Abstract
Background
Women do not receive appropriate surgical therapy for lung cancer as often as men. Patient gender may influence treatment recommendations; less is known about the effect of physician gender on recommendations.
Methods
Gender-neutral vignettes representing low-risk, average-risk, and high-risk candidates for lung resection were paired with concordant videos of standardized patients (SPs). Cardiothoracic trainees and practicing thoracic surgeons read a vignette, provided an initial estimate of the percentage risk of major adverse events after lung resection, viewed a video (randomized to male or female SP), provided a final estimate of risk, and ranked the importance of the video in the final risk estimate.
Results
Overall, 107 surgeons participated, of whom 90 were men. Initial estimated risks mirrored actual vignette risks: 10.4% ± 9.9 for low risk, 17.6% ± 13.2 for average risk, and 21.0% ± 14.7 for high risk (p < 0.001). After SP videos were viewed and final risk estimates were rendered, there was a significant difference between male and female physicians in the absolute change in estimated risk (p = 0.002), with male physicians having larger changes than female physicians. There was also an effect of SP gender that varied by vignette type (p < 0.001). Increasing video importance scores were directly associated with increasing change in risk scores for average-risk and high-risk vignette/video combinations (p < 0.001 for each).
Conclusions
Differences in estimating complication risk for lung resection candidates are related to physician and patient gender. This may influence recommendations for surgical treatment. Understanding such differences may help reduce inequities in treatment recommendations.
Lung cancer is the most common cause of cancer-related mortality in the United States for both men and women [1]. There are sex-related differences in the presentation and outcomes of treatment for non-small cell lung cancer (NSCLC). Women tend to present with earlier stages of cancer, have a higher percentage of adenocarcinomas compared with other histologic types, experience fewer postoperative adverse events, and have comparatively better long-term cancer survival [2–8].
An early report suggested that women do not receive appropriate surgical therapy for lung cancer as often as men do [9]. This finding was supported by data from the Surveillance, Epidemiology, and End Results Program indicating that, among patients with early-stage NSCLC, women are 25% less likely to receive timely surgical resection than are men [10]. The reasons for this are unclear, but the findings suggest that there may be a selection bias for surgical therapy based on patient sex.
Biases in surgical decision making also extend to physician sex. These may be related to differences in the development and tolerance of anxiety, variations in visual and spatial perception, and patient sex-related considerations [11–14]. Such differences may result in biases in risk assessment and surgical recommendations.
We recently demonstrated that clinical vignettes and short silent videos of standardized patients (SPs) trained to represent lung resection candidates are useful in identifying factors that influence surgeon risk estimation [15]. The present study used this approach to examine the hypothesis that physician and patient gender have an influence on risk estimation for major lung resection.
Patients and Methods
This protocol was approved by The University of Chicago Institutional Review Board, and written consent was waived. Implicit consent was assumed when participants enrolled in the study. Potential participants were identified from a list of practicing thoracic surgeons and cardiothoracic trainees provided by the Thoracic Surgery Directors Association, and they were offered $100 compensation for completing the entire study. Thoracic surgeon subjects were required to be involved in trainee education and to have an active clinical practice consisting of more than 50% of noncardiac thoracic surgery. Trainee subjects were required to be in a traditional program consisting of 2 to 3 years of exclusive cardiothoracic training, or to be in the final 3 years of a 6-year integrated (I-6) program.
With the use of histories of patients operated on for non-small cell lung cancer at our institution, clinical vignettes were developed that were gender neutral and represented patients aged 60 to 69. The Charlson score (for assessment of risk status related to comorbidities) and (a weighted total score for forced Expiratory Volume in 1 second, Age, and Diffusing capacity for carbon monoxide) (EVAD) score (for physiologic risk status specifically related to lung resection) were calculated for each vignette [16, 17]; the possible range of Charlson scores was 0 to 37, and the possible range of EVAD scores was 0 to 12. The Charlson and EVAD scores were summed to give a total risk score for each vignette. Two patient vignettes were created to be low risk with a total score of 3 to 5, two were created to be average risk with a total score of 7 to 9, and two patient vignettes were created to be high risk with a total score of 11 to 13. These scores were not revealed to the study subjects.
A group of geriatric specialists, using an iterative process, had previously developed a set of physical characteristics based on the phenotypic criteria developed by Fried and colleagues [18] that could be portrayed in a short, silent video, including weight loss, gait speed, strength, and fatigue [19]. Three levels of physical performance (“somewhat vigorous,” “neither vigorous nor frail,” and “somewhat frail”) were assigned performance characteristics with standard metrics (Table 1).
Table 1.
Performance Characteristics and Metrics for Videos of Standardized Patients
| Clinical Factor | Somewhat Vigorous | Neither Vigorous nor Frail | Somewhat Frail |
|---|---|---|---|
| Weight loss | None | Shirt may appear somewhat loose; wearing oversized shirt | Both shirt and pants may appear loose; wearing oversized shirt and pants |
| Gait | Normal pace, medium stride | Slower pace, normal stride length | Slower pace, shortened stride |
| Gait speed to chair 100 steps/min; 0.65 to 0.8 m/sec; 3–4 steps over 6-ft span | Gait speed to chair 85 steps/min; 0.5 to 0.6 m/sec; 4–5 steps over 6-ft span | Gait speed to chair 70 steps/min; 0.3 to 0.4 m/sec; 6–7 steps over 6-ft span | |
| Gait speed to table 90 steps/min; 0.6 to 0.7 m/sec; 2.5 steps over 4-ft span | Gait speed to table 75 steps/min; 0.5 to 0.6 m/sec; 3.5 steps over 4-ft span | Gait speed to table 60 steps/min; 0.4 to 0.5 m/sec; 4.5 steps over 4-ft span | |
| Strength | Uses one hand for balance when sitting, standing, or climbing onto a step; time to sit and rise 1.5 sec each | Uses two hands for balance and aid when sitting and standing; slightly slow to climb onto step and turn; time to sit and rise 2.0 sec each | Some difficulty sitting and standing despite use of two hands; rocks back and forth before rising from a seated position; some difficulty in climbing onto and turning on step in order to sit on table; time to sit and rise 3.0 sec each |
| Fatigue | Moves without effort from chair to table | Moves somewhat slowly from chair to table; no evident fatigue | Some rapid breathing with moderate effort, appears tired, moves slowly from chair to table |
Twelve standardized patients (SPs) appearing to be aged 60 to 69 years were selected so that a man and a woman who were similar in apparent age, of the same race, and similar in body mass index were paired for research purposes. Each SP was dressed in black slacks, black flat shoes, black belt, and cream-colored long-sleeved shirt. SPs portraying “neither vigorous nor frail” behavior wore a shirt that was oversized by one size. SPs portraying “somewhat frail” patients wore shirts and pants that were both oversized by one size to portray weight loss. Two pairs of SPs each portrayed one of the three levels of frailty in videos set in a mock outpatient clinic, replicating gait speed, time required to sit in a chair, time required to stand up from a chair, and time required to climb onto an examination table. A custom sound track indicating the timing of these elements was used so that the overall performances of the paired SPs were as similar as possible (Table 1).
One pair of SP videos was assigned to each vignette: videos of SPs portraying “somewhat vigorous” behavior were paired with low-risk vignettes, “neither vigorous nor frail” SP videos were paired with average-risk vignettes, and videos of SPs portraying “somewhat frail” behavior were paired with high-risk vignettes. In this way the videos and vignettes were considered to represent concordant levels of surgical risk.
Subjects were asked to read a clinical vignette and to estimate the patient’s risk for major postoperative adverse events after lobectomy from 0% to 100% using an anchored Likert-like scale. Participants then viewed one of the videos of the SPs paired with the clinical vignette, randomly selected between videos showing male and female SPs. Subjects provided a second estimate of surgical risk based on the vignette and the video combined. The subjects were asked to rate the relative importance of the vignette and the video to their second risk estimate on a scale of 0% to 100%, with the total summing to 100%. This process was repeated for a total of six vignettes.
Videos were hosted on Vimeo. Study data were collected and managed with the Research Electronic Data Capture (REDCap) tool hosted at the University of Chicago. REDCap is a secure, web-based application designed to support data capture for research studies [20].
To analyze the data, two-sample t tests, χ2 analysis, and repeated-measures analysis of variance with Greenhouse-Geisser adjustment were used as appropriate. For comparison of changes in risk estimates (final – initial) or importance of the SP video based on surgeon characteristics (eg, trainee status and gender) or SP gender, generalized estimating equation (GEE) linear regression models were fit. This type of model accounts for the correlation between observations from the same surgeon. An exchangeable correlation was assumed. For assessment of the relationship between video importance and changes in risk estimates, plots were generated based on marginal estimates from a GEE regression model that permitted a quadratic effect of video importance with the inclusion of a squared term. Analyses were performed with Stata Version 14 (StataCorp, College Station, TX).
Results
A total of 377 potential subjects were solicited to participate, including 139 practicing thoracic surgeons and 238 trainees. Overall, 107 (28.4%) subjects participated, including 60 (43.2%) of the practicing surgeons and 47 (19.7%) of the trainees. The distributions of subject gender, age, training status, and comfort level with lobectomy are listed in Table 2.
Table 2.
Characteristics of Physician Subjects Participating in Study
| Category | All Subjects | Practicing Surgeon | Trainee | p value |
|---|---|---|---|---|
| Number | 107 | 60 | 47 | … |
| Male | 90 (84%) | 53 (88%) | 37 (79%) | 0.177 |
| Age | 43.1 ± 11.1 | 50.7 ± 9.7 | 33.8 ± 2.1 | <0.001 |
| Experience level | … | |||
| Traditional program | … | … | 38 | |
| Integrated training program | … | … | 9 | |
| Years in practice | … | 16.6 ± 10.8 | … | |
| Comfort with assessing lobectomy risk | <0.001 | |||
| Beginner | 9 (8%) | 0 | 9 (19%) | |
| Advanced beginner | 10 (9%) | 0 | 10 (21%) | |
| Competent | 34 (32%) | 9 (15%) | 25 (53%) | |
| Proficient | 54 (50%) | 51 (85%) | 3 (6%) |
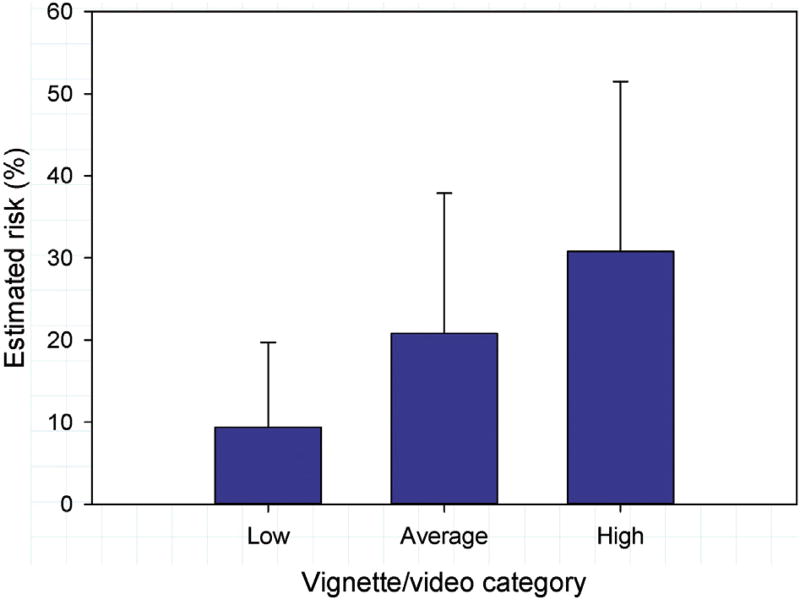
For the comparison of initial risk estimates across the three vignette categories (low risk, average risk, high risk), the mean for each subject of the two vignettes within each category was used. Initial risk estimates mirrored the level of risk designed in the vignettes (Table 3). There were no physician gender or training status interactions with vignette type (p = 0.33 and p = 0.77, respectively). Final risk estimates based on vignette/video combinations also mirrored the level of risk designed in the vignettes and portrayed in the videos (Fig 1).
Table 3.
Initial Risk Estimates Based on Vignettes Alone
| Category | Low-Risk Vignettes | Average-Risk Vignettes | High-Risk Vignettes | p value |
|---|---|---|---|---|
| All subjects | 10.4 ± 9.9 | 17.6 ± 13.2 | 21.0 ± 14.7 | <0.001 |
| Male physician subjects | 10.5 ± 9.7 | 17.6 ± 13.4 | 20.6 ± 13.8 | 0.78 |
| Female physician subjects | 10.3 ± 11.4 | 17.4 ± 12.8 | 23.6 ± 18.8 | |
| Trainees | 9.6 ± 10.4 | 16.0 ± 13.4 | 20.1 ± 15.5 | 0.39 |
| Practicing surgeons | 11.1 ± 9.5 | 18.7 ± 13.0 | 21.8 ± 14.1 |
Fig 1.
Average final risk estimates by vignette/video category comparing male and female physician estimates. Error bars represent +1 SD.
Relationship between physician gender and changes in risk estimates
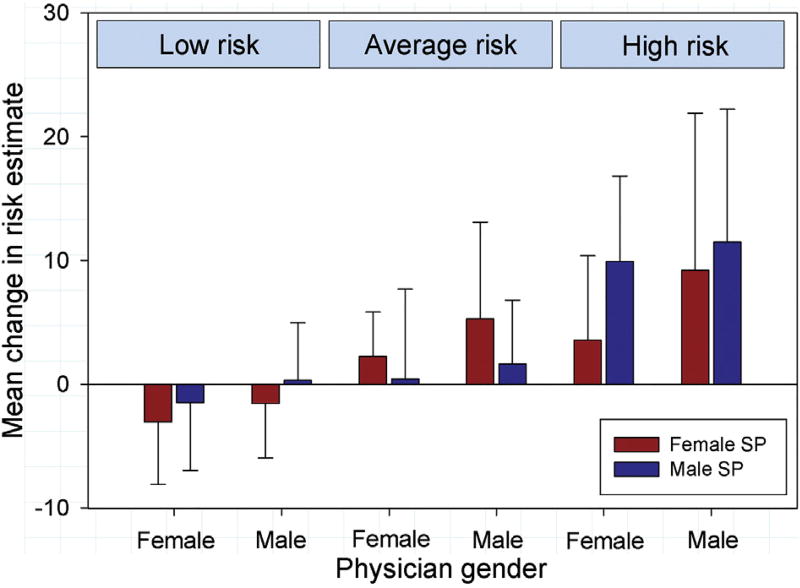
There was a significant difference in the absolute change in risk estimates between male and female physicians (β = 2.48 for male vs female physicians, 95% confidence interval [CI], 0.88 to 4.08, p = 0.002), with male physicians having larger changes in scores than female physicians–an effect that was consistent across the different vignette categories and genders of the SPs (Fig 2). There was no significant difference based on physician status (β = −0.45 for trainee vs practicing surgeon, 95% CI −2.17 to 1.27, p = 0.61), which also did not vary by vignette type. Similar relationships were found when the changes in risk scores were evaluated as a percentage of initial risk scores (data not shown).
Fig 2.
Average changes in estimated risk after viewing video according to level of risk based on vignette/video combination and physician gender, comparing changes for male standardized patients with those for female standardized patients. Error bars represent ±1 SD. (SP = standardized patient.)
Relationship between standardized patient gender and changes in risk estimates
The effect of SP gender varied by vignette type (p < 0.001 for the SP gender by vignette type interaction). For the low-risk vignette/video combination, male SPs had significantly greater changes than did female SPs (β = 1.82 for male vs female SPs, 95% CI 0.28 to 3.35, p = 0.02). For the average-risk vignettes/videos, female SPs had significantly greater changes than did male SPs (β = −2.75 for male vs female SPs, 95% CI −4.51 to −0.98, p = 0.002). For the high-risk vignettes/videos, male SPs had significantly greater changes than did female SPs (β = 2.87 for male vs female SPs, 95% CI 0.11 to 5.62, p = 0.04).
Whether male physicians responded differently to female versus male SPs compared with female physicians was assessed separately for each vignette/video type (low, average, and high risk) by testing the SP gender by physician gender interaction (p = 0.91, p = 0.77, and p = 0.09, respectively).
Relationship between video importance rating and changes in risk estimates
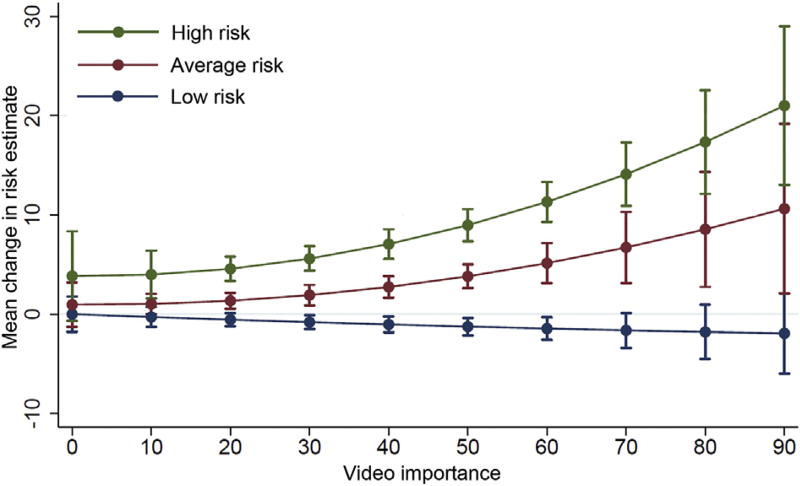
Overall video importance was greater for high-risk videos than for low-risk or average-risk videos (49.9 ± 19.6% vs 39.3 ± 21.0% and 39.6 ± 20.1%, respectively; p < 0.001 from GEE regression model). The importance of the video clips in making the final risk estimates was related to the video/vignette combinations in a complex manner. Video importance was related to the amount of change in risk estimates, especially for average-risk and high-risk vignettes, for which increasing video importance was associated with increasing change in risk scores (p < 0.001 for each). Low-risk videos were associated with a moderate negative change (ie, decrease) in risk estimates as video importance increased (p = 0.44) (Fig 3). There was no statistically significant difference in video importance based on physician status (p = 0.45) or physician gender (p = 0.68) when vignette type was controlled for.
Fig 3.
Relation of video importance and changes in risk estimates according to vignette/video risk level. Error bars represent 95% confidence intervals.
Comment
In the United States, women are approaching parity with men in relation to the incidence of new lung cancers [1, 21]. However, there is some evidence that women’s access to surgical care for early-stage lung cancer is less than that of men [9, 10], which suggests patient gender bias. In addition, there is growing evidence of physician gender-related differences in how clinical information is perceived and used in decision making [11–14]. The current randomized trial was designed to evaluate the hypothesis that physician and patient gender influence risk estimation for major lung resection.
We found that male physicians’ estimates of risk increased more in response to viewing a video of a standardized patient than did female physicians’ risk estimates. The relationship between standardized patient gender and changes in risk estimates was more complex. Although significant differences existed among the vignette/video combinations for different risk levels, these relationships did not reveal a consistent pattern of behavior. The influence of viewing videos on changes in risk estimates varied according to the risk level for the vignette; the importance of videos increased in association with increases in changes in estimated risk for average-risk and high-risk vignette/video combinations, whereas video importance was not significantly related to changes in risk estimates for low-risk vignette/video combinations. Understanding these interactions is important in developing educational processes that help mitigate gender differences in interpreting and using clinical information.
Patient gender disparities in surgical treatment of lung cancer are similar to findings regarding access to surgical care for vascular [22] and orthopedic [23] surgical treatment. These relationships are present after correction for confounders such as socioeconomic status and race. For lung cancer treatment, the disparity is present despite the fact that the short-term and long-term outcomes in women undergoing lung operations are superior to the outcomes in men [5, 8]. The reasons for these patient gender disparities are not known. The effect appears to exist primarily when physicians encounter patients (in person or in images or videos) rather than just when they are assessing patients’ paper or electronic records [24, 25]. To our knowledge, the characteristics of such encounters that are associated with bias have not been identified. They could include overt or subconscious prejudice or perhaps unconscious recognition of features in SP videos that were consistently recognized and unintended and that influenced risk estimates.
Physician gender differences in risk estimation and decision making are not well recognized or understood. Early studies demonstrated the existence of physician differences in clinical decision making, particularly related to testing and treatments that were patient gender specific [26, 27]. More recently, similar findings have been reported related to physician gender and bias in recommendations for surgical therapy and adjuvant radiation therapy for breast cancer [12, 13]. Some of these findings may be related to innate differences between men and women with regard to judgment and decision making. For example, uncertainty in pursuing shared decision making regarding treatment options can create anxiety, and the level of anxiety is significantly higher in female physicians than in male physicians, which may influence risk estimation [14]. Differences in visual and spatial perception between male and female physicians have been demonstrated, which could influence how videos of SPs are interpreted [11]. Simply put, female and male physicians may recognize different patterns among elements in video content and use them differently in judging risk.
We have previously shown that risk estimates for surgical treatment change considerably when vignette and video pairings are discordant [19]. Risk estimates decrease somewhat after subjects view a vigorous video paired with a high-risk vignette. By contrast, viewing a frail video paired with a low-risk vignette results in a substantial increase in risk estimates. These findings are true for vignettes and videos at the extremes of risk level and physical performance. The interactions of videos and vignettes in the middle range of risk are more nuanced and more difficult to model and characterize. At some point in the continuum of discordance, the direction of change in risk estimates transitions from negative to positive; the location of that inflection point is related to the vignette risk, the behavior portrayed in the video, the gender of the physician estimating risk, and the gender of the patient.
There are potential shortcomings in our study. The numbers of participants and vignette/video combinations were relatively small, limiting the statistical power of the study. There was considerable imbalance in the number of men versus women physicians, which may have prevented identification of additional behavioral differences. This imbalance reflects current workforce and trainee gender distribution. The videos were designed to exhibit specific levels of gait speed, weakness, fatigue, and weight loss to make the activities of the paired male and female SPs as similar as possible. Despite these efforts, there may have been features in the videos that could not be controlled for, resulting in mismatched content in paired videos that differently influenced surgeons’ perception of the level of vigor/frailty in the video and their subsequent risk estimates. We did not intentionally create discordant matches between vignettes and videos so that we could focus the outcomes on gender differences between the SPs, but such discordances may have existed and would be difficult to identify if present.
Our results indicate that physicians (and perhaps patients) need to be aware of the influences of physician and patient gender on decision making. Knowledge of these influences may assist in understanding disparities in the surgical treatment of lung cancer. Some of these effects may be mitigated through physician training. The use of appropriately designed shared decision making instruments may also help overcome some of the unconscious biases that appear to be involved.
In conclusion, we identified differences in estimation of surgical risk of lung resection related to physician and patient gender. Our results are preliminary and require further investigation to better define the nature and magnitude of these effects.
Acknowledgments
This study was supported in part by the Eugenia Dallas Fund for Thoracic Surgery Research. The REDCap project at The University of Chicago is managed by the Center for Research Informatics and funded by the Biological Sciences Division and by the Institute for Translational Medicine, CTSA grant number UL1 RR024999 from the National Institutes of Health.
Footnotes
Presented at the Twenty-fourth European Conference on General Thoracic Surgery, Naples, Italy, May 31, 2016.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson MK, Skosey C, Hoffman PC, Golomb HM. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson MK, Wang J, Hoffman PC, et al. Sex-associated differences in survival of patients undergoing resection for lung cancer. Ann Thorac Surg. 2000;69:245–50. doi: 10.1016/s0003-4975(99)01078-4. [DOI] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 5.LaPar DJ, Bhamidipati CM, Harris DA, et al. Gender, race, and socioeconomic status affects outcomes after lung cancer resections in the United States. Ann Thorac Surg. 2011;92:434–9. doi: 10.1016/j.athoracsur.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura H, Ando K, Shinmyo T, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17:469–80. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 7.Warwick R, Shackcloth M, Mediratta N, et al. Female sex and long-term survival post curative resection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44:624–30. doi: 10.1093/ejcts/ezt139. [DOI] [PubMed] [Google Scholar]
- 8.Tong BC, Kosinski AS, Burfeind WR, Jr, et al. Sex differences in early outcomes after lung cancer resection: analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg. 2014;148:13–8. doi: 10.1016/j.jtcvs.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jazieh AR, Kyasa MJ, Sethuraman G, Howington J. Disparities in surgical resection of early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:1173–6. doi: 10.1067/mtc.2002.122538. [DOI] [PubMed] [Google Scholar]
- 10.Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–81. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 11.Nair BK, Attia JR, Bowe SJ, Mears SR, Hitchcock KI. Interns are from Venus, consultants are from Mars: differential perception among clinicians. Med J Aust. 2003;179:659–61. doi: 10.5694/j.1326-5377.2003.tb05742.x. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Buono D, McBride RB, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100:199–206. doi: 10.1093/jnci/djm320. [DOI] [PubMed] [Google Scholar]
- 13.Hershman DL, Buono D, Jacobson JS, et al. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg. 2009;249:828–33. doi: 10.1097/SLA.0b013e3181a38f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Politi MC, Légaré F. Physicians’ reactions to uncertainty in the context of shared decision making. Patient Educ Couns. 2010;80:155–7. doi: 10.1016/j.pec.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MK, Farnan J, Hemmerich JA, Slawinski K, Acevedo J, Small S. The impact of perceived frailty on surgeons’ estimates of surgical risk. Ann Thorac Surg. 2014;98:210–6. doi: 10.1016/j.athoracsur.2014.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Sax FL, MacKenzie CR, Braham RL, Fields SD, Douglas RG., Jr Morbidity during hospitalization: can we predict it? J Chronic Dis. 1987;40:705–12. doi: 10.1016/0021-9681(87)90107-x. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson MK, Durkin AE. A comparison of three scoring systems for predicting complications after major lung resection. Eur J Cardiothorac Surg. 2003;23:35–42. doi: 10.1016/s1010-7940(02)00675-9. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson MK, Thompson K, Huisingh-Scheetz M, et al. Thoracic surgeons’ perception of frail behavior in videos of standardized patients. PLoS One. 2014;9:e98654. doi: 10.1371/journal.pone.0098654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sineshaw HM, Wu XC, Flanders WD, Osarogiagbon RU, Jemal A. Variations in receipt of curative-intent surgery for early-stage non-small cell lung cancer (NSCLC) by state. J Thorac Oncol. 2016;11:880–9. doi: 10.1016/j.jtho.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Amaranto DJ, Abbas F, Krantz S, Pearce WH, Wang E, Kibbe MR. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. 2009;50:1340–7. doi: 10.1016/j.jvs.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Borkhoff CM, Hawker GA, Kreder HJ, Glazier RH, Mahomed NN, Wright JG. The effect of patients’ sex on physicians’ recommendations for total knee arthroplasty. CMAJ. 2008;178:681–7. doi: 10.1503/cmaj.071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–26. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 25.Borkhoff CM, Hawker GA, Kreder HJ, Glazier RH, Mahomed NN, Wright JG. Patients’ gender affected physicians’ clinical decisions when presented with standardized patients but not for matching paper patients. J Clin Epidemiol. 2009;62:527–41. doi: 10.1016/j.jclinepi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Franks P, Clancy CM. Physician gender bias in clinical decision making: screening for cancer in primary care. Med Care. 1993;31:213–8. doi: 10.1097/00005650-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Lurie N, Margolis KL, McGovern PG, Mink PJ, Slater JS. Why do patients of female physicians have higher rates of breast and cervical cancer screening? J Gen Intern Med. 1997;12:34–43. doi: 10.1046/j.1525-1497.1997.12102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]