Summary
Extramedullary disease (EMD), defined as an infiltrate of clonal plasma cells at an anatomic site distant from the bone marrow, is an uncommon manifestation of multiple myeloma. Six hundred and sixty-three consecutive patients with multiple myeloma who underwent stem cell transplantation between January 2005 and December 2011 were assessed for the presence of EMD. A cohort of 55 patients with biopsy-proven EMD was identified, comprising 8.3% of the total study population. EMD was present at the time of diagnosis in 14.5% of cases and at the time of relapse in 76% of patients. The most common EMD presentations at relapse were liver involvement and pleural effusions. EMD specimens had high expression of CD44 (92%) and moderate expression of CXCR4. The median overall survival from time of myeloma diagnosis was 4.1 years (95% confidence interval: 3.1, 5.1) and the median overall survival from time of EMD diagnosis was 1.3 years (95% confidence interval: 0.8, 2.3). This report demonstrates that the incidence of EMD has not increased with the introduction of novel agents and stem cell transplantation. The most common EMD presentations in the relapsed setting were liver and pleural fluid. The presence of CD44 and CXCR4 expression may represent new markers of EMD that warrant further investigation.
Keywords: Multiple myeloma, extramedullary, Novel agents, stem cell transplantation, CD44
Introduction
Multiple myeloma (MM), a malignancy of terminally differentiated monoclonal plasma cells, continues to afflict a substantial proportion of the population, with an estimated incidence of 21,700 new cases diagnosed per year in the United States (Howlader et al. 2011). Although MM remains incurable, new therapeutic advances over the past 20 years have greatly improved clinical response rates, event-free survival and overall survival in myeloma (Rajkumar et al. 2006, Dimopoulos et al. 2007, Richardson et al. 2005, Attal et al. 1996).
Monoclonal plasma cells, which give rise to the clinical phenotype of MM, most often remain localized to the bone marrow environment. However, there exists a subset of patients with myeloma in whom pathogenic plasma cells can be found at distant anatomical sites, such as the liver, kidney, pleura, breast, testes, skin and meninges, among other tissues. These patients with extramedullary disease (EMD), strictly defined as a clonal plasmacytic infiltrate at an anatomic site distant from the bone marrow or adjacent soft tissue, appear to account for 6–7.5% of the total myeloma population in small series, and tend to have an unfavourable prognosis relative to MM with marrow-only disease (Bartel et al. 2009, Usmani et al. 2012).
At present, there are limited data regarding the basic characteristics of EMD, including incidence, prevalence, clinical characteristics, laboratory features and response to novel therapies. Several previous series investigating EMD have been hampered by definitional inconsistencies, as some authors have deemed EMD to reflect any extension of plasma cells from the marrow to adjacent soft tissues, while others have strictly defined it as the proliferation of plasma cells at anatomical sites physically separated from the bone marrow. For example, in the largest series of EMD to date (Varettoni et al. 2010) EMD was defined as extension from the marrow to the adjacent soft tissues in 85% of cases studied, while in recent analyses, EMD has been defined more narrowly, as evidence of plasma cells at distant locations (Short et al. 2011).
We have previously proposed that EMD should be defined strictly as the presence of an infiltrate of clonal plasma cells at an anatomic site distant from the bone marrow or adjacent soft tissue in a patient with underlying MM (Weinstock & Ghobrial 2012). Adherence to this definition will aid in the ongoing study of EMD and will permit accurate analysis of this entity’s basic epidemiological, clinical, pathogenic, immunophenotypic, cytogenetic and prognostic features.
Here, we report an analysis of EMD incidence, laboratory features and response to therapy among a group of patients with MM who underwent autologous or allogeneic stem cell transplantation at a single, large, academic medical centre in the United States.
Methods
Six-hundred and sixty-three consecutive patients with MM who were treated with either autologous or allogeneic haematopoietic stem cell transplantation at the Dana-Farber Cancer Institute (DFCI) between January 2005 and December 2011 were analysed for the presence or absence of EMD, as well as their laboratory characteristics, specific treatment regimens and response to therapy. Approval for this protocol was obtained from DFCI and was in accordance with the Declaration of Helsinki. All patients were treated with novel therapeutic agents, including thalidomide, lenalidomide or bortezomib. This cohort was chosen in order to most accurately reflect the current state of myeloma therapy, in which novel agents are routinely used in tandem with stem cell transplantation.
The diagnosis of plasma cell neoplasm was rendered on tissue or cytology sections as part of routine clinical care in accordance with the 2008 World Health Organization Classification system (McKenna & Kroft 2008). EMD relapse was defined as pathological or radiological evidence of EMD at any time following the initial diagnosis of MM. In accordance with the strict definitional criteria noted above, those patients with pathological or radiological evidence of neoplastic plasma cells in the soft tissues adjacent to the axial skeleton, including the epidural space, paraspinal soft tissue and calvarium, were deemed to have locally-advanced myeloma, but not EMD. Patients with plasma cell leukaemia were specifically excluded from this analysis.
Immunohistochemical studies, performed as part of this analysis, utilized the following antibodies: anti-CD138 (Mouse mAb, MI15, Dako, Carpinteria, CA), anti-CXCR4 (Rabbit mAb, UMB2, Abcam, Cambridge, MA), anti-CD56 (Mouse mAb, 123C3, Dako), anti-CD44 (Rat mAb, IM7, eBioscience, San Diego, CA), and anti-CCR6 (Mouse mAb, 53103, R&D Systems, Minneapolis, MN).
Statistical analysis: Patient characteristics were summarized using descriptive statistics. Kaplan Meier methods were used to estimate survival from the time of myeloma diagnosis and time from EMD diagnosis. We used R version 3.0.2 and the survival package for the analysis (http://www.R-project.org/).
Results
Baseline demographics and characteristics of EMD presentation
Of the entire study cohort of 663 patients who underwent autologous or allogeneic stem cell transplantation for MM at a single, large academic medical centre in the United States between January 2005 and December 2011, 55 were found to have EMD at any time during their disease course (8.3%). The cohort of EMD patients showed a male predominance (63.6%). At the time of MM diagnosis, the cohort of EMD patients had a median haemoglobin of 117 g/l, a median creatinine of 88.4 µmol/l, median calcium of 2.4 mmol/l, median albumin of 39 g/l, median LDH of 183.5 us/l, and median beta-2 microglobulin of 2.79 mg/l (Table I).
Table I.
Disease Characteristics of EMD patients at Time of MM Diagnosis
| Variable | N | % | Reference | ||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 35 | 63.6 | |||
| Female | 20 | 36.4 | |||
| Ig Heavy Chain | 55 | ||||
| A | 12 | 21.8 | |||
| G | 29 | 52.7 | |||
| n/a | 14 | 25.4 | |||
| Ig Light Chain | 61 | ||||
| λ | 22 | 40.0 | |||
| κ | 33 | 60.0 | |||
| Hb (g/l) | 39 | ||||
| Median | 117 | ||||
| Range | 65–151 | 132 −167 | |||
| Creatinine (µmol/l) | 44 | ||||
| Median | 88.4 | ||||
| Range | 44.2–884 | 61.88–114.92 | |||
| Calcium (mmol/l) | 41 | ||||
| Median | 2.4 | ||||
| Range | 0.925–5 | 2.2–2.625 | |||
| Albumin (g/l) | 31 | ||||
| Median | 39 | ||||
| Range | 29–50 | 37–54 | |||
| LDH (u/l) | 20 | ||||
| Median | 183.5 | ||||
| Range | 105–622 | 107–231 | |||
| β-2 microglobulin (mg/l) | 30 | ||||
| Median | 2.79 | ||||
| Range | 1–20.8 | 0.2–7 |
EMD, extramedullary disease; MM, multiple myeloma; Ig, immunoglobulin; Hb, haemoglobin; LDH, lactate dehydrogenase
The median age at the time of diagnosis of EMD was 52 years (range 34–66). The median haemoglobin was 104 g/l and median LDH elevation was 283.5 u/l at the time of EMD diagnosis . Fifty-three % had immunoglobin G (IgG) heavy chain restriction and 21.8% had immunoglobin A (IgA) heavy chain restriction; 40.0% had lambda light chain restriction and 60.0% had kappa light chain restriction.
EMD was present at the time of MM diagnosis in 8/55 of these patients (14.5% of all EMD cases). Another 5/55 (9%) patients had EMD at the time of diagnosis and at relapse and 42/55 (76%) patients developed EMD at the time of disease relapse.
The most common locations for EMD at the time of diagnosis of MM were the head and neck soft tissue (31.6%), abdomen (26.3%), chest (21.1%) and central nervous system (12%) (Table II) and included cervical lymph nodes and oropharynx involvement. Involvement in the abdomen included myeloma involvement in the pancreas, peritoneum, kidney and ileum.
Table II.
Anatomical Location of EMD
| EMD at Diagnosis of MM | EMD at Relapse | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total locations (n) | 19 | Total locations (n) | 92 | ||
| Head and Neck Soft Tissue | 6 | 31.6 | Head and Neck Soft Tissue | 12 | 13.0 |
| Cervical Lymph Node | 2 | 10.5 | Orbit | 3 | 3.3 |
| Scalp | 1 | 5.3 | Scapula | 2 | 2.2 |
| Lip | 1 | 5.3 | Gingiva | 2 | 2.2 |
| Pharynx | 1 | 5.3 | Sinus | 1 | 1.1 |
| Tonsil | 1 | 5.3 | Cranial Nerve | 1 | 1.1 |
| Parotid | 1 | 1.1 | |||
| Larynx | 1 | 1.1 | |||
| Cervical Lymph Node | 1 | 1.1 | |||
| Central Nervous System | 3 | 15.9 | Central Nervous System | 13 | 14.1 |
| Cerebrospinal Fluid | 1 | 5.3 | Dura | 4 | 4.3 |
| Spinal Cord | 1 | 5.3 | Brain | 2 | 2.2 |
| Brain | 1 | 5.3 | Frontal extra-axial mass | 2 | 2.2 |
| Cerebrospinal Fluid | 1 | 1.1 | |||
| Spinal canal | 1 | 1.1 | |||
| Leptomeninges | 1 | 1.1 | |||
| Thecal | 1 | 1.1 | |||
| Subcortical white matter | 1 | 1.1 | |||
| Chest | 4 | 21.1 | Chest | 22 | 23.9 |
| Lung and pleural effusion | 4 | 21.1 | Lung & pleural effusion | 15 | 16.3 |
| Mediastinum | 3 | 3.3 | |||
| Breast | 2 | 2.2 | |||
| Tracheal arch | 1 | 1.1 | |||
| Axillary Lymph Node | 1 | 1.1 | |||
| Abdomen | 5 | 26.3 | Abdomen | 40 | 43.5 |
| Pancreas | 1 | 5.3 | Liver | 14 | 15.1 |
| Peritoneum | 1 | 5.3 | Peritoneal surface | 7 | 7.5 |
| Kidney | 1 | 5.3 | Kidney | 6 | 6.5 |
| Spleen | 1 | 5.3 | Mesentery | 3 | 3.2 |
| Ileum | 1 | 5.3 | Pancreas | 2 | 2.2 |
| Spleen | 2 | 2.2 | |||
| Para-aortic Lymph Node | 1 | 1.1 | |||
| Pelvic Lymph Node | 1 | 1.1 | |||
| Ureter | 1 | 1.1 | |||
| Iliac Lymph Node | 1 | 1.1 | |||
| Buttock | 1 | 1.1 | |||
| Paravertebral | 1 | 1.1 | |||
| Other Soft Tissue | 1 | 5.3 | Other Soft Tissue | 5 | 5.4 |
EMD, extramedullary disease; MM, multiple myeloma
The most common locations for EMD at relapse were the abdomen (40%) and the chest (23.9%). The most common sites of involvement were lung and pleural effusions (16%) followed by liver involvement in 15% of the total EMD sites of disease involvement (Table II, Figures 1, 2).
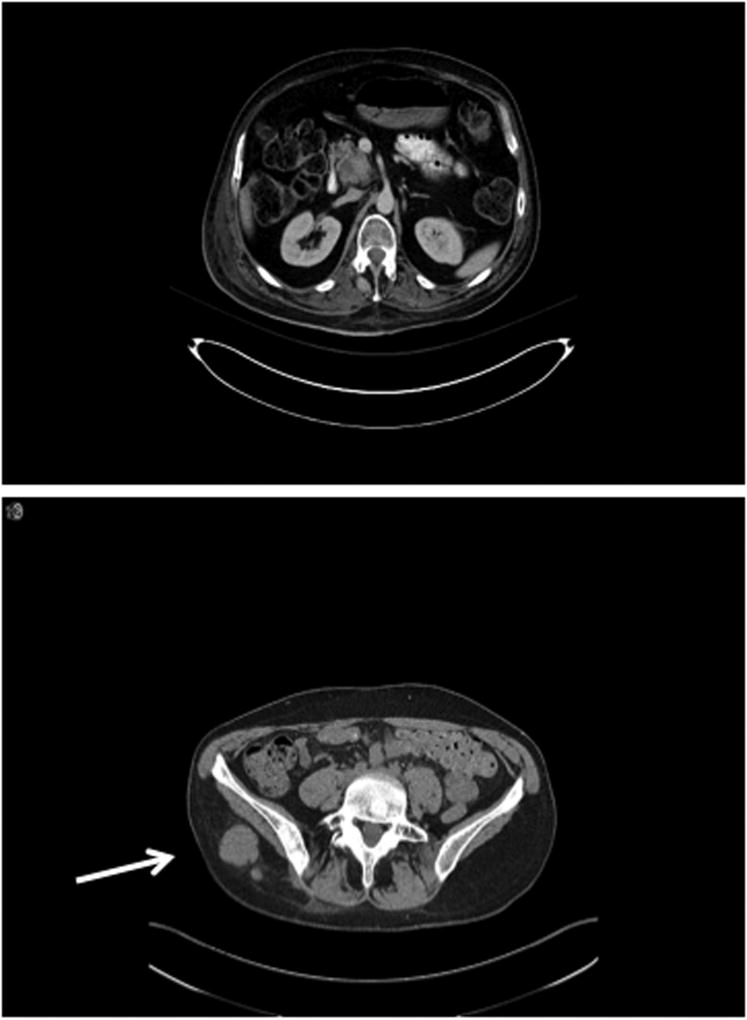
Figure 1.
Right gluteal soft tissue mass, with biopsy-proven plasma cells.
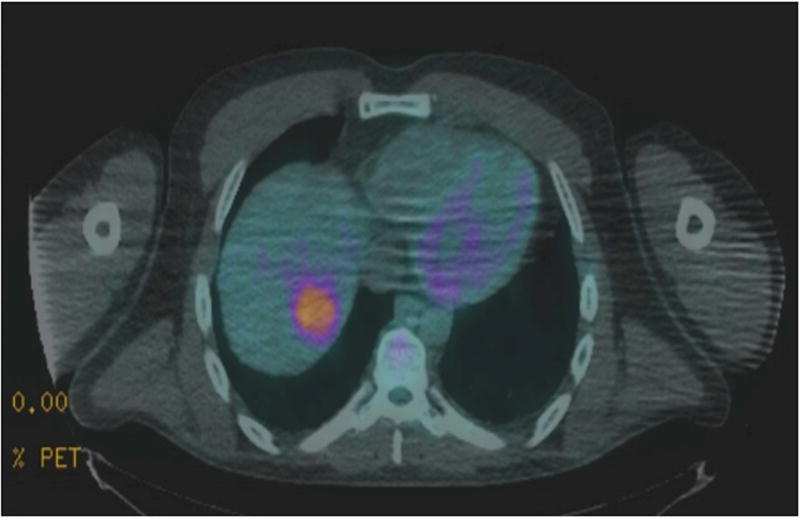
Figure 2.
Positron emission tomography (PET) scan with liver mass demonstrating liver involvement with extramedullary myeloma.
Cytogenetic and Molecular characteristics
Cytogenetic analysis of clonal plasma cells in the bone marrow was available for 29/55 (52%) of the patients with EMD at the time of MM diagnosis. Of these, 15/29 had normal cytogenetics (51.7%) and 14/29 had abnormal cytogenetics (48.3%). Hyperdiploidy occurred in 4/14 (28.5%) cases. The most common cytogenetic abnormalities included deletion 13q or monosomy 13 that were present in 9/14 (64%) of cases. IGH rearrangements occurred in 6 cases (43%). Two cases (14%) presented with t(11;14), 1q amplification occurred in 1 case (7%) and 17p deletion occurred in 1 case (7%).
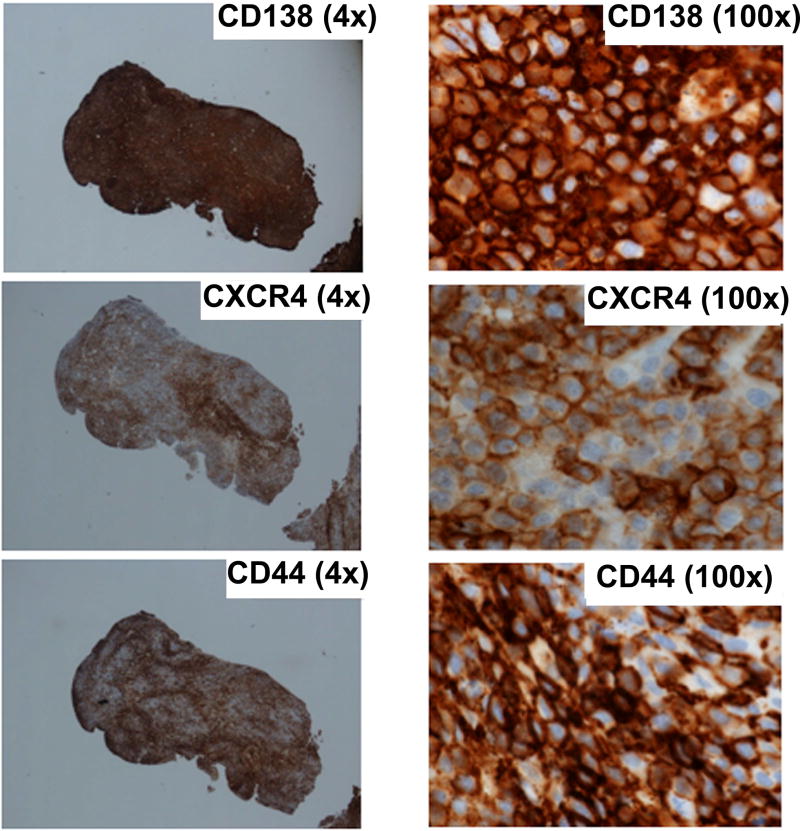
Immunohistochemical analysis was available for 11 patients with EMD, and 13 total samples were analysed. These specimens were obtained from various anatomical sites, including the liver, chest wall, abdomen, breast, oral mucosa, pleural fluid, brain and maxillary sinus. Results of immunohistochemical staining confirmed the presence of CD138-positive plasma cells in all examined EMD specimens. Twelve of the 13 EMD biopsy sites were strongly positive for CD44 (92.3%, 90% confidence interval [CI]: 86.4–99.6), 5/13 of the EMD biopsy sites were positive for CXCR4 (38.5%, 90% CI: 16.6–64.5) and 5 for CD56 staining (38.5%, 90% CI 16.6–64.5) (Table III, Figure 3).
Table III.
Immunohistochemical Characteristics of a subset of 11 MM patients with EMD.
| Patient | Location | CD138 | CXCR4 | CD56 | CD44 | CCR6 |
|---|---|---|---|---|---|---|
| 1 | Liver | POS | NEG | NEG | POS | NEG |
| 1 | Chest Wall | POS | NEG | NEG | POS | NEG |
| 2 | Liver | POS | NEG | NEG | POS | NEG |
| 3 | Liver | POS | POS | NEG | POS | NEG |
| 4 | Chest Wall | POS | NEG | POS | POS | NEG |
| 5 | Chest Wall | POS | NEG | POS | POS | NEG |
| 6 | Abdominal mass | POS | NEG | NEG | POS | NEG |
| 7 | Abdominal mass | POS | POS | NEG | POS | NEG |
| 8 | Breast | POS | POS | NEG | NEG | NEG |
| 9 | Oral mucosa | POS | NEG | POS | POS | NEG |
| 9 | Pleural fluid | POS | NEG | POS | POS | NEG |
| 10 | Brain | POS | POS | NEG | POS | NEG |
| 11 | Maxillary sinus | POS | POS | POS | POS | NEG |
| Percentage Positive (%) | 100 | 38.46 | 38.46 | 92.31 | 0 | |
Samples from 2 biopsy sites were analysed for Patients 1 and 9.
EMD, extramedullary disease; MM, multiple myeloma
Figure 3.
Immunohistochemistry straining of CD138, CD44 and CXCR4 of an extramedullary myeloma sample showing strong positive expression of CD138, CD44 and CXCR4. CD56 and CCR6 were negative in this sample (not shown).
Therapeutic interventions
The 55 EMD patients were treated with a median of 4 different treatment regimens prior to the development of EMD and a median of 5 total treatment regimens (Table IV). All 55 patients underwent autologous stem cell transplantation and 15 also underwent allogeneic stem cell transplantation (27.3%). The median age at autologous stem cell transplantation was 54.2 years (range 35–69). The most commonly used combination therapy was dexamethasone/thalidomide (45.5%). Other common regimens included RVD (lenalidomide, bortezomib, dexamethasone; 41.8%), maintenance lenalidomide (36.3%), VD (bortezomib/dexamethasone; 27.3%) and lenalidomide/dexamethasone (25.4%).
Table IV.
Treatment of patients with EMD
| N | % | Median | |
|---|---|---|---|
| Autologous SCT | 55 | 100 | |
| Allogeneic SCT | 15 | 27.3 | |
| Dexamethasone/Thalidomide | 25 | 45.5 | |
| RVD | 23 | 41.8 | |
| Maintenance Lenalidomide | 20 | 36.3 | |
| Dexamethasone/Bortezomib | 15 | 27.3 | |
| Dexamethasone/Lenalidomide | 14 | 25.4 | |
| Lines of Therapy prior to EMD (n) | 4 | ||
| Total lines of Therapy (n) | 5 | ||
| Age at Autologous Transplant, years | 54.2 (range 35–69) |
EMD, extramedullary disease; SCT, stem cell transplantation; RVD, lenalidomide, bortezomib, dexamethasone
Survival analysis
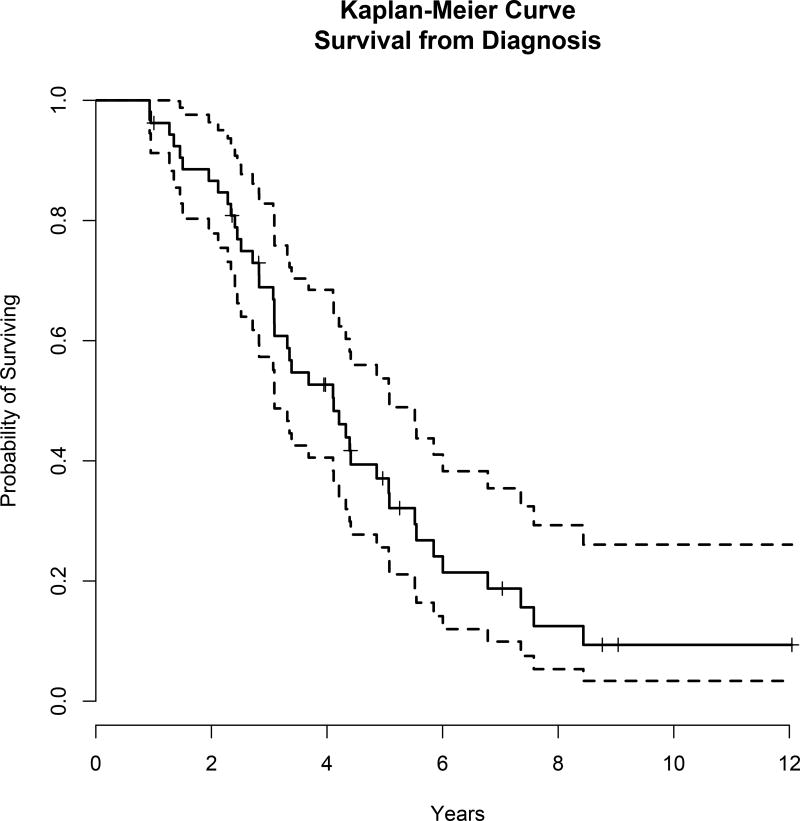
At a median follow-up from time of MM diagnosis of 8.8 years, 41 patients have died. The median overall survival from time of myeloma diagnosis was 4.1 years, (95% CI: 3.1, 5.1), Figure 4A. The median follow-up time from EMD diagnosis was 4.4 years. The median overall survival from time of EMD diagnosis was 1.3 years (95% CI: 0.8,2.3), Figure 4B.
Figure 4.
Survival data. A) The median overall survival from time of myeloma diagnosis was 4.1 years, (95% confidence interval [CI]: 3.1, 5.1). B) The median overall survival from time of extramedullary disease (EMD) diagnosis was 1.3 years (95% CI: 0.8,2.3).
Discussion
It has been known for many years that EMD portends a worse prognosis relative to marrow-localized MM (Blade et al. 1996, Blade et al. 1994). Several characteristic laboratory, cytogenetic and immunophenotypic features of EMD have been identified in small series (Blade et al 2011). However, all studies of EMD that have been published to date have either been small, limited largely to the era prior to the introduction of novel immunomodulatory therapies for MM or have been hampered by inconsistencies in the definition of EMD itself. Our cohort represents a large series of patients with EMD that adheres to a strict definition of biopsy-proven EMD and spans the time of novel therapeutic agents and stem cell transplantation.
Previous series have suggested that EMD may be present in 15–20% of MM cases at the time of diagnosis, and another 15% during the course of their disease (Blade et al. 1996, Blade et al. 1994). However, these early studies have been limited to patients with rare and more aggressive MM phenotypes (such as less than 40 years of age at presentation or IgD myeloma), which may be inherently and artificially enriched for a higher proportion of cases of EMD. Other studies of EMD (Varettoni et al. 2010) may also overestimate the incidence of EMD because of an overly inclusive definition of this clinical entity.
Our series of EMD patients was selected by using a strict definition of EMD at anatomical sites that were non-contiguous with the bone marrow cavity, and included only those patients with biopsy-proven clonal plasma cell infiltrates. From this series, we determined an EMD incidence of 8.3% among all MM patients. This is consistent with several smaller, recent studies, which indicate an EMD incidence of 6–7.5% at the time of diagnosis (Short et al. 2011, Bartel et al. 2009).
Other smaller studies have suggested that patients with EMD may share characteristic laboratory features relative to patients with marrow-localized MM, including lower haemoglobin levels, higher LDH levels and increased rates of thrombocytopenia (Barlogie et al. 1989, Usmani et al. 2012). Our cohort of 55 patients presented with mild anaemia and mildly elevated LDH at the time of MM diagnosis and at the time of EMD development, suggesting that neither the haemoglobin level nor the LDH level is a reliable predictor of MM patients who may already harbour EMD or will proceed to develop EMD during the course of their disease.
Interestingly, the presentation of EMD at the time of diagnosis was mostly in the head and neck area. Lung and pleural effusions as well as abdominal involvement, such as pancreatic or renal involvement, occurred in some cases. However, liver involvement did not occur in any cases at the time of diagnosis. This is in contrast with cases presenting after prior therapeutic interventions. In the relapsed EMD setting, the most common site of involvement was the liver followed by pleural fluid. These presentations may indicate specific tropism or homing of extramedullary myeloma clones that are more prone to trafficking to these sites. Further investigations into the mechanisms of specific tropism of these aggressive myeloma clones are required. Clinically, attention to liver involvement in patients with myeloma should be considered and routine abdominal imaging may be considered to detect those lesions before significant tumour progression and liver dysfunction occurs.
Several bone marrow cytogenetic abnormalities identified in this EMD cohort, particularly deletion of 13q, have been observed in another small, retrospective study of patients with EMD (Rasche et al. 2012). Our cytogenetic data were obtained from patients at the time of myeloma diagnosis and not at the time of development of EMD. Cytogenetic or fluorescence in situ hybridization studies from the EMD samples or the bone marrow were not available at the time of EMD diagnosis . Given the recent data of clonal evolution and heterogeneity, it would not be surprising to identify specific subclones that have a higher propensity for development of EMD. Therefore, further studies are necessary to refine and differentiate the genomic profile of EMD from that of marrow-localized MM.
The immunophenotypic characteristics of EMD have, as yet, remained poorly defined. A previous small series of seven patients with extramedullary MM reported that CD56, a membrane glycoprotein in the immunoglobulin family, is variably expressed in plasma cells resident in the bone marrow, but is absent in extramedullary plasma cells (Dahl et al. 2002). Several reports have therefore suggested that CD56 down-regulation may have a pathogenic role in the development of EMD (Blade et al 2011). In our series, 5 of the 13 analysed EMD specimens (38.5%) were found to be positive for CD56. Although the sample size is small, our findings suggest that CD56 down-regulation may not be as closely linked to EMD pathogenesis as previously described.
Other immunohistochemical studies have reported up-regulation of the cell adhesion molecule CD44 in EMD (Dahl et al. 2002). CD44 mediates binding of tumour cells to stroma and regulates interleukin-6 production (Stauder et al. 1996). Prior studies have shown variable expression of CD44 with about 73% of the cases showing 20% or more positive expression in one study (Zheng et al, 2013). Higher expression was present in patients with recurrent or more aggressive disease. In addition, expression of variant isoforms containing the 9v domain was shown to be associated with an advanced stage and progressive disease with shorter overall survival in MM (Stauder et al.1996).
In our series we confirm that CD44 appears to be reliably over-expressed in EMD specimens (92.3%). However, we did not have matching bone marrow samples for all the EMD samples to compare the relative expression of CD44 in plasma cells present in the bone marrow compared to those present in the EMD sites. Therefore, future studies are required to determine the role of CD44 expression and the different isoforms in the localization of malignant plasma cells in EMD sites.
Additionally, in our series, we note that an increased number of EMD specimens (38.5%) were positive for the presence of CXCR4. This stands in contrast to pre-clinical murine data, in which CXCR4 and other chemokine receptors have been found to be down-regulated in the setting of EMD (Stessman et al. 2013). Further study of the immunohistochemical characteristics of EMD will be necessary for complete elucidation of the mechanisms that underpin the “metastatic” transition of marrow-localized MM into EMD.
Previous studies have suggested that the introduction of novel therapeutic agents may have contributed to an increased incidence of EMD over the past two decades (Raanani et al. 2007). However, it is unclear whether this observation is related to improved methods of radiological detection, increased overall survival of the entire MM population in general or greater awareness of EMD as a distinct clinical entity. Our series of cases gathered entirely within the era of routine stem cell transplantation, proteasome inhibition and immunomodulatory therapy, suggests that the incidence of EMD has remained fairly consistent in the time of modern MM therapy.
The survival of the patients in this series remains poor, with a median overall survival of 4.1 years and a median overall survival of 1.3 years from the time of diagnosis of EMD. This indicates an urgent need for the development of better therapeutic modalities that target this unique subset of patients.
In summary, EMD is an uncommon, but by no means rare, manifestation of MM. This cohort of patients 55 patients represents a large group of patients with EMD who were treated in the era of novel therapeutic strategies and autologous or allogeneic haematopoietic stem cell transplantation for MM at a single academic medical centre. The presence of surface markers of CXCR4 and CD44 may represent new biological markers for EMD but need further confirmation. Further study of these patients’ molecular characteristics and responses to therapy will be necessary so that this distinct disease entity can continue to be characterized.
Acknowledgments
This work was supported by Leukemia and Lymphoma Society and NIH/NCI R01CA181683
Footnotes
Competing interests: the authors have no competing interests
Authorship contribution
Mathew Weinstock: performed the research, designed the research, analysed the data and wrote the paper.
Yosra Aljawai: performed the research, designed the research, analysed the data and wrote the paper.
Elizabeth A. Morgan: performed the research, analysed the data and wrote the paper.
Jacob Laubach: designed the research and wrote the paper
Muriel Gannon: performed the research
Aldo M. Roccaro: designed the research and analysed the data
Cindy Varga: performed the research
Constantine S. Mitsiades: analysed the data and wrote the paper
Claudia Paba-Prada: analysed the data and wrote the paper
Robert Schlossman: analysed the data and wrote the paper
Nikhil Munshi: analysed the data and wrote the paper
Kenneth C. Anderson: analysed the data and wrote the paper
Paul P. Richardson: analysed the data and wrote the paper
Edie Weller: analysed the data and wrote the paper
Irene M. Ghobrial: performed the research, designed the research, analysed the data and wrote the paper.
References
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneauve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. New England Journal of Medicine. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Smallwood L, Smith T, Alexanian R. High serum levels of lactic dehydrogenase identify a high-grade lymphoma-like myeloma. Annals of Internal Medicine. 1989;110:521–525. doi: 10.7326/0003-4819-110-7-521. [DOI] [PubMed] [Google Scholar]
- Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, Alpe T, Angtuaco E, Walker R, Epstein J, Crowley J, Barlogie B. F-18 fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blade J, Lust JA, Kyle RA. Immuglobulin D multiple myeloma: presenting features, reponse to therapy, and survival in a series of 53 cases. Journal of Clinical Oncology. 1994;12:2398–2404. doi: 10.1200/JCO.1994.12.11.2398. [DOI] [PubMed] [Google Scholar]
- Blade J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. British Journal of Haematology. 1996;93:345–351. doi: 10.1046/j.1365-2141.1996.5191061.x. [DOI] [PubMed] [Google Scholar]
- Blade J, Fernandez de Larrea C, Rosinol L, Cibeira MT, Jimenez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. Journal of Clinical Oncology. 2011;29:3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- Dahl IM, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. British Journal of Haematology. 2002;116:273–277. doi: 10.1046/j.1365-2141.2002.03258.x. [DOI] [PubMed] [Google Scholar]
- Dimopoulous M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, Corso A, Masliak Z, Olesyckyj M, Yu Z, Patin J, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. New England Journal of Medicine. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. http://seer.cancer.gov/csr/1975_2009_pops09/ based on Novemner 2011 SEER data submission, posted to the SEER website, 2012. [Google Scholar]
- McKenna RW, Kroft SH. Plasma cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. 2008. pp. 194–195. [Google Scholar]
- Rajkumar SV, Blood E, Vessole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- Raanani P, Shpilberg O, Ben-Bassat I. Extramedullary disease and targeted therapies for hematologic malignancies—is the association real? Annals of Oncology. 2007;18:7–12. doi: 10.1093/annonc/mdl129. [DOI] [PubMed] [Google Scholar]
- Rasche L, Bernard C, Topp M, Kapp M, Duell J, Wesemeier C, Haralambieva E, Maeder U, Einsele H, Knop S. Features of extramedulllary myeloma relapse: high proliferation, minimal marrow involvement, adverse cytogenetics: a retrospective single-center study of 24 cases. Annals of Hematology. 2012;91:1031–1037. doi: 10.1007/s00277-012-1414-5. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, Gertz M, Kumar S, Mikhael J, Roy V, Kyle RA, Lacy MQ. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of Pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder R, Van Driel M, Schwärzler C, Thaler J, Lokhorst HM, Kreuser ED, Bloem AC, Günthert U, Eisterer W. Different CD44 splicing patterns define prognostic subgroups in multiple myeloma. Blood. 1996 Oct 15;88(8):3101–8. [PubMed] [Google Scholar]
- Stessman HA, Mansoor A, Zhan F, Janz S, Linden MA, Baughn LB, Van Ness B. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia. 2013 Oct;27(10):2075–7. doi: 10.1038/leu.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, Alsayed Y, Waheed S, Haider S, Restrepo A, Van Rhee F, Crowley J, Barlogie B. Extramedullary disease portends poor prognosis in multiple myeloma and is overrepresented in high risk disease even in the era of novel agents. Haematologica. 2012;97:1761–1767. doi: 10.3324/haematol.2012.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Annals of Oncology. 2010;21:325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leukemia and Lymphoma. 2012;54:1135–1141. doi: 10.3109/10428194.2012.740562. [DOI] [PubMed] [Google Scholar]
- Zheng W, Liu D, Fan X, Powers L, Goswami M, Hu Y, Lin P, Medeiros LJ, Wang SA. Potential therapeutic biomarkers in plasma cell myeloma: a flow cytometry study. Cytometry B Clin Cytom. 2013 Jul-Aug;84(4):222–8. doi: 10.1002/cyto.b.21083. [DOI] [PubMed] [Google Scholar]