Abstract
Background
The presence of frailty or prefrailty in older adults is a risk factor for postsurgical complications. The frailty phenotype can be improved through long-term resistance and aerobic training. It is unknown whether short-term preoperative interventions targeting frailty will help to mitigate surgical risk. The purpose of this study was to determine the proportion of frail and prefrail patients presenting to a thoracic surgical clinic who could benefit from a frailty reduction intervention.
Methods
A prospective cohort study was performed at a single-site thoracic surgical clinic. Starting October 1, 2014, surgical candidates 60 years of age or older who consented to be screened were included. Patients were screened using an adapted version of Fried’s phenotypic frailty criteria: weakness (grip strength), slow gait (15-foot walk), unintentional weight loss, self-reported exhaustion, and low self-reported physical activity (Physical Activity Scale for the Elderly). Prefrailty was identified when participants demonstrated one to two frailty characteristics; frailty was identified when participants demonstrated three to five frailty characteristics.
Results
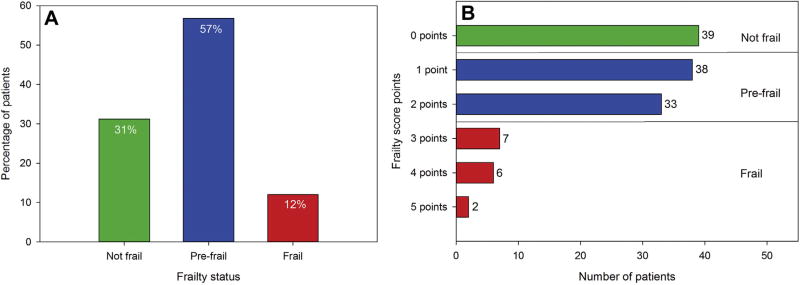
Of 180 eligible patients, 126 consented, and 125 completed screening. Thirty-nine participants (31%) were not frail, 71 (57%) were prefrail, and 15 (12%) were frail. Exhaustion was the most common frailty symptom (34%). Frailty prevalence did not significantly differ among men and women (men: 10%, women: 14%; p = 0.75).
Conclusions
We found a high proportion of prefrail and frail patients among patients deemed candidates for thoracic surgical procedures. This finding indicates that frailty may be underrecognized. Substantial numbers of patients may be considered for a presurgical frailty reduction intervention.
Frailty is defined as a state of increased vulnerability to physiologic stressors [1, 2]. Although no single operational definition exists [3], phenotypic frailty has been shown to predict falls, disability, hospitalization, and death [4]. As more patients of advanced age present for surgical treatment, there has been growing interest in assessing frailty as a surgical risk factor [5–11]. Phenotypic frailty has been shown to predict surgical complications, increased hospital length of stay, and postdischarge institutionalization [5, 6]. Research has begun to focus on interventions to mitigate the risks of frailty [12]. Some frailty activity interventions were able to improve frailty measures in as little time as 6 weeks [13, 14], thus indicating that a presurgical frailty intervention may also be feasible.
The prevalence of frailty in thoracic surgical candidates is not known. In the study originally defining the phenotypic frailty criteria, the prevalence of frailty and prefrailty in a community dwelling sample was 7% and 47%, respectively [4]. A systematic review showed frailty prevalence ranging from 4.0% to 59.1%, with the overall weighted prevalence of frailty at 10.7%, or 9.9% when focusing on physical frailty [15]. Frailty is more prevalent in the presence of acute and chronic disease, a finding suggesting that frailty prevalence may be higher in surgical groups [4]. In a study of 594 patients presenting for elective surgical procedures, 10.4% were frail and 31.3% were prefrail using Fried’s frailty index [5]. Thoracic surgical candidates may represent a group with increased comorbidity and frailty, and they may be an ideal group to target for an intervention.
The objective of this study was to determine the proportion of frail and prefrail patients presenting to a thoracic surgical clinic as potential surgical candidates who could benefit from a preoperative frailty intervention. Results from this study will inform an intervention designed to reduce frailty and frailty-related surgical complications in this population.
Patients and Methods
Participants
Patients seen in the University of Chicago Thoracic Surgery Clinic in Chicago were actively recruited to participate in frailty screening from October 1, 2014 through January 6, 2016. These patients were recruited and consented to participate in screening during their first or second clinic visit if they were deemed to be candidates for major thoracic surgical procedures. Inclusion criteria were age 60 years or older, ability to consent, willingness to participate in frailty screening, no obvious contraindication to surgical intervention, and thoracic disease that could require major operation (major lung resection, esophagectomy, repair of giant paraesophageal hernia, chest wall resection, extended pleurectomy or decortication, or sternotomy for thymectomy or other mediastinal process). Contraindications to surgical treatment were assessed by participating surgeons based on an overview of the patients’ condition, which included their physical status, comorbidities, and cancer stage, as appropriate.
Frailty Assessment
Once consent was obtained, subjects were screened using an adapted version of Fried’s phenotypic frailty criteria: (1) unintentional weight loss, (2) weakness, (3) exhaustion, (4) low physical activity, and (5) slowness [4]. Unintentional weight loss was assessed using measured weight loss (if available) or self-reported unintentional weight loss over the previous year. A frailty point was assigned if the participant reported a decline of 10 pounds or more or 5% body weight in the past year. Weakness was assessed by measuring the grip strength of the dominant hand by using a dynamometer (JAMAR Plus+ Hand Dynamometer, Instrument M3–200, Patterson Medical, Warrenville, IL). The average of three measurements was recorded. A frailty point was assigned if strength was in the lowest quintile for sex and body mass index category by using previously established cutpoints [4].
Exhaustion was assessed using two self-reported questions: In the last week, “I felt that everything I did was an effort;” and “I could not get going.” Answer options included: rarely or none of the time (<1 day), some or a little of the time (1 to 2 days), a moderate amount of the time (3 to 4 days), or most of the time (5 to 7 days). Exhaustion was identified if either answer was a moderate amount of time (3 to 4 days per week) or most of the time (5 to 7 days per week). Low physical activity level was assessed using the Physical Activity Scale for the Elderly score. A point was assigned if the participant scored in lowest quartile by sex by using previously established cutpoints [16].
Slowness was assessed by measuring gait speed over a distance of 15 feet at a normal pace, averaged over three trials. A frailty point was assigned if the participant scored in lowest quintile by sex and height by using previously established cut-points [4]. The presence of one to two criteria indicated prefrailty; three or more criteria indicated frailty.
Covariates
Data were also collected on subjects’ age, body mass index (kg/m2), sex, race, and referral diagnosis.
Statistical Analysis
The primary outcome studied was the proportion of prefrail and frail patients in the study sample. Means (continuous) and frequencies (categorical) were generated for baseline characteristics and frailty status. Frailty status and Eastern Cooperative Oncology Group (ECOG) performance status were compared [17]. ECOG status was dichotomized as 0 to 1 (normal) and 2 to 3 (low performance status). Frequencies were also assessed across sex subgroups. χ2 tests were used to identify correlation between frailty status and ECOG status and to identify significant differences among sex subgroups.
Results
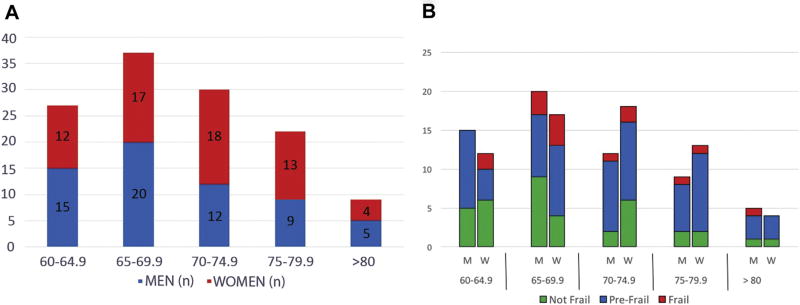
Of the 180 eligible patients, 126 consented, 21 deferred, 13 declined, 10 did not follow up in clinic, and 10 were not approached. The average age of the participants was 70.4 years. In this sample there was a slightly higher proportion of female patients (51.2%), and most patients were white (72.8%). The most common referral diagnosis was for a lung lesion (including lung mass and lung nodule; 68.8%). Complete demographic data are presented in Table 1. Age breakdown by sex is shown in Figure 1.
Table 1.
Demographic Characteristics of Screened Patients
| Characteristic | Value |
|---|---|
| Age (mean [range]) | 70.4 (60–88) |
| BMI (mean [range]) | 27.6 (14–48) |
| Sex (n [%]) | |
| Women | 64 (51.2%) |
| Men | 61 (48.8%) |
| Race (n [%]) | |
| White | 91 (72.8%) |
| Black | 27 (21.6%) |
| Asian | 6 (4.0%) |
| Referral diagnosis (n [%]) | |
| Lung nodule | 46 (36.8%) |
| Lung cancer | 40 (32.0%) |
| Mediastinal mass | 9 (7.2%) |
| Paraesophageal hernia | 7 (5.6%) |
| Esophageal cancer | 7 (5.6%) |
| Mesothelioma | 6 (4.8%) |
| Metastatic, nonlung primary tumor | 3 (2.4%) |
| Chest wall tumor | 2 (1.6%) |
| Pleural effusion | 2 (1.6%) |
| Emphysema | 2 (1.6%) |
| Pneumothorax | 1 (0.8%) |
BMI = body mass index.
Fig 1.
(A) Age breakdown by sex. (B) Frailty status by age and sex. (M = men; W = women.)
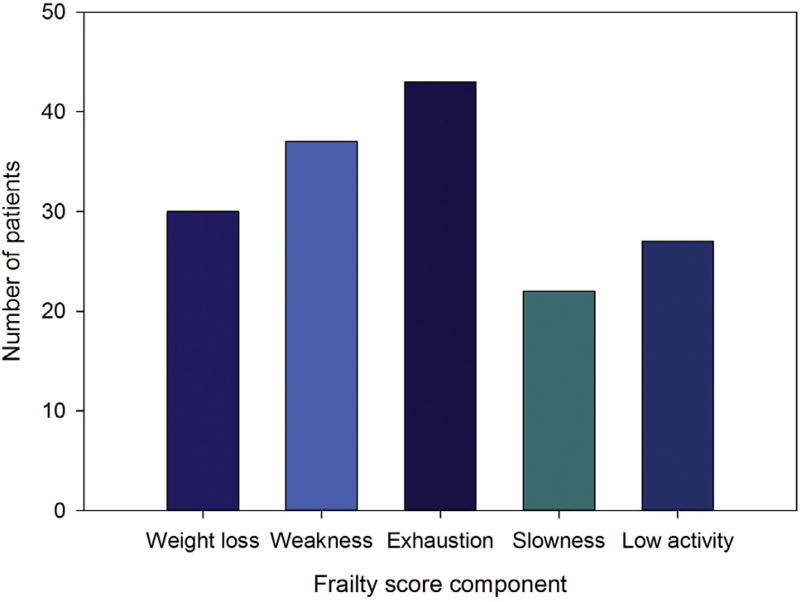
In this sample, 68.8% of patients were prefrail or frail (Fig 2). Of the five phenotypic frailty characteristics, the most commonly identified characteristic was exhaustion. The least commonly identified characteristic was slowness (Fig 3). Frailty status and ECOG status were not significantly correlated (p = 0.080; Table 2). Frailty prevalence was not significantly different across sex subgroups (men: 9.8%, women: 14.1%; p = 0.75). Women were more likely to demonstrate weak grip strength than men (women: 37.5% vs men: 21.31%; p = 0.046. Men and women were equally likely to demonstrate the remaining four frailty criteria (p value range, 0.07 to 0.46).
Fig 2.
(A) Percentage of screened surgical candidates according to frailty status. (B) Number of surgical candidates scoring 0 to 5 on adapted Fried’s frailty index.
Fig 3.
Number of surgical candidates scoring each frailty component.
Table 2.
Frailty Status Versus Eastern Cooperative Oncology Group Status
| ECOG Status | ||
|---|---|---|
|
|
||
| Frailty Status | Normal (0–1) | Low (2–3) |
| Frail | 11 | 4 |
| Prefrail | 63 | 8 |
| Not frail | 37 | 2 |
| All | 111 | 14 |
Pearson χ2 = 5.054; degrees of freedom = 2; p value = 0.080.
ECOG = Eastern Cooperative Oncology Group.
Comment
In this pilot sample of preoperative thoracic surgical patients, we found that most (68.8%) surgical candidates were prefrail or frail. The higher prevalence noted in our sample is not surprising given that most patients were referred to a surgical clinic for a diagnosis of lung lesions suggestive of malignancy. The high level of frailty in our sample (12.0%) suggests that frailty may be underrecognized by clinicians as a surgical risk factor and that mitigating options are needed for this population.
The most common frailty criterion in our sample was exhaustion. This was a unique finding and possibly specific to patients with cancer who were assessed for frailty. The high prevalence of this symptom among thoracic surgical candidates suggests two key considerations: (1) cancer and frailty may share common pathways, and (2) thoracic surgical candidates may benefit from better depression screening and presurgical treatment. Exhaustion is a common complaint in patients with cancer [18], and it may result from a hypermetabolic state, which has also been demonstrated in a subset of frail patients [19, 20]. Fatigue and depression are difficult to distinguish in patients with cancer [21], and exhaustion may indicate higher levels of depression. In either case, the high prevalence of exhaustion may be clinically significant. A previous study of patients undergoing pancreaticoduodenectomy found exhaustion to be an independent predictor of major surgical complications, longer hospital stays, and surgical intensive care admissions [22]. In addition, depression has been shown to be a risk factor for death after coronary artery bypass procedures [23], and it affects outcomes in other types of operations as well [24, 25].
Frailty status and ECOG performance status were not strongly correlated in this population. Many more patients were categorized as prefrail or frail (68.8%) compared with having low performance status (11.2%). Frailty measurement likely provides additional information missed by ECOG performance status. Although ECOG performance status has also been correlated with worse surgical outcomes [26], frailty assessment may be a better marker for surgical risk than ECOG performance status alone. Earlier studies of older oncology patients indicated that performance status may miss a considerable amount of geriatric health syndromes [27, 28].
Frailty prevalence has been previously demonstrated to be higher in women [4]. We found a slightly higher prevalence of frailty among women than among men, but this difference was not statistically significant. Women with lung cancer appear to have better survival than men in all stages and treatment groups [29]. This finding suggests that lung cancer in women may have a different natural history. Nonsignificant differences in frailty between the sexes, as previously described, may be explained by our small sample size, or alternatively, they may reflect this difference in the natural history of lung cancer between the sexes.
The high prevalence of frailty in our sample is an especially important finding given the impact of frailty as a surgical risk factor predicting postoperative morbidity and death [5–11]. In a study of surgical outcomes by Makary and colleagues [5], patients who were prefrail or frail had higher rates of postoperative complications, increased length of stay, and increased discharge institutionalization. In addition, frailty was shown to improve predictive power of traditional risk assessment models. Other investigators have identified similar findings in older patients with cancer who were undergoing surgical treatment [30–32]. In a study by Tsiouris and associates [8], mortality rates were higher in patients who underwent open lobectomy who had an increased modified frailty index. The high prevalence of prefrailty and frailty in our population suggests that thoracic surgeons are caring for a particularly high-risk group of older adults. Frailty screening becomes particularly important in such a group to aid in preoperative discussions of risk and to provide objective targets for risk reduction.
Our study shows not only that frailty, a significant surgical risk factor, is prevalent in a thoracic surgical clinic, but also that screening for frailty is feasible. Patients were able to complete the Physical Activity Scale for the Elderly activity questionnaire while waiting for their appointment, and screening for the other four frailty criteria typically took less than 10 minutes of clinic time. In addition, a nonphysician staff member was trained to complete the screening, so provider time was not constrained. The greatest limitation to widespread screening is the requirement of a dynamometer to measure grip strength. We chose to use a frailty screening tool requiring such a device because of the published reports showing that a strong association between the phenotypic frailty criteria and surgical outcomes is well-established. However, other validated screening tools that do not use this equipment do exist, and alternatives for measuring grip strength are being developed [33, 34].
Although screening for frailty is feasible and important, it is still unclear whether preoperative frailty interventions will reduce frailty-related poor surgical outcomes [35–37]. Early work evaluated frailty reduction programs in nonsurgical samples. The most consistently positive results have come from physical activity interventions, with the largest improvements noted among prefrail patients [12]. Although some interventions have occurred over a 6- to 12-month period [38, 39], other studies have shown positive change in physical performance tests over as little as 6 weeks [13]. In a study targeting all candidates for surgical resection of colorectal cancer, rather than just candidates with frailty, a prehabilitation program consisting of exercise, nutrition, and anxiety reduction components (average duration, 33 days) significantly improved postoperative function recovery as measured by the 6-minute walk test [40]. The ability to improve physical performance over a short time is especially important in designing presurgical interventions that would be time sensitive.
A limitation of our study was the deferral rate. Subjects were allowed to defer decision to their next clinic visit, and some subjects elected this option. It is unclear whether the majority of those who deferred would later choose to consent to participation in the study. Further investigation is needed to understand reasons for deferral because this may have skewed the results. Other limitations include a small sample size, a single site, and a single surgical specialty.
The impressive levels of frailty and prefrailty in our study indicate a need to study the impact of a presurgical frailty intervention trial and that thoracic surgical candidates comprise an ideal group for study of such an intervention. No frailty intervention studies have evaluated whether a preoperative frailty reduction program also reduces surgical morbidity and mortality rates. Future work may examine how a brief strength and resistance training intervention for prefrail and frail patients affects frailty status, surgical risk estimation, and surgical outcomes.
Acknowledgments
This research was supported by the Jeanie Dallas Thoracic Surgery Research Fund.
References
- 1.Fried LP, Walston JD, Ferrucci L. Frailty. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard’s Geriatric Medicine and Gerontology. 6. New York, NY: McGraw-Hill Medical; 2009. pp. 631–45. [Google Scholar]
- 2.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older person: a consensus report. J Am Geriatr Soc. 2004;52:625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsiouris A, Hammoud ZT, Velanovich V, et al. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183:40–6. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Sündermann SH, Dademasch A, Seifert B, et al. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact Cardiovasc Thorac Surg. 2014;18:580–5. doi: 10.1093/icvts/ivu006. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta M, Rolfson DB, Stolee P, et al. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–8. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 12.Bibas L, Levia M, Bendayan M, et al. Therapeutic interventions for frail elderly patients: Part I. Published randomized trials. Prog Cardiovasc Dis. 2014;52:134–43. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Marsh AP, Chmelo EA, Katula JA, et al. Should physical activity programs be tailored when older adults have compromised function? J Aging Phys Act. 2009;17:294–306. doi: 10.1123/japa.17.3.294. [DOI] [PubMed] [Google Scholar]
- 14.Drey M, Zech A, Freiberger E, et al. Effects of strength training versus power training on physical performance in prefrail community-dwelling older adults. Gerontology. 2012;58:197–204. doi: 10.1159/000332207. [DOI] [PubMed] [Google Scholar]
- 15.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 16.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 18.Lawrence DP, Kupelnick B, Miller K, et al. Evidence report on the occurrence assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 19.Weiss CO, Cappola AR, Varadhan R, Fried LP. Resting metabolic rate in old-old women with and without frailty: variability and estimation of energy requirements. J Am Geriatr Soc. 2012;60:1695–700. doi: 10.1111/j.1532-5415.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Welsh DA, Ravussin E, et al. An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively. J Gerontol A Biol Sci Med Sci. 2014;69:650–6. doi: 10.1093/gerona/glt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter K, Harter M. The concepts of fatigue and depression in cancer. Eur J Cancer Care (Engl) 2004;13:127–34. doi: 10.1111/j.1365-2354.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 22.Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improved prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy. Ann Surg. 2014;259:960–5. doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–9. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 24.Ellis HB, Howard KJ, Khaleel MA, et al. Effect of psychopathology on patient perceived outcomes of total knee arthroplasty within an indigent population. J Bone Joint Surg Am. 2012;94:841. doi: 10.2106/JBJS.K.00888. [DOI] [PubMed] [Google Scholar]
- 25.Stundner O, Kirksey M, Chiu YOL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population based study. Psychosomatics. 2013;54:149–57. doi: 10.1016/j.psym.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 26.PACE participants. Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–63. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Schulkes KJ, Hamaker ME, van den Bos F, et al. Relevance of geriatric assessment for elderly patients with lung cancer: a systematic review. Clin Lung Cancer. 2016 Jun 2; doi: 10.1016/j.cllc.2016.05.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Ferguson MK, Skosey C, Hoffman PC, et al. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- 30.Tan KY, Kawamura YJ, Tokomitsu A, et al. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–43. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Courtney-Brooks M, Tellawi AR, Scalici J, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126:20–4. doi: 10.1016/j.ygyno.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Ronning B, Wyller TB, Jordhoy MS, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol. 2014;5:26–32. doi: 10.1016/j.jgo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Adams P, Ghanem T, Stachler R, et al. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139:783–9. doi: 10.1001/jamaoto.2013.3969. [DOI] [PubMed] [Google Scholar]
- 34.Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41:844–51. doi: 10.1016/j.ejso.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 2012;11:CD010118. doi: 10.1002/14651858.CD010118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33:17–33. doi: 10.1016/j.anclin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Halloway S, Buchholz SW, Wilbur J, Schoeny ME. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res. 2015;37:103–23. doi: 10.1177/0193945914551006. [DOI] [PubMed] [Google Scholar]
- 38.Gill TM, Baker DI, Gottschalk M, et al. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–74. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 39.LIFE study investigators. Pahor M, Blair SN, et al. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–82. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]