Abstract
Stress and anxiety have a negative impact on working memory systems by competing for executive resources and attention. Broad memory deficits, anxiety, and elevated stress have been reported in individuals with chromosome 22q11.2 deletion syndrome (22q11.2DS). We investigated anxiety and physiological stress reactivity in relation to visuospatial working memory impairments in 20 children with 22q11.2DS and 32 typically developing (TD) children ages 7 to 16. Children with 22q11.2DS demonstrated poorer working memory, reduced post-stress respiratory sinus arrhythmia recovery, and overall increased levels of cortisol in comparison to TD children. Anxiety, but not physiological stress responsivity, mediated the relationship between 22q11.2DS diagnosis and visuospatial working memory impairment. Findings indicate that anxiety exacerbates impaired working memory in children with 22q11.2DS.
Keywords: Anxiety, Chromosome 22q11.2DS, DiGeorge Syndrome, Stress, Velocardiofacial Syndrome, Working memory
Introduction
Chromosome 22q11.2 deletion syndrome (22q11.2DS), previously known as velocardiofacial or DiGeorge syndrome, is a neurodevelopmental disorder that arises from a 1.5 to 3 megabase microdeletion on the long arm of chromosome 22. Prevalence rates range from 1:2000 and 1:4000 live births (Howley et al. 2012). The syndrome is associated with a broad psychiatric phenotype (Fabbro et al. 2012; Shashi et al. 2012). Social and communication deficits are common and become more obvious as the child ages, leading them to become increasingly anxious and socially withdrawn (Swillen et al. 2000).
Distinct cognitive patterns also have been noted in this population. Children and adolescents with 22q11.2DS display full-scale IQ (FSIQ) scores in the borderline/very low range, with verbal IQ in the low average range (Moss et al. 1999). Specific deficits are seen in visual-spatial memory and complex forms of verbal recall (Woodin et al. 2001). The disparity between visuospatial and verbal memory abilities in children with 22q11.2DS does not appear to be related to FSIQ, suggesting that general intellectual ability is not a comprehensive explanation (Bearden et al. 2001). Furthermore, anxiety has been shown to be more predictive than intellectual function of age-appropriate daily living skills in children with 22q11.2DS (Angkustsiri et al. 2014;Stephenson et al. 2015). Thus, while impairments in general intellectual functioning might be expected to explain these deficits at least in-part, this is not a foregone conclusion given the probable contributing role of anxiety and stress.
Several studies report working memory impairments in children and adolescents with 22q11.2DS compared to their typically developing peers (Bearden et al. 2001; Kates et al. 2007; Lajiness-O’Neill et al. 2005; Montojo et al. 2014; Simon et al. 2005). However, these impairments have not been examined in connection with children’s affect. Eysenck and Calvo (1992) report that anxiety exhausts cognitive capacity with deleterious effects on performance and processing efficiency. Attentional control theory would suggest that anxiety disturbs the goal-driven attentional system while amplifying the stimulus-driven attentional system (Derakshan and Eysenck 2009). The former refers to top-down control of attention, while the latter refers to bottom-up processes that favor behaviorally relevant and salient stimuli (Corbetta and Shulman 2002). These are not mutually exclusive with impaired attentional control likely exacerbated by anxiety.
In contrast to passive longer-term storage, working memory relies on the ability to efficiently update and manipulate task-relevant information (Miyake et al. 2000). Anxiety reduces the efficiency of executive functions associated with working memory updating such as appropriate inhibition and attention shifting (Derakshan and Eysenck 2009; Eysenck and Derakshan 2011). As proposed by attentional control theory, anxiety reduces these top-down mechanisms, thereby resulting in less attentional control. Anxious individuals have a higher probability of diverting attentional resources to extraneous stimuli. These distractors can either be external or internal (e.g. worry and perseveration), which ultimately degrades processing efficiency (Eysenck et al. 2007). Therefore, tasks that involve greater attentional discipline also require individuals with higher levels of anxiety to employ more cognitive resources (Wright et al. 2014).
Anxiety and stress have an impact on general cognitive and memory performance in a variety of human and animal model studies (Contarino et al. 1999; Dalgleish et al. 2003; Harrison et al. 2009; Robert and Hockey 1997;Roozendaal 2002). Anxiety and memory impairments are well documented in people with 22q11.2DS. Given the heterogeneous, complex, and sometimes unpredictable course of 22q11.2DS, stress likely arises from and contributes to symptomatology and impairments in this population (Beaton and Simon 2011). Nevertheless, there has been little attention on the role of anxiety and stress on cognitive function in people with 22q11.2DS.
Respiratory sinus arrhythmia (RSA) refers to the oscillations in heart periods that occur in conjunction with a breathing cycle (Berntson et al. 1993). RSA is a widely used measure of cardiac vagal control. The vagus nerve (i.e. the tenth cranial nerve) is controlled by the parasympathetic nervous system and inhibits sympathetic upregulation of heart contraction (Rottenberg et al. 2002). In situations perceived as stressful or threatening, an individual experiences vagal withdrawal. With the resolution of the stressor, sympathetic influences decrease by vagal induction and the organism returns back to a state of rest. This “vagal brake” allows mammals to quickly respond to their environment and return to a state of homeostasis (Scott and Weems 2014). Studies have linked lower RSA to poor social and emotional regulation, including social anxiety and depression (Friedman and Thayer 1998; McLaughlin et al. 2015; Porges et al. 1996; Rottenberg et al. 2005). Forexample, McLaughlin et al. (2015) reported an interaction between vagal tone and psychosocial stressors. After exposure to a stressor, adolescents with low vagal tone were more likely to display internalizing symptoms than adolescents with high vagal tone.
Chronic stress is also reflected in major biochemical pathways that regulate metabolic processes. Miller and colleagues (2007) suggest that the hypothalamic–pituitary– adrenal (HPA) axis is initially activated in the presence of a stressor, resulting in increased levels of cortisol. As time since the onset of a stressor increases, the system returns to homeostasis. There appears to be significant variability in the stress-response system as a function of underlying psychopathology. Increased basal cortisol levels have been noted in youth at ultra-high risk for psychosis, while cortisol response to acute stress is blunted compared to healthy controls (Chaumette et al. 2016; Pruessner et al. 2013). Elevated salivary cortisol has been reported in children with 22q11.2DS. Beaton and colleagues (2014) found that 73 children with 22q11.2DS had higher levels of anxiety and elevated salivary cortisol compared 50 TD control children in response to the relatively mild stress of training to remain still in a MRI mock-up apparatus prior to a MRI scanning session.
Working memory impairments are well-characterized in children with 22q11.2DS (Bearden et al. 2001; Lajiness-O’Neill et al. 2005; Montojo et al. 2014; Simon et al. 2005). While these studies have largely focused on neuroanatomical differences, there is a paucity of research investigating the role of anxiety and stress as possible exacerbating factors on memory impairments in these children. In one study, while salivary cortsiol levels were significantly higher in 11 children with 22q11.2DS compared to age-matched controls, cortisol levels prior to the start of cognitive testing was not correlated with performance on Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2) in the 22q11.2DS group. Interestingly, higher cortisol levels did predict lower WRAML-2 scores in the TD control group (Jacobson et al. 2016).
As outlined by Eysenck and Derakshan (2009), anxiety has deliterious effects on on the goal-driven attentional system of working memory. This ultimately breaks down the central executive system, making it difficult to ignore irrelevant stimuli. Additionally, cortisol activity interacts with brain regions that are relevant to memory function. The hippocampus, which plays a crucial role in feedback regulation of cortisol release, shows an increased rate of cell death and blunted cell development in the presence of cortisol (Al’Absi et al. 2002). These compounding elements are, therefore, necessary to investigate as possible factors resulting in poor working memory in children with 22q11.2DS.
Anxiety disorders and ADHD are two of the most common diagnoses in children with 22q11.2DS, occuring in approximately 35.6 and 37% of these youth, respectively (Schneider et al. 2014). A comorbid diagnosis is not uncommon in the general population, with the Multimodal Treatment Study of Children with ADHD (1999) reporting a co-occurance with anxiety at 33.5%. Children with ADHD commonly show working memory deficits, as attentional processes are degraded (Barkley 2014). Yet, as mentioned, theory suggests that anxiety interrupts goal-driven attentional systems (Eysenck and Derakshan 2011). As ADHD is expected to be a source of homogenous variance, we decided to use it as a covariate in our analyses in order to directly assess the heterogeneous sources of variance in the relationship between group, anxiety, stress physiology, and working memory impairment.
The Current Study
The overarching aim of the current study was to investigate the roles of anxiety and stress on working memory function in children with 22q11.2DS. As discussed, research shows that these children have higher levels of anxiety and a lower working memory capacity. Yet, can anxiety and stress partially explain working memory deficits in children with 22q11.2DS? While anxiety is considered a cognitive component of anticipatory fear, worry, and threat, examining stress physiology allows us to corraborate evidence suggesting that these children experience a significantly greater amount of stress and gives us potential insight into glucocorticoid mechanisms in this population.
We hypothesized that: (1) Children with 22q11.2DS would have greater levels of anxiety and poorer performance on a visuospatial working memory task than TD children; (2a) Children with 22q11.2DS would have lower baseline RSA and higher baseline cortisol than TD children; (2b) Children with 22q11.2DS would have an elevated stress response and blunted stress recovery compared to TD children as measured by RSA, salivary cortisol, and temperature; and, (3) Anxiety and stress physiology would explain the degree of visuospatial working memory impairment in children with 22q11.2DS, controlling for symptoms of ADHD.
Methods
All methods were approved by the Institutional Review Board at the University of New Orleans.
Participants
As part of a larger ongoing study, families were recruited via the state and national chromosome 22q11.2 deletion / velocardiofacial / DiGeorge syndrome community support networks, social media, fliers posted around the Greater New Orleans area, and word-of-mouth. Families spent two and a half days participating in the research activities. Upon arrival, families were briefed on all tasks and procedures to be conducted and gave informed consent. Children signed assent forms to indicate that they understood and wanted to continue with the study.
Participants were children aged 7 to 16 years (M = 11.4, SD = 2.52) with (n = 20, 10 females) and without (n = 32, 13 female) 22q11.2DS and their parents. The majority of participants were Caucasian (82%), while parents identified the remaining participants as Creole (7%), Hispanic (7%) and African American (4%). The presence of a 22q11.2 deletion was confirmed by fluorescence in situ hybridization. Groups did not differ based on sex composition, Χ2(1, N = 52) = 0.44, p = 0.51 or age, t(50) = 0.12, p = 0.09. Participants were excluded if they had a prior head injury, central nervous system infection, and other focal neurologic abnormalities. Exclusion criteria for the typically developing participant included a known genetic disorder, learning disorder and behavioral or other known psychiatric disorder. See Table 1 for the demographic characteristics of the sample.
Table 1.
Demographic characteristics and IQ scores of the sample
| Typically developing | 22q11.2 deletion syndrome |
|
|---|---|---|
| (n = 32) | (n = 20) | |
| Mean age in years (SD) | 10.9 (2.5) | 12.2 (2.4) |
| Gender | ||
| %Female | 41 | 50 |
| Ethnicity % | ||
| Caucasian | 74.1 | 94.7 |
| Creole | 11.1 | 0 |
| African American | 7.4 | 0 |
| Hispanic | 7.1 | 5.3 |
| Mean FSIQ (SD) | 107.1 (13.4) | 72.2 (9.2) |
Note: FSIQ Full-scale IQ
Procedure
Psychological Measures
The Multidimensional anxiety scale for children second edition: Parent MASC 2-P (MASC 2-P March 2012) is a 50-item parent report questionnaire that assesses the presence of anxiety-related symptoms in children aged 8 to 19 using a four-point scale (0 = never to 3 = often). The form yields a total score, an anxiety probability score, and scales for problems including: Separation Anxiety/ Phobias, GAD Index, Social Anxiety, Obsessions & Compulsions, Physical Symptoms, and Harm Avoidance.
Swanson, Nolan, and Pelham Questionnaire (SNAPIV) The SNAP-IV Rating Scale (Swanson 1983) is a parent-report questionnaire using DSM-IV criteria for Attention Deficit/Hyperactivity Disorder (ADHD) and Oppositional Defiant Disorder (ODD). Statements are rated on a four-point scale (0 = not at all to 3 = very much). For this study, the sum of inattentive and hyperactive/impulsive scores were used as an indicator of ADHD-like symptoms.
Wechsler intelligence scale for children—fourth edition (WISC-IV) The WISC-IV (Wechsler 2003) is a cognitive ability assessment of verbal comprehension, perceptual reasoning, working memory, and processing speed. The WISC-IV was used to assess general intellectual functioning in this study.
Physiological Measures
Cardiac and hemodynamic measures were continuously collected during task participation with Biopac MP150 hardware and AcqKnowledge 4 (BIOPAC Systems, Inc., Goleta, CA) software and was sampled at 1000 Hz. Electrocardiogram (ECG) recordings were collected using a lead II configuration (right clavicle, left clavicle, and left lower torso) and three pre-gelled disposable Ag/AgCl electrodes. A respiration belt transducer was strapped around the abdomen of the participant in order to obtain a respiration signal. Respiratory sinus arrhythmia was calculated with AcqKnowledge 4 software using the time-domain peak-and-valley method. A band pass filter between 0.5 and 35 Hz, using 8000 coefficients was applied to the ECG waveform. Heart beats were detected using the Find Cycle tool. To identify artifacts and missed beats in the R-R interval, a raw tachogram was generated and visually inspected. If an R-peak was undetected, the long beat was split into two equivalent R-R intervals (Berntson et al. 1997). Temperature readings were collected using a Biopac TSD202B temperature probe adhered to the participant’s non-dominant hand.
Hormone Measures
Participants were instructed to refrain from eating, brushing their teeth, and drinking any liquids other than water in the 60 minutes prior to starting the study. Saliva collection began in the afternoon (M = 1:02 pm, SD = 1 h 18 min). Five saliva samples were collected (1) prior to beginning the tasks, (2) prior to beginning the Math Countdown Task, (3) after the Math Countdown Task/prior to the Math Problems Task, (4) after the Math Problems Task, and (5) after a 15 min calming period. Participants deposited saliva in 2 mL microcentrifuge tubes. Samples were frozen immediately at −80 °C. Saliva was assayed for cortisol using Salimetrics enzyme-linked immunosorbent assay (ELISA) kits and were run in duplicate. Salivary cortisol was measured in µg/dL.
Math Stress Computer Task
The Math Task was divided into two parts: the Math Countdown Task and the Math Problems Task. Each section began with a practice trial before continuing to the real task. The Math Countdown Task required the participant to begin at 300 and mentally subtract seven from each correct answer given. Three possible answers were displayed and a countdown clock ranging from 30 to 15 s was shown at the bottom of the screen. If the participant selected an incorrect answer or went over time, they were required to return to the beginning of the task and start again. This section lasted 8 min. During the Math Problems Task, the participant completed a series of math problems ranging from simple addition and subtraction to geometry and trigonometry. As with the Math Countdown Task, three possible answer choices were given, with a countdown clock ranging from 15 to 30 s displayed at the bottom. This section was dynamically adjusted for performance and lasted a total of 8 min. For this study, physiological and hormone measures taken during the task were used as an indicator of stress response and recovery.
Shapes Working Memory Computer Task
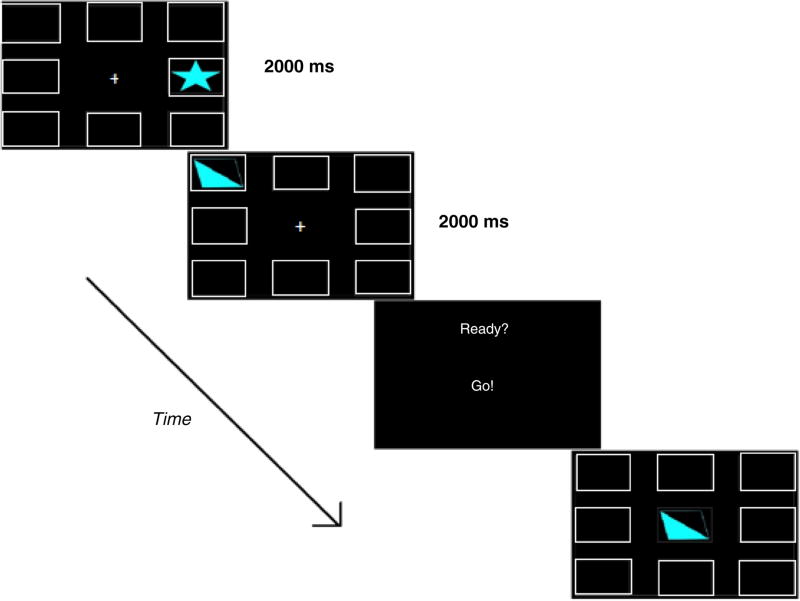
The Shapes Working Memory Task presented the participant with eight empty squares surrounding a central focus (+) on a 27-in touchscreen monitor (Planar PCT-2785) using E-prime Software (Psychology Software Tools, Inc., Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002). Stimuli consisted of blue (R:128 G:255 B:255) geometric shapes (6 cm tall×10 cm wide) saturated at varying degrees individually presented within any of the eight white square borders (6 cm tall×10 cm wide). Trial blocks proceeded in the following event order (Fig. 1): Fixation (2000 ms), Stimulus (2000 ms), Prompt (1000 ms), Cue (infinite), Response. During ‘Response’ events, a ninth white square border appeared in the center of the screen and replaced the fixation. Previously encountered shapes during that trial appeared within this border, and participants were instructed to tap the empty bordered square they last remembered the shape to reside. Figure 1 depicts the event order for a practice round consisting of two shapes. Levels began at 2-shape blocks and increased by one additional shape until a maximum of 8-shape blocks were achieved. Each level was dynamically adjusted to participant accuracy such that 65% block performance or better advanced participants to the next level, while less than 65% block performance discontinued the task. Scores were formatted as Level Attained.Percent Accuracy (i.e. 5.23) as our measure of spatial working memory capacity.
Fig. 1.
Shapes task scheme
Baseline Motor Task
The Baseline Motor Task presented participants with six landscapes. Birds appeared at random locations on a screen. Participants were instructed to press a green button on a response box whenever they observed a bird on the screen. Obradović and colleagues (2011) indicated that “stress reactivity” adjusted for psychomotor activity was incongruent with traditional, resting reactivity. The stress task in this study required the subject to elicit a motor response (i.e. pressing a button). Therefore, this study implemented the baseline motor task instead of a resting baseline in order to account for the possible confound of psychomotor activity.
Stress Recovery Period
The Stress Recovery Period consisted of a 15-min resting state. Participants remained seated while watching a calming video of underwater scenes. Saliva was collected in 10 to 15 min intervals post-stressor to allow for cortisol recovery to baseline levels (Dickerson and Kemeny 2004; Jacobson et al. 2016). To reduce potential frustration due to bore-dom, we limited our recovery period to 15 min.
Statistical Analyses
Prior to analysis, the variables: anxiety, ADHD, IQ, working memory capacity, RSA, skin temperature, and cortisol were screened for missing data, normality of distribution, univariate outliers, and multivariate outliers. A series of independent samples t-test were conducted to examine whether children with 22q11.2DS differed from typical controls on levels of anxiety, ADHD, FSIQ, working memory capacity, baseline RSA, and baseline cortisol using IBM SPSS Statistics version 23. Three 2 (group) by 3 (time) repeated measures ANOVAs were conducted to investigate differences in RSA, salivary cortisol, and temperature reactivity and recovery in children with 22q11.2DS and controls.
A mixed variable structural equation model was created in Mplus (v. 7.4, Muthen & Muthen) to test the mediating roles of anxiety and physiology in the association between group and working memory impairment. The three observed variables of cortisol, RSA, and temperature reactivity were used to create the latent variable of Stress Response, while the MASC 2-P Total T-score was used as the observed variable of Anxiety. Sex, Age, and ADHD symptoms were entered as covariates in the model. Parameter estimation was assessed through maximum likelihood, and overall fit of the model was measured by examining various fit indices, including Chi square, comparative fit index (CFI), the root mean square of approximation (RMSEA) and 90% confidence intervals. Indirect effects were tested using a 95% bootstrap confidence interval set to 1000 iterations (Bollen and Stine 1990; Shrout and Bolger 2002).
Results
Data Cleaning
Prior to analysis, the variables: anxiety, ADHD, IQ, working memory capacity, RSA, temperature, and cortisol were screened for missing data, normality of distribution, univariate outliers, and multivariate outliers. Analysis of cases with missing data indicated that the missing data appeared to be random, as the missing values were not related to other variable scores. To inspect normality and identify outliers, analysis of univariate distribution was performed. Two extreme outliers were identified: one in the salivary cortisol distribution and one in the anxiety score distribution. These values were removed, as they did not appear to be representative of the sample. Finally, the Mahalanobis distance test, using p < 0.001, (i.e. χ2(6) = 22.5) was conducted to test for multivariate outliers among the scales. No multivariate outliers were identified.
Psychological and Psychophysiological Results
To examine group differences in symptoms of anxiety, ADHD, FSIQ, and working memory capacity, a series of independent samples t-tests were conducted. To correct for multiple comparisons, a Bonferroni correction was applied (p < 0.008). Parents of children with 22q11.2DS reported significantly greater levels of anxiety and ADHD symptomatology in their children compared to parents of typically developing children. Additionally, children with 22q11.2DS scored significantly lower on the WISC-IV FSIQ and shapes working memory task.
To examine group differences in baseline levels of RSA and cortisol, a series of independent samples t-tests were conducted. Consistent with the hypothesis, children with 22q11.2DS had significantly higher levels of baseline cortisol compared to controls. Yet, there was no significant difference in baseline levels of RSA between the two groups (see Table 2).
Table 2.
Independent samples t-test comparing variable means between groups
| Typically developing | 22q11.2 deletion syndrome |
t | df | p | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | SD | Mean | SD | ||||
| ADHD | 8.24 | 7.15 | 26.28 | 10.3 | −6.43 | 37 | 0.000 |
| FSIQ | 107.07 | 13.42 | 72.21 | 9.24 | 9.92 | 47 | 0.000 |
| Anxiety | 49.18 | 10.68 | 64.95 | 10.89 | −5.00 | 46 | 0.000 |
| Working memory score | 4.26 | 1.50 | 2.97 | 1.10 | 3.43 | 45.38 | 0.001 |
| Baseline cortisol | 0.09 | 0.03 | 0.12 | 0.04 | −2.69 | 46 | 0.010 |
| Baseline RSA | 3.96 | 0.45 | 3.85 | 0.90 | 0.49 | 22.15 | 0.631 |
Note: ADHD = SNAP-IV ADHD score; FSIQ Full-scale IQ; Anxiety = MASC 2-P total anxiety score. RSA values were log transformed. p < 0.008. Cortisol concentrations are in micrograms per deciliter (µg/dL)
Skin Temperature Results
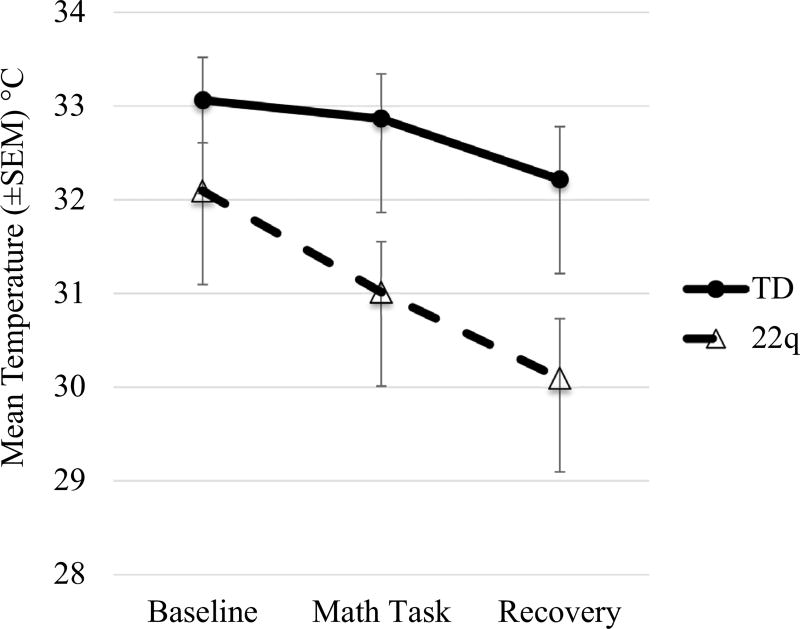
To characterize the difference in skin temperature stress reactivity and recovery, a 2 (group) by 3 (time) repeated measures ANOVA was conducted. Mauchly’s test indicated sphericity could be assumed. Results indicated a significant main effect of time, F(2,82) = 23.99, p < 0.001, and a significant effect of group, F(1,41) = 5.25, p = 0.03. Additionally, there was a significant group by time interaction, F(2,82) = 4.27, p = 0.02. Children with 22q11.2DS displayed lower skin temperature during the math task and recovery period than controls. Follow-up pairwise comparisons indicated a significant decrease in temperature from the math task to the recovery for controls (p = 0.03), and a significant decrease in temperature from baseline to the math task (p < 0.001) and math task to recovery (p = 0.01) for children with 22q11.2DS (see Fig. 2).
Fig. 2.
Temperature during baseline, math task, and recovery period
Salivary Cortisol Results
Salivary cortisol for both groups was within the normal range typically reported in children (McCarthy et al. 2009). To test the hypothesis that children with 22q11.2DS had blunted cortisol reactivity and recovery compared to typically developing children, a 2 (group) by 3 (time) repeated measures ANOVA was conducted. Mauchly’s test indicated that sphericity could not be assumed. Therefore, the Greenhouse-Geisser procedure was used to modify degrees of freedom. Contrary to the hypothesis, results indicated no significant main effect of time, F(1.58,66.35) = 0.37, p = 0.64. However, between-subjects results indicated a significant effect of group, F(1,42) = 28.01, p < 0.001, with children with 22q11.2DS showing higher overall levels of cortisol concentration at each time point. There was no group by time interaction, F(1.58,66.35) = 0.88, p = 0.40 (see Fig. 3).
Fig. 3.
Cortisol levels during baseline, math task, and recovery period
Respiratory Sinus Arrythmia Results
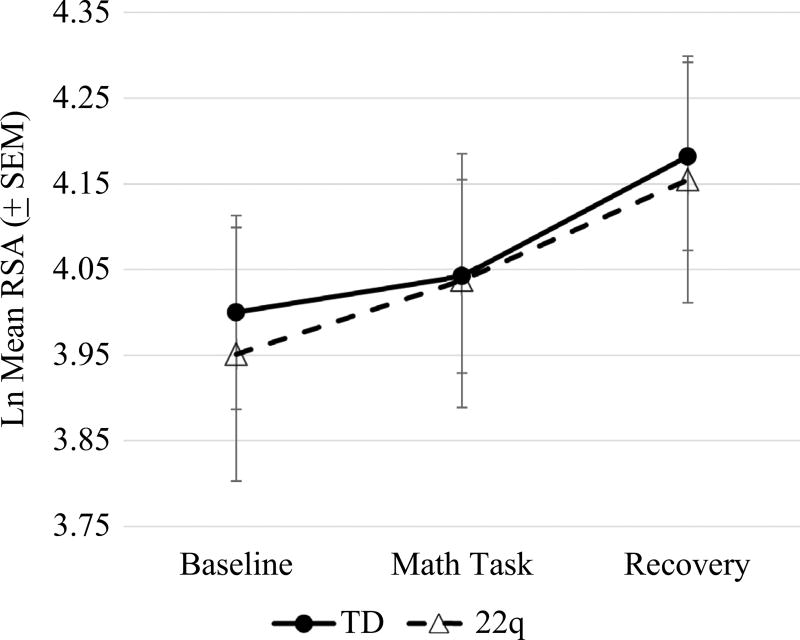
A 2 (group) by 3 (time) repeated measures ANOVA was performed in order to test the hypothesis that children with 22q11.2DS will show blunted RSA reactivity and recovery compared to controls. Mauchly’s test indicated that sphericity could be assumed. Results indicated a significant main effect of time, F(2,88) = 7.37, p < 0.01, but no main effect of group, F(1,44) = 0.23, p = 0.88. Additionally, there was no group by time interaction, F(2,88) = 0.09, p = 0.91. Follow-up pairwise comparisons indicated a significant RSA recovery for typically developing controls (p < 0.05) but not for children with 22q11.2DS (p = 0.16) (see Fig. 4).
Fig. 4.
RSA levels during baseline, math task, and recovery period
Math Task Accuracy and Physiology
To examine group differences in Math Task errors, a series of independent samples t-tests were conducted. Typically developing children scored significantly higher on the Math Task than children with 22q11.2DS, t(42) = 2.80, p < 0.01. Follow up multiple regression analyses were conducted to determine if there was a relationship between Math Task errors and physiological reactivity. For typically developing children, there was a relationship between Math Task accuracy and RSA reactivity, β = −1.07, t(26) = −3.23, p = 0.003. As their accuracy increased, RSA reactivity decreased. However, there was no relationship for children with 22q11.2DS, β = −1.38, t(12) = −1.61, p = 0.136. There was no relationship between Math Task accuracy and cortisol reactivity for either group.
Anxiety and Stress Physiology Results
Due to the theoretical relationship of attention and working memory, as well as the commonality of ADHD in children with 22q11.2DS, an alternative model was investigated. We examined the indirect effect of group on working memory capacity through ADHD symptomology. Though fit indices were good, Χ2(15) = 16.54, p = 0.35; RMSEA = 0.05; CFI = 0.96; SRMR = 0.08, results indicated that ADHD did not mediate the relationship between group and working memory capacity, β = −0.40, p = 0.61.
To test the mediating roles of anxiety and latent stress response in the association between group and working memory impairment, a mixed structural equation model was created in Mplus Version 7.4 (Muthen & Muthen). The three observed variables of cortisol reactivity, RSA reactivity, and temperature reactivity were used to create the latent variable of Stress Response, while the MASC 2-P Total T-score was used as the observed variable of Anxiety. Sex, Age, and ADHD symptoms were entered as covariates in the model. Parameter estimation of the model was conducted using Maximum Likelihood (ML) estimates. Overall fit of the model was assessed using the various fit indices, Χ2(12) = 15.08, p = 0.24; RMSEA = 0.08; CFI = 0.93; SRMR = 0.09. These values indicate good fit for the model.
The total effect of group on working memory capacity, controlling for age, sex, and ADHD symptomology, was not significant, β = −1.15, p = 0.09. Kenny and Judd (2013) indicate that since c (i.e. the total effect) and c’ (i.e. the direct effect) have relatively low power compared to the indirect effect, it is highly possible that c will not be statistically significant. The direct effect of group on working memory capacity was also not significant, β = −0.24, p = 0.78. However, the specific indirect effect of group on working memory capacity via anxiety was significant, β = −1.15, p = 0.04, with a 95% bootstrapped confidence interval (CI) −2.05 to −0.23. The specific indirect effect of group on working memory capacity via stress response, however, was not significant, β = 0.24, p = 0.60, with a 95% bootstrapped CI −0.20 to −1.21. Results indicate that, contrary to the hypothesis, latent stress response did not mediate the relationship between group and working memory capacity. However, complete mediation through anxiety was demonstrated (see Fig. 5).
Fig. 5.
Mixed variable SEM with standardized coefficients. Note: ADHD = SNAP-IV ADHD score; Cort cortisol reactivity, RSA respiratory sinus arrhythmia reactivity, Temp temperature reactivity, Working memory shapes working memory task accuracy; Anxiety MASC 2-Parent report total anxiety score
Discussion
This study was designed to address gaps in the existing research investigating associations among anxiety, physiology, and working memory capacity in children with 22q11.2DS. Consistent with hypotheses, parents of children with 22q11.2DS reported significantly higher levels of anxiety and ADHD symptomatology in their children compared to the control group and those parents’ perceptions of their typically developing children. These findings are in accordance with past studies that report a heightened rate of anxiety disorders and ADHD in individuals with 22q11.2DS (Angkustsiri et al. 2012; Antshel et al. 2010;Gothelf et al. 2013; Green et al. 2009; Hooper et al. 2013;Niklasson et al. 2009; Philip and Bassett 2011; Schneider et al. 2014). As noted previously, the commonality of anxiety disorders in children with 22q11.2DS has been linked to stressors associated with early traumatic exposure, difficulties in social assimilation, and differential genetic and neural profiles (Beaton and Simon 2011). Candidate genes and neurological abnormalities, such as COMT Val/Met variations and patterns of reduced white matter integrity in the frontal and parietal lobes, have also been associated with the increased prevalence of ADHD in this population (Gothelf et al. 2007; Simon et al. 2008).
Children with 22q11.2DS also scored significantly lower on FSIQ and the shapes working memory task, consistent with previous research (Kates et al. 2007; Lajiness-O’Neill et al. 2005; McDonald-McGinn and Sullivan 2011; Moss et al. 1999; Simon et al. 2005; Woodin et al. 2001). The neuropsychological profile of children with 22q11.2DS is markedly varied. While gross intelligence scores range from moderately deficient to average, verbal IQ scores are significantly higher than performance IQ scores (Simon et al. 2002; Sobin et al. 2005; Swillen et al. 1997; Woodin et al. 2001). This pattern is echoed by documented visuospatial working memory deficits. Campbell et al. (2010) reported that, compared to FSIQ scores, children with 22q11.2DS scored significantly lower than expected on visual but not verbal memory tasks. To date, research has primarily focused on altered brain networks as the possible cause of memory deficits in this population, while little attention has been paid to the potential exacerbating roles of anxiety and stress.
Variations in skin temperature were observed. Temperature reactivity from the baseline task to the math task was only significant for children with 22q11.2DS. Vasoconstriction typically occurs during activation of the sympathetic nervous system. When faced with acute stress, blood flow towards the skeletal muscles increases while peripheral blood flow decreases. This conserves heat and allows for increased muscular output due to readily available energy (Charkoudian 2010; Kistler et al. 1998; Kushki et al. 2013). Temperature decrease from the math task to the recovery period was seen for both children with 22q11.2DS and controls. This result was unexpected, as recovery from stress reduces sympathetically-mediated processes. Typically, this would increase peripheral blood flow thereby increasing skin temperature. However, this effect was not observed in either children with 22q11.2DS or typically developing controls.
A particularly novel aspect of this study is the characterization of physiological and hormonal profiles in children with 22q11.2DS. While the findings did show significantly higher baseline cortisol levels in these children, baseline RSA values did not differ from TD controls. Additionally, we found no significant changes in either RSA or cortisol reactivity, or cortisol recovery in the two groups. Nevertheless, the data indicates that RSA recovery from the math stressor task was only significant for typical controls, but not for children with 22q11.2DS. Typically, when a stressful situation is resolved, parasympathetic activation takes over to return the body back to homeostasis. This quick return to baseline is indicative of a flexible and efficient stress recovery system. However, an inability to return to homeostasis is suggestive of allostatic load, which results from a persistently overactive or underactive stress response system (McEwen 1998) Additionally, reduced cardiovascular recovery is associated with negative affect and chronic stress and anxiety (Chida and Hamer 2008).
Though no differences in cortisol reactivity and recovery in relation to the tasks were found, the overall increase in salivary cortisol levels at all three time points suggests potentially abnormal HPA axis functioning. Cognitive deficits, such as selective attention and memory impairment, have repeatedly been associated with corticosteroids (Lupien et al. 1994, 1998; Starkman et al. 1992; Vedhara et al. 2000). While varying causes have been linked to elevated cortisol levels, a variety of studies indicate that chronic stress exposure and anxiety lead to HPA axis dysregulation (Boudarene et al. 2001; Ruttle et al. 2011; Takahashi et al. 2005; van Eck et al. 1996). The present salivary cortisol findings are thus likely biomarkers of allostatic load in children with 22q11.2DS. While short-term release of cortisol is beneficial in response to stress, chronic excess leads to a compromised immune system and hippocampal dysfunction (McEwen 1998). Additionally, increased basal cortisol levels contribute to a persistent state of hypervigilance, where the individual is on constant alert to detect danger. Ultimately, this may inhibit the ability to discern between threatening and non-threatening stimuli (Sapolsky 1990).
A relationship between Math Task accuracy and RSA reactivity was observed for TD children. These results suggested that as math performance increased, physiological stress reactivity decreased. TD children who made more errors experienced a vagal induction, as seen by increased RSA reactivity. However, this pattern was not seen for children with 22q11.2DS. This suggests that regardless of math performance, children with 22q11.2DS experienced a steady state of arousal. This relationship was not observed in either group for cortisol reactivity.
Finally, we investigated the mediating roles of anxiety and stress reactivity in the relationship between 22q11.2DS diagnosis and impaired working memory. Physiological stress reactivity did not mediate 22q11.2DS diagnosis and working memory capacity. However, our results suggest that anxiety does mediate this relationship. These findings are congruent with existing theories which suggest that anxiety has deleterious effects on attentional control and processing efficiency (Derakshan and Eysenck 2009;Eysenck and Calvo 1992). Anxious individuals show difficulties in attending to task-relevant stimuli. A lack of inhibition results in extraneous stimuli, whether it be internal or external, having greater salience (Eysenck et al. 2007). Shackman et al. (2006) suggest that anxious arousal uniquely disturbs visuospatial working memory, as both compete for resources in the right prefrontal cortex and posterior parietal cortex. This effect may be further intensified, as reductions in gray matter volume have been noted in regions of the posterior parietal lobes and prefrontal cortex in children with 22q11.2DS (Gothelf et al. 2011; Shapiro et al. 2012).
Limitations and Future Directions
Results of this study should be interpreted within the context of known limitations. First, the relatively small and unequal sample sizes may have negatively impacted the ability to detect significant differences both between and within groups. However, this constraint is not uncommon, as recruitment of participants from special population of children with neurodevelopmental disorders poses significant challenges. Second, we used parental report measures, instead of self-report. Since our sample consisted of a wide age range (7–16 years), we believe that parent-reports likely give a more reliable account of children’s behavior. However, this data is subject to potential bias, and the inclusion of a secondary reporter (e.g. teacher) could provide additional information. Additionally, future research comparing self- and parent-report data may provide insight into differences of self and observer recognition in children with 22q11.2DS.
Third, as noted previously, our sample age range extended from middle childhood to early adolescence. Developmental changes associated with puberty could be controlled for in future investigations using age-matched participants and controls in order to efficiently account for these variations. Fourth, using a motor-matched baseline may have activated sympathetically-mediated processes that are not reflective of a true pre-stress state. RSA values for both children with 22q11.2DS and typically developing controls during baseline were lower than during the math task. This outcome may have been due to the effect of attentional demand, as Porges and Raskin (1969) reported reduced heart rate variability during tasks requiring continued attention. Therefore, our results may not have fully characterized physiological stress responses in the absence of a true resting baseline rather than the non-stressful motor baseline task used in the present study. Also, the task may not have been that stressful compared to other unaccounted for stressors resulting in a ceiling effect in the salivary cortisol measures. The lack of clear differences in physiological stress reactivity in the context of higher levels of anxiety may indicate that the physiological costs of higher anxiety are not yet evident in these children as a group.
Children with 22q11.2DS are often treated with a variety of medications that could affect physiological or psychological measures. Some of these medications cannot be abruptly stopped. This variability adds to the complexity of studying this population, and controlling for each type of medication would greatly reduce statistical power. Nonetheless, this is an important limitation of our design.
Theory suggests that state anxiety and trait anxiety affect cognitive processes differently. Sorg and Whitney (1992) suggest that these two subtypes of anxiety interact to reduce working memory performance. Pacheco-Unguetti and colleagues (2010) propose that while state anxiety influences bottom-up processes, trait anxiety interferes with top-down processes. Furthermore, they conclude that a general reduction in cognitive capacity is primarily due to trait anxiety but not state anxiety. While we did not incorporate a state anxiety self-report or interview measure, future studies would benefit from this addition.
Implications
These findings have important implications for future research and translational work into anxiety, stress and allostatic load in people with 22q11.DS. While impaired memory has been well documented in children with 22q11.2DS (Bearden et al. 2001; Kates et al. 2007; Lajiness-O’Neill et al. 2005; Montojo et al. 2014; Simon et al. 2005; Woodin et al. 2001), the current study is unique in identifying the potential exacerbating effect of anxiety on working memory. The importance of an efficient working memory in children with 22q11.2DS extends beyond impact on academic performance. In typically developing children, brain systems that subserve attention, memory, and emotion regulation become more efficient over time as the brain develops. This is guided by genetic programming but also experience and epigenetic factors. Thus in children with 22q11.2DS, atypical brain development combined with more anxiety and diminished coping capacity can impede gains in efficiency and integration of these brain systems. Furthermore, because of the interdependency of these systems, efficiency and integration can be undermined by atypical development in any one subsystem such as working memory. As noted above, efficient and capacitive working and long-term memory is needed for mental representation and perception of experiences in the context of past, present, and future. Disruption of systems such as working memory interacting with or driving atypical development in other brain systems that underpin autonoetic consciousness such as salience and long-term memory are likely part of the etiopathology of schizophrenia (Sonntag et al. 2003;Wheeler et al. 1997).
It is therefore crucial to identify factors that are both deleterious and beneficial to working memory efficiency and capacity. A meta-analysis conducted by Fusar-Poli et al. (2012) found that, while individuals at ultra-high risk (UHR) for psychosis had significant impairments in working memory compared to healthy controls, those UHR individuals who later transitioned into psychosis displayed an even more diminished working memory capacity than UHR individuals who did not. Given the 25- to 30-fold increased risk for schizophrenia in individuals with 22q11.2DS, the identification of mediating factors that are amenable to treatment, such as anxiety, is particularly significant. These findings have great potential to inform clinical research into interventions and intervention efficacy that specifically target anxiety in children with 22q11.DS and other neurodevelopmental disorders.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
This research was funded by a grant from the National Institute of Mental Health awarded to Dr. Elliott Beaton (R00MH086616-05) and via laboratory start-up funding from the Louisiana Board of Regents and the University of New Orleans. We especially thank the families who donated their time and effort to this study.
Footnotes
Author contributions AFPS contributed to experimental design; data collection, reduction, and coding; salivary hormone assays; statistical analyses; and she wrote the first draft of the manuscript. DAH contributed to experimental design; computer task design and coding; and data collection. DDS contributed to computer task design, data collection, and manuscript editing. RDL contributed to statistical plan, analyses, and interpretation. EAB contributed to experimental design, computer task design, salivary hormone assays, data interpretation, manuscript editing, and aquired the research funding.
Compliance with Ethical Standards
Conflict of interest Dr. Beaton reports having received research funding from the National Institute of Mental Health and the Louisiana Board of Regents and reports no biomedical financial interests or potential conflicts of interest. Ms. Sanders, Ms. Hobbs, Mr. Stephenson, and Dr. Laird report no biomedical financial interests or potential conflicts of interest.
References
- Al’Absi M, Hugdahl K, Lovallo WR. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39(1):95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Angkustsiri K, Goodlin-Jones B, Deprey L, Brahmbhatt K, Harris S, Simon TJ. Social impairments in chromosome 22q11. 2 deletion syndrome (22q11. 2DS): Autism spectrum disorder or a different endophenotype? Journal of Autism and Developmental Disorders. 2014;44:739–746. doi: 10.1007/s10803-013-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angkustsiri K, Leckliter I, Tartaglia N, Beaton EA, Enriquez J, Simon TJ. An examination of the relationship of anxiety and intelligence to adaptive functioning in children with chromosome 22q11. 2 deletion syndrome. Journal of Developmental and Behavioral Pediatrics. 2012;33(9):713. doi: 10.1097/DBP.0b013e318272dd24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(4):333–344. [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: Guilford Publications; 2014. [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Cannon TD. The neurocognitive phenotype of the 22q11. 2 deletion syndrome: selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Angkustsiri K, Leckliter I, Cabaral MH, McLennan YA, Enriquez J, Simon TJ. Elevated anxiety, depression, and salivary cortisol in children with chromosome 22q11.2 deletion syndrome during a mild novel stressor. Paper presented at the Biological Psychiatry.2014. [Google Scholar]
- Beaton EA, Simon TJ. How might stress contribute to increased risk for schizophrenia in children with chromosome 22q11. 2 deletion syndrome? Journal of Neurodevelopmental Disorders. 2011;3(1):68–75. doi: 10.1007/s11689-010-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Stone PH. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Stine R. Direct and indirect effects: Classical and bootstrap estimates of variability. Sociological Methodology. 1990;20(1):15–140. [Google Scholar]
- Boudarene M, Legros J, Timsit-Berthier M. [Study of the stress response: role of anxiety, cortisol and DHEAs] L’Encephale. 2001;28(2):139–146. [PubMed] [Google Scholar]
- Campbell LE, Azuma R, Ambery F, Stevens A, Smith A, Morris RG, Murphy KC. Executive functions and memory abilities in children with 22q11. 2 deletion syndrome. Australian and New Zealand Journal of Psychiatry. 2010;44(4):364–371. doi: 10.3109/00048670903489882. [DOI] [PubMed] [Google Scholar]
- Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. Journal of Applied Physiology. 2010;109(4):1221–1228. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumette B, Kebir O, Mam-Lam-Fook C, Morvan Y, Bourgin J, Godsil BP, Krebs M-O. Salivary cortisol in early psychosis: New findings and meta-analysis. Psychoneuroendocrinology. 2016;63:262–270. doi: 10.1016/j.psyneuen.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134(6):829. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee K-F, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Research. 1999;835(1):1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: A comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32(1):10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist. 2009;14(2):168–176. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition & Emotion. 1992;6(6):409–434. [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50(7):955–960. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fabbro A, Rizzi E, Schneider M, Debbane M, Eliez S. Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS) European Child & Adolescent Psychiatry. 2012;21(7):379–385. doi: 10.1007/s00787-012-0273-x. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998;44(1):133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, McGuire P. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11. 2 deletion syndrome. Journal of Psychiatric Research. 2011;45(3):322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M, Shohat M. Association of the low-activity COMT 158 Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. The International Journal of Neuropsychopharmacology. 2007;10(03):301–308. doi: 10.1017/S1461145706006699. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbané M, Frisch A, Glaser B, Eliez S. Risk factors and the evolution of psychosis in 22q11. 2 deletion syndrome: a longitudinal 2-site study. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(11):1192–1203. e1193. doi: 10.1016/j.jaac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Harrison F, Hosseini A, McDonald M. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural Brain Research. 2009;198(1):247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Curtiss K, Schoch K, Keshavan MS, Allen A, Shashi V. A longitudinal examination of the psychoeducational, neurocognitive, and psychiatric functioning in children with 22q11. 2 deletion syndrome. Research in Developmental Disabilities. 2013;34(5):1758–1769. doi: 10.1016/j.ridd.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley SA, Prasad SE, Pender NP, Murphy KC. Relationship between reaction time, fine motor control, and visual–spatial perception on vigilance and visual-motor tasks in 22q11. 2 Deletion Syndrome. Research in Developmental Disabilities. 2012;33(5):1495–1502. doi: 10.1016/j.ridd.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Jacobson D, Bursch M, Lajiness-O’Neill R. Potential role of cortisol in social and memory impairments in individuals with 22q11. 2 deletion syndrome. Journal of Pediatric Genetics. 2016;5(03):150–157. doi: 10.1055/s-0036-1584549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Krauss BR, AbdulSabur N, Colgan D, Antshel KM, Higgins AM, Shprintzen RJ. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11. 2 deletion syndrome) Neuropsychologia. 2007;45(12):2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, Judd CM. Power anomalies in testing mediation. Psychological Science. 2013 doi: 10.1177/0956797613502676. 0956797613502676. [DOI] [PubMed] [Google Scholar]
- Kistler A, Mariauzouls C, von Berlepsch K. Fingertip temperature as an indicator for sympathetic responses. International Journal of Psychophysiology. 1998;29(1):35–41. doi: 10.1016/s0167-8760(97)00087-1. [DOI] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Mobarak MP, Tanel N, Dupuis A, Chau T, Anagnostou E. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE. 2013;8(4):e59730. doi: 10.1371/journal.pone.0059730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness-O’Neill RR, Beaulieu I, Titus JB, Asamoah A, Bigler ED, Bawle EV, Pollack R. Memory and learning in children with 22q11. 2 deletion syndrome: evidence for ventral and dorsal stream disruption? Child Neuropsychology. 2005;11(1):55–71. doi: 10.1080/09297040590911202. [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair N, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. The Journal of Neuroscience. 1994;14(5):2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- March JS. Mulidimensional anxiety scale for children. 2. Toronto, Ontario: MHS; 2012. [Google Scholar]
- McCarthy AM, Hanrahan K, Kleiber C, Zimmerman MB, Lutgendorf S, Tsalikian E. Normative salivary cortisol values and responsivity in children. Applied Nursing Research. 2009;22(1):54–62. doi: 10.1016/j.apnr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11. 2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine. 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Rith-Najarian L, Dirks MA, Sheridan MA. Low vagal tone magnifies the association between psychosocial stress exposure and internalizing psychopathology in adolescents. Journal of Clinical Child and Adolescent Psychology. 2015;44(2):314–328. doi: 10.1080/15374416.2013.843464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133(1):25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Montojo CA, Ibrahim A, Karlsgodt KH, Chow C, Hilton A, Jonas RK, Bearden C. Disrupted working memory circuitry and psychotic symptoms in 22q11. 2 deletion syndrome. NeuroImage: Clinical. 2014;4:392–402. doi: 10.1016/j.nicl.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Wang PP. Psychoeducational profile of the 22q11. 2 microdeletion: A complex pattern. The Journal of Pediatrics. 1999;134(2):193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Óskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30(4):763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23(01):101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety different attentional functioning under state and trait anxiety. Psychological science. 2010;21(2):298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Philip N, Bassett A. Cognitive, behavioural and psychiatric phenotype in 22q11. 2 deletion syndrome. Behavior Genetics. 2011;41(3):403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental psychobiology. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Raskin DC. Respiratory and heart rate components of attention. Journal of Experimental Psychology. 1969;81(3):497. doi: 10.1037/h0027921. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Béchard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophrenia Research. 2013;146(1–3):79–86. doi: 10.1016/j.schres.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Robert G, Hockey J. Compensatory control in the regulation of human performance under stress and high workload: A cognitive-energetical framework. Biological Psychology. 1997;45(1):73–93. doi: 10.1016/s0301-0511(96)05223-4. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78(3):578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. Journal of Affective Disorders. 2002;71(1):265–272. doi: 10.1016/s0165-0327(01)00406-2. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DBD, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress in the wild. Scientific American. 1990;262:116–123. doi: 10.1038/scientificamerican0190-116. [DOI] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Menghetti S, Glaser B, Debbané M, Eliez S. Predominant negative symptoms in 22q11. 2 deletion syndrome and their associations with cognitive functioning and functional outcome. Journal of Psychiatric Research. 2014;48(1):86–93. doi: 10.1016/j.jpsychires.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Scott BG, Weems CF. Resting vagal tone and vagal response to stress: associations with anxiety, aggression, and perceived anxiety control among youths. Psychophysiology. 2014;51(8):718–727. doi: 10.1111/psyp.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6(1):40. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Takarae Y, Harvey DJ, Cabaral MH, Simon TJ. A cross-sectional study of the development of volitional control of spatial attention in children with chromosome 22q11. 2 deletion syndrome. Journal of Neurodevelopmental Disorders. 2012;4(1):1. doi: 10.1186/1866-1955-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Veerapandiyan A, Schoch K, Kwapil T, Keshavan M, Ip E, Hooper S. Social skills and associated psychopathology in children with chromosome 22q11. 2 deletion syndrome: implications for interventions. Journal of Intellectual Disability Research. 2012;56(9):865–878. doi: 10.1111/j.1365-2788.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7(4):422. [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22Q11.2 deletion syndrome. Cortex. 2005;41(2):145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Moss EM, McDonald-McGinn D, Zackai E, Wang PP. Cognitive development in VCFS. Progress in Pediatric Cardiology. 2002;15(2):109–117. [Google Scholar]
- Simon TJ, Wu Z, Avants B, Zhang H, Gee JC, Stebbins GT. Atypical cortical connectivity and visuospatial cognitive impairments are related in children with chromosome 22q11. 2 deletion syndrome. Behavioral and Brain Functions. 2008;4(1):1. doi: 10.1186/1744-9081-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, Blundell M, Karayiorgou M. Neuropsychological characteristics of children with the 22q11 deletion syndrome: A descriptive analysis. Child Neuropsychology. 2005;11(1):39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag P, Gokalsing E, Olivier C, Robert P, Burglen F, Kauffmann-Muller F, Danion J-M. Impaired strategic regulation of contents of conscious awareness in schizophrenia. Consciousness and Cognition. 2003;12(2):190–200. doi: 10.1016/s1053-8100(03)00016-3. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Whitney P. The effect of trait anxiety and situational stress on working memory capacity. Journal of Research in Personality. 1992;26(3):235–241. [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stephenson DD, Beaton EA, Weems CF, Angkustsiri K, Simon TJ. Identifying patterns of anxiety and depression in children with chromosome 22q11. 2 deletion syndrome: Comorbidity predicts behavioral difficulties and impaired functional communications. Behavioural Brain Research. 2015;276:190–198. doi: 10.1016/j.bbr.2014.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. Swanson, nolan, and pelham questionnaire. Irvine, CA: University of California; 1983. [Google Scholar]
- Swillen A, Devriendt K, Ghesquière P, Fryns J-P. Children with a 22q11 deletion versus children with a speech-language impairment and learning disability: behavior during primary school age. Genetic counseling. 2000;12(4):309–317. [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns J-P. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. Journal of Medical Genetics. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, Kameda T. Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuroendocrinology Letters. 2005;26(4):351–354. [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Hyde J, Gilchrist I, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology. 2000;25(6):535–549. doi: 10.1016/s0306-4530(00)00008-1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-WISCIV. Psychological corporation; 2003. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121(3):331. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11. 2 microdeletion. Genetics in Medicine. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Wright CA, Dobson KS, Sears CR. Does a high working memory capacity attenuate the negative impact of trait anxiety on attentional control? Evidence from the antisaccade task. Journal of Cognitive Psychology. 2014;26(4):400–412. [Google Scholar]