except the 4-month-ID group, where N=6. *p<0.05, iron sufficient vs. iron deficient. Abbreviations: IS, iron sufficient; ID, iron deficient; Pyr, pyruvate; Gln, glutamine; PCr, phosphocreatine; Cr, creatine.
Table 2.
The CSF citrate/pyruvate, citrate/lactate and citrate/glutamine ratios in the iron-sufficient and iron-deficient infant monkeys
| Metabolite Ratio | Group | Age | ||
|---|---|---|---|---|
| 4 months | 8 months | 12 months | ||
| Cit/Pyr | Iron-sufficient | 1.92±0.36 | 1.30±0.08 | 1.45±0.05 |
| Iron-deficient | 1.38±0.19 | 1.51±0.15 | 1.13±0.14 | |
| Cit/Lac | Iron-sufficient | 0.07±0.00 | 0.05±0.00 | 0.06±0.00 |
| Iron-deficient | 0.06±0.01 | 0.06±0.00 | 0.05±0.01 | |
| Cit/Gln | Iron-sufficient | 0.28±0.01 | 0.24±0.02 | 0.26±0.01 |
| Iron-deficient | 0.31±0.02 | 0.29±0.02 | 0.22±0.03 | |
Values are mean±SEM; N=7, iron-sufficient group and N=7, iron-deficient group, except at 4 months, where N=6. No significant differences between the two groups for any of the metabolite ratios at the three ages. Abbreviations: CSF, cerebrospinal fluid; Cit, citrate; Gln, glutamine; Lac, lactate; Pyr, pyruvate.
Relationship between hematological parameters and CSF metabolomic indices
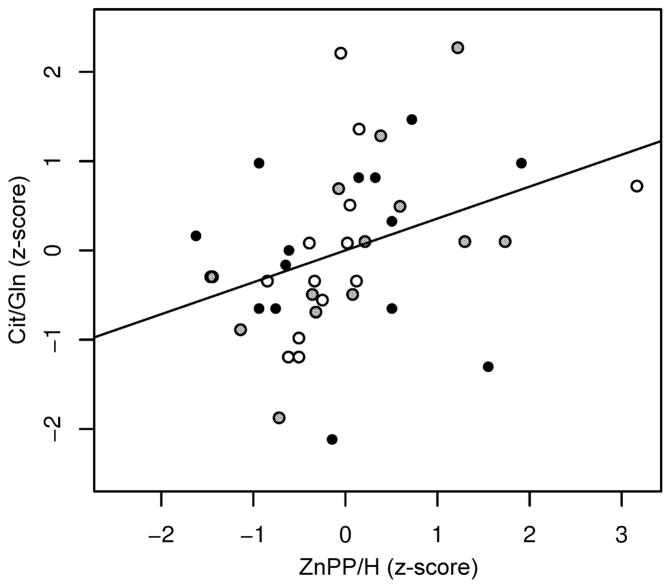
The relationship between hematological parameters and the CSF metabolomic indices in the entire cohort was also examined, controlling for within subject dependence and multiplicity of comparison. ZnPP/H was positively correlated with the CSF Cit/Gln ratio (marginal correlation, 0.34; p<0.001; FWER = 0.001; Fig 4). No other associations were significant at the FWER<0.05 level.
Fig 4.
The relationship between serum zinc protoporphyrin/heme and cerebrospinal fluid citrate/glutamine ratio in the entire set of infant rhesus monkeys. Paired specimens (N=41) from 4-month-old (open circles), 8-month-old (gray circles) and 12-month-old (filled, black circles) iron-sufficient and iron-deficient infants demonstrate that there is a positive correlation between serum zinc protoporphyrin/heme and cerebrospinal fluid citrate/glutamine ratio (marginal correlation, 0.34; p<0.001; family-wise error rate = 0.001). Abbreviations: Cit/Gln, citrate/glutamine; ZnPP/H, zinc protoporphyrin/heme.
Discussion
Our study indicates that ID alters the CSF metabolomic profile of infant monkeys before traditional red blood cell measures, including Hgb levels, would indicate a clinical diagnosis of IDA. Similar to a previous evaluation of anemic infant monkeys (29), metabolomic abnormalities reflecting impaired energy metabolism were present in the intrathecal compartment. However, unlike the prior CSF findings that identified metabolomic changes primarily during but not after the period of anemia, the current study characterized metabolomic abnormalities present in the pre-anemic stage, which then persisted after the resolution of anemia. Because CSF was collected at the cervical level, drawing CSF primarily from the ventricles, these metabolite differences likely reflect changes in iron-dependent energy pathways in the brain parenchyma. Thus, this study contributes two important findings to our understanding of early-life iron deficiency and the developing brain. It suggests that brain metabolism is compromised early in the course of ID prior to the presentation of anemia, which is the currently used biomarker to detect ID in human infants. By demonstrating that brain metabolism is altered concurrently with serum transferrin saturation and ZnPP/H, the study indicates that these non-hematological biomarkers would be better screening tools to detect brain risk than the standard hematological parameters (i.e., Hgb and MCV). Our study also found that brain energy metabolism remains abnormal even as the older, weaned infant acquires more dietary iron from iron-enriched solid foods and the peripheral anemia resolves. Because the monkey is considered to be an excellent model of human brain development and the nutritional needs and metabolism of the human infant (38–42), our findings on sustained differences in cerebral energy metabolism may help to explain the reports of persistent long-term cognitive and behavioral deficits in children who had received iron treatment after they were identified as anemic (4, 43).
The TCA cycle is at the center of cellular metabolism. Pyruvate, a product of glycolysis, enters the TCA cycle after conversion to acetyl-CoA. Glutamate and glutamine synthesis is coupled to the TCA cycle at the α-ketoglutarate step. In cell cultures, ID downregulates TCA cycle enzymes, citrate synthase, aconitase, isocitrate dehydrogenase and succinate dehydrogenase, and upregulates glycolysis (44). Glutamate dehydrogenase, the enzyme responsible for glutamate synthesis from α-ketoglutarate is decreased in the ID brain (45). Although similar TCA cycle enzyme abnormalities have yet to be determined in the CSF, the previously demonstrated lower Cit/Pyr and Cit/Lac ratios, and higher Pyr/Gln ratio in infant monkeys with established IDA indicate that ID impairs TCA cycle activity in the intrathecal compartment (29). Abnormal TCA cycle activity in the CSF is associated with and predates cognitive deficits in adult humans with neurological disorders (for example, in Alzheimer’s disease) (20).
The higher CSF Pyr/Gln ratio in the ID group at 4 months in the present study suggests that the TCA cycle activity in the intrathecal compartment is impaired prior to the onset of anemia. The CSF Pyr/Gln ratio was also higher in the ID infants at 4-months in the previous study using this NHP model (29). However, unlike the present study where only one infant was anemic (Hgb<110 g/L and MCV <60 fL), all the infants in the prior report were already anemic at 4 months, which may also explain the larger increase in CSF Pyr/Gln ratio (60% increase vs. the 40% increase in the present study). It has been posited that increased CSF Pyr/Gln ratio at 4 months could serve as a sensitive marker of the pressure on body iron stores based on the observation that IDA has a greater impact on the Pyr/Gln ratio at 4 months of age than at 7 months of age (60% increase vs. a 9% increase, respectively, relative to the IS group) (29). A relatively higher CSF Pyr/Gln ratio in animals with more emergent iron deficiency at 4 months of age (Hgb<110 g/L) in the present study corroborates this speculation. The positive relationship between ZnPP/H and CSF Cit/Gln ratio further supports the occurrence of impaired TCA cycle activity in ID. Increased ZnPP/H is a sensitive indicator of non-anemic ID (46). Although ID downregulates citrate synthase, the magnitude of downregulation is much lower (8% lower) compared with that of aconitase (51% lower), an iron-containing enzyme responsible for converting citrate to isocitrate (44), which may explain the increased Cit/Gln ratio.
The lower CSF PCr/Cr ratio at 4 months of age suggests lower cerebral energy reserves in the pre-anemic period and concurs with the impairment in brain energy metabolism demonstrated in rodent models of non-anemic brain ID (16). PCr is a readily available cytosolic high-energy phosphate store for buffering decreased ATP synthesis when there is inadequate oxidative phosphorylation (47). The persistence of lower PCr/Cr ratio in the 8-month old infant monkey, even after they have been consuming iron-enriched solid food, indicates that cerebral energy metabolism remains subnormal even as the ID in red blood cells resolves. Previous studies in developing rodents with IDA have demonstrated that the PCr/Cr ratio is altered in several brain regions during the period of anemia, but that it does not persist into the post-anemic period (18, 48, 49). Compared with the rodent brain, the primate brain has a much higher metabolic rate, and thus iron requirement, and is more similar to the rapidly growing brain of the human infant (39). Thus, the impact of ID on cerebral energy metabolism is likely to be magnified, which may explain the longer duration of the altered PCr/Cr ratio, a finding that is likely of potential relevance to the human infant.
This evidence for persistent metabolomic changes at 8 months of age also differs from a prior report on CSF metabolites in iron deficient monkeys, but in that report the follow-up evaluation was on older animals at one year of age (29). It is known that hepcidin levels are particularly low in anemic monkeys (30) and thus they would be actively absorbing iron from consumed foods across this whole period of 6–12 months of age. It appears that by 2 months after weaning from the mother the eating of solid foods is adequate to correct anemia, but not yet of sufficient duration to entirely correct metabolic dysfunction in the brain, likely due to the prioritization of iron to the red blood cells over all other tissues during iron supplementation (13, 15). Using the same NHP model of anemia, proteomic analyses of CSF documented that some differences in specific proteins do still persist at one year of age, most notably higher levels of beta-amyloid beta precursor-like protein and decreased prostaglandin D2 synthase (50).
The previous study also identified a low Cit/Pyr ratio in CSF as a sensitive bioindicator of ID (29), but that effect was not evident in this study. One parsimonious explanation is that the infants in the current ID group were less iron deficient compared with the ones assessed in the prior study. Whereas all of those infants were anemic at the time of the metabolomic analyses at 4- and 7-months of age in that study (29), only one of the seven infants met the criteria for anemia (Hgb <110 g/L and MCV <60 fL) at 4-months of age in the current study. Despite this less severe ID, which progressed to anemia only by 6 months of age, the magnitude of the Cit/Pyr ratio reduction in the CSF (28% decrease) in the present study is comparable to the previous report (26% decrease at 4 months and 22% decrease at 7 months) (29). Fewer animals with severe ID may also account for the fact that we did not see differences in another CSF metabolite ratio, Cit/Lac, in the current study. It is known that the activity of lactate dehydrogenase, the enzyme responsible for lactate production, increases in proportion to the severity of ID (51).
In summary, we have obtained additional evidence that ID alters the metabolomic profile of CSF. These changes in the intrathecal compartment suggest that energy metabolism was altered, presumably in the brain parenchyma, before clinical anemia would have been identified with standard tests, such as Hgb levels, and persisted after the common red cell-based tests suggested that the anemia had resolved. Without more frequent sampling it is not possible to know how early brain energy metabolism was affected before the onset of clinical anemia and for exactly how long it remained abnormal after the anemia had resolved. Similarly, without more concurrent information on iron status in the CSF and plasma metabolomics, it is not possible to know whether the CSF metabolomic changes were primarily mediated by brain ID or were a component of the global metabolic effects of ID on multiple organ systems. Nevertheless, the results of the present study have clinical relevance. Currently, in pediatric practice most infants are first screened for ID at one year of age (7), which would mean that their brains are likely iron deficient and metabolically impaired before detection of their anemia. Preterm infants and infants born to mothers with certain gestational complications, such as maternal ID, hypertension and diabetes mellitus, as well as to other high-risk mothers, including adolescent mothers and certain racial groups, are particularly vulnerable to becoming iron deficient early in infancy (1, 8, 52–57). Our results reinforce the recommendations for screening these high-risk infants at an earlier age, sooner than one year of age and likely using pre-anemic markers, and initiating iron supplementation in the risk period, prior to the appearance of anemia.
Acknowledgments
Funding details: This work was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development under grant numbers HD057064, HD080201 and HD039386. Funding for NMR instrumentation at University of Minnesota was provided by the Office of the Vice President for Research, the University of Minnesota Medical School, College of Biological Science, National Institutes of Health, National Science Foundation, and the Minnesota Medical Foundation.
The authors acknowledge the Minnesota Supercomputing Institute (MSI) at the University of Minnesota for providing resources that contributed to the research results within the paper, and Gulin Oz, PhD for helpful comments on the manuscript.
Biographies
Raghavendra Rao, MD investigates cerebral energy metabolism under adverse conditions in an attempt to mechanistically link cerebral metabolic changes with brain structure/function and develop sensitive biomarkers for intervention.
Kathleen Ennis, BA, a Senior Scientist in the Rao lab is an expert on metabolomic analysis of biofluids, and multimodal analyses in animal models.
Eric F. Lock, PhD develops methods for the analysis of complex high-dimensional data and multi-source data integration.
Michael K. Georgieff, MD focuses on fetal/neonatal nutrition, specifically, the effect of fetal/neonatal iron nutrition on brain development in human infants and animal models.
Christopher Coe, PhD focuses on how psychological, environmental and dietary factors, especially iron deficiency, influence health and immunity in infancy and old age.
Gabriele R Lubach, PhD, a Senior Scientist at University of Wisconsin-Madison, collaborates on the primate research with Dr. Coe.
Footnotes
Geolocation information: United States of America; states of Minnesota and Wisconsin.
Disclosure statement: The authors report no declarations of interest. This work was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development under grant numbers HD057064, HD080201 and HD039386.
References
- 1.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92(2):73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34(7):513–8. doi: 10.1038/jp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–57. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 4.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13(2):54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol. 2009;34(6):762–79. doi: 10.1080/87565640903265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozoff B, Armony-Sivan R, Kaciroti N, Jing Y, Golub M, Jacobson SW. Eye-blinking rates are slower in infants with iron-deficiency anemia than in nonanemic iron-deficient or iron-sufficient infants. J Nutr. 2010;140(5):1057–61. doi: 10.3945/jn.110.120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126(5):1040–50. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 8.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121(1):109–14. doi: 10.1016/s0022-3476(05)82554-5. [DOI] [PubMed] [Google Scholar]
- 9.Guiang SF, Georgieff MK, Lambert DJ, Schmidt RL, Widness JA. Intravenous iron supplementation effect on tissue iron and hemoproteins in chronically phlebotomized lambs. Am J Physiol. 1997;273(6 Pt 2):R2124–31. doi: 10.1152/ajpregu.1997.273.6.R2124. [DOI] [PubMed] [Google Scholar]
- 10.Georgieff MK, Mills MM, Gordon K, Wobken JD. Reduced neonatal liver iron concentrations after uteroplacental insufficiency. J Pediatr. 1995;127(2):308–4. doi: 10.1016/s0022-3476(95)70317-9. [DOI] [PubMed] [Google Scholar]
- 11.Dallman PR, Siimes MA, Manies EC. Brain iron: persistent deficiency following short-term iron deprivation in the young rat. Br J Haematol. 1975;31(2):209–15. doi: 10.1111/j.1365-2141.1975.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 12.Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, et al. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117(3):455–61. doi: 10.1016/s0022-3476(05)81097-2. [DOI] [PubMed] [Google Scholar]
- 13.Geguchadze RN, Coe CL, Lubach GR, Clardy TW, Beard JL, Connor JR. CSF proteomic analysis reveals persistent iron deficiency-induced alterations in non-human primate infants. J Neurochem. 2008;105(1):127–36. doi: 10.1111/j.1471-4159.2007.05113.x. [DOI] [PubMed] [Google Scholar]
- 14.Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol. 1992;262(3 Pt 2):R485–91. doi: 10.1152/ajpregu.1992.262.3.R485. [DOI] [PubMed] [Google Scholar]
- 15.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133(10):3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 16.Carlson ES, Fretham SJ, Unger E, O'Connor M, Petryk A, Schallert T, et al. Hippocampus specific iron deficiency alters competition and cooperation between developing memory systems. J Neurodev Disord. 2010;2(3):133–43. doi: 10.1007/s11689-010-9049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22(8):1691–702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward KL, Tkac I, Jing Y, Felt B, Beard J, Connor J, et al. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutr. 2007;137(4):1043–9. doi: 10.1093/jn/137.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27(4):729–40. doi: 10.1038/sj.jcbfm.9600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One. 2013;8(5):e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller M, Enot DP, Hodson MP, Igwe EI, Deigner HP, Dean J, et al. Inflammatory-induced hibernation in the fetus: priming of fetal sheep metabolism correlates with developmental brain injury. PLoS One. 2011;6(12):e29503. doi: 10.1371/journal.pone.0029503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair AJ, Viant MR, Ball AK, Burdon MA, Walker EA, Stewart PM, et al. NMR-based metabolomic analysis of cerebrospinal fluid and serum in neurological diseases--a diagnostic tool? NMR Biomed. 2010;23(2):123–32. doi: 10.1002/nbm.1428. [DOI] [PubMed] [Google Scholar]
- 23.Lexcen DR, Lusczek ER, Witowski NE, Mulier KE, Beilman GJ. Metabolomics classifies phase of care and identifies risk for mortality in a porcine model of multiple injuries and hemorrhagic shock. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S147–55. doi: 10.1097/TA.0b013e3182609821. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Liu H, Zhang L, Bhakoo K, Lu L. An insight into the metabolic responses of ultra-small superparamagnetic particles of iron oxide using metabonomic analysis of biofluids. Nanotechnology. 2010;21(39):395101. doi: 10.1088/0957-4484/21/39/395101. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Litt L, Segal MR, Kelly MJ, Pelton JG, Kim M. Metabolomics of oxidative stress in recent studies of endogenous and exogenously administered intermediate metabolites. Int J Mol Sci. 2011;12(10):6469–501. doi: 10.3390/ijms12106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanos V, Antonucci R, Barberini L, Atzori L. Urinary metabolomics in newborns and infants. Adv Clin Chem. 2012;58:193–223. doi: 10.1016/b978-0-12-394383-5.00013-8. [DOI] [PubMed] [Google Scholar]
- 27.Fanos V, Antonucci R, Barberini L, Noto A, Atzori L. Clinical application of metabolomics in neonatology. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):104–9. doi: 10.3109/14767058.2012.663198. [DOI] [PubMed] [Google Scholar]
- 28.Wishart DS, Lewis MJ, Morrissey JA, Flegel MD, Jeroncic K, Xiong Y, et al. The human cerebrospinal fluid metabolome. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871(2):164–73. doi: 10.1016/j.jchromb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Rao R, Ennis K, Oz G, Lubach GR, Georgieff MK, Coe CL. Metabolomic analysis of cerebrospinal fluid indicates iron deficiency compromises cerebral energy metabolism in the infant monkey. Neurochem Res. 2013;38(3):573–80. doi: 10.1007/s11064-012-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coe CL, Lubach GR, Busbridge M, Chapman RS. Optimal iron fortification of maternal diet during pregnancy and nursing for investigating and preventing iron deficiency in young rhesus monkeys. Res Vet Sci. 2013;94(3):549–54. doi: 10.1016/j.rvsc.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson IA, Ponnuru P, Klinger ME, Myers RL, Devraj K, Coe CL, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab. 2015;35(1):48–57. doi: 10.1038/jcbfm.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallin DJ, Tkac I, Stucker S, Ennis KM, Sola-Visner M, Rao R, et al. Phlebotomy-induced anemia alters hippocampal neurochemistry in neonatal mice. Pediatr Res. 2015;77(6):765–71. doi: 10.1038/pr.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Dev Psychobiol. 2009;51(3):301–9. doi: 10.1002/dev.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes TM, Coe CL. The proinflammatory cytokine network: interactions in the CNS and blood of rhesus monkeys. Am J Physiol. 1998;274(1 Pt 2):R139–44. doi: 10.1152/ajpregu.1998.274.1.R139. [DOI] [PubMed] [Google Scholar]
- 35.Fernie S, Wrenshall E, Malcolm S, Bryce F, Arnold DL. Normative hematologic and serum biochemical values for adult and infant rhesus monkeys (Macaca mulatta) in a controlled laboratory environment. J Toxicol Environ Health. 1994;42(1):53–72. doi: 10.1080/15287399409531863. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz DJ, Datta S, Harkema SJ. Marginal association measures for clustered data. Stat Med. 2011;30(27):3181–91. doi: 10.1002/sim.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sture H. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 38.O'Sullivan A, He X, McNiven EM, Hinde K, Haggarty NW, Lonnerdal B, et al. Metabolomic phenotyping validates the infant rhesus monkey as a model of human infant metabolism. J Pediatr Gastroenterol Nutr. 2013;56(4):355–63. doi: 10.1097/MPG.0b013e31827e1f07. [DOI] [PubMed] [Google Scholar]
- 39.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;(Suppl 27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 40.Lonnerdal B. Preclinical assessment of infant formula. Ann Nutr Metab. 2012;60(3):196–9. doi: 10.1159/000338209. [DOI] [PubMed] [Google Scholar]
- 41.Rhesus Macaque Genome Sequencing and Analysis Consortium. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316(5822):222–34. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 42.Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, et al. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27(4):615–27. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Pt 2):S34–43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochimica et biophysica acta. 1999;1413(3):99–107. doi: 10.1016/s0005-2728(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 45.Taneja V, Mishra K, Agarwal KN. Effect of early iron deficiency in rat on the gamma-aminobutyric acid shunt in brain. J Neurochem. 1986;46:1670–1674. doi: 10.1111/j.1471-4159.1986.tb08483.x. [DOI] [PubMed] [Google Scholar]
- 46.Hastka J, Lasserre JJ, Schwarzbeck A, Hehlmann R. Central role of zinc protoporphyrin in staging iron deficiency. Clin Chem. 1994;40(5):768–73. [PubMed] [Google Scholar]
- 47.Buck L, Espanol M, Litt L, Bickler P. Reversible decreases in ATP and PCr concentrations in anoxic turtle brain. Comp Biochem Physiol A Mol Integr Physiol. 1998;120(4):633–9. doi: 10.1016/s1095-6433(98)10079-x. [DOI] [PubMed] [Google Scholar]
- 48.Rao R, Tkac I, Schmidt AT, Georgieff MK. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr Neurosci. 2011;14(2):59–65. doi: 10.1179/1476830511Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao R, Tkac I, Unger EL, Ennis K, Hurst A, Schallert T, et al. The iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of frontal cortex and hippocampus in adult rats. Pediatr Res. 2013;73(1):31–7. doi: 10.1038/pr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Dev Neurosci. 2012;34(4):354–65. doi: 10.1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stangl GI, Kirchgessner M. Effect of different degrees of moderate iron deficiency on the activities of tricarboxylic acid cycle enzymes, and the cytochrome oxidase, and the iron, copper, and zinc concentrations in rat tissues. Z Ernahrungswiss. 1998;37(3):260–8. doi: 10.1007/s003940050025. [DOI] [PubMed] [Google Scholar]
- 52.Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36(1):27–42. doi: 10.1016/j.clp.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–9. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern Fetal Neonatal Med. 2014;27(9):887–91. doi: 10.3109/14767058.2013.845161. [DOI] [PubMed] [Google Scholar]
- 55.Bora R, Akhtar SS, Venkatasubramaniam A, Wolfson J, Rao R. Effect of 40-cm segment umbilical cord milking on hemoglobin and serum ferritin at 6 months of age in full-term infants of anemic and non-anemic mothers. J Perinatol. 2015;35(10):832–6. doi: 10.1038/jp.2015.92. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1–1):42–8. doi: 10.1038/pr.2015.183. [DOI] [PubMed] [Google Scholar]
- 57.Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in >/= 35 weeks gestational age infants. J Pediatr. 2013;163(5):1267–71. doi: 10.1016/j.jpeds.2013.06.020. [DOI] [PubMed] [Google Scholar]